The Emerging Role of LPA as an Oncometabolite
Abstract
1. Introduction
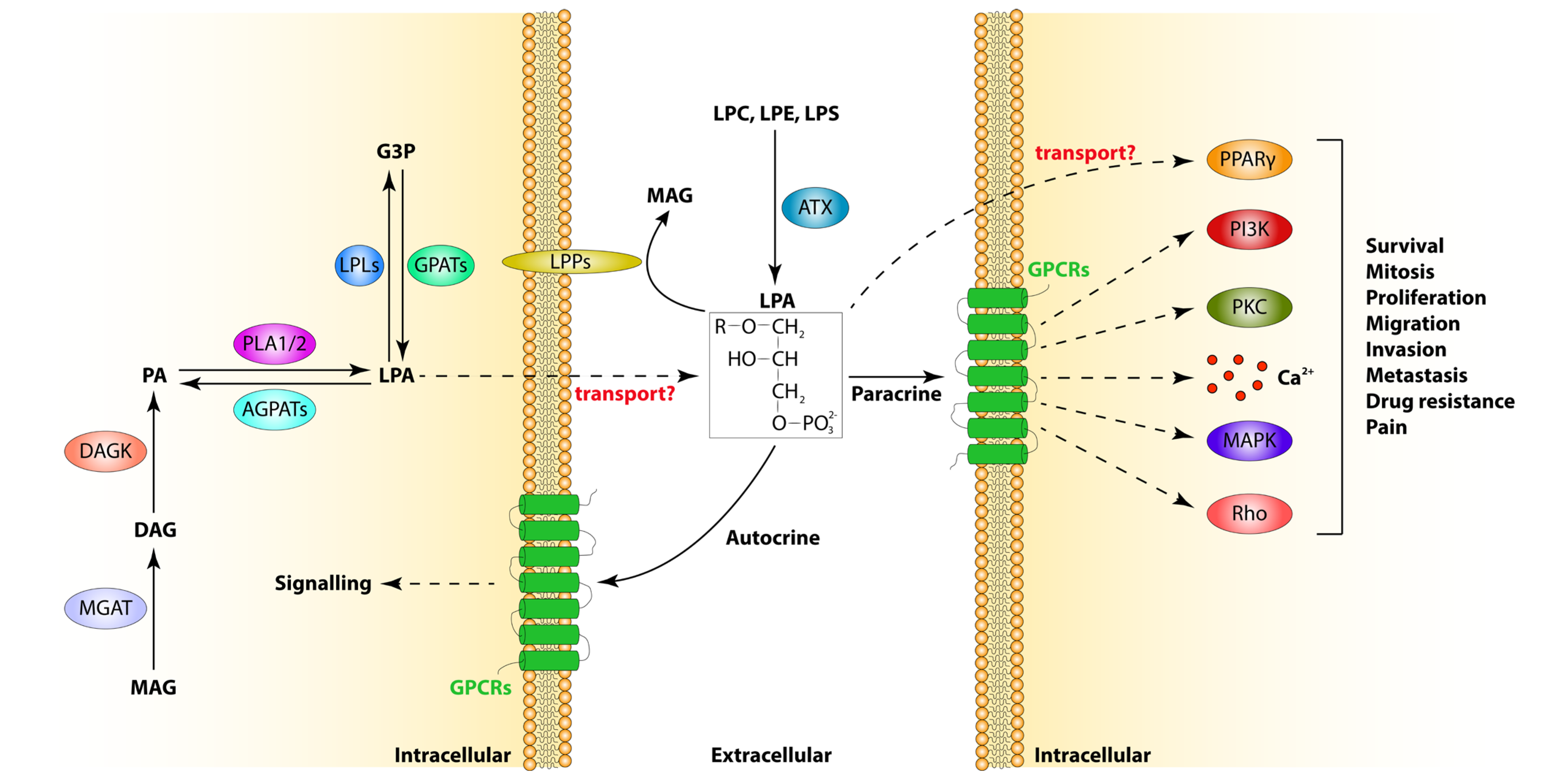
2. LPA Metabolism
2.1. LPA Synthesis
2.2. LPA Catabolism
3. LPA Receptors
4. LPA in Human Physiology
5. LPA in Human Cancers
5.1. LPA in Migration/Invasion/Metastasis
5.1.1. LPA in Gynaecological Malignancies
5.1.2. LPA in Other Tumour Types
5.2. LPA in Proliferation
5.3. LPA in Anti-Tumour Immunity
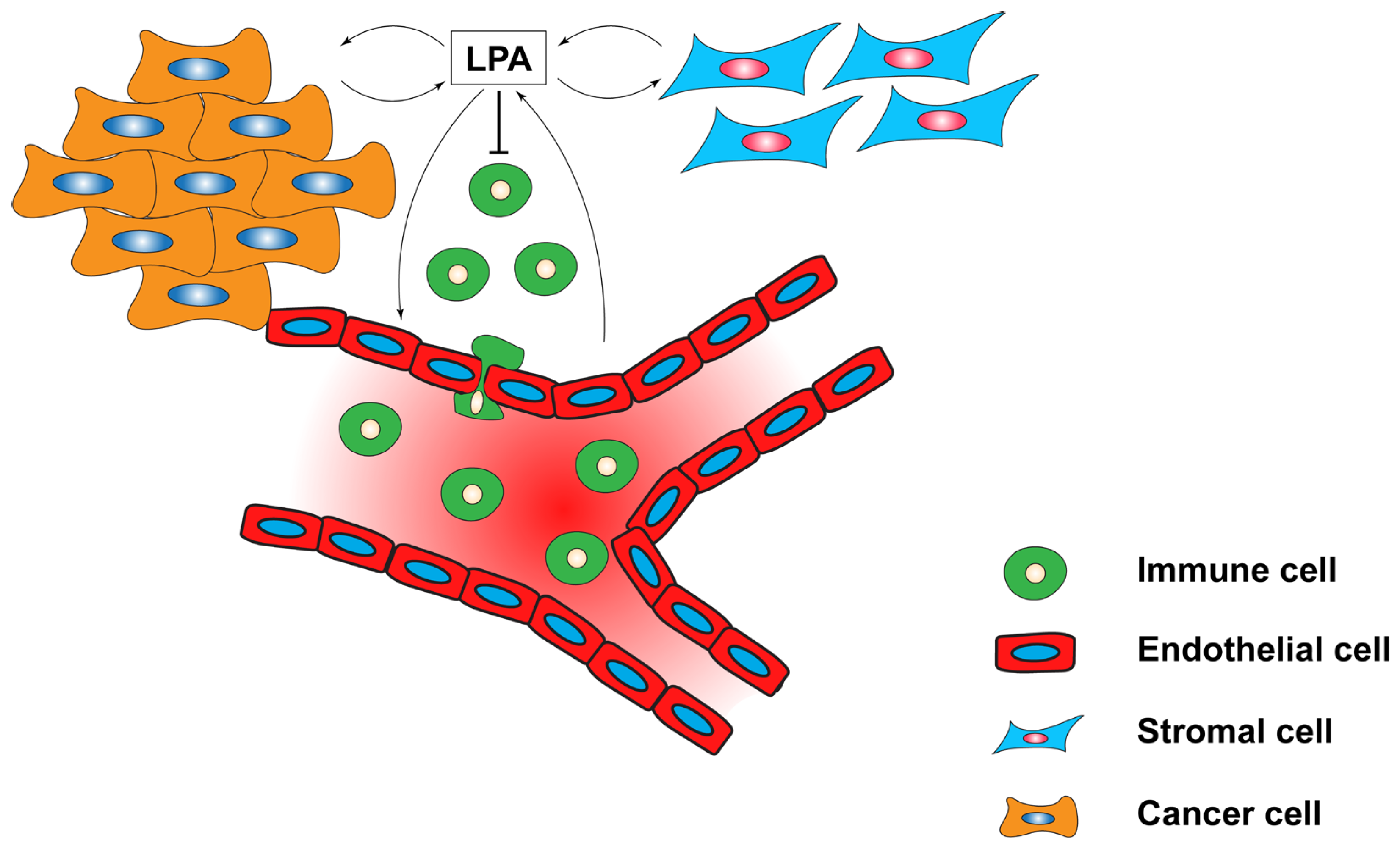
5.4. LPA in the Tumour Microenvironment (TME)
5.5. LPA in Drug and Radiotherapy Resistance
5.6. Other Functions of LPA in Tumours
6. Targeting LPA for Cancer Treatment
| Drug Name | Target | Tumour Type | Effect | Reference |
|---|---|---|---|---|
| IOA-289 | ATX | Pancreatic | Restores gemcitabine and galunisertib sensitivity | [136] |
| IOA-289 | ATX | Breast | Decreases tumour growth | [157] |
| IOA-289 | ATX | Gastrointestinal | Reduces migration and 2D/3D growth | [156] |
| BrP-LPA | ATX | Non-small-cell lung | Restores anti-PD-1 treatment sensitivity | [116] |
| S32826 | ATX | Pancreatic | Induces apoptosis, reduces proliferation and migration | [158] |
| AS2717638 | LPAR5 | Non-small-cell lung | Restores anti-PD-1 treatment sensitivity | [116] |
| Ki-16425 | LPAR1 | Hepatocellular carcinoma | Promotes oncogene-induced senescence | [151] |
| Ki-16425 | LPAR3 | Ovarian | Inhibits proliferation and migration | [79] |
| Ki-16425 | LPARs | T-cell lymphoma | Induces apoptosis, inhibits glycolysis and activates immune system | [161] |
| Ki-16425 | LPARs | Breast | Reduces inflammatory cytokine expression | [84] |
| ONO-7300243 | LPAR1 | Osteosarcoma | Decreases lung metastasis | [100] |
| BMS-986020 | LPAR1 | Oesophageal squamous cell carcinoma | Reduces tumour growth | [103] |
| XAV393 | LPAR2 | Gastric | Inhibits ATP production, glycolysis and oxidative phosphorylation | [152] |
| XAA | LPAR6 | Hepatocellular carcinoma | Increases sensitivity to sorafenib | [143] |
| Resverastrol | - | Ovarian | Reduces growth, migration and platinum resistance | [164] |
| Acacetin | - | Ovarian | Prevents mesothelial cell-initiated malignancy | [163] |
| Berberine | - | Liver | Antagonises ATX/LPA/LPAR2/p38/leptin axis | [97] |
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Chen, G.X. Production of extracellular lysophosphatidic acid in the regulation of adipocyte functions and liver fibrosis. World J. Gastroenterol. 2018, 24, 4132–4151. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, R.; Zhang, Q.X.; Pilquil, C.; Singh, I.; Xu, J.; Dewald, J.; Dillon, D.A.; Berthiaume, L.G.; Carman, G.M.; Waggoner, D.W.; et al. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 1999, 340 Pt 3, 677–686. [Google Scholar] [CrossRef]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Clair, T.; Lee, H.Y.; Liotta, L.A.; Stracke, M.L. Autotaxin is an exoenzyme possessing 5’-nucleotide phosphodiesterase/ATP pyrophosphatase and ATPase activities. J. Biol. Chem. 1997, 272, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Loh, K.; Song, Z.Y.; Yang, H.Q.; Zhang, Y.; Lin, S. Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes 2018, 8, 34. [Google Scholar] [CrossRef]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.L.; Li, D.; Tobin, J.F.; Gimeno, R.E. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19695–19700. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.A.; Vergnes, L.; Dejong, H.; Wang, S.; Lewin, T.M.; Reue, K.; Coleman, R.A. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6−/− mice. J. Lipid Res. 2008, 49, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Kuo, M.S.; Li, S.; Bui, H.H.; Peake, D.A.; Sanders, P.E.; Thibodeaux, S.J.; Chu, S.; Qian, Y.W.; Zhao, Y.; et al. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J. Biol. Chem. 2008, 283, 10048–10057. [Google Scholar] [CrossRef]
- Richmond, G.S.; Smith, T.K. Phospholipases A(1). Int. J. Mol. Sci. 2011, 12, 588–612. [Google Scholar] [CrossRef]
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef]
- Fourcade, O.; Simon, M.F.; Viode, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournie, B.; Sarda, L.; Chap, H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef] [PubMed]
- le Balle, F.; Simon, M.F.; Meijer, S.; Fourcade, O.; Chap, H. Membrane sidedness of biosynthetic pathways involved in the production of lysophosphatidic acid. Adv. Enzyme Regul. 1999, 39, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Uchida, H.; Nagai, J.; Inoue, M.; Aoki, J.; Ueda, H. Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 2010, 333, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kalari, S.K.; Usatyuk, P.V.; Gorshkova, I.; He, D.; Watkins, T.; Brindley, D.N.; Sun, C.; Bittman, R.; Garcia, J.G.; et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: Role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 2007, 282, 14165–14177. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.Z.; Morris, A.J. Role of phosphatidic acid phosphatase 2a in uptake of extracellular lipid phosphate mediators. Biochim. Biophys. Acta 2000, 1487, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Hooks, S.B.; Ragan, S.P.; Lynch, K.R. Identification of a novel human phosphatidic acid phosphatase type 2 isoform. FEBS Lett. 1998, 427, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Sciorra, V.A.; Morris, A.J. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998, 273, 22059–22067. [Google Scholar] [CrossRef]
- Kai, M.; Wada, I.; Imai, S.; Sakane, F.; Kanoh, H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J. Biol. Chem. 1997, 272, 24572–24578. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, A.H.; Berdyshev, E.V.; Skobeleva, A.; Mataya, C.; Natarajan, V.; Brindley, D.N.; Lynch, K.R. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 2009, 419, 611–618. [Google Scholar] [CrossRef]
- Kok, B.P.; Venkatraman, G.; Capatos, D.; Brindley, D.N. Unlike two peas in a pod: Lipid phosphate phosphatases and phosphatidate phosphatases. Chem. Rev. 2012, 112, 5121–5146. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.D. Lysophospholipids in development: Miles apart and edging in. J. Cell Biochem. 2004, 92, 967–992. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N.; Pilquil, C. Lipid phosphate phosphatases and signaling. J. Lipid Res. 2009, 50, S225–S230. [Google Scholar] [CrossRef] [PubMed]
- Subauste, A.R.; Das, A.K.; Li, X.; Elliott, B.G.; Evans, C.; El Azzouny, M.; Treutelaar, M.; Oral, E.; Leff, T.; Burant, C.F. Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations. Diabetes 2012, 61, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Yvone, G.M.; Brown, W.J. Membrane topology of human AGPAT3 (LPAAT3). Biochem. Biophys. Res. Commun. 2010, 397, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.S.; Garg, A.; Agarwal, A.K. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: Localization of AGPAT5 to mitochondria. J. Lipid Res. 2011, 52, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.; Campbell, R.D. Characterization of a human lysophosphatidic acid acyltransferase that is encoded by a gene located in the class III region of the human major histocompatibility complex. J. Biol. Chem. 1998, 273, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dennis, E.A. Mammalian lysophospholipases. Biochim. Biophys. Acta 1999, 1439, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pete, M.J.; Exton, J.H. Purification of a lysophospholipase from bovine brain that selectively deacylates arachidonoyl-substituted lysophosphatidylcholine. J. Biol. Chem. 1996, 271, 18114–18121. [Google Scholar] [CrossRef]
- Leslie, C.C. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J. Biol. Chem. 1991, 266, 11366–11371. [Google Scholar] [CrossRef]
- Reynolds, L.J.; Hughes, L.L.; Louis, A.I.; Kramer, R.M.; Dennis, E.A. Metal ion and salt effects on the phospholipase A2, lysophospholipase, and transacylase activities of human cytosolic phospholipase A2. Biochim. Biophys. Acta 1993, 1167, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, Y.; Kudo, I.; Fujita, K.; Inoue, K. Characteristics of lysophospholipase activity expressed by cytosolic phospholipase A2. Eur. J. Biochem. 1993, 218, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Gross, R.W. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J. Biol. Chem. 1996, 271, 30879–30885. [Google Scholar] [CrossRef] [PubMed]
- Lio, Y.C.; Dennis, E.A. Interfacial activation, lysophospholipase and transacylase activity of group VI Ca2+-independent phospholipase A2. Biochim. Biophys. Acta 1998, 1392, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Oh, Y.S.; Jun, H.S. Lysophosphatidic Acid Signaling in Diabetic Nephropathy. Int. J. Mol. Sci. 2019, 20, 2850. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Contos, J.J.; Fukushima, N.; Chun, J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 2000, 58, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Huang, Y.; Johansson, S. G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling. Int. J. Mol. Sci. 2016, 17, 215. [Google Scholar] [CrossRef]
- Yanagida, K.; Masago, K.; Nakanishi, H.; Kihara, Y.; Hamano, F.; Tajima, Y.; Taguchi, R.; Shimizu, T.; Ishii, S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem. 2009, 284, 17731–17741. [Google Scholar] [CrossRef]
- Noguchi, K.; Ishii, S.; Shimizu, T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 2003, 278, 25600–25606. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Rivera, R.; Dubin, A.E.; Chun, J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 2007, 282, 4310–4317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Kharode, Y.; Bodine, P.V.; Yaworsky, P.J.; Robinson, J.A.; Billiard, J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J. Cell Biochem. 2010, 109, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Polo, F.; Borza, R.; Matsoukas, M.T.; Marsais, F.; Jagerschmidt, C.; Waeckel, L.; Moolenaar, W.H.; Ford, P.; Heckmann, B.; Perrakis, A. Autotaxin facilitates selective LPA receptor signaling. Cell Chem. Biol. 2023, 30, 69–84.e14. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Baba, K.; Shiraishi, A.; Ito, M.; Fujita, N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2007, 363, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Ota, R.; Shima, M.; Yamashita, A.; Sugiura, T. GPR35 is a novel lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2010, 395, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Shiraishi, A.; Tabata, K.; Fujita, N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2008, 371, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Toure, F.; Chitayat, S.; Pei, R.; Song, F.; Li, Q.; Zhang, J.; Rosario, R.; Ramasamy, R.; Chazin, W.J.; et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J. Exp. Med. 2012, 209, 2339–2350. [Google Scholar] [CrossRef]
- Nieto-Posadas, A.; Picazo-Juarez, G.; Llorente, I.; Jara-Oseguera, A.; Morales-Lazaro, S.; Escalante-Alcalde, D.; Islas, L.D.; Rosenbaum, T. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat. Chem. Biol. 2011, 8, 78–85. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136. [Google Scholar] [CrossRef]
- Sheng, X.; Yung, Y.C.; Chen, A.; Chun, J. Lysophosphatidic acid signalling in development. Development 2015, 142, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, S.; Oikonomou, N.; Grigorieva, E.; Nikitopoulou, I.; Paparountas, T.; Thanassopoulou, A.; Zhao, Z.; Xu, Y.; Kontoyiannis, D.L.; Remboutsika, E.; et al. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 2010, 339, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okudaira, S.; Kishi, Y.; Ohkawa, R.; Iseki, S.; Ota, M.; Noji, S.; Yatomi, Y.; Aoki, J.; Arai, H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 2006, 281, 25822–25830. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.; Fukushima, N.; Weiner, J.A.; Kaushal, D.; Chun, J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA 2000, 97, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H.; Noguchi, K.; Kihara, Y.; Abe, M.; Yanagida, K.; Hamano, F.; Sato, S.; Tamaki, K.; Morishita, Y.; Kano, M.R.; et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 2010, 116, 5060–5070. [Google Scholar] [CrossRef]
- Ye, X.; Hama, K.; Contos, J.J.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.B.; Jin, M.; Xu, X.; Song, Y.Q.; Wu, C.P.; Poo, M.M.; Duan, S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003, 5, 38–45. [Google Scholar] [CrossRef]
- Fukushima, N.; Weiner, J.A.; Chun, J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev. Biol. 2000, 228, 6–18. [Google Scholar] [CrossRef]
- Fukushima, N.; Weiner, J.A.; Kaushal, D.; Contos, J.J.; Rehen, S.K.; Kingsbury, M.A.; Kim, K.Y.; Chun, J. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol. Cell Neurosci. 2002, 20, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Fujita, R.; Ma, Y.; Ueda, H. Lysophosphatidic acid-induced membrane ruffling and brain-derived neurotrophic factor gene expression are mediated by ATP release in primary microglia. J. Neurochem. 2008, 107, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.; Contos, J.J.; Musante, D.B.; Chun, J.; Ransom, B.R. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J. Biol. Chem. 2001, 276, 25946–25952. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Repp, H.; Richter, H.; Koschinski, A.; Heinemann, U.; Dreyer, F.; Eder, C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca(2+)-dependent K(+) channels. Neuroscience 2002, 109, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Tham, C.S.; Lin, F.F.; Rao, T.S.; Yu, N.; Webb, M. Microglial activation state and lysophospholipid acid receptor expression. Int. J. Dev. Neurosci. 2003, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.A.; Hecht, J.H.; Chun, J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 1998, 398, 587–598. [Google Scholar] [CrossRef]
- Yuelling, L.W.; Waggener, C.T.; Afshari, F.S.; Lister, J.A.; Fuss, B. Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain. Glia 2012, 60, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.; Amaral, R.F.D.; Fonseca, A.; Garcia, C.; Mendes, F.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, T.E.; Mattsson, C.L.; Wang, Y.; Iakovleva, I.; Petrovic, N.; Nedergaard, J. Non-transactivational, dual pathways for LPA-induced Erk1/2 activation in primary cultures of brown pre-adipocytes. Exp. Cell Res. 2010, 316, 2664–2675. [Google Scholar] [CrossRef]
- Valet, P.; Pages, C.; Jeanneton, O.; Daviaud, D.; Barbe, P.; Record, M.; Saulnier-Blache, J.S.; Lafontan, M. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J. Clin. Investig. 1998, 101, 1431–1438. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.C.; Krummel, M.F.; Rosen, S.D. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 2012, 189, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kong, Y.; Voice, J.K. Cutting edge: Differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J. Immunol. 2000, 164, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Voice, J.K.; Kong, Y.; Goetzl, E.J. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J. 2000, 14, 2387–2389. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, D.M.; Tanabe, N.; Pereverzev, A.; Spencer, M.; Shugg, R.P.; Dixon, S.J.; Sims, S.M. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J. Biol. Chem. 2010, 285, 25792–25801. [Google Scholar] [CrossRef]
- Davoudian, K.; Bhattacharya, S.; Thompson, D.; Thompson, M. Coupled Electrostatic and Hydrophobic Destabilisation of the Gelsolin-Actin Complex Enables Facile Detection of Ovarian Cancer Biomarker Lysophosphatidic Acid. Biomolecules 2023, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Lotay, N.; Thompson, M. Affinity-based electrochemical biosensor with antifouling properties for detection of lysophosphatidic acid, a promising early-stage ovarian cancer biomarker. Bioelectrochemistry 2023, 153, 108466. [Google Scholar] [CrossRef] [PubMed]
- Ojasalu, K.; Lieber, S.; Sokol, A.M.; Nist, A.; Stiewe, T.; Bullwinkel, I.; Finkernagel, F.; Reinartz, S.; Muller-Brusselbach, S.; Grosse, R.; et al. The lysophosphatidic acid-regulated signal transduction network in ovarian cancer cells and its role in actomyosin dynamics, cell migration and entosis. Theranostics 2023, 13, 1921–1948. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Suh, P.G. O-GlcNAcylation regulates lysophosphatidic acid-induced cell migration by regulating ERM family proteins. FEBS Open Bio 2022, 12, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yun, Q.; Li, A.; Li, R.; Yan, Y.; Wang, Y.; Sun, H.; Damirin, A. LPA3 is a precise therapeutic target and potential biomarker for ovarian cancer. Med. Oncol. 2022, 39, 17. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Cho, K.H.; Yoon, S.H.; Park, C.G.; Park, H.W.; Lee, H.Y. Discoidin Domain Receptor 2 Mediates Lysophosphatidic Acid-Induced Ovarian Cancer Aggressiveness. Int. J. Mol. Sci. 2021, 22, 5374. [Google Scholar] [CrossRef]
- Wu, D.; Wu, F.; Li, B.; Huang, W.; Wang, D. EZH2 promotes the expression of LPA1 by mediating microRNA-139 promoter methylation to accelerate the development of ovarian cancer. Cancer Cell Int. 2020, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Dai, X.; Fang, W.; Zheng, Y.; Zhang, J.; Liu, Y.; Gu, D. Overexpression of microRNA-367 inhibits angiogenesis in ovarian cancer by downregulating the expression of LPA1. Cancer Cell Int. 2020, 20, 476. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, Y.; Bos, B.; Campbell, L.; Loughran, E.; Liu, Y.; Yang, J.; Kim, O.; Stack, M.S. Lysophosphatidic acid modulates ovarian cancer multicellular aggregate assembly and metastatic dissemination. Sci. Rep. 2020, 10, 10877. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.; Kadam, S.; Heinz, K.; Garcia Peraza, M.; Schmid, R.; Kremer, A.E.; Wolf, K.; Bauer, A.; Horch, R.E.; Arkudas, A.; et al. Influence of the autotaxin-lysophosphatidic acid axis on cellular function and cytokine expression in different breast cancer cell lines. Sci. Rep. 2022, 12, 5565. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Lee, J.W.; Seen, D.S.; Jeong, J.Y.; Huh, W.K. LPA1-mediated inhibition of CXCR4 attenuates CXCL12-induced signaling and cell migration. Cell Commun. Signal 2023, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Drosouni, A.; Fanidis, D.; Karaglani, M.; Balgkouranidou, I.; Xenidis, N.; Aidinis, V.; Chatzaki, E. ENPP2 Promoter Methylation Correlates with Decreased Gene Expression in Breast Cancer: Implementation as a Liquid Biopsy Biomarker. Int. J. Mol. Sci. 2022, 23, 3717. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Fanidis, D.; Aidinis, V.; Chatzaki, E. ENPP2 Methylation in Health and Cancer. Int. J. Mol. Sci. 2021, 22, 11958. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Wu, R.; Tang, X.; Brindley, D.N.; Ishikawa, T.; Takabe, K. Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression. Int. J. Mol. Sci. 2023, 24, 9812. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Guo, S.; Li, K.; Tian, J.; Zong, B.; Ai, T.; Peng, Y.; Zhang, Y.; Liu, S. Lysophosphatidic acid receptor 6 regulated by miR-27a-3p attenuates tumor proliferation in breast cancer. Clin. Transl. Oncol. 2022, 24, 503–516. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Gangoiti, P.; Camacho, L.; Presa, N.; Martin, C.; Gomez-Munoz, A. Phosphatidic Acid Stimulates Lung Cancer Cell Migration through Interaction with the LPA1 Receptor and Subsequent Activation of MAP Kinases and STAT3. Biomedicines 2023, 11, 1804. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, C.; Ning, Z.; Hua, Y.; Li, Y.; Chen, L.; Liu, L.; Chen, Z.; Meng, Z. CMTM8 as an LPA1-associated partner mediates lysophosphatidic acid-induced pancreatic cancer metastasis. Ann. Transl. Med. 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Ghil, S. Crosstalk between cannabinoid receptor 2 and lysophosphatidic acid receptor 5. Biochem. Biophys. Res. Commun. 2023, 666, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Long, Y.; Wang, Y.; Yin, N.; Zhang, X.; Zhang, J. Hypoxia Increases ATX Expression by Histone Crotonylation in a HIF-2alpha-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 7031. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Wang, W.; Miller, M.R.; Boucher, M.; Reynold, J.E.; Daurio, N.A.; Li, D.; Hirenallur-Shanthappa, D.; Ahn, Y.; Beebe, D.A.; et al. GPAT1 Deficiency in Mice Modulates NASH Progression in a Model-Dependent Manner. Cell Mol. Gastroenterol. Hepatol. 2023, 17, 279–291. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sugiyama, A.; Snyder, N.W.; Teneche, M.G.; Liu, X.; Maner-Smith, K.M.; Goessling, W.; Hagen, S.J.; Ortlund, E.A.; Najafi-Shoushtari, S.H.; et al. Acyl-CoA thioesterase 12 suppresses YAP-mediated hepatocarcinogenesis by limiting glycerolipid biosynthesis. Cancer Lett. 2023, 565, 216210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, T.; Zhao, Z.; Zhang, H.; Yuan, P.; Wang, G.; Zhao, Z.; An, J.; Lyu, Z.; Xing, J.; et al. Down-regulation of BMAL1 by MiR-494-3p Promotes Hepatocellular Carcinoma Growth and Metastasis by Increasing GPAM-mediated Lipid Biosynthesis. Int. J. Biol. Sci. 2022, 18, 6129–6144. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Guo, J.H.; Feng, C.L.; Ding, Y.W.; Dong, B.; Han, Y.X.; Li, Y.H.; Wang, L.L.; Jiang, J.D. Berberine inhibits carcinogenesis through antagonizing the ATX-LPA-LPAR2-p38-leptin axis in a mouse hepatoma model. Mol. Ther. Oncolytics 2022, 26, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, Z.; Li, Z.; Bai, S.; Damirin, A. LPAR2-mediated action promotes human renal cell carcinoma via MAPK/NF-kappaB signaling to regulate cytokine network. J. Cancer Res. Clin. Oncol. 2023, 149, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, M.A.; Lee, S.C.; Shin, Y.; Norman, D.D.; Lin, K.H.; E, S.; Yue, J.; Benyo, Z.; Tigyi, G.J. Prometastatic Effect of ATX Derived from Alveolar Type II Pneumocytes and B16-F10 Melanoma Cells. Cancers 2022, 14, 1586. [Google Scholar] [CrossRef]
- Takagi, S.; Sasaki, Y.; Koike, S.; Takemoto, A.; Seto, Y.; Haraguchi, M.; Ukaji, T.; Kawaguchi, T.; Sugawara, M.; Saito, M.; et al. Platelet-derived lysophosphatidic acid mediated LPAR1 activation as a therapeutic target for osteosarcoma metastasis. Oncogene 2021, 40, 5548–5558. [Google Scholar] [CrossRef]
- Tong, C.; Wang, C.; Wang, Y.; Xiao, X. TNRC6C-AS1 Promotes Thyroid Cancer Progression by Upregulating LPAR5 via miR-513c-5p. Cancer Manag. Res. 2021, 13, 6141–6155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Zhu, L.L.; Yang, W.Q.; Xu, S.J.; Chen, J.; Ding, X.F.; Liang, Y.; Chen, G. LPAR5 promotes thyroid carcinoma cell proliferation and migration by activating class IA PI3K catalytic subunit p110beta. Cancer Sci. 2021, 112, 1624–1632. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, H.; Min, L.; Ning, T.; Xu, J.; Wang, T.; Wang, X.; Zhang, Q.; Cao, R.; Zhang, S.; et al. Lysophosphatidic acid mediated PI3K/Akt activation contributed to esophageal squamous cell cancer progression. Carcinogenesis 2021, 42, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahman, M.; Tan, M.L.; Johnson, S.P.; Hollows, R.J.; Chai, W.L.; Mansell, J.P.; Yap, L.F.; Paterson, I.C. Deregulation of lysophosphatidic acid metabolism in oral cancer promotes cell migration via the up-regulation of COX-2. PeerJ 2020, 8, e10328. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Zhang, J.; Zhang, X. NSun2 promotes cell migration through methylating autotaxin mRNA. J. Biol. Chem. 2020, 295, 18134–18147. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.F.D.; Geraldo, L.H.M.; Einicker-Lamas, M.; Spohr, T.C.L.d.S.e.; Mendes, F.; Lima, F.R.S. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA(1) receptor. J. Neurochem. 2021, 156, 499–512. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, P.; Nanding, K.; Wu, M.; Bai, X.; Morigen, M.; Fan, L. Lysophosphatidic acid suppresses apoptosis of high-grade serous ovarian cancer cells by inducing autophagy activity and promotes cell-cycle progression via EGFR-PI3K/Aurora-A(Thr288)-geminin dual signaling pathways. Front. Pharmacol. 2022, 13, 1046269. [Google Scholar] [CrossRef]
- Zhao, H.; Gezi, G.; Tian, X.; Jia, P.; Morigen, M.; Fan, L. Lysophosphatidic Acid-Induced EGFR Transactivation Promotes Gastric Cancer Cell DNA Replication by Stabilizing Geminin in the S Phase. Front. Pharmacol. 2021, 12, 706240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Chen, Q.K.; Xu, J.F. LPAR5 stimulates the malignant progression of non-small-cell lung carcinoma by upregulating MLLT11. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8902–8910. [Google Scholar]
- Chen, J.; Li, H.; Xu, W.; Guo, X. Evaluation of serum ATX and LPA as potential diagnostic biomarkers in patients with pancreatic cancer. BMC Gastroenterol. 2021, 21, 58. [Google Scholar] [CrossRef]
- Han, L.; Jiang, Y.; Shi, M.; Gan, L.; Wu, Z.; Xue, M.; Zhu, Y.; Xiong, C.; Wang, T.; Lin, X.; et al. LIPH contributes to glycolytic phenotype in pancreatic ductal adenocarcinoma by activating LPA/LPAR axis and maintaining ALDOA stability. J. Transl. Med. 2023, 21, 838. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, M.; Cao, G.; Liu, H.; Li, Y.; Wang, S.; Zijtveld, S.; Delvoux, B.; Xanthoulea, S.; Romano, A.; et al. Human monocytes differentiate into tumor-associated macrophages upon SKOV3 cells coculture and/or lysophosphatidic acid stimulation. J. Inflamm. 2022, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, M.; Zhang, Z.; Cui, R.; Jiang, X.; Wang, S.; Bai, H.; Liu, C.; Zhang, Z. Potential interaction between lysophosphatidic acid and tumor-associated macrophages in ovarian carcinoma. J. Inflamm. 2020, 17, 23. [Google Scholar] [CrossRef]
- Chae, C.S.; Sandoval, T.A.; Hwang, S.M.; Park, E.S.; Giovanelli, P.; Awasthi, D.; Salvagno, C.; Emmanuelli, A.; Tan, C.; Chaudhary, V.; et al. Tumor-Derived Lysophosphatidic Acid Blunts Protective Type I Interferon Responses in Ovarian Cancer. Cancer Discov. 2022, 12, 1904–1921. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Mukherjee, A.; He, T.; Wang, X.Y.; Ma, Y.; Fang, X. Lysophosphatidic acid induces tumor necrosis factor-alpha to regulate a pro-inflammatory cytokine network in ovarian cancer. FASEB J. 2020, 34, 13935–13948. [Google Scholar] [CrossRef] [PubMed]
- Konen, J.M.; Rodriguez, B.L.; Wu, H.; Fradette, J.J.; Gibson, L.; Diao, L.; Wang, J.; Schmidt, S.; Wistuba, I.I.; Zhang, J.; et al. Autotaxin suppresses cytotoxic T cells via LPAR5 to promote anti-PD-1 resistance in non-small cell lung cancer. J. Clin. Investig. 2023, 133, e163128. [Google Scholar] [CrossRef]
- Turner, J.A.; Fredrickson, M.A.; D’Antonio, M.; Katsnelson, E.; MacBeth, M.; Van Gulick, R.; Chimed, T.S.; McCarter, M.; D’Alessandro, A.; Robinson, W.A.; et al. Lysophosphatidic acid modulates CD8 T cell immunosurveillance and metabolism to impair anti-tumor immunity. Nat. Commun. 2023, 14, 3214. [Google Scholar] [CrossRef]
- Torres, R.M.; Turner, J.A.; D’Antonio, M.; Pelanda, R.; Kremer, K.N. Regulation of CD8 T-cell signaling, metabolism, and cytotoxic activity by extracellular lysophosphatidic acid. Immunol. Rev. 2023, 317, 203–222. [Google Scholar] [CrossRef]
- Kremer, K.N.; Buser, A.; Thumkeo, D.; Narumiya, S.; Jacobelli, J.; Pelanda, R.; Torres, R.M. LPA suppresses T cell function by altering the cytoskeleton and disrupting immune synapse formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118816119. [Google Scholar] [CrossRef]
- He, J.; Gao, R.; Meng, M.; Yu, M.; Liu, C.; Li, J.; Song, Y.; Wang, H. Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma. Int. J. Environ. Res. Public Health 2021, 18, 11038. [Google Scholar] [CrossRef]
- He, J.; Meng, M.; Wang, H. A Novel Prognostic Biomarker LPAR6 in Hepatocellular Carcinoma via Associating with Immune Infiltrates. J. Clin. Transl. Hepatol. 2022, 10, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; Frijlink, E.; van der Haar Avila, I.; Menegakis, A.; van Zon, M.; Morris, A.J.; Koster, J.; Salgado-Polo, F.; de Kivit, S.; Lanca, T.; et al. Autotaxin impedes anti-tumor immunity by suppressing chemotaxis and tumor infiltration of CD8(+) T cells. Cell Rep. 2021, 37, 110013. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, R.X.; Li, J.Z.; Luo, Z.X. LPAR2 correlated with different prognosis and immune cell infiltration in head and neck squamous cell carcinoma and kidney renal clear cell carcinoma. Hereditas 2022, 159, 16. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.; Wu, R.; Tang, X.; Brindley, D.N.; Ishikawa, T.; Takabe, K. Autotaxin production in the human breast cancer tumor microenvironment mitigates tumor progression in early breast cancers. Am. J. Cancer Res. 2023, 13, 2790–2813. [Google Scholar]
- Jeong, B.Y.; Cho, K.H.; Jeong, K.J.; Cho, S.J.; Won, M.; Kim, S.H.; Cho, N.H.; Hur, G.M.; Yoon, S.H.; Park, H.W.; et al. Lysophosphatidic acid-induced amphiregulin secretion by cancer-associated fibroblasts augments cancer cell invasion. Cancer Lett. 2022, 551, 215946. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Wu, R.; Tang, X.; Brindley, D.N.; Ishikawa, T.; Takabe, K. Decreased Lipid Phosphate Phosphatase 1/3 and Increased Lipid Phosphate Phosphatase 2 Expression in the Human Breast Cancer Tumor Microenvironment Promotes Tumor Progression and Immune System Evasion. Cancers 2023, 15, 2299. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G.; Lin, K.H.; Jang, I.H.; Lee, S.C. Revisiting the role of lysophosphatidic acid in stem cell biology. Exp. Biol. Med. 2021, 246, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, Y.H.; Kim, J.H. The Role of Lysophosphatidic Acid in Adult Stem Cells. Int. J. Stem Cells 2020, 13, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Yamada, S.; Yanagida, S.; Ono, A.; Yasuhiko, Y.; Nishida, M.; Kanda, Y. Lysophosphatidic Acid Promotes the Expansion of Cancer Stem Cells via TRPC3 Channels in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 1967. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Y.; Hao, J.; Guenter, R.; Lathia, J.; Beck, A.W.; Hattaway, R.; Hurst, D.; Wang, Q.J.; Liu, Y.; et al. Development of an arteriolar niche and self-renewal of breast cancer stem cells by lysophosphatidic acid/protein kinase D signaling. Commun. Biol. 2021, 4, 780. [Google Scholar] [CrossRef]
- Ray, R.; Jangde, N.; Singh, S.K.; Sinha, S.; Rai, V. Lysophosphatidic acid-RAGE axis promotes lung and mammary oncogenesis via protein kinase B and regulating tumor microenvironment. Cell Commun. Signal 2020, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Ueda, N.; Ishimoto, K.; Kurisu, R.; Takamoto, M.; Ikeda, H.; Tsujiuchi, T. Roles of endothelial cells in the regulation of cell motility via lysophosphatidic acid receptor-2 (LPA(2)) and LPA(3) in osteosarcoma cells. Exp. Mol. Pathol. 2021, 118, 104596. [Google Scholar] [CrossRef]
- Takai, M.; Takamoto, M.; Amano, Y.; Yamamoto, M.; Hara, K.; Yashiro, N.; Tsujiuchi, T. Induction of lysophosphatidic acid (LPA) receptor-mediated signaling regulates cell motility and survival to anticancer drugs in cancer cells treated with hydrogen peroxide. Adv. Biol. Regul. 2023, 89, 100978. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Minami, A.; Minami, K.; Ueda, N.; Tsujiuchi, T. Different effects of lysophosphatidic acid receptor-2 (LPA(2)) and LPA(5) on the regulation of chemoresistance in colon cancer cells. J. Recept. Signal Transduct. Res. 2021, 41, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Y.; Qu, M.; Zhang, D.; Zhang, X.; Zhang, J. Combined inhibition of EZH2 and the autotaxin-LPA-LPA2 axis exerts synergistic antitumor effects on colon cancer cells. Cancer Lett. 2023, 566, 216226. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Sabbadini, F.; Bertolini, M.; Mangiameli, D.; De Vita, V.; Fazzini, F.; Lunardi, G.; Casalino, S.; Scarlato, E.; Merz, V.; et al. Autotaxin secretion is a stromal mechanism of adaptive resistance to TGFbeta inhibition in pancreatic ductal adenocarcinoma. Cancer Res. 2023, 84, 118–132. [Google Scholar] [CrossRef]
- Okuda, A.; Takai, M.; Kurisu, R.; Takamoto, M.; Ikeda, H.; Tsujiuchi, T. Roles of lysophosphatidic acid (LPA) receptor-2 (LPA(2)) in the regulation of cellular responses induced by X-ray irradiation and hydrogen peroxide in pancreatic cancer cells. Int. J. Radiat. Biol. 2023, 99, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Okuda, A.; Amano, Y.; Yashiro, N.; Hara, K.; Yamamoto, M.; Tsujiuchi, T. Effects of LPA receptor-mediated signaling on the modulation of cellular functions of pancreatic cancer cells cultured in fibroblast supernatants under hypoxic conditions. J. Bioenerg. Biomembr. 2023, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Takauchi, M.; Kuribayashi, M.; Tsujiuchi, T. LPA receptor-mediated signaling regulates cell motility and survival to anticancer drug of pancreatic cancer cells under glucose-deprived and hypoxic conditions. Biochem. Biophys. Res. Commun. 2023, 661, 21–27. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, D.; Yang, S.; Zhang, X.; Wang, J.; Liu, Y.; Sun, Y.; Lu, Y.; Yang, K. LPAR1, Correlated With Immune Infiltrates, Is a Potential Prognostic Biomarker in Prostate Cancer. Front. Oncol. 2020, 10, 846. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Luo, L.P.; Deng, K.C. Circular RNA LPAR3 targets JPT1 via microRNA-513b-5p to facilitate glycolytic activation but repress prostate cancer radiosensitivity. Acta Biochim. Pol. 2023, 70, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, Z.; Wu, Y.; Zhan, Q.; Huang, H.; Fan, J. Circular RNA lysophosphatidic acid receptor 3 (circ-LPAR3) enhances the cisplatin resistance of ovarian cancer. Bioengineered 2022, 13, 3739–3750. [Google Scholar] [CrossRef]
- Gnocchi, D.; Kurzyk, A.; Mintrone, A.; Lentini, G.; Sabba, C.; Mazzocca, A. Inhibition of LPAR6 overcomes sorafenib resistance by switching glycolysis into oxidative phosphorylation in hepatocellular carcinoma. Biochimie 2022, 202, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Ueda, N.; Ishimoto, K.; Kurisu, R.; Takamoto, M.; Ikeda, H.; Tsujiuchi, T. Cooperation of G12/13 and Gi proteins via lysophosphatidic acid receptor-2 (LPA(2)) signaling enhances cancer cell survival to cisplatin. Biochem. Biophys. Res. Commun. 2020, 532, 427–432. [Google Scholar] [CrossRef]
- Chhabra, R.; Nanjundan, M. Lysophosphatidic acid reverses Temsirolimus-induced changes in lipid droplets and mitochondrial networks in renal cancer cells. PLoS ONE 2020, 15, e0233887. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Mou, X.; Xu, Y.; Han, W.; Huang, A.; Li, Y.; Jiang, H.; Yang, X.; Hu, Z. Lysophosphatidic acid protects cervical cancer HeLa cells from apoptosis induced by doxorubicin hydrochloride. Oncol. Lett. 2022, 24, 267. [Google Scholar] [CrossRef]
- Sun, X.; Bai, C.; Li, H.; Xie, D.; Chen, S.; Han, Y.; Luo, J.; Li, Y.; Ye, Y.; Jia, J.; et al. PARP1 modulates METTL3 promoter chromatin accessibility and associated LPAR5 RNA m(6)A methylation to control cancer cell radiosensitivity. Mol. Ther. 2023, 31, 2633–2650. [Google Scholar] [CrossRef]
- Wu, C.; Weis, S.M.; Cheresh, D.A. Upregulation of fibronectin and its integrin receptors—An adaptation to isolation stress that facilitates tumor initiation. J. Cell Sci. 2023, 136, jcs261483. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rakhshandehroo, T.; Wettersten, H.I.; Campos, A.; von Schalscha, T.; Jain, S.; Yu, Z.; Tan, J.; Mose, E.; Childers, B.G.; et al. Pancreatic cancer cells upregulate LPAR4 in response to isolation stress to promote an ECM-enriched niche and support tumour initiation. Nat. Cell Biol. 2023, 25, 309–322. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Khasabov, S.G.; Johns, M.; Juliette, J.; Zheng, A.; Morgan, H.; Flippen, A.; Allen, K.; Golovko, M.Y.; Golovko, S.A.; et al. Exosome-associated lysophosphatidic acid signaling contributes to cancer pain. Pain 2023, 164, 2684–2695. [Google Scholar] [CrossRef]
- Konopa, A.; Meier, M.A.; Franz, M.J.; Bernardinelli, E.; Voegele, A.L.; Atreya, R.; Ribback, S.; Roessler, S.; Aigner, A.; Singer, K.; et al. LPA receptor 1 (LPAR1) is a novel interaction partner of Filamin A that promotes Filamin A phosphorylation, MRTF-A transcriptional activity and oncogene-induced senescence. Oncogenesis 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ara, H.; Subedi, U.; Sharma, P.; Bhattarai, S.; Sharma, S.; Manikandan, S.; Yu, X.; Bhuiyan, M.S.; Sun, H.; Miriyala, S.; et al. Alteration of Cellular Energy Metabolism through LPAR2-Axin2 Axis in Gastric Cancer. Biomolecules 2022, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Guo, X.Z. KAI1/CD82 gene and autotaxin-lysophosphatidic acid axis in gastrointestinal cancers. World J. Gastrointest. Oncol. 2022, 14, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, H.G.; Lee, M. Paradoxical downregulation of LPAR3 exerts tumor-promoting activity through autophagy induction in Ras-transformed cells. BMC Cancer 2022, 22, 969. [Google Scholar] [CrossRef] [PubMed]
- Deken, M.A.; Niewola-Staszkowska, K.; Peyruchaud, O.; Mikulcic, N.; Antolic, M.; Shah, P.; Cheasty, A.; Tagliavini, A.; Nizzardo, A.; Pergher, M.; et al. Characterization and translational development of IOA-289, a novel autotaxin inhibitor for the treatment of solid tumors. Immunooncol. Technol. 2023, 18, 100384. [Google Scholar] [CrossRef] [PubMed]
- Centonze, M.; Di Conza, G.; Lahn, M.; Fabregat, I.; Dituri, F.; Gigante, I.; Serino, G.; Scialpi, R.; Carrieri, L.; Negro, R.; et al. Autotaxin inhibitor IOA-289 reduces gastrointestinal cancer progression in preclinical models. J. Exp. Clin. Cancer Res. 2023, 42, 197. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Morris, A.J.; Deken, M.A.; Brindley, D.N. Autotaxin Inhibition with IOA-289 Decreases Breast Tumor Growth in Mice Whereas Knockout of Autotaxin in Adipocytes Does Not. Cancers 2023, 15, 2937. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, L.; Yang, J.; Shi, Z. ATX/LPA axis regulates FAK activation, cell proliferation, apoptosis, and motility in human pancreatic cancer cells. In Vitro Cell Dev. Biol. Anim. 2022, 58, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, Y.; Ohhata, A.; Nakatani, S.; Hisaichi, K.; Okabe, Y.; Hiramatsu, A.; Watanabe, T.; Yamamoto, S.; Nishiyama, T.; Kobayashi, J.; et al. ONO-8430506: A Novel Autotaxin Inhibitor That Enhances the Antitumor Effect of Paclitaxel in a Breast Cancer Model. ACS Med. Chem. Lett. 2020, 11, 1335–1341. [Google Scholar] [CrossRef]
- Jinno, N.; Yoshida, M.; Hayashi, K.; Naitoh, I.; Hori, Y.; Natsume, M.; Kato, A.; Kachi, K.; Asano, G.; Atsuta, N.; et al. Autotaxin in ascites promotes peritoneal dissemination in pancreatic cancer. Cancer Sci. 2021, 112, 668–678. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, A. Targeting lysophosphatidic acid receptor with Ki16425 impedes T cell lymphoma progression through apoptosis induction, glycolysis inhibition, and activation of antitumor immune response. Apoptosis 2022, 27, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Abdelmessih, R.G.; Xu, J.; Hung, F.R.; Auguste, D.T. Integration of an LPAR1 Antagonist into Liposomes Enhances Their Internalization and Tumor Accumulation in an Animal Model of Human Metastatic Breast Cancer. Mol. Pharm. 2023, 20, 5500–5514. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Tang, Y.; Huang, T.; Liu, Y.; Pan, Y. Amelioration of human peritoneal mesothelial cell co-culture-evoked malignant potential of ovarian cancer cells by acacetin involves LPA release-activated RAGE-PI3K/AKT signaling. Cell Mol. Biol. Lett. 2021, 26, 51. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213. [Google Scholar] [CrossRef] [PubMed]

| Tumour Type | Process |
|---|---|
| Ovarian | Promotes proliferation, migration, invasion, metastasis, tumour progression, suppresses immune system activation |
| Breast | Promotes inflammation, cancer stemness, angiogenesis, tumour progression |
| Lung | Promotes immunotherapy resistance, migration, proliferation, tumour growth, inhibits anti-tumour immunity |
| Colon | Promotes drug resistance, migration, proliferation |
| Pancreatic | Promotes proliferation, migration, invasion, tumour growth, inflammatory CAF phenotype, drug resistance |
| Hepatocellular | Promotes tumour initiation, growth, metastasis, chemotherapy resistance, protects against oncogene-induced senescence |
| Fibrosarcoma | Promotes drug resistance, pain |
| Prostate | Promotes resistance to radiotherapy, decreases anti-tumour immunity |
| Gastric | Reprograms cellular metabolism |
| Renal | Promotes tumour growth, metastasis, drug resistance |
| Cervical | Promotes drug and irradiation resistance |
| Melanoma | Promotes lung metastasis, inhibits anti-tumour immunity |
| Osteosarcoma | Induces lung metastasis, migration |
| Thyroid | Promotes proliferation, migration |
| Oesophageal | Promotes proliferation, migration |
| Oral | Promotes migration |
| Glioma | Promotes migration, tumour growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalis, T.; Poulogiannis, G. The Emerging Role of LPA as an Oncometabolite. Cells 2024, 13, 629. https://doi.org/10.3390/cells13070629
Karalis T, Poulogiannis G. The Emerging Role of LPA as an Oncometabolite. Cells. 2024; 13(7):629. https://doi.org/10.3390/cells13070629
Chicago/Turabian StyleKaralis, Theodoros, and George Poulogiannis. 2024. "The Emerging Role of LPA as an Oncometabolite" Cells 13, no. 7: 629. https://doi.org/10.3390/cells13070629
APA StyleKaralis, T., & Poulogiannis, G. (2024). The Emerging Role of LPA as an Oncometabolite. Cells, 13(7), 629. https://doi.org/10.3390/cells13070629






