The Influence of Myeloid-Derived Suppressor Cell Expansion in Neuroinflammation and Neurodegenerative Diseases
Abstract
:1. Introduction
1.1. Phenotype of MDSCs
1.2. The Dual Role of MDSCs
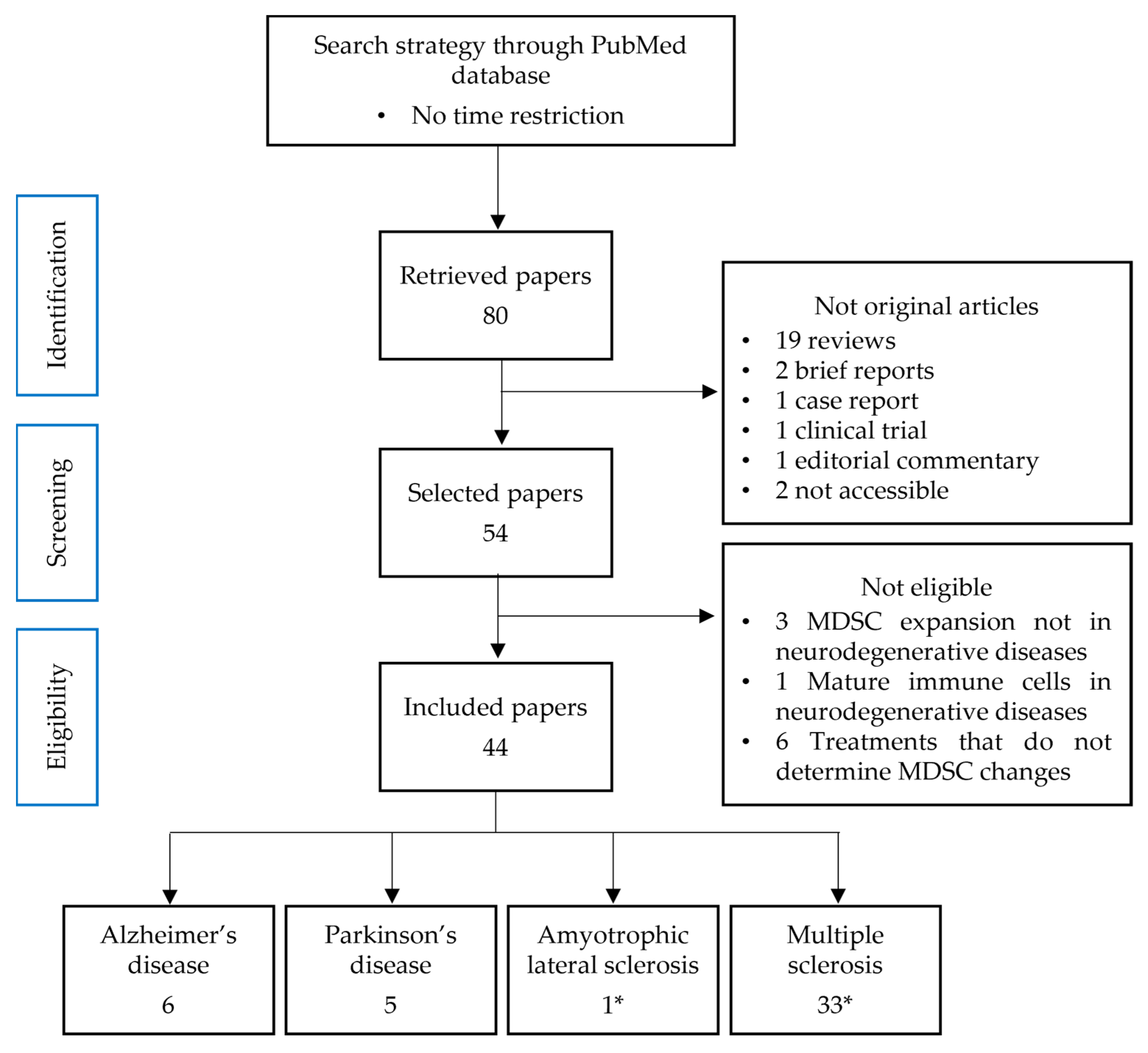
2. Materials and Methods
2.1. Source and Search Strategy
2.2. Data Collection and Sorting
2.3. Study Selection Process
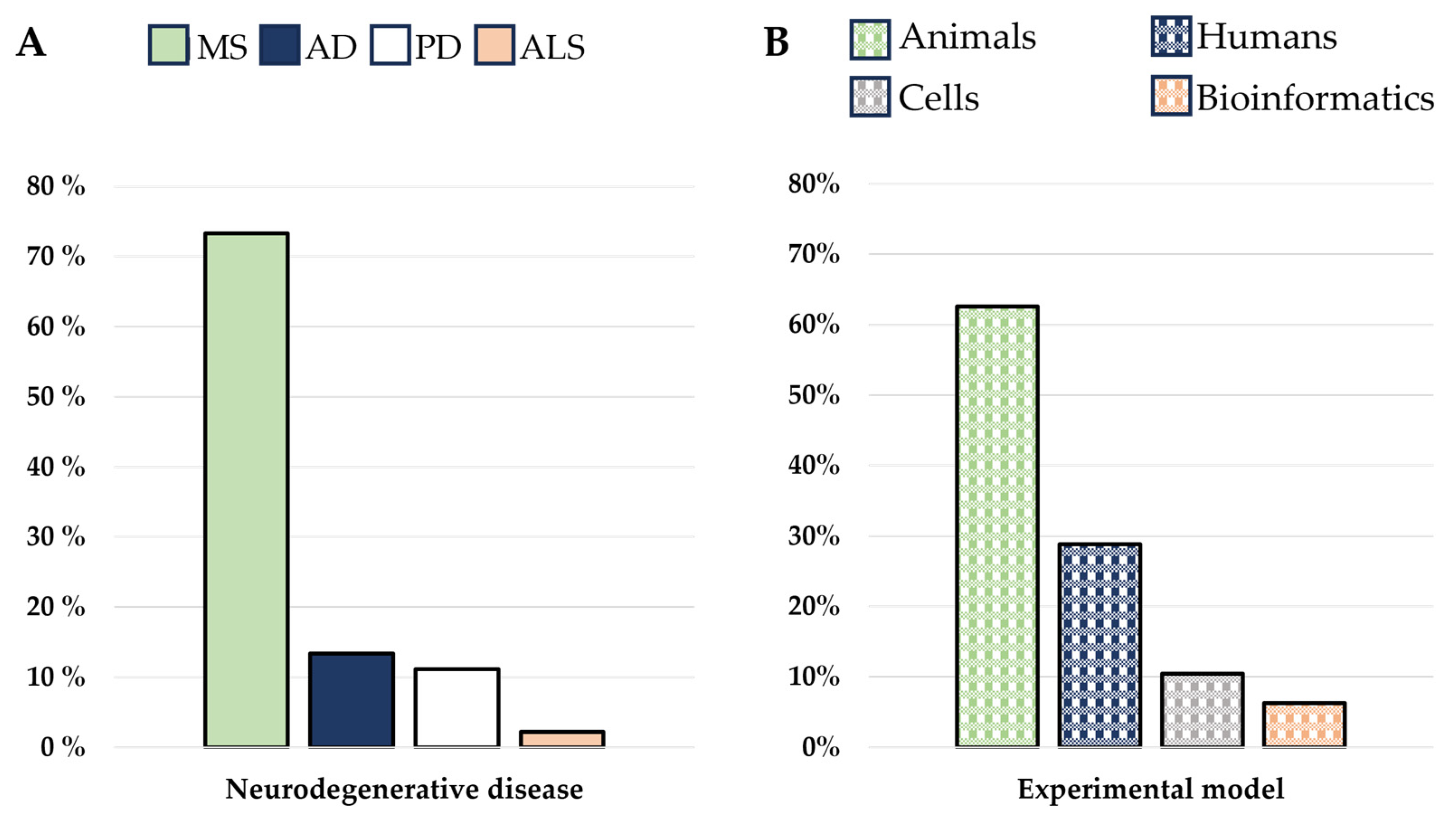
3. Results
3.1. The Immune System Participation in Neurodegenerative Diseases
3.2. MDSCs in Alzheimer’s Disease
3.3. MDSCs in Parkinson’s Disease
3.4. MDSCs in Amyotrophic Lateral Sclerosis
3.5. MDSCs in Multiple Sclerosis
MDSCs Modulation via Treatments in MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Recent advances in myeloid-derived suppressor cell biology. Front. Med. 2021, 15, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Rébé, C.; Végran, F.; Berger, H.; Ghiringhelli, F. STAT3 activation: A key factor in tumor immunoescape Cédric. JAK-STAT 2013, 2, e23010. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.G.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A.J. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef]
- Shi, H.; Qin, Y.; Tian, Y.; Wang, J.; Wang, Y.; Wang, Z.; Lv, J. Interleukin-1beta triggers the expansion of circulating granulocytic myeloid-derived suppressor cell subset dependent on Erk1/2 activation. Immunobiology 2022, 227, 152165. [Google Scholar] [CrossRef]
- Park, S.J.; Nam, D.E.; Seong, H.C.; Hahn, Y.S. New Discovery of Myeloid-Derived Suppressor Cell’s Tale on Viral Infection and COVID-19. Front. Immunol. 2022, 13, 842535. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Lee, C.R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.H.; Ye, M.B.; Lee, W.; Sim, K.Y.; Kang, J.A.; Kim, Y.C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-β during Murine Colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.S.; Feng, M.X.; Xu, J.; Xu, Y.G.; Song, C.Y.; Lin, L.Y.; Li, L.; Lu, Y.Q. Early Activation of Myeloid-Derived Suppressor Cells Participate in Sepsis-Induced Immune Suppression via PD-L1/PD-1 Axis. Front. Immunol. 2020, 11, 1299. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Safi, S.; Blattner, C.; Rathinasamy, A.; Umansky, L.; Juenger, S.; Warth, A.; Eichhorn, M.; Muley, T.; Herth, F.J.F.; et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, J.S.; Jeong, Y.H.; Son, J.; Ban, Y.H.; Lee, B.-H.; Chen, L.; Chang, J.; Chung, D.H.; Choi, I.; et al. PD-1 Upregulated on Regulatory T Cells during Chronic Virus Infection Enhances the Suppression of CD8+ T Cell Immune Response via the Interaction with PD-L1 Expressed on CD8+ T Cells. J. Immunol. 2015, 194, 5801–5811. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Liu, C.; Whartenby, K.A. Frontline Science: Induction of experimental autoimmune encephalomyelitis mobilizes Th17-promoting myeloid derived suppressor cells to the lung. J. Leukoc. Biol. 2019, 105, 829–841. [Google Scholar] [CrossRef]
- Vijitha, N.; Engel, D.R. Remote control of Th17 responses: The lung-CNS axis during EAE. J. Leukoc. Biol. 2019, 105, 827–828. [Google Scholar] [CrossRef]
- Yi, H.; Guo, C.; Yu, X.; Zuo, D.; Wang, X.-Y. Mouse CD11b+Gr-1+ Myeloid Cells Can Promote Th17 Cell Differentiation and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2012, 189, 4295–4304. [Google Scholar] [CrossRef]
- Moseley, T.A.; Haudenschild, D.R.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Ciric, B.; Marek, R.; Sadhukhan, S.; Caruso, M.L.; Shafagh, J.; Fitzgerald, D.C.; Shindler, K.S.; Rostami, A.M. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2009, 6, 14. [Google Scholar] [CrossRef]
- Di Filippo, M.; Mancini, A.; Bellingacci, L.; Gaetani, L.; Mazzocchetti, P.; Zelante, T.; La Barbera, L.; De Luca, A.; Tantucci, M.; Tozzi, A.; et al. Interleukin-17 affects synaptic plasticity and cognition in an experimental model of multiple sclerosis. Cell Rep. 2021, 37, 110094. [Google Scholar] [CrossRef]
- Moynes, D.M.; Vanner, S.J.; Lomax, A.E. Participation of interleukin 17A in neuroimmune interactions. Brain Behav. Immun. 2014, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Maaser-Hecker, A.; Tanzi, R.E. The neuroimmune axis of Alzheimer’s disease. Genome Med. 2023, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Dupuis, G.; Frost, E.H.; Larbi, A.; Pawelec, G.; Witkowski, J.M.; Fulop, T. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp. Gerontol. 2018, 107, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, Y.; Tan, J.; Chen, F.; Lei, P.; Zhang, Q. Identification of mitochondrial related signature associated with immune microenvironment in Alzheimer’s disease. J. Transl. Med. 2023, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Garneau, H.; Dupuis, G.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Pawelec, G.; Fulop, T. Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild Alzheimer diseased patients. Front. Immunol. 2017, 8, 783. [Google Scholar] [CrossRef]
- Thome, A.D.; Faridar, A.; Beers, D.R.; Thonhoff, J.R.; Zhao, W.; Wen, S.; Pascual, B.; Masdeu, J.C.; Appel, S.H. Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 61. [Google Scholar] [CrossRef]
- Xing, Z.; Zuo, Z.; Hu, D.; Zheng, X.; Wang, X.; Yuan, L.; Zhou, L.; Qi, F.; Yao, Z. Influenza vaccine combined with moderate-dose PD1 blockade reduces amyloid-β accumulation and improves cognition in APP/PS1 mice. Brain Behav. Immun. 2021, 91, 128–141. [Google Scholar] [CrossRef]
- Li, C.; Liu, K.; Zhu, J.; Zhu, F. The effects of high plasma levels of Aβ1-42 on mononuclear macrophage in mouse models of Alzheimer’s disease. Immun. Ageing 2023, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chi, L.; Lin, T.; Liang, F.; Pei, Z.; Sun, J.; Teng, W. Exogenous monocyte myeloid-derived suppressor cells ameliorate immune imbalance, neuroinflammation and cognitive impairment in 5xFAD mice infected with Porphyromonas gingivalis. J. Neuroinflamm. 2023, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Lozano, A.M. Parkinson’s Disease. N. Engl. J. Med. Rev. 1998, 339, 1130–1143. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Niu, Y.; Xu, Y.; Zhou, Q.; Xu, X.; Wang, J.; Yu, M. Increased abundance of myeloid-derived suppressor cells and Th17 cells in peripheral blood of newly-diagnosed Parkinson’s disease patients. Neurosci. Lett. 2017, 648, 21–25. [Google Scholar] [CrossRef]
- Yang, L.; Guo, C.; Zhu, J.; Feng, Y.; Chen, W.; Feng, Z.; Wang, D.; Sun, S.; Lin, W.; Wang, Y. Increased levels of pro-inflammatory and anti-inflammatory cellular responses in parkinson’s disease patients: Search for a disease indicator. Med. Sci. Monit. 2017, 23, 2972–2978. [Google Scholar] [CrossRef]
- Gopinath, A.; Mackie, P.; Hashimi, B.; Buchanan, A.M.; Smith, A.R.; Bouchard, R.; Shaw, G.; Badov, M.; Saadatpour, L.; Gittis, A.; et al. DAT and TH expression marks human Parkinson’s disease in peripheral immune cells. npj Park. Dis. 2022, 8, 72. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Huang, J.; Hu, B.; Huang, W. Identification of Immune-Related Hub Genes in Parkinson’s Disease. Front. Genet. 2022, 13, 914645. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Ning, W.; Huang, X.; Liu, X.; Deng, Y.; Franceschi, D.; Ogbuehi, A.C.; Lethaus, B.; Savkovic, V.; et al. Identifying crosstalk genetic biomarkers linking a neurodegenerative disease, Parkinson’s disease, and periodontitis using integrated bioinformatics analyses. Front. Aging Neurosci. 2022, 14, 1032401. [Google Scholar] [CrossRef]
- Graves, M.C.; Fiala, M.; Dinglasan, L.A.V.; Liu, N.Q.; Sayre, J.; Chiappelli, F.; van Kooten, C.; Vinters, H.V. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and t cells. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 213–219. [Google Scholar] [CrossRef]
- Hooten, K.G.; Beers, D.R.; Zhao, W.; Appel, S.H. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 364–375. [Google Scholar] [CrossRef]
- Vaknin, I.; Kunis, G.; Miller, O.; Butovsky, O.; Bukshpan, S.; Beers, D.R.; Henkel, J.S.; Yoles, E.; Appel, S.H.; Schwartz, M. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PLoS ONE 2011, 6, e26921. [Google Scholar] [CrossRef] [PubMed]
- Iacobaeus, E.; Douagi, I.; Jitschin, R.; Marcusson-Ståhl, M.; Andrén, A.T.; Gavin, C.; Lefsihane, K.; Davies, L.C.; Mougiakakos, D.; Kadri, N.; et al. Phenotypic and functional alterations of myeloid-derived suppressor cells during the disease course of multiple sclerosis. Immunol. Cell Biol. 2018, 96, 820–830. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.; Zanghì, A.; Parrinello, N.L.; Romano, A.; Palumbo, G.A.; Chisari, C.G.; Toscano, S.; Di Raimondo, F.; Zappia, M.; Patti, F. Immunological Subsets Characterization in Newly Diagnosed Relapsing–Remitting Multiple Sclerosis. Front. Immunol. 2022, 13, 819136. [Google Scholar] [CrossRef]
- Ioannou, M.; Alissafi, T.; Lazaridis, I.; Deraos, G.; Matsoukas, J.; Gravanis, A.; Mastorodemos, V.; Plaitakis, A.; Sharpe, A.; Boumpas, D.; et al. Crucial Role of Granulocytic Myeloid-Derived Suppressor Cells in the Regulation of Central Nervous System Autoimmune Disease. J. Immunol. 2012, 188, 1136–1146. [Google Scholar] [CrossRef]
- Knier, B.; Hiltensperger, M.; Sie, C.; Aly, L.; Engleitner, T.; Garg, G.; Muschaweckh, A.; Koedel, U.; Höchst, B.; Knolle, P.; et al. Myeloid-derived suppressor cells control B cell accumulation in the CNS during autoimmunity. Nat. Immunol. 2018, 19, 1341–1351. [Google Scholar] [CrossRef]
- Ortega, M.C.; Lebrón-Galán, R.; Machín-Díaz, I.; Naughton, M.; Pérez-Molina, I.; García-Arocha, J.; Garcia-Dominguez, J.M.; Goicoechea-Briceño, H.; Vila-del Sol, V.; Quintanero-Casero, V.; et al. Central and peripheral myeloid-derived suppressor cell-like cells are closely related to the clinical severity of multiple sclerosis. Acta Neuropathol. 2023, 146, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.L.; Olson, J.K. Innate Immune CD11b+Gr-1+ Cells, Suppressor Cells, Affect the Immune Response during Theiler’s Virus-Induced Demyelinating Disease. J. Immunol. 2009, 183, 6971–6980. [Google Scholar] [CrossRef]
- Moliné-Velázquez, V.; Cuervo, H.; Vila-Del Sol, V.; Ortega, M.C.; Clemente, D.; De Castro, F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011, 21, 678–691. [Google Scholar] [CrossRef]
- Hertzenberg, D.; Lehmann-Horn, K.; Kinzel, S.; Husterer, V.; Cravens, P.D.; Kieseier, B.C.; Hemmer, B.; Brück, W.; Zamvil, S.S.; Stüve, O.; et al. Developmental maturation of innate immune cell function correlates with susceptibility to central nervous system autoimmunity. Eur. J. Immunol. 2013, 43, 2078–2088. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; Costa, C.; Eixarch, H.; Mansilla, M.J.; López-Estévez, S.; Martorell, L.; Parés, M.; Montalban, X.; Espejo, C.; Barquinero, J. Myeloid-derived suppressor cells expressing a self-antigen ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 2016, 286, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Melero-Jerez, C.; Alonso-Gómez, A.; Moñivas, E.; Lebrón-Galán, R.; Machín-Díaz, I.; de Castro, F.; Clemente, D. The proportion of Myeloid-Derived Suppressor Cells in the spleen is related to the severity of the clinical course and tissue damage extent in a murine model of Multiple Sclerosis. Neurobiol. Dis. 2020, 140, 104869. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Oguz, C.; Metidji, A.; Dahlstrom, E.; Barbian, K.; Kanakabandi, K.; Sykora, L.; Shevach, E.M. Type I IFN signaling in T regulatory cells modulates chemokine production and Myeloid Derived Suppressor Cells trafficking during EAE. J. Autoimmun. 2020, 115, 102525. [Google Scholar] [CrossRef] [PubMed]
- Melero-Jerez, C.; Fernández-Gómez, B.; Lebrón-Galán, R.; Ortega, M.C.; Sánchez-de Lara, I.; Ojalvo, A.C.; Clemente, D.; de Castro, F. Myeloid-derived suppressor cells support remyelination in a murine model of multiple sclerosis by promoting oligodendrocyte precursor cell survival, proliferation, and differentiation. Glia 2021, 69, 905–924. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Cignarella, F.; Ghezzi, L.; Mikesell, B.; Bollman, B.; Berrien-Elliott, M.M.; Ireland, A.R.; Fehniger, T.A.; Wu, G.F.; Piccio, L. Mir-223 regulates the number and function of myeloid-derived suppressor cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2017, 133, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Ghezzi, L.; Choi, J.; Cross, A.H.; Piccio, L. Targeting miR-223 enhances myeloid-derived suppressor cell suppressive activities in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2023, 76, 104839. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.V.; Wu, L.; Olivares-Villagómez, D.; Wilson, K.T.; Van Kaer, L. Activated Invariant NKT Cells Control Central Nervous System Autoimmunity in a Mechanism That Involves Myeloid-Derived Suppressor Cells. J. Immunol. 2013, 190, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Moliné-Velázquez, V.; Ortega, M.C.; Vila del Sol, V.; Melero-Jerez, C.; de Castro, F.; Clemente, D. The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Neurobiol. Dis. 2014, 67, 149–164. [Google Scholar] [CrossRef]
- Alabanza, L.M.; Esmon, N.L.; Esmon, C.T.; Bynoe, M.S. Inhibition of Endogenous Activated Protein C Attenuates Experimental Autoimmune Encephalomyelitis by Inducing Myeloid-Derived Suppressor Cells. J. Immunol. 2013, 191, 3764–3777. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Dopkins, N.; Miranda, K.; Wilson, K.; Holloman, B.L.; Nagarkatti, P.; Nagarkatti, M. Effects of Orally Administered Cannabidiol on Neuroinflammation and Intestinal Inflammation in the Attenuation of Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2022, 17, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.; Peter, B.; Rebeaud, J.; Vigne, S.; Bressoud, V.; Roumain, M.; Wyss, T.; Yersin, Y.; Wagner, I.; Kreutzfeldt, M.; et al. Endothelial cell-derived oxysterol ablation attenuates experimental autoimmune encephalomyelitis. EMBO Rep. 2023, 24, e55328. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Verhagen, J.; Wraith, D.C. Myeloid-derived suppressor cells mediate tolerance induction in autoimmune disease. Immunology 2017, 151, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Toledano, C.; Machín-Díaz, I.; Calahorra, L.; Cabañas-Cotillas, M.; Otaegui, D.; Castillo-Triviño, T.; Villar, L.M.; Costa-Frossard, L.; Comabella, M.; Midaglia, L.; et al. Peripheral myeloid-derived suppressor cells are good biomarkers of the efficacy of fingolimod in multiple sclerosis. J. Neuroinflamm. 2022, 19, 277. [Google Scholar] [CrossRef] [PubMed]
- Melero-Jerez, C.; Suardíaz, M.; Lebrón-Galán, R.; Marín-Bañasco, C.; Oliver-Martos, B.; Machín-Díaz, I.; Fernández, Ó.; de Castro, F.; Clemente, D. The presence and suppressive activity of myeloid-derived suppressor cells are potentiated after interferon-β treatment in a murine model of multiple sclerosis. Neurobiol. Dis. 2019, 127, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Radojević, D.; Bekić, M.; Gruden-Movsesijan, A.; Ilić, N.; Dinić, M.; Bisenić, A.; Golić, N.; Vučević, D.; Đokić, J.; Tomić, S. Myeloid-derived suppressor cells prevent disruption of the gut barrier, preserve microbiota composition, and potentiate immunoregulatory pathways in a rat model of experimental autoimmune encephalomyelitis. Gut Microbes 2022, 14, 2127455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, G.; Li, G.; Wang, M.; Ma, Z.; Li, H.; Wang, X.Y.; Yi, H. Methylprednisolone alleviates multiple sclerosis by expanding myeloid-derived suppressor cells via glucocorticoid receptor β and S100A8/9 up-regulation. J. Cell. Mol. Med. 2020, 24, 13703–13714. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, B.; Tan, G.J.; Gai, X.L.; Xing, J.N.; Wang, J.Q.; Quan, M.Y.; Zhang, N.; Guo, L. NAD+ attenuates experimental autoimmune encephalomyelitis through induction of CD11b+ gr-1+ myeloid-derived suppressor cells. Biosci. Rep. 2020, 40, BSR20200353. [Google Scholar] [CrossRef]
- Dagkonaki, A.; Papalambrou, A.; Avloniti, M.; Gkika, A.; Evangelidou, M.; Androutsou, M.E.; Tselios, T.; Probert, L. Maturation of circulating Ly6ChiCCR2+ monocytes by mannan-MOG induces antigen-specific tolerance and reverses autoimmune encephalomyelitis. Front. Immunol. 2022, 13, 972003. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, X.; Yang, F.; Li, J.; Leng, X.; Mo, C.; Li, L.; Wang, Y. Pseudolycorine chloride ameliorates Th17 cell-mediated central nervous system autoimmunity by restraining myeloid-derived suppressor cell expansion. Pharm. Biol. 2022, 60, 899–908. [Google Scholar] [CrossRef]
- Ishihara, A.; Ishihara, J.; Watkins, E.A.; Tremain, A.C.; Nguyen, M.; Solanki, A.; Katsumata, K.; Mansurov, A.; Budina, E.; Alpar, A.T.; et al. Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis. Nat. Biomed. Eng. 2021, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Trager, N.; Smith, A.; Wallace, G., IV; Azuma, M.; Inoue, J.; Beeson, C.; Haque, A.; Banik, N.L. Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J. Neurochem. 2014, 130, 268–279. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Type of Treatment | Peripheral Blood | Spleen | dNLs | CNS | Myelination State | MS Severity | Ref. |
|---|---|---|---|---|---|---|---|---|
| a-GalCer | Immunostimulant glycolipid derived from marine sponge. | - | ↑ M-MDSCs ↓ CD4+ T | = MDSCs = CD4+ T | ↑ MDSCs ↓ CD4+ T | - | Amelioration | [57] |
| Am80 | Differentiation agent | - | ↓ MDSCs ↑ CD4+ T ↓ CD8+ T | - | ↓ MDSCs ↑ CD4+ T ↓ CD8+ T | - | Worsening | [58] |
| anti-PC | Ab against anticoagulant Protein C | - | ↑ MDSCs ↑ Tregs ↓ CD4+ T | - | ↑ Tregs ↑ IL-10 ↓ Il-17 | Improved | Amelioration | [59] |
| CBD | Non-psychoactive cannabinoid | - | ↑ MDSCs ↑ IL-10 ↓ IL-17 | - | ↓ MDSCs - | - | Amelioration | [60] |
| CBD | Non-psychoactive cannabinoid | - | ↑ M-MDSCs = PMN-MDSCs | = MDSCs | ↑ PMN-MDSCs = M-MDSCs | - | Amelioration | [61] |
| Ch25h | Ablation of oxidoreductase enzyme | - | - | - | ↑ PMN-MDSCs | - | Amelioration | [62] |
| EDI with MBPAc1-9(4Y) | Myelin basic protein MBPAc1-9(4Y) | - | ↑ PMN-MDSCs ↑ Tregs | ↑ MDSCs | ↑ MDSCs | - | Amelioration | [63] |
| Fingolimod | Immuno- suppressive drug | Treatment results in a greater MS amelioration in patients having higher M-MDSCs amount at the start of the treatment. | Improved | Amelioration | [64] | |||
| Gemcitabine | Antineoplastic drug | ↓ MDSCs | ↓ MDSCs ↓ Th17 | ↓ Th17 | ↓ MDSCs ↓ Th17 | Improved | Amelioration | [17] |
| IFN-β | Interferon | - | ↑ MDSCs | - | ↑ MDSCs | - | Amelioration | [65] |
| MDSC-PGE2 | MDSCs differentiated with prostaglandin (PG)E2 | - | = Th17 | - | ↓ Th17/IL-17 ↑ Tregs | - | Amelioration | [66] |
| MPPT (humans) | Methylpredniso-lone, a synthetic glucocorticoid | ↑ PMN-MDSCs ↓ M-MDSCs ↑ ARG1 | - | - | - | - | Amelioration | [67] |
| NAD+ | Nicotinamide adenine dinucleotide | - | ↑ MDSCs ↑ ARG1 ↓ Th17/IL-17 | - | ↑ MDSCs ↑ ARG1 ↓ Th17/IL-17 | - | Amelioration | [68] |
| OM-MOG | Oxidized form of fungal mannan polysaccharide conjugated to myelin antigen | = MDSCs | = MDSCs | - | ↓ MDSCs | Improved | Amelioration | [69] |
| PLY | Antiviral agent with antileukemic activity | ↓ M-MDSCs = PMN-MDSCs | - | - | ↓ MDSCs ↓ Th17/IL-17 | - | Amelioration | [70] |
| RB6-8C5 | Anti Gr-1 Ab | - | ↓ MDSCs | - | - | - | Worsening | [60] |
| SA-IL-4 | Serum albumin fused to IL-4 | - | ↑ PMN-MDSCs ↓ Th17/IL-17 | ↑ PMN-MDSCs ↓ M-MDSCs ↓ Th17 | ↑ PMN-MDSCs ↓ Th17 | Improved | Amelioration | [71] |
| SNJ-1945 | Calpain inhibitor | - | ↑ Tregs ↓ Th17 ↓ IL-17 | ↑ MDSCs ↑ Tregs ↑ IL-10 ↓ Th17/IL-17 | - | Improved | Amelioration | [72] |
| Disease | Stage of Disease | Cells | Function | |
|---|---|---|---|---|
| Alzheimer’s disease | aMCI | ↑ PMN-MDSCs = M-MDSCs | Anti-inflammatory in the early phase. Not involved in later stages | |
| CDR0.5 | ↑M-MDSCs | |||
| mAD | = M-MDSCs | |||
| CDR1 | ↑ M-MDSCs | |||
| CDR2/3 | ↓ M-MDSCs | |||
| Parkinson’s diseases | Late stages | ↑ M-MDSCs | May increase the inflammation for a limited time | |
| Amyotrophic lateral sclerosis | Any stage | ↑ MDSCs | Worsen disease progression | |
| Multiple sclerosis | RRMS | Relapse | ↑ PMN-MDSCs ↑ M-MDSCs | The greater the number of M-MDSCs in the relapse phase, the faster the recovery |
| Remitting | ↓ PMN-MDSCs ↓ M-MDSCs | Establishment of an anti-inflammatory state | ||
| PPMS (chronic) | MDSCs detectable | M-MDSCs are associated with chronic inflammation and show reduced suppressive activity | ||
| SPMS (chronic) | MDSCs detectable | M-MDSCs are associated with chronic inflammation and show reduced suppressive activity | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamberi, L.; Belloni, A.; Pugnaloni, A.; Rippo, M.R.; Olivieri, F.; Procopio, A.D.; Bronte, G. The Influence of Myeloid-Derived Suppressor Cell Expansion in Neuroinflammation and Neurodegenerative Diseases. Cells 2024, 13, 643. https://doi.org/10.3390/cells13070643
Tamberi L, Belloni A, Pugnaloni A, Rippo MR, Olivieri F, Procopio AD, Bronte G. The Influence of Myeloid-Derived Suppressor Cell Expansion in Neuroinflammation and Neurodegenerative Diseases. Cells. 2024; 13(7):643. https://doi.org/10.3390/cells13070643
Chicago/Turabian StyleTamberi, Lorenza, Alessia Belloni, Armanda Pugnaloni, Maria Rita Rippo, Fabiola Olivieri, Antonio Domenico Procopio, and Giuseppe Bronte. 2024. "The Influence of Myeloid-Derived Suppressor Cell Expansion in Neuroinflammation and Neurodegenerative Diseases" Cells 13, no. 7: 643. https://doi.org/10.3390/cells13070643






