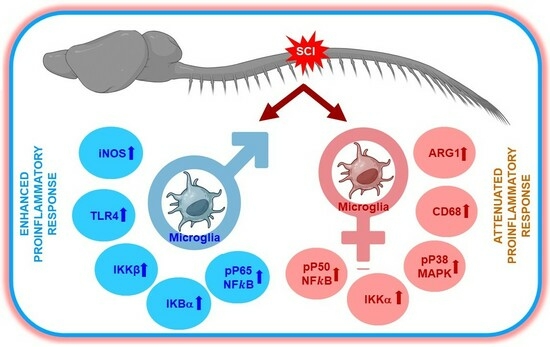
Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Pre-Operative Preparation
2.4. Moderate Thoracic Spinal Cord Contusion Injury and Post-Operative Care
2.5. Animal Perfusion and Tissue Extraction
2.6. Microglia Isolation
2.7. Flow Cytometry
2.8. Immunohistochemistry
2.9. Image Analysis
2.10. Statistical Analysis
3. Results
3.1. Analysis of Pro- and Anti-Inflammatory Markers in Male and Female after SCI
3.1.1. Morphological Analysis of Iba1+ Microglia
3.1.2. Elevated iNOS and Decreased ARG-1 in SCI Male Compared to Female Microglia
3.1.3. CD86 Expression in Female Microglia Is Markedly Higher Than That of Males after Subacute SCI
3.1.4. Male Microglia Exhibit More Potent TLR4 Immunoreactivity Than Females after Subacute SCI
3.1.5. Female SCI Rats Show Increased Expression of the Phagocytic Marker CD68 Compared to Males following Subacute SCI
3.1.6. No Sex-Dependent Differences in Microglial MRC1 after Subacute SCI
3.2. Differential Induction and Subcellular Localization of Phosphorylated P38 MAPK among Sexes after SCI
3.3. Sex Disparaties in the NFκB Pathway in Microglia after SCI
Female Microglia Show Marked Differences in the Induction and Subcellular Localization of Components of the NF-κB Signal Transduction Pathway after Subacute SCI Compared to Males
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datto, J.P.; Bastidas, J.C.; Miller, N.L.; Shah, A.K.; Arheart, K.L.; Marcillo, A.E.; Dietrich, W.D.; Pearse, D.D. Female Rats Demonstrate Improved Locomotor Recovery and Greater Preservation of White and Gray Matter after Traumatic Spinal Cord Injury Compared to Males. J. Neurotrauma 2015, 32, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Mohammed, Y.; Lee, I.; Pearse, D.D. Effect of gender on recovery after spinal cord injury. Transl. Stroke Res. 2013, 4, 447–461. [Google Scholar] [CrossRef]
- Sipski, M.L.; Jackson, A.B.; Gomez-Marin, O.; Estores, I.; Stein, A. Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 2004, 85, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Farooque, M.; Suo, Z.; Arnold, P.M.; Wulser, M.J.; Chou, C.T.; Vancura, R.W.; Fowler, S.; Festoff, B.W. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006, 44, 182–187. [Google Scholar] [CrossRef]
- Hauben, E.; Mizrahi, T.; Agranov, E.; Schwartz, M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: A gender gap in beneficial autoimmunity? Eur. J. Neurosci. 2002, 16, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Osimanjiang, W.; Allgood, J.E.; Van Sandt, R.L.; Burns, D.T.; Bushman, J.S. Sexual Dimorphism in Lesion Size and Sensorimotor Responses Following Spinal Cord Injury. Front. Neurol. 2022, 13, 925797. [Google Scholar] [CrossRef]
- Li, Y.; Ritzel, R.M.; Lei, Z.; Cao, T.; He, J.; Faden, A.I.; Wu, J. Sexual dimorphism in neurological function after SCI is associated with disrupted neuroinflammation in both injured spinal cord and brain. Brain Behav. Immun. 2022, 101, 1–22. [Google Scholar] [CrossRef]
- Stewart, A.N.; Lowe, J.L.; Glaser, E.P.; Mott, C.A.; Shahidehpour, R.K.; McFarlane, K.E.; Bailey, W.M.; Zhang, B.; Gensel, J.C. Acute inflammatory profiles differ with sex and age after spinal cord injury. J. Neuroinflamm. 2021, 18, 113. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering sex differences of rodent microglia. J. Neuroinflamm. 2021, 18, 74. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2020, 68, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ojeda, W.; Hurley, R.A. Sexual Dimorphism in Brain Development: Influence on Affective Disorders. J. Neuropsychiatry Clin. Neurosci. 2021, 33, A485–A489. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.L.; Nissen, J.C. The Pathological Activation of Microglia Is Modulated by Sexually Dimorphic Pathways. Int. J. Mol. Sci. 2023, 24, 4739. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.T.; Yin, Z.; Guneykaya, D.; Gauthier, C.D.; Hayes, J.P.; D’Hary, A.; Butovsky, O.; Moalem-Taylor, G. Sex-specific transcriptome of spinal microglia in neuropathic pain due to peripheral nerve injury. Glia 2022, 70, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.; Dietrich, D.W.; Bramlett, H.M.; Raval, A.P. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci. Ther. 2019, 25, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Loane, D.J.; Burns, M.P. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 2017, 65, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.J.; Ritzel, R.M.; Glaser, E.P.; Henry, R.J.; Faden, A.I.; Loane, D.J. Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. J. Neurotrauma 2019, 36, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Nichols, E.; Aslanyan, V.; Simone, S.M.; Rabin, J.S.; La Joie, R.; Brickman, A.M.; Dams-O’Connor, K.; Palta, P.; Kumar, R.G.; et al. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain 2022, 145, 3536–3545. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, M.B.; Zhang, L.; Zhang, B.; Cai, D. Sex Differences in Alzheimer’s Disease: Insights From the Multiomics Landscape. Biol. Psychiatry 2022, 91, 61–71. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef]
- Spivia, W.; Magno, P.S.; Le, P.; Fraser, D.A. Complement protein C1q promotes macrophage anti-inflammatory M2-like polarization during the clearance of atherogenic lipoproteins. Inflamm. Res. 2014, 63, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Xu, Y.; Pearse, D.D. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J. Neuroinflamm. 2016, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Frolinger, T.; Iqbal, U.; Estill, M.; Shen, L.; Trageser, K.J.; Pasinetti, G.M. The role of the Toll like receptor 4 signaling in sex-specific persistency of depression-like behavior in response to chronic stress. Brain Behav. Immun. 2024, 115, 169–178. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Ahmad, A.; Di Paola, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of Toll like receptor 4 signaling pathway in the secondary damage induced by experimental spinal cord injury. Immunobiology 2015, 220, 1039–1049. [Google Scholar] [CrossRef]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef] [PubMed]
- Francos-Quijorna, I.; Sanchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; de Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J. Chondroitin sulfate proteoglycans prevent immune cell phenotypic conversion and inflammation resolution via TLR4 in rodent models of spinal cord injury. Nat. Commun. 2022, 13, 2933. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Ghosh, S. Regulation of the NF-kappaB-Mediated Transcription of Inflammatory Genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, Y.; Umezawa, H.; Hatano, M. Stress-Activated Protein Kinases in Spinal Cord Injury: Focus on Roles of p38. Int. J. Mol. Sci. 2018, 19, 867. [Google Scholar] [CrossRef] [PubMed]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.P.; Ahel, H.I.; Ittner, A. Functions of p38 MAP Kinases in the Central Nervous System. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.M.; Richman, A.R.; Boyd, J.R.; Sabikunnahar, B.; Lahue, K.G.; Montgomery, T.L.; Caldwell, S.; Varnum, S.; Frietze, S.; Krementsov, D.N. p38 MAP Kinase Signaling in Microglia Plays a Sex-Specific Protective Role in CNS Autoimmunity and Regulates Microglial Transcriptional States. Front. Immunol. 2021, 12, 715311. [Google Scholar] [CrossRef] [PubMed]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A.; Zangrossi, H., Jr. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef]
- Pearse, D.D.; Bastidas, J.; Izabel, S.S.; Ghosh, M. Schwann Cell Transplantation Subdues the Pro-Inflammatory Innate Immune Cell Response after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2550. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Hill, C.E. Transplantation of Adult Rat Schwann Cells into the Injured Spinal Cord. Methods Mol. Biol. 2018, 1739, 409–438. [Google Scholar] [CrossRef]
- Schaal, S.M.; Garg, M.S.; Ghosh, M.; Lovera, L.; Lopez, M.; Patel, M.; Louro, J.; Patel, S.; Tuesta, L.; Chan, W.M.; et al. The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS ONE 2012, 7, e43634. [Google Scholar] [CrossRef]
- Lee, J.K.; Tansey, M.G. Microglia isolation from adult mouse brain. Methods Mol. Biol. 2013, 1041, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.E.; Huang, D.; Sasse, M.E.; Ransohoff, R.M. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 2006, 1, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Hefley, A.B.; Morales, A.A.; Ghosh, M. Comparative Profiling of TG2 and Its Effectors in Human Relapsing Remitting and Progressive Multiple Sclerosis. Biomedicines 2022, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Kobayashi, M.; Kunisawa, T.; Imai, K.; Sayo, A.; Malissen, B.; Crocker, P.R.; Sato, K.; Kiyama, H. Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia 2017, 65, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Manzotti, C.N.; Liu, M.; Burke, F.; Mead, K.I.; Sansom, D.M. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 2004, 172, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Muka, T.; Glisic, M. Sex and gender gap in spinal cord injury research: Focus on cardiometabolic diseases. A mini review. Maturitas 2021, 147, 14–18. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Vegeto, E.; Pollio, G.; Ciana, P.; Maggi, A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp. Gerontol. 2000, 35, 1309–1316. [Google Scholar] [CrossRef]
- Dimayuga, F.O.; Reed, J.L.; Carnero, G.A.; Wang, C.; Dimayuga, E.R.; Dimayuga, V.M.; Perger, A.; Wilson, M.E.; Keller, J.N.; Bruce-Keller, A.J. Estrogen and brain inflammation: Effects on microglial expression of MHC, costimulatory molecules and cytokines. J. Neuroimmunol. 2005, 161, 123–136. [Google Scholar] [CrossRef]
- Vegeto, E.; Belcredito, S.; Ghisletti, S.; Meda, C.; Etteri, S.; Maggi, A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 2006, 147, 2263–2272. [Google Scholar] [CrossRef]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Roof, R.L.; Hall, E.D. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000, 17, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, H.M.; Dietrich, W.D. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma 2001, 18, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef]
- Mills, C.D. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: A life or death issue. Crit. Rev. Immunol. 2001, 21, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; You, Z. Switching of the Microglial Activation Phenotype Is a Possible Treatment for Depression Disorder. Front. Cell Neurosci. 2018, 12, 306. [Google Scholar] [CrossRef]
- Yue, Y.; Yang, X.; Feng, K.; Wang, L.; Hou, J.; Mei, B.; Qin, H.; Liang, M.; Chen, G.; Wu, Z. M2b macrophages reduce early reperfusion injury after myocardial ischemia in mice: A predominant role of inhibiting apoptosis via A20. Int. J. Cardiol. 2017, 245, 228–235. [Google Scholar] [CrossRef]
- Zhang, B.; Bailey, W.M.; Braun, K.J.; Gensel, J.C. Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury. Exp. Neurol. 2015, 273, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Wang, R.; Wang, J.; Vadlamudi, R.K.; Brann, D.W. 17beta-Estradiol Regulates Microglia Activation and Polarization in the Hippocampus Following Global Cerebral Ischemia. Oxid. Med. Cell Longev. 2018, 2018, 4248526. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Rajan, R.; Choudhary, H.; Vrionis, F.; Hanafy, K.A. Gender differences in Alzheimer’s may be associated with TLR4-LYN expression in damage associated microglia and neuronal phagocytosis. J. Cell Physiol. 2022; early view. [Google Scholar] [CrossRef]
- Loiola, R.A.; Wickstead, E.S.; Solito, E.; McArthur, S. Estrogen Promotes Pro-resolving Microglial Behavior and Phagocytic Cell Clearance Through the Actions of Annexin A1. Front. Endocrinol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Yanguas-Casas, N.; Crespo-Castrillo, A.; Arevalo, M.A.; Garcia-Segura, L.M. Aging and sex: Impact on microglia phagocytosis. Aging Cell 2020, 19, e13182. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.E.; O’Connell, R.M.; Miranda, G.A.; Vaidya, S.A.; Chow, E.K.; Liu, P.T.; Suzuki, S.; Suzuki, N.; Modlin, R.L.; Yeh, W.C.; et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 2004, 199, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Georgakopoulos, D.; Lu, G.; Hester, L.; Kass, D.A.; Hasday, J.; Wang, Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 2005, 111, 2494–2502. [Google Scholar] [CrossRef]
- Saha, R.N.; Jana, M.; Pahan, K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J. Immunol. 2007, 179, 7101–7109. [Google Scholar] [CrossRef]
- Guma, M.; Hammaker, D.; Topolewski, K.; Corr, M.; Boyle, D.L.; Karin, M.; Firestein, G.S. Antiinflammatory functions of p38 in mouse models of rheumatoid arthritis: Advantages of targeting upstream kinases MKK-3 or MKK-6. Arthritis Rheum. 2012, 64, 2887–2895. [Google Scholar] [CrossRef]
- Hammaker, D.; Boyle, D.L.; Topolewski, K.; Firestein, G.S. Differential regulation of anti-inflammatory genes by p38 MAP kinase and MAP kinase kinase 6. J. Inflamm. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Reyskens, K.M.; Arthur, J.S. Emerging Roles of the Mitogen and Stress Activated Kinases MSK1 and MSK2. Front. Cell Dev. Biol. 2016, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, L.; Herranz, S.; Luque, A.; Hortelano, S. Critical role of p38 MAPK in IL-4-induced alternative activation of peritoneal macrophages. Eur. J. Immunol. 2015, 45, 273–286. [Google Scholar] [CrossRef]
- Bethea, J.R.; Castro, M.; Keane, R.W.; Lee, T.T.; Dietrich, W.D.; Yezierski, R.P. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J. Neurosci. 1998, 18, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Bebien, M.; Liu, G.Y.; Nizet, V.; Karin, M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 2005, 434, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Luedde, T.; Schmidt-Supprian, M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006, 13, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, T.; Perkins, N.D.; Wilson, C.L. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- Beg, A.A.; Baldwin, A.S., Jr. The I kappa B proteins: Multifunctional regulators of Rel/NF-kappa B transcription factors. Genes. Dev. 1993, 7, 2064–2070. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Huang, C. The biological functions of NF-kappaB1 (p50) and its potential as an anti-cancer target. Curr. Cancer Drug Targets 2009, 9, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, X.; Edwards, J.P.; Mosser, D.M. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 2006, 281, 26041–26050. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Rimoldi, M.; Raes, G.; Brys, L.; Ghezzi, P.; Di Liberto, D.; Dieli, F.; Ghisletti, S.; Natoli, G.; De Baetselier, P.; et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl. Acad. Sci. USA 2009, 106, 14978–14983. [Google Scholar] [CrossRef] [PubMed]
- Oakley, F.; Mann, J.; Nailard, S.; Smart, D.E.; Mungalsingh, N.; Constandinou, C.; Ali, S.; Wilson, S.J.; Millward-Sadler, H.; Iredale, J.P.; et al. Nuclear factor-kappaB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am. J. Pathol. 2005, 166, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Guan, H.; Ricciardi, R.P. Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding. J. Biol. Chem. 2003, 278, 45994–45998. [Google Scholar] [CrossRef]
- Elsharkawy, A.M.; Oakley, F.; Lin, F.; Packham, G.; Mann, D.A.; Mann, J. The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J. Hepatol. 2010, 53, 519–527. [Google Scholar] [CrossRef]












| Antibodies Used for Flow Cytometry | ||||
|---|---|---|---|---|
| Primary Antibody | Host | Manufacturer | Catalog Number | Dilution |
| Anti-Rat CD11b:Pacific Blue | Mouse | Bio-Rad (Hercules, CA, USA) | MCA275PB | 1:50 |
| PE anti-mouse CX3CR1 | Mouse | BioLegend (San Diego, CA, USA) | 149006 | 1:100 |
| Human/Mouse anti-Arginase 1-APC | Sheep | R&D Systems (Minneapolis, MN, USA) | IC5868A | 1:10 |
| Anti-mouse iNOS-Alexa Fluor 488 | Rat | Invitrogen (Waltham, MA, USA) | 53-5920-82 | 60 µg/mL |
| Anti-rat CD163:FITC | Mouse | Bio-rad | MCA342F | 1:10 |
| Anti- MMR/CD206-APC conjugated | Goat | R&D Systems | FAB2535A | 1:10 |
| Anti-rat CD68-Alexa Fluor 647 | Mouse | Bio-rad | MCA341A647 | 1:10 |
| FITC anti-rat CD86 | Mouse | BioLegend | 200305 | 6.25 µg/mL |
| FITC anti-rat CD38 | Mouse | BioLegend | 250503 | 1:50 |
| Anti-rat CD38 (14.27) eFluor660 | Mouse | Invitrogen | 50-0380-80 | 2.5 µg/mL |
| pAb anti-EGR2 [FITC] | Rabbit | Novus Biologicals (Centennial, CO, USA) | NB110-59723F | 1:10 |
| Anti-NOS2 (C-11) FITC | Mouse | Santa Cruz Biotechnology | SC-7271 FITC | 10 µg/mL |
| SIGLEC H Monoclonal Antibody (eBio440c), PE | Rat | Fisher Scientific (Hampton, NH, USA) | 50-103-25 | 1.25 μg/mL |
| Antibodies Used for Immunohistochemistry | ||||
| Primary Antibody | Host | Company | Catalog Number | Dilution |
| Anti-Arginase 1 | Rabbit | Genetex (Irvine, CA, USA) | CTX109242 | 1:100 |
| Anti-MRC1 | Rabbit | Sigma-Aldrich (St. Louis, MO, USA) | HPA045134 | 1:100 |
| anti-Phospho-p38 MAPK (Thr180/Tyr182) (D3F9) | Rabbit | Cell Signaling Technology (Danvers, MA, USA) | 4511S | 1:400 |
| Phospho-p38 MAPK (Thr180/Tyr182) | Rabbit | Cell Signaling Technology | 9211 | 1:200 |
| Anti-NOS2 (C-11) FITC | Mouse | Santa Cruz Biotechnology | sc-7271 | 1:50 |
| Anti-TLR4 | Mouse | Abcam (Waltham, MA, USA) | ab22048 | 1:100 |
| Anti-Iba1 | Goat | Abcam | ab5076 | 1:500 |
| Anti Rat CD68, clone ED1 | Mouse | Bio-rad | MCA341R | 1:100 |
| Anti-rat CD163 | Rabbit | Bio-rad | MCA342R | 1:100 |
| Phospho-IKKα (Ser176, Ser180) | Rabbit | ThermoFisher Scientific (Waltham, MA, USA) | 44-714 | 1:200 |
| Phospho-IKKβ (Tyr188) Polyclonal | Rabbit | Invitrogen | PA5-104693 | 1:100 |
| Anti-IL-1 beta | Rabbit | Abcam | ab2105 | 1:100 |
| Anti-IL-1 beta (E7-2-hIL1β) | Mouse | Santa Cruz Biotechnology | sc-32294 | 1:100 |
| Anti Iba1, Rabbit (for ICC) | Rabbit | Wako/Fuji (Richmond, VA, USA) | 019-19741 | 1:1000 |
| Chicken Polyclonal to Iba1 | Chicken | EnCor Biotechnology (Gainesville, FL, USA) | CPCA-Iba1 | 1:1000 |
| Goat pAb to Iba1 | Goat | Abcam | ab5076 | 1:500 |
| Pro-Inflammatory | Male | Female |
|---|---|---|
| iNOS |  |  |
| CD86 |  |  |
| TLR4 |  |  |
| pP38 MAPK |  |  |
| IKKβ |  |  |
| IKBα |  |  |
| NFκB pp65 |  |  |
| Anti-Inflammatory | Male | Female |
| ARG1 |  |  |
| CD68 |  |  |
| MRC1/CD206 |  |  |
| pP38 MAPK |  |  |
| IKKα |  |  |
| NFκB pp50 |  |  |
 indicates increased expression level and
indicates increased expression level and  suggests decreased levels of target proteins.
suggests decreased levels of target proteins.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, M.; Lee, J.; Burke, A.N.; Strong, T.A.; Sagen, J.; Pearse, D.D. Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat. Cells 2024, 13, 645. https://doi.org/10.3390/cells13070645
Ghosh M, Lee J, Burke AN, Strong TA, Sagen J, Pearse DD. Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat. Cells. 2024; 13(7):645. https://doi.org/10.3390/cells13070645
Chicago/Turabian StyleGhosh, Mousumi, Jinyoung Lee, Ashley N. Burke, Thomas A. Strong, Jacqueline Sagen, and Damien D. Pearse. 2024. "Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat" Cells 13, no. 7: 645. https://doi.org/10.3390/cells13070645
APA StyleGhosh, M., Lee, J., Burke, A. N., Strong, T. A., Sagen, J., & Pearse, D. D. (2024). Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat. Cells, 13(7), 645. https://doi.org/10.3390/cells13070645