Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning
Abstract
1. Introduction
2. Methods
2.1. Donor Specifications
2.2. Isolation of ADSCs
2.3. Culture of Human ADSCs
2.4. Flow Cytometry
2.5. Treatment with FBS, IL-1β, TGF-β1 and Hypoxia
2.6. RNA Isolation and Bulk Sequencing
2.7. Data Analysis
3. Results
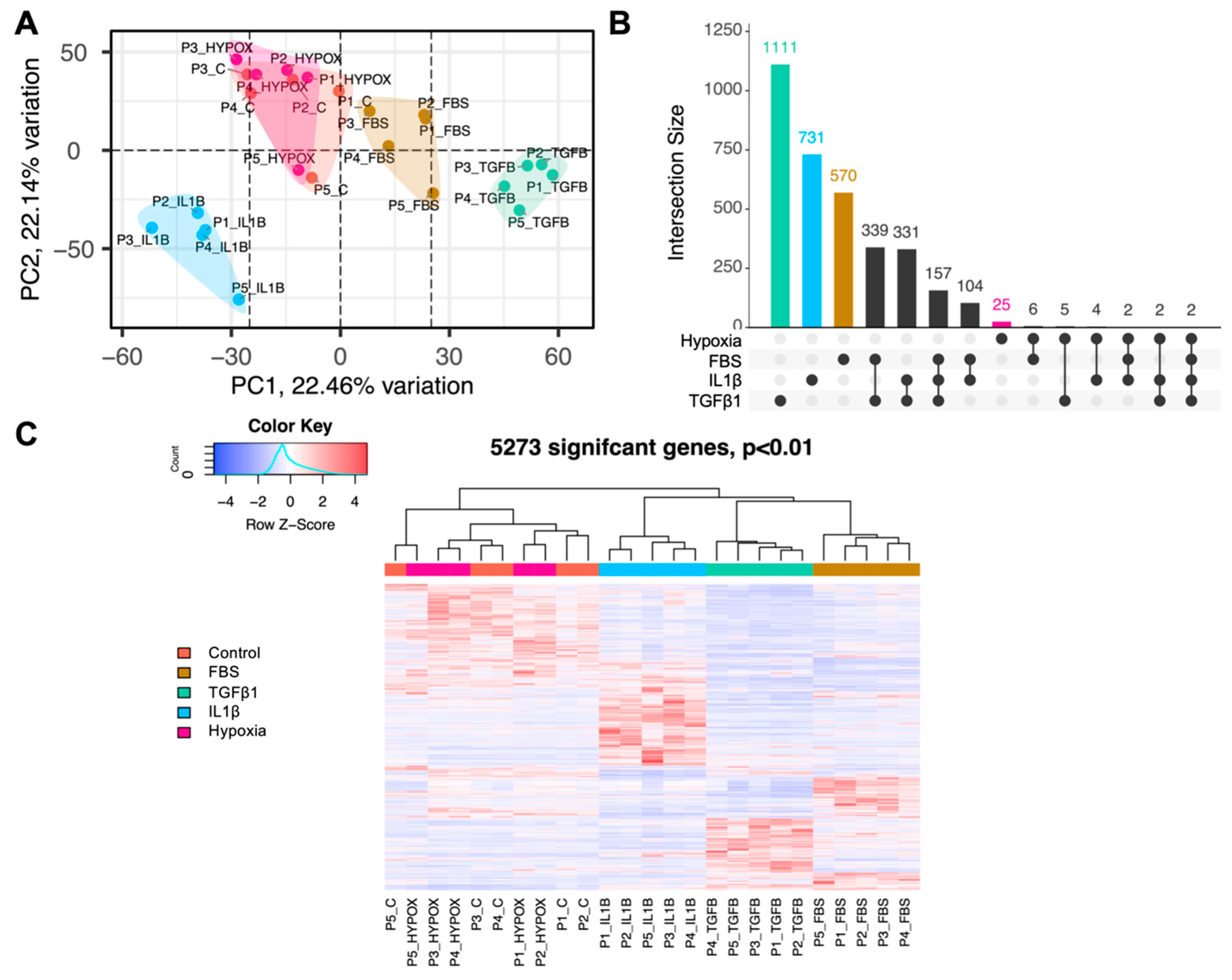
3.1. ADSCs Exhibit Distinct Transcriptomic Profiles following In Vitro Treatments
3.2. The Most Highly Differentially Expressed ADSC Genes form Unique Clusters Depending on the Type of Treatment and Map to Selective Cellular Pathways
3.3. Pathway Analysis Reveals Changes in the Biological Behaviour of ADSCs after Treatment
3.4. Genes of Selected Pathways Highlight the Association of ADSCs with Key Features Underlying Skin Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Lokmic, Z.; Musyoka, J.; Hewitson, T.D.; Darby, I.A. Chapter three-Hypoxia and Hypoxia Signaling in Tissue Repair and Fibrosis. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 296, pp. 139–185. [Google Scholar]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Ruthenborg, R.J.; Ban, J.J.; Wazir, A.; Takeda, N.; Kim, J.W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells 2014, 37, 637–643. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Smith, G.P.; Chan, E.S.L. Molecular Pathogenesis of Skin Fibrosis: Insight from Animal Models. Curr. Rheumatol. Rep. 2010, 12, 26–33. [Google Scholar] [CrossRef]
- Amin, K.; Clarke, A.; Sivakumar, B.; Puri, A.; Fox, Z.; Brough, V.; Denton, C.P.; Peter, E.M.; Butler, M.D. The psychological impact of facial changes in scleroderma. Psychol. Health Med. 2011, 16, 304–312. [Google Scholar] [CrossRef]
- Griffin, M.F.; Drago, J.; Almadori, A.; Kalavrezos, N.; Butler, P.E. Evaluation of the efficacy of lipotransfer to manage radiation-induced fibrosis and volume defects in head and neck oncology. Head Neck 2019, 41, 3647–3655. [Google Scholar] [CrossRef]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Almadori, A.; Hansen, E.; Boyle, D.; Zenner, N.; Swale, V.; Reid, W.; Maclane, A.; Butler, P.E.M. Fat Grafting Improves Fibrosis and Scarring in Vulvar Lichen Sclerosus: Results from a Prospective Cohort Study. J. Low. Genit. Tract Dis. 2020, 24, 305–310. [Google Scholar] [CrossRef]
- Klinger, M.; Caviggioli, F.; Klinger, F.M.; Giannasi, S.; Bandi, V.; Banzatti, B.; Forcellini, D.; Maione, L.; Catania, B.; Vinci, V. Autologous Fat Graft in Scar Treatment. J. Craniofacial Surg. 2013, 24, 1610–1615. [Google Scholar] [CrossRef]
- Borovikova, A.A.; Ziegler, M.E.; Banyard, D.A.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Adipose-Derived Tissue in the Treatment of Dermal Fibrosis: Antifibrotic Effects of Adipose-Derived Stem Cells. Ann. Plast. Surg. 2018, 80, 297–307. [Google Scholar] [CrossRef]
- Stachura, A.; Paskal, W.; Pawlik, W.; Mazurek, M.J.; Jaworowski, J. The Use of Adipose-Derived Stem Cells (ADSCs) and Stromal Vascular Fraction (SVF) in Skin Scar Treatment—A Systematic Review of Clinical Studies. J. Clin. Med. 2021, 10, 3637. [Google Scholar] [CrossRef]
- Spiekman, M.; van Dongen, J.A.; Willemsen, J.C.; Hoppe, D.L.; van der Lei, B.; Harmsen, M.C. The power of fat and its adipose-derived stromal cells: Emerging concepts for fibrotic scar treatment. J. Tissue Eng. Regen. Med. 2017, 11, 3220–3235. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 2012, 53, 227–246. [Google Scholar] [CrossRef]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Frommer, M.L.; Langridge, B.J.; Awad, L.; Jasionowska, S.; Denton, C.P.; Abraham, D.J.; Abu-Hanna, J.; Butler, P.E.M. Single-Cell Analysis of ADSC Interactions with Fibroblasts and Endothelial Cells in Scleroderma Skin. Cells 2023, 12, 1784. [Google Scholar] [CrossRef]
- Pattani, N.; Sanghera, J.; Langridge, B.J.; Frommer, M.L.; Abu-Hanna, J.; Butler, P. Exploring the mechanisms behind autologous lipotransfer for radiation-induced fibrosis: A systematic review. PLoS ONE 2024, 19, e0292013. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Rodríguez, T.M.; Saldías, A.; Irigo, M.; Zamora, J.V.; Perone, M.J.; Dewey, R.A. Effect of TGF-β1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2015, 4, 894–898. [Google Scholar] [CrossRef]
- van Dalen, S.C.M.; Blom, A.B.; Walgreen, B.; Slöetjes, A.W.; Helsen, M.M.A.; Geven, E.J.W.; ter Huurne, M.; Vogl, T.; Roth, J.; van de Loo, F.A.J.; et al. IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology. Front. Immunol. 2019, 10, 1075. [Google Scholar] [CrossRef]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. 2021, 2, 100141. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Iyer, V.R.; Eisen, M.B.; Ross, D.T.; Schuler, G.; Moore, T.; Lee, J.C.F.; Trent, J.M.; Staudt, L.M.; Hudson, J.; Boguski, M.S.; et al. The Transcriptional Program in the Response of Human Fibroblasts to Serum. Science 1999, 283, 83–87. [Google Scholar] [CrossRef]
- Langridge, B.J.; Jasionowska, S.; Khan, H.; Awad, L.; Turner, B.R.H.; Varghese, J.; Butler, P.E.M. Achieving optimal clinical outcomes in autologous fat grafting: A systematic review of processing techniques. J. Plast. Reconstr. Aesthetic Surg. 2023, 81, 9–25. [Google Scholar] [CrossRef]
- Mrozikiewicz-Rakowska, B.; Szabłowska-Gadomska, I.; Cysewski, D.; Rudziński, S.; Płoski, R.; Gasperowicz, P.; Konarzewska, M.; Zieliński, J.; Mieczkowski, M.; Sieńko, D.; et al. Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact. Int. J. Mol. Sci. 2023, 24, 1472. [Google Scholar] [CrossRef]
- Forcheron, F.; Agay, D.; Scherthan, H.; Riccobono, D.; Herodin, F.; Meineke, V.; Drouet, M. Autologous Adipocyte Derived Stem Cells Favour Healing in a Minipig Model of Cutaneous Radiation Syndrome. PLoS ONE 2012, 7, e31694. [Google Scholar] [CrossRef]
- Huang, S.-P.; Huang, C.-H.; Shyu, J.-F.; Lee, H.-S.; Chen, S.-G.; Chan, J.Y.-H.; Huang, S.-M. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J. Biomed. Sci. 2013, 20, 51. [Google Scholar] [CrossRef]
- Ejaz, A.; Greenberger, J.S.; Rubin, P.J. Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacol. Ther. 2019, 204, 107399. [Google Scholar] [CrossRef]
- Beyer, C.; Schett, G.; Gay, S.; Distler, O.; Distler, J.H.W. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res. Ther. 2009, 11, 220. [Google Scholar] [CrossRef]
- Garza, R.M.; Paik, K.J.; Chung, M.T.; Duscher, D.; Gurtner, G.C.; Longaker, M.T.; Wan, D.C. Studies in Fat Grafting: Part III. Fat Grafting Irradiated Tissue—Improved Skin Quality and Decreased Fat Graft Retention. Plast. Reconstr. Surg. 2014, 134, 249–257. [Google Scholar] [CrossRef]
- Luan, A.; Duscher, D.; Whittam, A.J.; Paik, K.J.; Zielins, E.R.; Brett, E.A.; Atashroo, D.A.; Hu, M.S.; Lee, G.K.; Gurtner, G.C.; et al. Cell-Assisted Lipotransfer Improves Volume Retention in Irradiated Recipient Sites and Rescues Radiation-Induced Skin Changes. Stem Cells 2016, 34, 668–673. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Cao, R.; Eriksson, A.; Kubo, H.; Alitalo, K.; Cao, Y.; Thyberg, J. Comparative Evaluation of FGF-2, VEGF-A, and VEGF-C: Induced Angiogenesis, Lymphangiogenesis, Vascular Fenestrations, and Permeability. Circ. Res. 2004, 94, 664–670. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine Effect of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Bone Regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Huo, Y.; Yang, Y.; Wang, Y. Hypoxia-Pretreated Human MSCs Attenuate Acute Kidney Injury through Enhanced Angiogenic and Antioxidative Capacities. BioMed Res. Int. 2014, 2014, 462472. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Wang, M.; Crisostomo, P.R.; Herring, C.; Meldrum, K.K.; Meldrum, D.R. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R880–R884. [Google Scholar] [CrossRef]
- Suga, H.; Eto, H.; Shigeura, T.; Inoue, K.; Aoi, N.; Kato, H.; Nishimura, S.; Manabe, I.; Gonda, K.; Yoshimura, K. IFATS Collection: Fibroblast Growth Factor-2-Induced Hepatocyte Growth Factor Secretion by Adipose-Derived Stromal Cells Inhibits Postinjury Fibrogenesis through a c-Jun N-Terminal Kinase-Dependent Mechanism. Stem Cells 2009, 27, 238–249. [Google Scholar] [CrossRef]
- Vittal, R.; Fisher, A.J.; Thompson, E.L.; Cipolla, E.M.; Gu, H.; Mickler, E.A.; Varre, A.; Agarwal, M.; Kim, K.K.; Vasko, M.R.; et al. Overexpression of Decay Accelerating Factor Mitigates Fibrotic Responses to Lung Injury. Am. J. Respir. Cell Mol. Biol. 2022, 67, 459–470. [Google Scholar] [CrossRef]
- Pilny, E.; Smolarczyk, R.; Jarosz-Biej, M.; Hadyk, A.; Skorupa, A.; Ciszek, M.; Krakowczyk, Ł.; Kułach, N.; Gillner, D.; Sokół, M.; et al. Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue. Stem Cell Res. Ther. 2019, 10, 93. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Christiaens, V.; Van Hul, M.; Lijnen, H.R.; Scroyen, I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 949–956. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef]
- Smith, L.T.; Holbrook, K.A.; Byers, P.H. Structure of the dermal matrix during development and in the adult. J. Investig. Dermatol. 1982, 79 (Suppl. 1), 93s–104s. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Tanaka, K.; Arao, T.; Maegawa, M.; Matsumoto, K.; Kaneda, H.; Kudo, K.; Fujita, Y.; Yokote, H.; Yanagihara, K.; Yamada, Y.; et al. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int. J. Cancer 2009, 124, 1072–1080. [Google Scholar] [CrossRef]
- Leask, A. Conjunction junction, what’s the function? CCN proteins as targets in fibrosis and cancers. Am. J. Physiol.-Cell Physiol. 2020, 318, C1046–C1054. [Google Scholar] [CrossRef]
- Chinnapaka, S.; Yang, K.S.; Surucu, Y.; Bengur, F.B.; Arellano, J.A.; Tirmizi, Z.; Malekzadeh, H.; Epperly, M.W.; Hou, W.; Greenberger, J.S.; et al. Human adipose ECM alleviates radiation-induced skin fibrosis via endothelial cell-mediated M2 macrophage polarization. iScience 2023, 26, 107660. [Google Scholar] [CrossRef]
- Pinheiro-Machado, E.; Getova, V.E.; Harmsen, M.C.; Burgess, J.K.; Smink, A.M. Towards standardization of human adipose-derived stromal cells secretomes. Stem Cell Rev. Rep. 2023, 19, 2131–2140. [Google Scholar] [CrossRef]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.-C.; Davies, M.J.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci. Rep. 2020, 10, 17300. [Google Scholar] [CrossRef]
- Lepsenyi, M.; Algethami, N.; Al-Haidari, A.A.; Algaber, A.; Syk, I.; Rahman, M.; Thorlacius, H. CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin. Exp. Metastasis 2021, 38, 401–410. [Google Scholar] [CrossRef]
- Jürgensen, H.J.; Silva, L.M.; Krigslund, O.; van Putten, S.; Madsen, D.H.; Behrendt, N.; Engelholm, L.H.; Bugge, T.H. CCL2/MCP-1 signaling drives extracellular matrix turnover by diverse macrophage subsets. Matrix Biol. Plus 2019, 1, 100003. [Google Scholar] [CrossRef]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.-D.; Joo, S.T.; Choi, I.; et al. Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat. Food Sci. Anim. Resour. 2022, 42, 775–799. [Google Scholar] [CrossRef]
- Mark, P.; Kleinsorge, M.; Gaebel, R.; Lux, C.A.; Toelk, A.; Pittermann, E.; David, R.; Steinhoff, G.; Ma, N. Human Mesenchymal Stem Cells Display Reduced Expression of CD105 after Culture in Serum-Free Medium. Stem Cells Int. 2013, 2013, 698076. [Google Scholar] [CrossRef]
- Pilgrim, C.R.; McCahill, K.A.; Rops, J.G.; Dufour, J.M.; Russell, K.A.; Koch, T.G. A Review of Fetal Bovine Serum in the Culture of Mesenchymal Stromal Cells and Potential Alternatives for Veterinary Medicine. Front. Vet. Sci. 2022, 9, 859025. [Google Scholar] [CrossRef]
- Frazier, T.P.; Gimble, J.M.; Kheterpal, I.; Rowan, B.G. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 2013, 95, 2286–2296. [Google Scholar] [CrossRef]


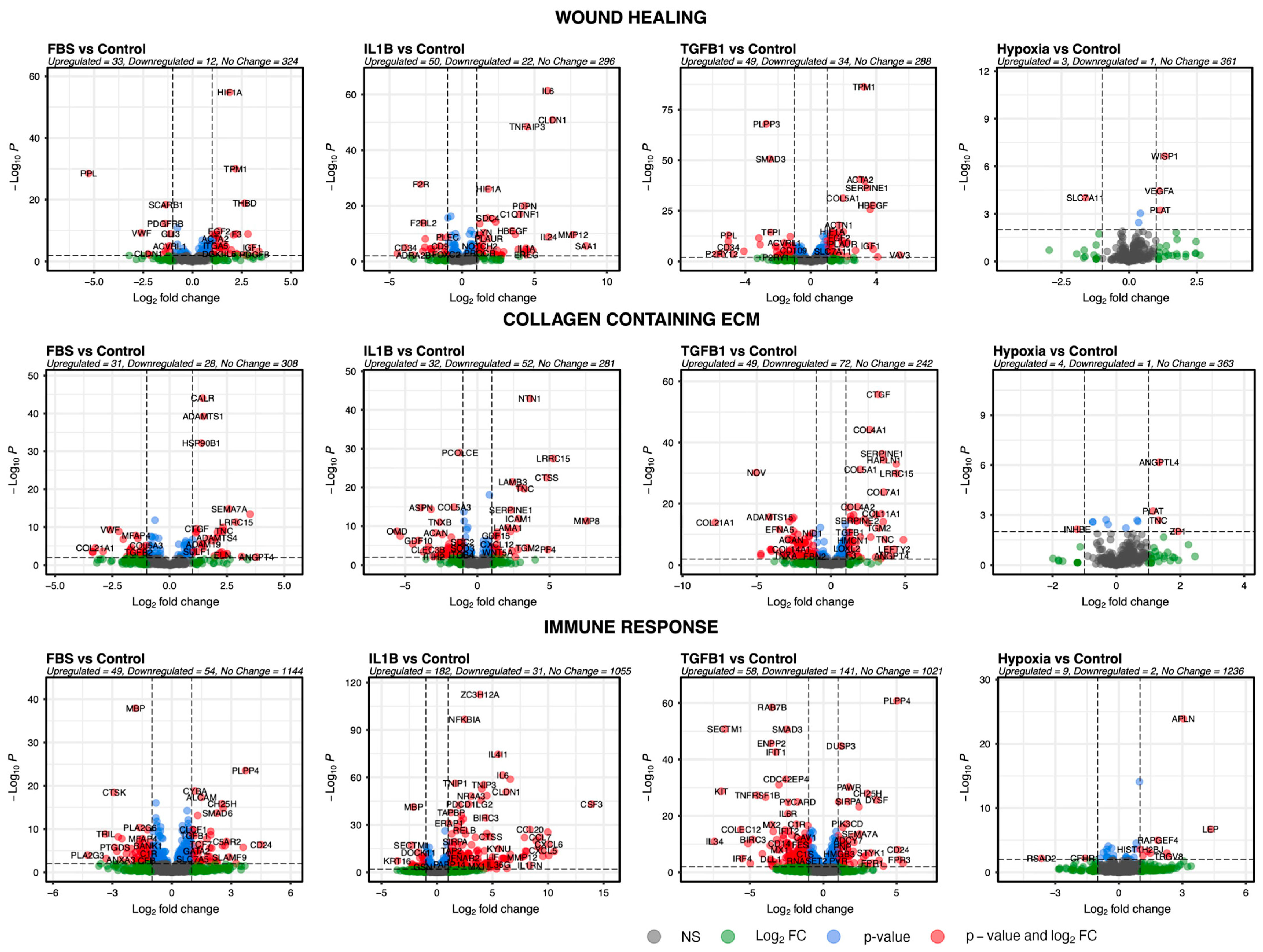
| Pathway | Genes |
|---|---|
| Immune Reaction | |
| Up- or downregulated in all 3 conditions | APLN (↑) |
| Upregulated in TGF-β1 and IL1-β | ST3GAL1, STYK1, IL4R, ABL1, IL21R, DUSP3, BTN2A1, PDE4D, PXDN, IL6, CH25H, EPG5, PIK3AP1, LRP8, FCER1G, IL12A, PIK3CD, RASGRP1, CLCF1, IL1RAP, CD55, PDCD1LG2, ENTPD7, SIRPA, PNP, STMP1 |
| Downregulated in TGF-β1 and IL1-β | SECTM1, DOCK11, RAB7B, MEF2C, CD36, JAG1, MAP2K6, TRIL, FES, ACKR4, CRIP1 |
| ECM Organisation | |
| Up- or downregulated in all 3 conditions | None |
| Upregulated in TGF-β1 and IL1-β | TNC, SRPX2, SERPINE1, COL7A1, COL10A1, CYR61, ADAMTS4, LRRC15, TGM2 |
| Downregulated in TGF-β1 and IL1-β | COL5A3, NOV, TNXB, OMD, ACAN, MMRN2, COL21A1, KAZALD1, OGN, S100A10, ADAMTS15, ADAMTSL4, SOD3, CLEC3B, S100A4, TMEFF2, SBSPON, ECM2 |
| Wound Healing | |
| Up- or downregulated in all 3 conditions | VEGFA (↑) |
| Upregulated in TGF-β1 and IL1-β | TNFRSF12A, PLAUR, B4GALT1, HIF1A, SERPINE1, HBEGF, IL6, FGF2, CYR61, FCER1G |
| Downregulated in TGF-β1 and IL1-β | F2R, ACVRL1, F10, CD34, CD36, PPL, TMEFF2, ENTPD1, P2RY12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frommer, M.L.; Langridge, B.J.; Beedie, A.; Jasionowska, S.; Awad, L.; Denton, C.P.; Abraham, D.J.; Abu-Hanna, J.; Butler, P.E.M. Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning. Cells 2024, 13, 693. https://doi.org/10.3390/cells13080693
Frommer ML, Langridge BJ, Beedie A, Jasionowska S, Awad L, Denton CP, Abraham DJ, Abu-Hanna J, Butler PEM. Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning. Cells. 2024; 13(8):693. https://doi.org/10.3390/cells13080693
Chicago/Turabian StyleFrommer, Marvin L., Benjamin J. Langridge, Alexandra Beedie, Sara Jasionowska, Laura Awad, Christopher P. Denton, David J. Abraham, Jeries Abu-Hanna, and Peter E. M. Butler. 2024. "Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning" Cells 13, no. 8: 693. https://doi.org/10.3390/cells13080693
APA StyleFrommer, M. L., Langridge, B. J., Beedie, A., Jasionowska, S., Awad, L., Denton, C. P., Abraham, D. J., Abu-Hanna, J., & Butler, P. E. M. (2024). Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning. Cells, 13(8), 693. https://doi.org/10.3390/cells13080693






