Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro
Abstract
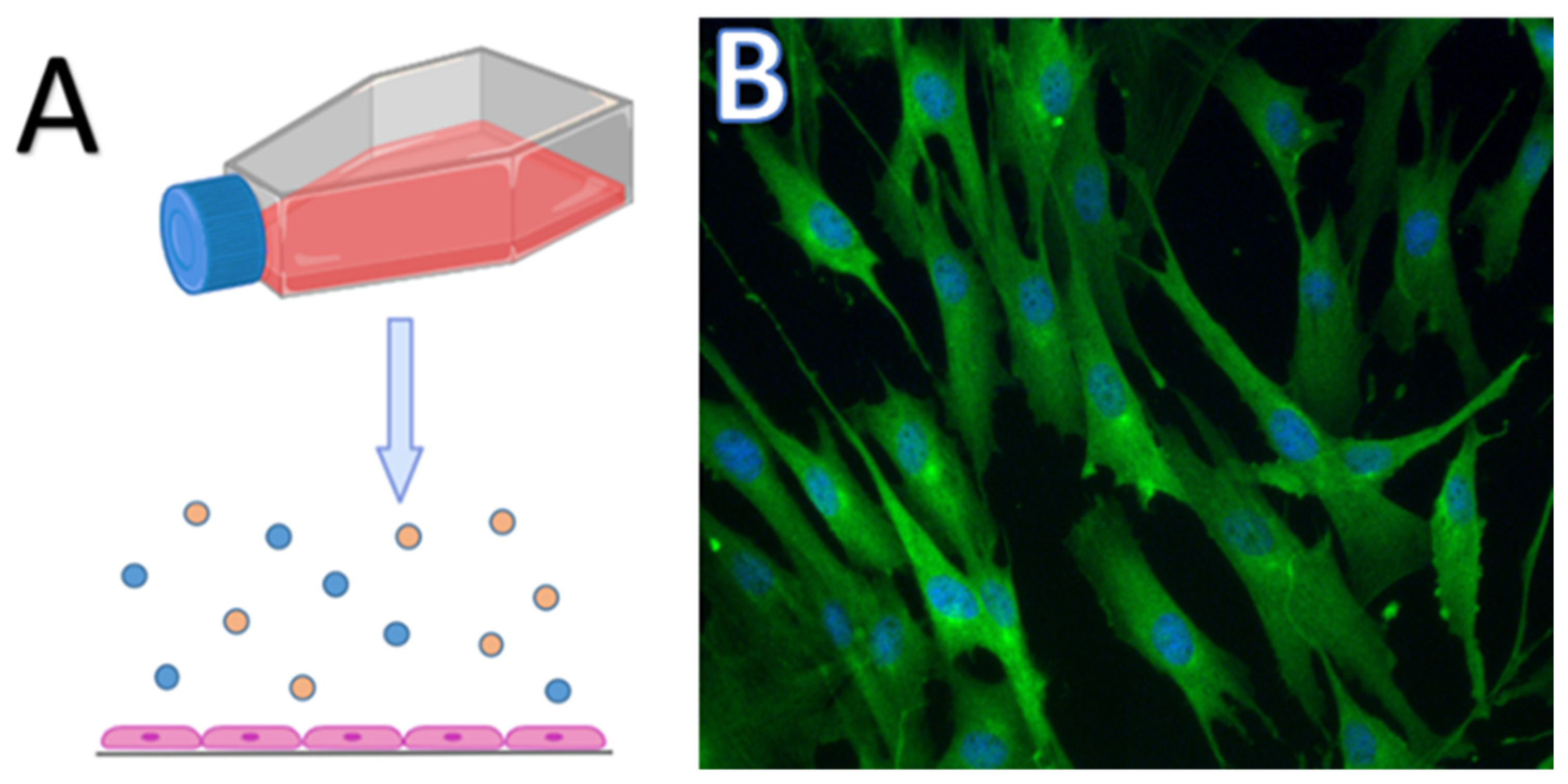
:1. Introduction
2. Cell Cultures for Cancer Research
3. Two-Dimensional Culture, Pros and Cons
4. Three-Dimensional Models for Cancer Study
4.1. Scaffold-Free 3D Systems
4.2. Scaffold-Based 3D Systems
4.2.1. Biological 3D Scaffolds
4.2.2. Synthetic 3D Scaffolds
4.2.3. Decellularised Matrices
4.3. Specialised 3D Cell Culture Platforms
4.3.1. Microfluidic Devices
4.3.2. Organ-on-Chip
4.3.3. Three-Dimensional Bioprinting
5. Two-Dimensional and Three-Dimensional Model Systems in CMT Research
Microenvironment/Cellular Components in 3D Culture
6. Application of Different 3D Cultivation Models in CMT Research
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; de Rooster, H.; Veldhuis Kroeze, E.J.B.; Van Ginneken, C.; Van Brantegem, L. Canine Mammary Tumours, an Overview. Reprod. Domest. Anim. 2011, 46, 1112–1131. [Google Scholar] [CrossRef] [PubMed]
- Allen, A. Tumors in Domestic Animals, 4th Edition. Can. Vet. J. 2003, 44, 684. [Google Scholar]
- Tavasoly, A.; Golshahi, H.; Rezaie, A.; Farhadi, M. Classification and Grading of Canine Malignant Mammary Tumors. Vet. Res. Forum. 2013, 4, 25–30. [Google Scholar] [PubMed]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, Anatomy, Histology, Lymphatic Drainage, Clinical Features, and Cell Differentiation Markers of Canine Mammary Gland Neoplasms. Vet. Pathol. 2011, 48, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Angeli, D.; Salvi, S.; Tedaldi, G. Genetic Predisposition to Breast and Ovarian Cancers: How Many and Which Genes to Test? Int. J. Mol. Sci. 2020, 21, 1128. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Melin, M.; Biagi, T.; Fall, T.; Häggström, J.; Lindblad-Toh, K.; von Euler, H. Mammary Tumor Development in Dogs Is Associated with BRCA1 and BRCA2. Cancer Res. 2009, 69, 8770–8774. [Google Scholar] [CrossRef] [PubMed]
- Mouser, P.; Miller, M.A.; Antuofermo, E.; Badve, S.S.; Mohammed, S.I. Prevalence and Classification of Spontaneous Mammary Intraepithelial Lesions in Dogs without Clinical Mammary Disease. Vet. Pathol. 2010, 47, 275–284. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.-M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine Mammary Gland Tumours; a Histological Continuum from Benign to Malignant; Clinical and Histopathological Evidence. Vet. Comp. Oncol. 2009, 7, 162–172. [Google Scholar] [CrossRef]
- Rutteman, G.R. Hormones and Mammary Tumour Disease in the Female Dog: An Update. In Vivo 1990, 4, 33–40. [Google Scholar] [PubMed]
- Queiroga, F.L.; Pérez-Alenza, M.D.; Silvan, G.; Peña, L.; Lopes, C.; Illera, J.C. Role of Steroid Hormones and Prolactin in Canine Mammary Cancer. J. Steroid. Biochem. Mol. Biol. 2005, 94, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, N.; Failing, K.; Richter, A.; Wehrend, A. Mammary Tumor Recurrence in Bitches after Regional Mastectomy. Vet. Surg. 2008, 37, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Grossmann, M.E.; Ray, A. Effect of Obesity on Breast Cancer Development. Vet. Pathol. 2010, 47, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Pires, I.; Lobo, L.; Lopes, C.S. The Role of Cox-2 Expression in the Prognosis of Dogs with Malignant Mammary Tumours. Res. Vet. Sci. 2010, 88, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Hunley, D.W.; Mauldin, G.N.; Shiomitsu, K.; Mauldin, G.E. Clinical Outcome in Dogs with Nasal Tumors Treated with Intensity-Modulated Radiation Therapy. Can. Vet. J. 2010, 51, 293–300. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.A. Introduction to Animal Cell Culture. Tech. Bull. 2008. [Google Scholar]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular Cues. J. Cell. Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Harrison, R.G.; Greenman, M.J.; Mall, F.P.; Jackson, C.M. Observations of the Living Developing Nerve Fiber. Anat. Record. 1907, 1, 116–128. [Google Scholar] [CrossRef]
- Harrison, R.G. The Outgrowth of the Nerve Fiber as a Mode of Protoplasmic Movement. J. Exp. Zool. 1910, 9, 787–846. [Google Scholar] [CrossRef]
- Cox, M.C.; Reese, L.M.; Bickford, L.R.; Verbridge, S.S. Toward the Broad Adoption of 3D Tumor Models in the Cancer Drug Pipeline. ACS Biomater. Sci. Eng. 2015, 1, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Design of Spherically Structured 3D in Vitro Tumor Models -Advances and Prospects. Acta Biomater. 2018, 75, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Biology’s New Dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue Architecture: The Ultimate Regulator of Breast Epithelial Function. Curr. Opin. Cell Biol. 2003, 15, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between Cell Shape and Type of Collagen Synthesised as Chondrocytes Lose Their Cartilage Phenotype in Culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Lelièvre, S.; Lakins, J.N.; Chrenek, M.A.; Jones, J.C.R.; Giancotti, F.; Werb, Z.; Bissell, M.J. Β4 Integrin-Dependent Formation of Polarized Three-Dimensional Architecture Confers Resistance to Apoptosis in Normal and Malignant Mammary Epithelium. Cancer Cell 2002, 2, 205–216. [Google Scholar] [CrossRef]
- Meyers, J.; Craig, J.; Odde, D.J. Potential for Control of Signaling Pathways via Cell Size and Shape. Curr. Biol. 2006, 16, 1685–1693. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Gilbert, P.; Havenstrite, K.; Magnusson, K.; Sacco, A.; Leonardi, N.; Kraft, P.; Nguyen, N.; Thrun, S.; Lutolf, M.; Blau, H. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Greilberger, J.; Herwig, R.; Greilberger, M.; Stiegler, P.; Wintersteiger, R. Alpha-Ketoglutarate and 5-HMF: A Potential Anti-Tumoral Combination against Leukemia Cells. Antioxidants 2021, 10, 1804. [Google Scholar] [CrossRef]
- Wang, F.; Awan, U.F.; Wang, Y.; Wang, L.; Qing, H.; Ma, H.; Deng, Y. Damage of Neuroblastoma Cell SH-SY5Y Mediated by MPP+ Inhibits Proliferation of T-Cell Leukemia Jurkat by Co-Culture System. Int. J. Mol. Sci. 2014, 15, 10738–10750. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Marone, G.; Galdiero, M.R.; Guglielmi, G.; Foddis, R.; et al. Primary Cell Cultures for the Personalized Therapy in Aggressive Thyroid Cancer of Follicular Origin. Semin. Cancer Biol. 2022, 79, 203–216. [Google Scholar] [CrossRef]
- Raulf, M. T Cell: Primary Culture from Peripheral Blood. In Allergy: Methods and Protocols; Lympany, P., Jones, M.G., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 17–31. ISBN 978-1-4939-9591-2. [Google Scholar]
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266. [Google Scholar] [CrossRef]
- Nosalova, N.; Keselakova, A.; Kello, M.; Martinkova, M.; Fabianova, D.; Pilatova, M.B. Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro. Int. J. Mol. Sci. 2023, 24, 11696. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking Cell-Matrix Adhesions to the Third Dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in Establishment and Analysis of Three-Dimensional Tumor Spheroid-Based Functional Assays for Target Validation and Drug Evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef]
- Hamburger, A.W.; Salmon, S.E. Primary Bioassay of Human Tumor Stem Cells. Science 1977, 197, 461–463. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 334617. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Multicellular Spheroids. A Review on Cellular Aggregates in Cancer Research. J. Cancer Res. Clin. Oncol. 1987, 113, 101–122. [Google Scholar] [CrossRef]
- Doublier, S.; Belisario, D.C.; Polimeni, M.; Annaratone, L.; Riganti, C.; Allia, E.; Ghigo, D.; Bosia, A.; Sapino, A. HIF-1 Activation Induces Doxorubicin Resistance in MCF7 3-D Spheroids via P-Glycoprotein Expression: A Potential Model of the Chemo-Resistance of Invasive Micropapillary Carcinoma of the Breast. BMC Cancer 2012, 12, 4. [Google Scholar] [CrossRef]
- Ekert, J.E.; Johnson, K.; Strake, B.; Pardinas, J.; Jarantow, S.; Perkinson, R.; Colter, D.C. Three-Dimensional Lung Tumor Microenvironment Modulates Therapeutic Compound Responsiveness in Vitro--Implication for Drug Development. PLoS ONE 2014, 9, e92248. [Google Scholar] [CrossRef]
- Stadler, M.; Walter, S.; Walzl, A.; Kramer, N.; Unger, C.; Scherzer, M.; Unterleuthner, D.; Hengstschläger, M.; Krupitza, G.; Dolznig, H. Increased Complexity in Carcinomas: Analyzing and Modeling the Interaction of Human Cancer Cells with Their Microenvironment. Semin. Cancer Biol. 2015, 35, 107–124. [Google Scholar] [CrossRef]
- Shri, M.; Agrawal, H.; Rani, P.; Singh, D.; Onteru, S.K. Hanging Drop, A Best Three-Dimensional (3D) Culture Method for Primary Buffalo and Sheep Hepatocytes. Sci. Rep. 2017, 7, 1203. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS Inflammation and Neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-Dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Jaganathan, H.; Gage, J.; Leonard, F.; Srinivasan, S.; Souza, G.R.; Dave, B.; Godin, B. Three-Dimensional in Vitro Co-Culture Model of Breast Tumor Using Magnetic Levitation. Sci. Rep. 2014, 4, 6468. [Google Scholar] [CrossRef]
- Leonard, F.; Godin, B. 3D in Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods Mol. Biol. 2016, 1406, 239–251. [Google Scholar] [CrossRef]
- Quagliarini, E.; Digiacomo, L.; Caputo, D.; Coppola, A.; Amenitsch, H.; Caracciolo, G.; Pozzi, D. Magnetic Levitation of Personalized Nanoparticle-Protein Corona as an Effective Tool for Cancer Detection. Nanomaterials 2022, 12, 1397. [Google Scholar] [CrossRef]
- Digiacomo, L.; Quagliarini, E.; Marmiroli, B.; Sartori, B.; Perini, G.; Papi, M.; Capriotti, A.L.; Montone, C.M.; Cerrato, A.; Caracciolo, G.; et al. Magnetic Levitation Patterns of Microfluidic-Generated Nanoparticle-Protein Complexes. Nanomaterials 2022, 12, 2376. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A Historical Perspective of Thinking in Three Dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Purves, D.; Lichtman, J.W. Principles of Neural Development; Sinauer Associates: Sunderland, MA, USA, 1985; ISBN 978-0-87893-744-8. [Google Scholar]
- Jung, P.; Sato, T.; Merlos-Suárez, A.; Barriga, F.M.; Iglesias, M.; Rossell, D.; Auer, H.; Gallardo, M.; Blasco, M.A.; Sancho, E.; et al. Isolation and in Vitro Expansion of Human Colonic Stem Cells. Nat. Med. 2011, 17, 1225–1227. [Google Scholar] [CrossRef]
- Zaret, K.S. Regulatory Phases of Early Liver Development: Paradigms of Organogenesis. Nat. Rev. Genet. 2002, 3, 499–512. [Google Scholar] [CrossRef]
- Heavner, W.; Pevny, L. Eye Development and Retinogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008391. [Google Scholar] [CrossRef]
- Jamieson, P.R.; Dekkers, J.F.; Rios, A.C.; Fu, N.Y.; Lindeman, G.J.; Visvader, J.E. Derivation of a Robust Mouse Mammary Organoid System for Studying Tissue Dynamics. Development 2017, 144, 1065–1071. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Efraim, Y.; Schoen, B.; Zahran, S.; Davidov, T.; Vasilyev, G.; Baruch, L.; Zussman, E.; Machluf, M. 3D Structure and Processing Methods Direct the Biological Attributes of ECM-Based Cardiac Scaffolds. Sci. Rep. 2019, 9, 5578. [Google Scholar] [CrossRef]
- Shamir, E.R.; Ewald, A.J. Three-Dimensional Organotypic Culture: Experimental Models of Mammalian Biology and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef]
- Peng, Y.; Zhuang, Y.; Liu, Y.; Le, H.; Li, D.; Zhang, M.; Liu, K.; Zhang, Y.; Zuo, J.; Ding, J. Bioinspired Gradient Scaffolds for Osteochondral Tissue Engineering. Exploration 2023, 3, 20210043. [Google Scholar] [CrossRef]
- Unnikrishnan, K.; Thomas, L.V.; Ram Kumar, R.M. Advancement of Scaffold-Based 3D Cellular Models in Cancer Tissue Engineering: An Update. Front. Oncol. 2021, 11, 733652. [Google Scholar] [CrossRef]
- Jordahl, S.; Solorio, L.; Neale, D.B.; McDermott, S.; Jordahl, J.H.; Fox, A.; Dunlay, C.; Xiao, A.; Brown, M.; Wicha, M.; et al. Engineered Fibrillar Fibronectin Networks as Three-Dimensional Tissue Scaffolds. Adv. Mater. 2019, 31, e1904580. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, C.; Yao, R.; Sui, A.; Sun, L.; Liu, X.; Wu, S.; Su, Z.; Li, T.; Liu, S.; et al. 3D Culture Enhances Chemoresistance of ALL Jurkat Cell Line by Increasing DDR1 Expression. Exp. Ther. Med. 2019, 17, 1593–1600. [Google Scholar] [CrossRef]
- Worthington, P.; Drake, K.M.; Li, Z.; Napper, A.D.; Pochan, D.J.; Langhans, S.A. Beta-Hairpin Hydrogels as Scaffolds for High-Throughput Drug Discovery in Three-Dimensional Cell Culture. Anal. Biochem. 2017, 535, 25–34. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Jakubikova, J.; Cholujova, D.; Hideshima, T.; Gronesova, P.; Soltysova, A.; Harada, T.; Joo, J.; Kong, S.-Y.; Szalat, R.E.; Richardson, P.G.; et al. A Novel 3D Mesenchymal Stem Cell Model of the Multiple Myeloma Bone Marrow Niche: Biologic and Clinical Applications. Oncotarget 2016, 7, 77326–77341. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Maniglia, B.C.; Buchet, R.; Millán, J.L.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Three-Dimensional Cell-Laden Collagen Scaffolds: From Biochemistry to Bone Bioengineering. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 967–983. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen Scaffolds for Tissue Engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Glowacki, J. A Review of Osteoinductive Testing Methods and Sterilization Processes for Demineralized Bone. Cell Tissue Bank 2005, 6, 3–12. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Knight, E.; Murray, B.; Carnachan, R.; Przyborski, S. Alvetex®: Polystyrene Scaffold Technology for Routine Three Dimensional Cell Culture. Methods Mol. Biol. 2011, 695, 323–340. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R.; Orive, G. Endogenous Morphogens and Fibrin Bioscaffolds for Stem Cell Therapeutics. Trends Biotechnol. 2013, 31, 364–374. [Google Scholar] [CrossRef]
- Kural, M.H.; Billiar, K.L. Regulating Tension in Three-Dimensional Culture Environments. Exp. Cell Res. 2013, 319, 2447–2459. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-Based Biomaterials: Modulation of Macroscopic Properties through Rational Design at the Molecular Level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-Fibrinogen Hydrogels for Three-Dimensional Breast Cancer Cell Culture. J. Biomed. Mater. Res. A 2017, 105, 236–252. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin Scaffolds in Tissue Engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.-C. Redox-Responsive Alginate Microsphere Containing Cystamine. J. Biomater. Sci. Polym. Ed. 2016, 27, 1520–1533. [Google Scholar] [CrossRef]
- Complex Coacervate-Based Materials for Biomedicine—Blocher—2017—WIREs Nanomedicine and Nanobiotechnology—Wiley Online Library. Available online: https://wires.onlinelibrary.wiley.com/doi/full/10.1002/wnan.1442 (accessed on 25 January 2024).
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Pan, Z.; Hao, Y.; Wang, C.; Dong, Z.; Li, Q.; Han, Y.; Tian, L.; Feng, L.; et al. Metallo-Alginate Hydrogel Can Potentiate Microwave Tumor Ablation for Synergistic Cancer Treatment. Sci. Adv. 2022, 8, eabo5285. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Martino, M.M.; Hubbell, J.A. The 12th–14th Type III Repeats of Fibronectin Function as a Highly Promiscuous Growth Factor-Binding Domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Haskins, K.; Anseth, K.S. The Effects of Cell-Matrix Interactions on Encapsulated Beta-Cell Function within Hydrogels Functionalized with Matrix-Derived Adhesive Peptides. Biomaterials 2007, 28, 3004–3011. [Google Scholar] [CrossRef]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kirita, Y.; Kami, D.; Kitani, T.; Ozaki, C.; Itakura, Y.; Toyoda, M.; Gojo, S. Novel Detergent for Whole Organ Tissue Engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3364–3373. [Google Scholar] [CrossRef]
- Simsa, R.; Padma, A.M.; Heher, P.; Hellström, M.; Teuschl, A.; Jenndahl, L.; Bergh, N.; Fogelstrand, P. Systematic in Vitro Comparison of Decellularization Protocols for Blood Vessels. PLoS ONE 2018, 13, e0209269. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised Scaffolds: Just a Framework? Current Knowledge and Future Directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef]
- Varinelli, L.; Guaglio, M.; Brich, S.; Zanutto, S.; Belfiore, A.; Zanardi, F.; Iannelli, F.; Oldani, A.; Costa, E.; Chighizola, M.; et al. Decellularized Extracellular Matrix as Scaffold for Cancer Organoid Cultures of Colorectal Peritoneal Metastases. J. Mol. Cell Biol. 2023, 14, mjac064. [Google Scholar] [CrossRef]
- Mao, J.; Yang, W.; Guo, H.; Dong, R.; Ren, L.; Li, S. A cervical cancer tissue-derived decellularized extracellular matrix scaffold for cervical cancer tissue reconstruction in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2023, 43, 157–165. [Google Scholar] [CrossRef]
- Liu, G.; Wang, B.; Li, S.; Jin, Q.; Dai, Y. Human Breast Cancer Decellularized Scaffolds Promote Epithelial-to-Mesenchymal Transitions and Stemness of Breast Cancer Cells in Vitro. J. Cell. Physiol. 2019, 234, 9447–9456. [Google Scholar] [CrossRef]
- Maia, F.R.; Reis, R.L.; Oliveira, J.M. Nanoparticles and Microfluidic Devices in Cancer Research. In Biomaterials- and Microfluidics-Based Tissue Engineered 3D Models; Oliveira, J.M., Reis, R.L., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 161–171. ISBN 978-3-030-36588-2. [Google Scholar]
- Aw Yong, K.M.; Li, Z.; Merajver, S.D.; Fu, J. Tracking the Tumor Invasion Front Using Long-Term Fluidic Tumoroid Culture. Sci. Rep. 2017, 7, 10784. [Google Scholar] [CrossRef]
- Montanez-Sauri, S.I.; Sung, K.E.; Berthier, E.; Beebe, D.J. Enabling Screening in 3D Microenvironments: Probing Matrix and Stromal Effects on the Morphology and Proliferation of T47D Breast Carcinoma Cells. Integr. Biol. 2013, 5, 631–640. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.A.; Zhang, H.; Zhang, Y.; Cui, W. Advanced Microfluidic Devices for Fabricating Multi-Structural Hydrogel Microsphere. Exploration 2021, 1, 20210036. [Google Scholar] [CrossRef]
- Microfluidics for Cancer Nanomedicine: From Fabrication to Evaluation—Zhang—2018—Small—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/smll.201800360 (accessed on 11 April 2024).
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-Dimensional in Vitro Tumor Models for Cancer Research and Drug Evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef]
- Yun, H.; Kim, K.; Lee, W.G. Cell Manipulation in Microfluidics. Biofabrication 2013, 5, 022001. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zou, J.; Jia, C.; Hu, Y.; Du, H.; Wang, H. Evaluation of Photodynamic Therapy Efficiency Using an in Vitro Three-Dimensional Microfluidic Breast Cancer Tissue Model. Lab Chip 2015, 15, 735–744. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yin, L.; Hu, R.; Qiu, T.; Yin, Y.; Xiong, X.; Zheng, H.; Wang, Q. A PH-Sensitive Nanosystem Based on Carboxymethyl Chitosan for Tumor-Targeted Delivery of Daunorubicin. J. Biomed. Nanotechnol. 2016, 12, 1688–1698. [Google Scholar] [CrossRef]
- Maia, F.R.; Reis, R.L.; Oliveira, J.M. Finding the Perfect Match between Nanoparticles and Microfluidics to Respond to Cancer Challenges. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102139. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J.; et al. Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan Nanoparticles Containing Covalently Entrapped 6-Mercaptopurine for Enhanced Intracellular Drug Delivery in Leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef]
- Reyes, D.R.; van Heeren, H.; Guha, S.; Herbertson, L.; Tzannis, A.P.; Ducrée, J.; Bissig, H.; Becker, H. Accelerating Innovation and Commercialization through Standardization of Microfluidic-Based Medical Devices. Lab Chip 2021, 21, 9–21. [Google Scholar] [CrossRef]
- Mastrangeli, M.; Millet, S.; Mummery, C.; Loskill, P.; Braeken, D.; Eberle, W.; Cipriano, M.; Fernandez, L.; Graef, M.; Gidrol, X.; et al. Building Blocks for a European Organ-on-Chip Roadmap. ALTEX 2019, 36, 481–492. [Google Scholar] [CrossRef]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O.; et al. Biology-Inspired Microphysiological System Approaches to Solve the Prediction Dilemma of Substance Testing. ALTEX 2016, 33, 272–321. [Google Scholar] [CrossRef]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach to Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-Chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Mastrangeli, M.; van den Eijnden-van Raaij, J. Organs-on-Chip: The Way Forward. Stem. Cell Rep. 2021, 16, 2037–2043. [Google Scholar] [CrossRef]
- Bērziņa, S.; Harrison, A.; Taly, V.; Xiao, W. Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Wan, L.; Neumann, C.A.; LeDuc, P.R. Tumor-on-a-Chip for Integrating a 3D Tumor Microenvironment: Chemical and Mechanical Factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef]
- Pires de Mello, C.P.; Carmona-Moran, C.; McAleer, C.W.; Perez, J.; Coln, E.A.; Long, C.J.; Oleaga, C.; Riu, A.; Note, R.; Teissier, S.; et al. Microphysiological Heart-Liver Body-on-a-Chip System with a Skin Mimic for Evaluating Topical Drug Delivery. Lab Chip 2020, 20, 749–759. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A Four-Organ-Chip for Interconnected Long-Term Co-Culture of Human Intestine, Liver, Skin and Kidney Equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef]
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D Vascularized Organotypic Microfluidic Assays to Study Breast Cancer Cell Extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. A Micro Cell Culture Analog (MicroCCA) with 3-D Hydrogel Culture of Multiple Cell Lines to Assess Metabolism-Dependent Cytotoxicity of Anti-Cancer Drugs. Lab Chip 2009, 9, 1385–1394. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent No. 638905, 1984. [Google Scholar]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles and Applications; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-803030-1. [Google Scholar]
- Shukla, P.; Yeleswarapu, S.; Heinrich, M.A.; Prakash, J.; Pati, F. Mimicking Tumor Microenvironment by 3D Bioprinting: 3D Cancer Modeling. Biofabrication 2022, 14, 032002. [Google Scholar] [CrossRef]
- Knowlton, S.; Onal, S.; Yu, C.H.; Zhao, J.J.; Tasoglu, S. Bioprinting for Cancer Research. Trends Biotechnol. 2015, 33, 504–513. [Google Scholar] [CrossRef]
- 3D Bioprinted Drug-Resistant Breast Cancer Spheroids for Quantitative In Situ Evaluation of Drug Resistance—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1742706121006978 (accessed on 9 February 2024).
- Rosanoff, E.I. Continuous Cultivation of Malignant Cells from a Canine Testicular Tumor. Proc. Soc. Exp. Biol. Med. 1959, 100, 384–387. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Leitner, N.; Hlavatý, J.; Ertl, R.; Gabner, S.; Fuchs-Baumgartinger, A.; Walter, I. Lipid Droplets and Perilipins in Canine Osteosarcoma. Investigations on Tumor Tissue, 2D and 3D Cell Culture Models. Vet. Res. Commun. 2022, 46, 1175–1193. [Google Scholar] [CrossRef]
- Inglebert, M.; Dettwiler, M.; Hahn, K.; Letko, A.; Drogemuller, C.; Doench, J.; Brown, A.; Memari, Y.; Davies, H.R.; Degasperi, A.; et al. A Living Biobank of Canine Mammary Tumor Organoids as a Comparative Model for Human Breast Cancer. Sci. Rep. 2022, 12, 18051. [Google Scholar] [CrossRef]
- San Miguel, Y.; Gomez, S.L.; Murphy, J.D.; Schwab, R.B.; McDaniels-Davidson, C.; Canchola, A.J.; Molinolo, A.A.; Nodora, J.N.; Martinez, M.E. Age-Related Differences in Breast Cancer Mortality According to Race/Ethnicity, Insurance, and Socioeconomic Status. BMC Cancer 2020, 20, 228. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 2: Immunophenotypes and Prognostic Significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Raposo, T.; Carvalho, M.I.; Prada, J.; Pires, I. Canine Mammary Tumours as a Model to Study Human Breast Cancer: Most Recent Findings. In Vivo 2011, 25, 455–465. [Google Scholar]
- Abdelmegeed, S.M.; Mohammed, S. Canine Mammary Tumors as a Model for Human Disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef]
- Zeng, L.; Li, W.; Chen, C.-S. Breast Cancer Animal Models and Applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef]
- Murphy, S. Mammary Tumours in Dogs and Cats. In Pract. 2008, 30, 334–339. [Google Scholar] [CrossRef]
- Kristiansen, V.M.; Peña, L.; Díez Córdova, L.; Illera, J.C.; Skjerve, E.; Breen, A.M.; Cofone, M.A.; Langeland, M.; Teige, J.; Goldschmidt, M.; et al. Effect of Ovariohysterectomy at the Time of Tumor Removal in Dogs with Mammary Carcinomas: A Randomized Controlled Trial. J. Vet. Intern. Med. 2016, 30, 230–241. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, C.; Xian, H.; Zhang, X.; Li, J.; Liang, Z.-X.; Zhi, Y.; Ma, Y.; Wang, H.-J. Toosendanin-Induced Apoptosis of CMT-U27 Is Mediated through the Mitochondrial Apoptotic Pathway. Vet. Comp. Oncol. 2023, 21, 315–326. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Liu, J.; Zhang, D.; Diao, H.; Lin, D. Establishment of Stable Expression of Firefly Luciferase and EGFP in a Canine Inflammatory Mammary Carcinoma Cell Line and Tumor-Bearing Model in Nude Mice. Front. Vet. Sci. 2022, 9, 935005. [Google Scholar] [CrossRef] [PubMed]
- Ustun-Alkan, F.; Bakırel, T.; Üstüner, O.; Anlas, C.; Cinar, S.; Yıldırım, F.; Gürel, A. Effects of Tyrosine Kinase Inhibitor-Masitinib Mesylate on Canine Mammary Tumour Cell Lines. J. Vet. Res. 2021, 65, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.B.; Nagamine, M.K.; Biondi, L.R.; Sanches, D.S.; Toyota, F.; Giovani, T.M.; de Jesus, I.P.; da Fonseca, I.I.M.; Queiroz-Hazarbassanov, N.; Diaz, B.L.; et al. Establishment of Primary Mixed Cell Cultures from Spontaneous Canine Mammary Tumors: Characterization of Classic and New Cancer-Associated Molecules. PLoS ONE 2017, 12, e0184228. [Google Scholar] [CrossRef]
- Tlsty, T.D.; Coussens, L.M. Tumor Stroma and Regulation of Cancer Development. Annu. Rev. Pathol. 2006, 1, 119–150. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, D.; Chakrabarty, S. Growth Factors in Tumor Microenvironment. Front. Biosci. 2010, 15, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, J.; Chen, S.; Ma, J.; Zhang, X.; Huang, S.; Hu, J.; Yue, T.; Zhang, J.; Wang, P.; et al. Low Expression of SPARC in Gastric Cancer-Associated Fibroblasts Leads to Stemness Transformation and 5-Fluorouracil Resistance in Gastric Cancer. Cancer Cell. Int. 2019, 19, 137. [Google Scholar] [CrossRef]
- Nasiraee, M.R.; Shahrivari, S.; Sayad, S.; Mahdavi, H.; Saraygord-Afshari, N.; Bagheri, Z. An Agarose-Alginate Microfluidic Device for the Study of Spheroid Invasion, ATRA Inhibits CAFs-Mediated Matrix Remodeling. Cytotechnology 2023, 75, 309–323. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F.; Baldini, N. Pre-Clinical Models for Studying the Interaction Between Mesenchymal Stromal Cells and Cancer Cells and the Induction of Stemness. Front. Oncol. 2019, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Devarasetty, M.; Wang, E.; Soker, S.; Skardal, A. Mesenchymal Stem Cells Support Growth and Organization of Host-Liver Colorectal-Tumor Organoids and Possibly Resistance to Chemotherapy. Biofabrication 2017, 9, 021002. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer Cells Enter Dormancy after Cannibalizing Mesenchymal Stem/Stromal Cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of Tumor-Associated Macrophages in Neoplastic Progression and Immune Surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Xiong, G.; St Clair, D.K.; Xu, W.; Xu, R. Increased ROS Production in Non-Polarized Mammary Epithelial Cells Induces Monocyte Infiltration in 3D Culture. J. Cell Sci. 2017, 130, 190–202. [Google Scholar] [CrossRef]
- Koeck, S.; Kern, J.; Zwierzina, M.; Gamerith, G.; Lorenz, E.; Sopper, S.; Zwierzina, H.; Amann, A. The Influence of Stromal Cells and Tumor-Microenvironment-Derived Cytokines and Chemokines on CD3+CD8+ Tumor Infiltrating Lymphocyte Subpopulations. Oncoimmunology 2017, 6, e1323617. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, X.; Wang, S.; Trinkle, C. Tumor Organoid Models in Precision Medicine and Investigating Cancer-Stromal Interactions. Pharmacol. Ther. 2021, 218, 107668. [Google Scholar] [CrossRef]
- Livesey, S.A.; Herndon, D.N.; Hollyoak, M.A.; Atkinson, Y.H.; Nag, A. Transplanted Acellular Allograft Dermal Matrix. Potential as a Template for the Reconstruction of Viable Dermis. Transplantation 1995, 60, 1–9. [Google Scholar] [CrossRef]
- Sutherland, R.S.; Baskin, L.S.; Hayward, S.W.; Cunha, G.R. Regeneration of Bladder Urothelium, Smooth Muscle, Blood Vessels and Nerves into an Acellular Tissue Matrix. J. Urol. 1996, 156, 571–577. [Google Scholar] [CrossRef]
- Drakos, S.G.; Terrovitis, J.V.; Nanas, J.N.; Charitos, E.I.; Ntalianis, A.S.; Malliaras, K.G.; Diakos, N.; Koudoumas, D.; Theodoropoulos, S.; Yacoub, M.H.; et al. Reverse Electrophysiologic Remodeling after Cardiac Mechanical Unloading for End-Stage Nonischemic Cardiomyopathy. Ann. Thorac. Surg. 2011, 91, 764–769. [Google Scholar] [CrossRef]
- Xiong, G.; Flynn, T.J.; Chen, J.; Trinkle, C.; Xu, R. Development of an Ex Vivo Breast Cancer Lung Colonization Model Utilizing a Decellularized Lung Matrix. Integr. Biol. 2015, 7, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.B.; Cukierman, E.; Artym, V.V. 3-D Extracellular Matrix from Sectioned Human Tissues. Curr. Protoc. Cell Biol. 2014, 62, 19.16.1–19.16.20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Shuler, M.L. Engineering a Bioartificial Human Colon Model Through Decellularization and Recellularization. Methods Mol. Biol. 2019, 1907, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, A.; Król, M. Identification and Characterization of Cancer Stem Cells in Canine Mammary Tumors. Acta Vet. Scand. 2016, 58, 86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Wang, S.B.; Liu, J.H.; Jin, L.; Liu, Y.; Li, C.Y.; Su, Y.R.; Liu, Y.R.; Sang, X.; Wan, Q.; et al. Modifying the Tumour Microenvironment and Reverting Tumour Cells: New Strategies for Treating Malignant Tumours. Cell Prolif. 2020, 53, e12865. [Google Scholar] [CrossRef] [PubMed]
- Trams, E.G.; Lauter, C.J.; Salem, N.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.C.-K.; Oentaryo, M.J.; Lee, C.W. MMP-Mediated Modulation of ECM Environment during Axonal Growth and NMJ Development. Neurosci. Lett. 2020, 724, 134822. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Das, A.; Sagi, I. Emerging Roles of ECM Remodeling Processes in Cancer. Semin. Cancer Biol. 2020, 62, 192–200. [Google Scholar] [CrossRef]
- Vuksanaj, K. Spheroids, Organoids Replacing Standard Cultures for Cell-Based Assays. Available online: https://www.genengnews.com/insights/spheroids-organoids-replacing-standard-cultures-for-cell-based-assays/ (accessed on 9 February 2024).
- Cardoso, T.C.; Sakamoto, S.S.; Stockmann, D.; Souza, T.F.B.; Ferreira, H.L.; Gameiro, R.; Vieira, F.V.; Louzada, M.J.Q.; Andrade, A.L.; Flores, E.F. A Three-Dimensional Cell Culture System as an in Vitro Canine Mammary Carcinoma Model for the Expression of Connective Tissue Modulators. Vet. Comp. Oncol. 2017, 15, 582–593. [Google Scholar] [CrossRef]
- Gama, A.; Gärtner, F.; Alves, A.; Schmitt, F. Immunohistochemical Expression of Epidermal Growth Factor Receptor (EGFR) in Canine Mammary Tissues. Res. Vet. Sci. 2009, 87, 432–437. [Google Scholar] [CrossRef]
- Gumuskaya, B.; Alper, M.; Hucumenoglu, S.; Altundag, K.; Uner, A.; Guler, G. EGFR Expression and Gene Copy Number in Triple-Negative Breast Carcinoma. Cancer Genet. Cytogenet. 2010, 203, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lamp, O.; Honscha, K.U.; Schweizer, S.; Heckmann, A.; Blaschzik, S.; Einspanier, A. The Metastatic Potential of Canine Mammary Tumours Can Be Assessed by MRNA Expression Analysis of Connective Tissue Modulators. Vet. Comp. Oncol. 2013, 11, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, F.; Di Cosimo, S.; Arpino, G. Human Epidermal Growth Factor Receptor 2 (HER2)-Positive and Hormone Receptor-Positive Breast Cancer: New Insights into Molecular Interactions and Clinical Implications. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Lamp, O.; Honscha, K.U.; Jakob, J.; Lamp, J.; Schweizer, S.; Reischauer, A.; Gottschalk, J.; Hahn, A.; Ebert, M.; Rothemund, S.; et al. Investigation of the Local Expression of the Relaxin System in Canine Mammary Tumours. Reprod. Domest. Anim. 2009, 44, 224–229. [Google Scholar] [CrossRef]
- WHO Classification of Tumours of the Breast—UQ ESpace. Available online: https://espace.library.uq.edu.au/view/UQ:8984059 (accessed on 8 January 2024).
- Sachs, N.; De Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Jacob, K. Invasion Assays. Curr. Protoc. Cell. Biol. 2001. [Google Scholar] [CrossRef]
- Manuali, E.; De Giuseppe, A.; Feliziani, F.; Forti, K.; Casciari, C.; Marchesi, M.C.; Pacifico, E.; Pawłowski, K.M.; Majchrzak, K.; Król, M. CA 15-3 Cell Lines and Tissue Expression in Canine Mammary Cancer and the Correlation between Serum Levels and Tumour Histological Grade. BMC Vet. Res. 2012, 8, 86. [Google Scholar] [CrossRef]
- Król, M.; Pawłowski, K.M.; Majchrzak, K.; Gajewska, M.; Majewska, A.; Motyl, T. Global Gene Expression Profiles of Canine Macrophages and Canine Mammary Cancer Cells Grown as a Co-Culture in Vitro. BMC Vet. Res. 2012, 8, 16. [Google Scholar] [CrossRef]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible Bioprinting: Adaptation of a Low-Cost 3D-Printer for Precise Cell Placement and Stem Cell Differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef]
- Mollica, P.A.; Creech, E.; Reid, J.A.; Zamponi, M.; Sullivan, S.M.; Palmer, X.-L.; Sachs, P.C.; Bruno, R.D. 3D Bioprinted Mammary Organoids and Tumoroids in Human Mammary Derived ECM Hydrogels. Acta Biomater. 2019, 95, 201–213. [Google Scholar] [CrossRef]
- Bruno, R.D.; Smith, G.H. Reprogramming Non-Mammary and Cancer Cells in the Developing Mouse Mammary Gland. Semin. Cell Dev. Biol. 2012, 23, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Palmer, X.-L.; Mollica, P.A.; Northam, N.; Sachs, P.C.; Bruno, R.D. A 3D Bioprinter Platform for Mechanistic Analysis of Tumoroids and Chimeric Mammary Organoids. Sci. Rep. 2019, 9, 7466. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2017. Available online: https://seer.cancer.gov/csr/1975_2017/index.html (accessed on 8 January 2024).
- Truong, D.; Puleo, J.; Llave, A.; Mouneimne, G.; Kamm, R.D.; Nikkhah, M. Breast Cancer Cell Invasion into a Three Dimensional Tumor-Stroma Microenvironment. Sci. Rep. 2016, 6, 34094. [Google Scholar] [CrossRef]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-Chip Platform to Investigate Progression and Drug Sensitivity in Cell Lines and Patient-Derived Organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef] [PubMed]
- Firatligil-Yildirir, B.; Bati-Ayaz, G.; Tahmaz, I.; Bilgen, M.; Pesen-Okvur, D.; Yalcin-Ozuysal, O. On-Chip Determination of Tissue-Specific Metastatic Potential of Breast Cancer Cells. Biotechnol. Bioeng. 2021, 118, 3799–3810. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huniadi, M.; Nosálová, N.; Almášiová, V.; Horňáková, Ľ.; Valenčáková, A.; Hudáková, N.; Cizkova, D. Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro. Cells 2024, 13, 695. https://doi.org/10.3390/cells13080695
Huniadi M, Nosálová N, Almášiová V, Horňáková Ľ, Valenčáková A, Hudáková N, Cizkova D. Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro. Cells. 2024; 13(8):695. https://doi.org/10.3390/cells13080695
Chicago/Turabian StyleHuniadi, Mykhailo, Natália Nosálová, Viera Almášiová, Ľubica Horňáková, Alexandra Valenčáková, Nikola Hudáková, and Dasa Cizkova. 2024. "Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro" Cells 13, no. 8: 695. https://doi.org/10.3390/cells13080695
APA StyleHuniadi, M., Nosálová, N., Almášiová, V., Horňáková, Ľ., Valenčáková, A., Hudáková, N., & Cizkova, D. (2024). Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro. Cells, 13(8), 695. https://doi.org/10.3390/cells13080695






