Selective Targeting of α4β7/MAdCAM-1 Axis Suppresses Fibrosis Progression by Reducing Proinflammatory T Cell Recruitment to the Liver
Abstract
:1. Introduction
2. Methods
2.1. Mouse Studies
2.2. Mouse Model of Liver Fibrosis and α4β7 and MAdCAM-1 Antibody Treatment
2.3. Human Tissue
2.4. Histopathology
2.5. Immunofluorescence Microscopy
2.6. Serological Analysis
2.7. Flow Cytometric Analysis
2.8. Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
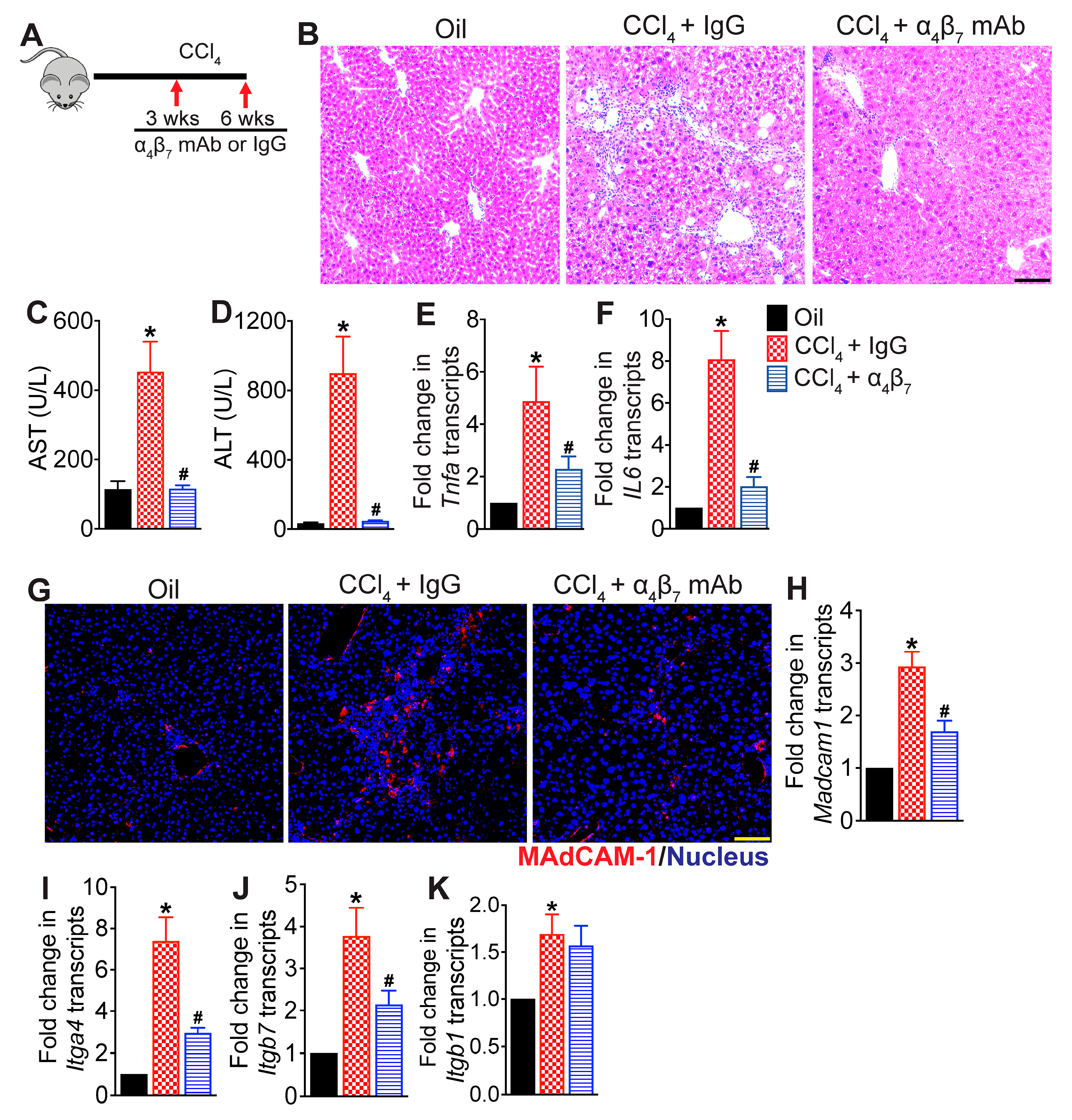
3.1. Integrin α4β7 Blockade Attenuates CCl4-Induced Hepatic Inflammation
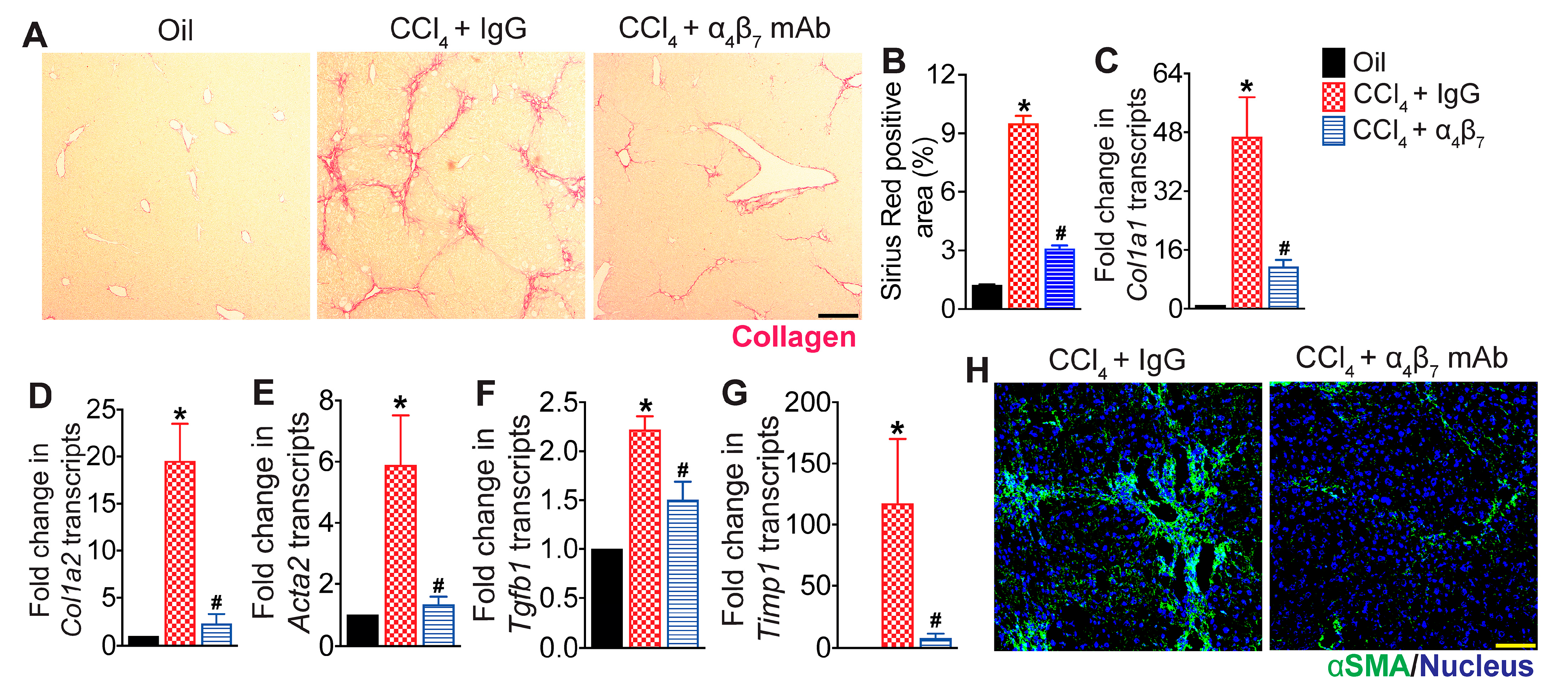
3.2. Integrin α4β7 Blockade Reduces Hepatic Fibrosis Progression
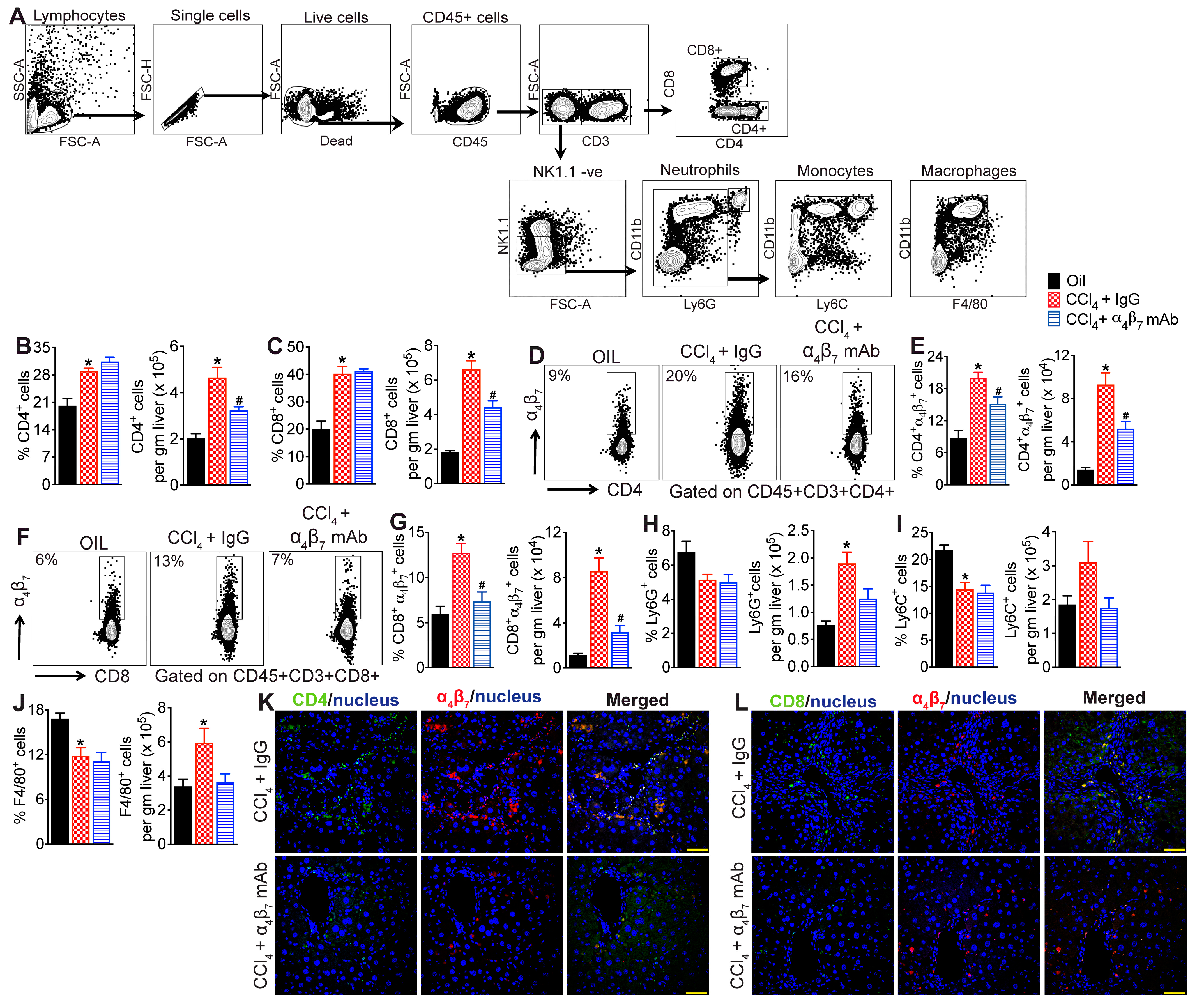
3.3. α4β7 mAb Treatment Reduces Accumulation of α4β7+ T Cells in the Fibrotic Liver
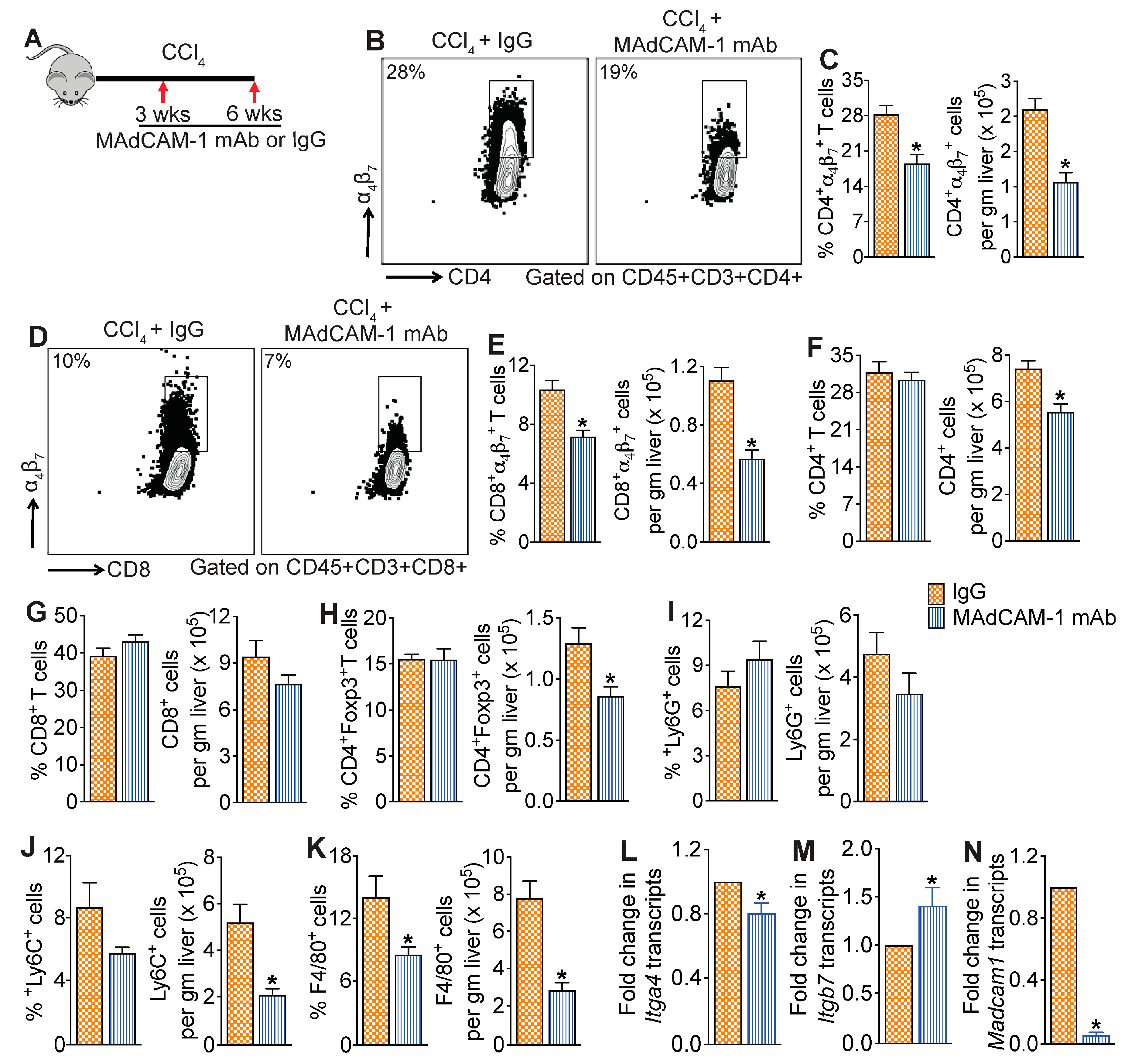
3.4. MAdCAM-1 Blockade Reduces CCl4-Induced Hepatic Infiltration of α4β7+ T Cells
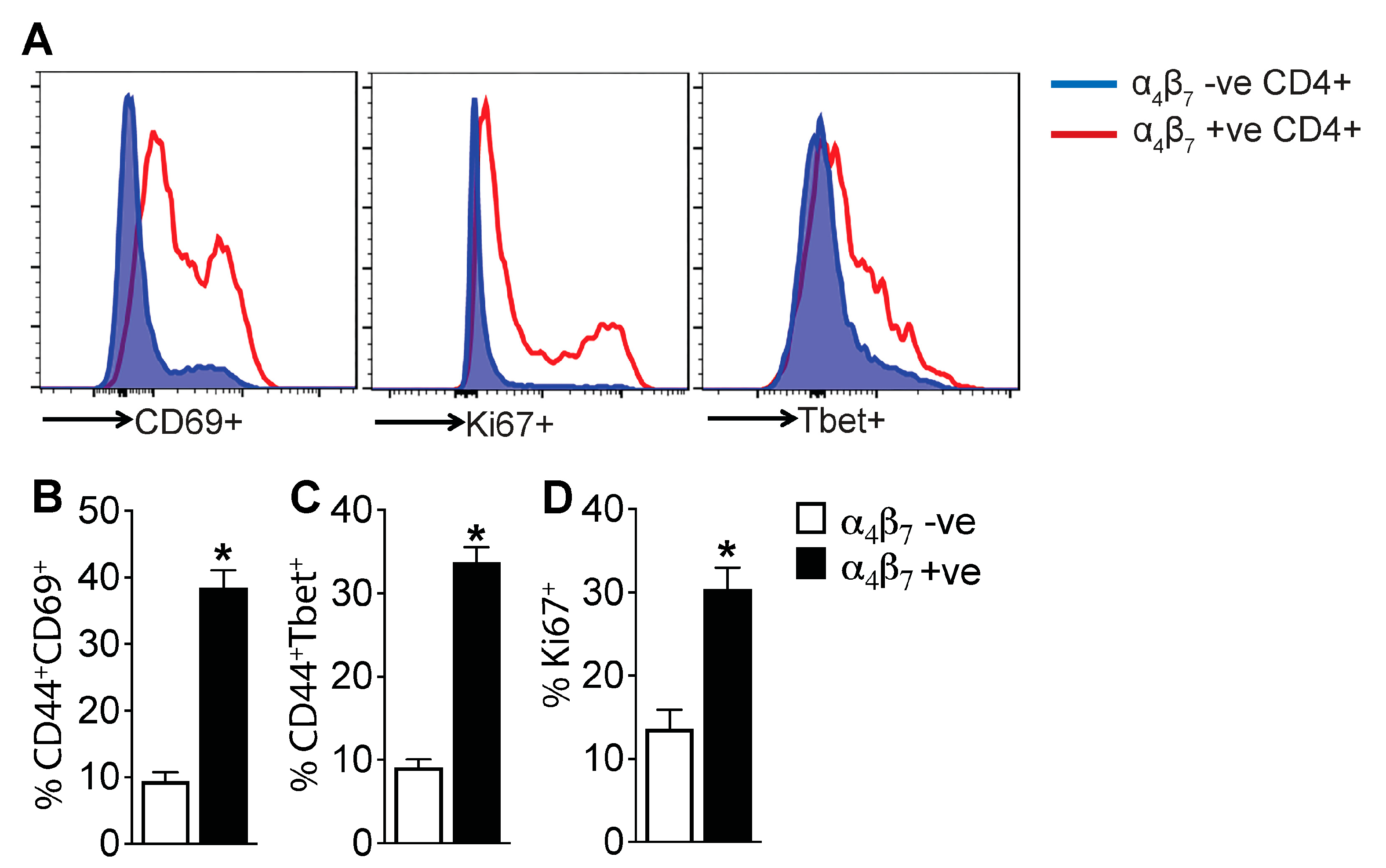
3.5. α4β7+ CD4 T Cells Are Enriched for Markers of Activation and Proliferation, Demonstrating an Effector Phenotype
3.6. MAdCAM-1 Blockade Attenuates CCl4-Induced Hepatic Injury and Fibrosis
3.7. Increased Infiltration of α4β7+ T Cells in the Livers of NASH- and ASH-Associated Cirrhosis Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019, 70, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; McCullough, A.J. Noninvasive Diagnosis of NASH and Liver Fibrosis Within the Spectrum of NAFLD. Gastroenterol. Hepatol. (N. Y.) 2012, 8, 661–668. [Google Scholar] [PubMed]
- Pinzani, M.; Rombouts, K.; Colagrande, S. Fibrosis in chronic liver diseases: Diagnosis and management. J. Hepatol. 2005, 42, S22–S36. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.P.; Liu, Y.; Iyer, S.S.; Liu, S.; Gupta, B.; Desai, C.; Kumar, P.; Smith, T.; Singhi, A.D.; Nusrat, A.; et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J. Hepatol. 2020, 73, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Ala, A.; Brown, D.; Khan, K.; Standish, R.; Odin, J.A.; Fiel, M.I.; Schiano, T.D.; Hillan, K.J.; Rahman, S.A.; Hodgson, H.J.F.; et al. Mucosal Addressin Cell Adhesion Molecule (MAdCAM-1) Expression Is Upregulated in the Cirrhotic Liver and Immunolocalises to the Peribiliary Plexus and Lymphoid Aggregates. Dig. Dis. Sci. 2013, 58, 2528–2541. [Google Scholar] [CrossRef]
- Grant, A.J.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Adams, D.H. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). J. Hepatol. 2001, 33, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Shimoda, S.; Bowlus, C.; Keen, C.L.; Gershwin, M.E. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin. Immunopathol. 2009, 31, 309–322. [Google Scholar] [CrossRef]
- Graham, J.J.; Mukherjee, S.; Yuksel, M.; Mateos, R.S.; Si, T.; Huang, Z.; Huang, X.; Abu Arqoub, H.; Patel, V.; McPhail, M.J.; et al. Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis. J. Hepatol. 2022, 75, 518–530. [Google Scholar] [CrossRef]
- Rahman, K.; Desai, C.; Iyer, S.S.; Thorn, N.E.; Kumar, P.; Liu, Y.; Smith, T.; Neish, A.S.; Li, H.; Tan, S.; et al. Loss of Junctional Adhesion Molecule a Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016, 151, 733–746.e12. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Karlmark, K.R.; E Wasmuth, H.; Trautwein, C.; Tacke, F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Karlmark, K.R.; Weiskirchen, R.; Zimmermann, H.W.; Gassler, N.; Ginhoux, F.; Weber, C.; Merad, M.; Luedde, T.; Trautwein, C.; Tacke, F. Hepatic recruitment of the inflammatory Gr1+monocyte subset upon liver injury promotes hepatic fibrosis. J. Hepatol. 2009, 50, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Hillan, K.J.; Hagler, K.E.; MacSween, R.N.M.; Ryan, A.M.; Renz, M.E.; Chiu, H.H.; Ferrier, R.K.; Bird, G.L.; Dhillon, A.P.; Ferrell, L.D.; et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver Int. 1999, 19, 509–518. [Google Scholar] [CrossRef]
- de Krijger, M.; Wildenberg, M.E.; de Jonge, W.J.; Ponsioen, C.Y. Return to sender: Lymphocyte trafficking mechanisms as contributors to primary sclerosing cholangitis. J. Hepatol. 2019, 71, 603–615. [Google Scholar] [CrossRef]
- Drescher, H.K.; Schippers, A.; Clahsen, T.; Sahin, H.; Noels, H.; Hornef, M.; Wagner, N.; Trautwein, C.; Streetz, K.L.; Kroy, D.C. β7-Integrin and MAdCAM-1 play opposing roles during the development of non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, 1251–1264. [Google Scholar] [CrossRef]
- Schippers, A.; Hübel, J.; Heymann, F.; Clahsen, T.; Eswaran, S.; Schlepütz, S.; Püllen, R.; Gaßler, N.; Tenbrock, K.; Tacke, F.; et al. MAdCAM-1/α4β7 Integrin-Mediated Lymphocyte/Endothelium Interactions Exacerbate Acute Immune-Mediated Hepatitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1227–1250.e1. [Google Scholar] [CrossRef]
- Wagner, N.; Löhler, J.; Kunkel, E.J.; Ley, K.; Leung, E.; Krissansen, G.; Rajewsky, K.; Muller, W. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 1996, 382, 366–370. [Google Scholar] [CrossRef]
- Sun, H.; Lagarrigue, F.; Gingras, A.R.; Fan, Z.; Ley, K.; Ginsberg, M.H. Transmission of integrin β7 transmembrane domain topology enables gut lymphoid tissue development. J. Cell Biol. 2018, 217, 1453–1465. [Google Scholar] [CrossRef]
- Tidswell, M.; Pachynski, R.; Wu, S.W.; Qiu, S.Q.; Dunham, E.; Cochran, N.; Briskin, M.J.; Kilshaw, P.J.; Lazarovits, A.I.; Andrew, D.P.; et al. Structure-function analysis of the integrin beta 7 subunit: Identification of domains involved in adhesion to MAdCAM-1. J. Immunol. 1997, 159, 1497–1505. [Google Scholar] [CrossRef]
- Soler, D.; Chapman, T.; Yang, L.-L.; Wyant, T.; Egan, R.; Fedyk, E.R. The Binding Specificity and Selective Antagonism of Vedolizumab, an Anti-α4β7 Integrin Therapeutic Antibody in Development for Inflammatory Bowel Diseases. J. Pharmacol. Exp. Ther. 2009, 330, 864–875. [Google Scholar] [CrossRef]
- Lazarovits, A.I.; A Moscicki, R.; Kurnick, J.T.; Camerini, D.; Bhan, A.K.; Baird, L.G.; Erikson, M.; Colvin, R.B. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J. Immunol. 1984, 133, 1857–1862. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173–183. [Google Scholar] [CrossRef]
- Arijs, I.; De Hertogh, G.; Lemmens, B.; Van Lommel, L.; De Bruyn, M.; Vanhove, W.; Cleynen, I.; Machiels, K.; Ferrante, M.; Schuit, F.; et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018, 67, 43–52. [Google Scholar] [CrossRef]
- Bargatze, R.F.; Jutila, M.A.; Butcher, E.C. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: The multistep model confirmed and refined. Immunity 1995, 3, 99–108. [Google Scholar] [CrossRef]
- Briskin, M.J.; McEvoy, L.M.; Butcher, E.C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature 1993, 363, 461–464. [Google Scholar] [CrossRef]
- Berg, E.L.; McEvoy, L.M.; Berlin, C.; Bargatze, R.F.; Butcher, E.C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 1993, 366, 695–698. [Google Scholar] [CrossRef]
- Wittner, M.; Schlicker, V.; Libera, J.; Bockmann, J.-H.; Horvatits, T.; Seiz, O.; Kummer, S.; Manthey, C.F.; Hüfner, A.; Kantowski, M.; et al. Comparison of the integrin α4β7 expression pattern of memory T cell subsets in HIV infection and ulcerative colitis. PLOS ONE 2019, 14, e0220008. [Google Scholar] [CrossRef]
- Kurmaeva, E.; Lord, J.D.; Zhang, S.; Bao, J.R.; Kevil, C.G.; Grisham, M.B.; Ostanin, D.V. T cell-associated α4β7 but not α4β1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal Immunol. 2014, 7, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, P.; Kam, S.; Heikenwalder, M. T cells: Friends and foes in NASH pathogenesis and hepatocarcinogenesis. J. Hepatol. 2022, 75, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef]
- Lee, H.-G.; Cho, M.-J.; Choi, J.-M. Bystander CD4+ T cells: Crossroads between innate and adaptive immunity. Exp. Mol. Med. 2020, 52, 1255–1263. [Google Scholar] [CrossRef]
- Campbell, C.; Rudensky, A. Roles of Regulatory T Cells in Tissue Pathophysiology and Metabolism. Cell Metab. 2020, 31, 18–25. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Kopf, M.; Bachmann, M.F.; Marsland, B.J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 2010, 9, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Fuschiotti, P.; Larregina, A.T.; Ho, J.; Feghali-Bostwick, C.; Medsger, T.A., Jr. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013, 65, 236–246. [Google Scholar] [CrossRef]
- Fuschiotti, P. Current perspectives on the role of CD8+ T cells in systemic sclerosis. Immunol. Lett. 2018, 195, 55–60. [Google Scholar] [CrossRef]
- Nakamura, K.; Amakawa, R.; Takebayashi, M.; Son, Y.; Miyaji, M.; Tajima, K.; Nakai, K.; Ito, T.; Matsumoto, N.; Zen, K.; et al. IL-4-producing CD8+ T cells may be an immunological hallmark of chronic GVHD. Bone Marrow Transplant. 2005, 36, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Teratani, T.; Chu, P.-S.; Hagihara, Y.; Mikami, Y.; Harada, Y.; Tsujikawa, H.; Miyamoto, K.; Suzuki, T.; Taniki, N.; et al. CD8+ tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, B.; Rai, R.P.; Pal, P.B.; Rossmiller, D.; Chaudhary, S.; Chiaro, A.; Seaman, S.; Singhi, A.D.; Liu, S.; Monga, S.P.; et al. Selective Targeting of α4β7/MAdCAM-1 Axis Suppresses Fibrosis Progression by Reducing Proinflammatory T Cell Recruitment to the Liver. Cells 2024, 13, 756. https://doi.org/10.3390/cells13090756
Gupta B, Rai RP, Pal PB, Rossmiller D, Chaudhary S, Chiaro A, Seaman S, Singhi AD, Liu S, Monga SP, et al. Selective Targeting of α4β7/MAdCAM-1 Axis Suppresses Fibrosis Progression by Reducing Proinflammatory T Cell Recruitment to the Liver. Cells. 2024; 13(9):756. https://doi.org/10.3390/cells13090756
Chicago/Turabian StyleGupta, Biki, Ravi Prakash Rai, Pabitra B. Pal, Daniel Rossmiller, Sudrishti Chaudhary, Anna Chiaro, Shannon Seaman, Aatur D. Singhi, Silvia Liu, Satdarshan P. Monga, and et al. 2024. "Selective Targeting of α4β7/MAdCAM-1 Axis Suppresses Fibrosis Progression by Reducing Proinflammatory T Cell Recruitment to the Liver" Cells 13, no. 9: 756. https://doi.org/10.3390/cells13090756
APA StyleGupta, B., Rai, R. P., Pal, P. B., Rossmiller, D., Chaudhary, S., Chiaro, A., Seaman, S., Singhi, A. D., Liu, S., Monga, S. P., Iyer, S. S., & Raeman, R. (2024). Selective Targeting of α4β7/MAdCAM-1 Axis Suppresses Fibrosis Progression by Reducing Proinflammatory T Cell Recruitment to the Liver. Cells, 13(9), 756. https://doi.org/10.3390/cells13090756







