TGFβ1 Restores Energy Homeostasis of Human Trophoblast Cells Under Hyperglycemia In Vitro by Inducing PPARγ Expression, AMPK Activation, and HIF1α Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
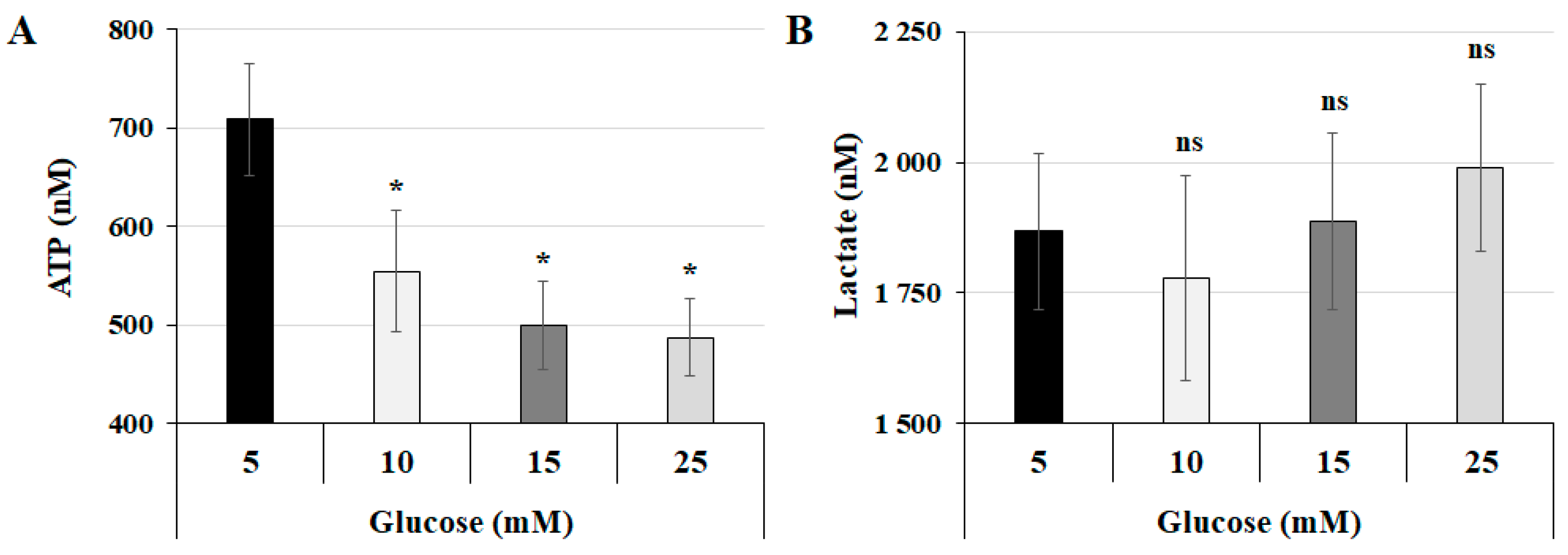
2.3. ATP Detection
2.4. Lactate Detection
2.5. Cell Viability/Proliferation
2.6. Subcellular Fractionation
2.7. Proteins Immunodetection
2.8. Statistical Analysis
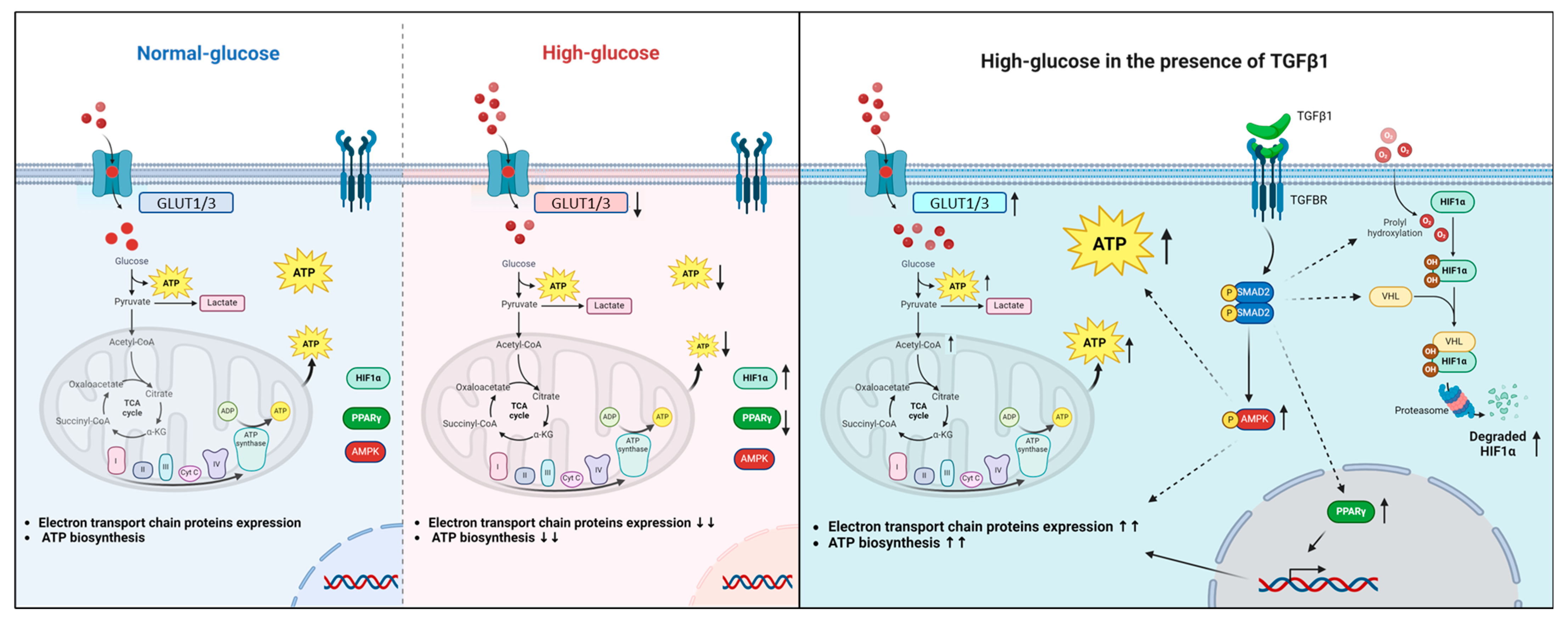
3. Results
3.1. The Interplay Between Glucose Concentration and Metabolic Changes in Human Trophoblast Cells
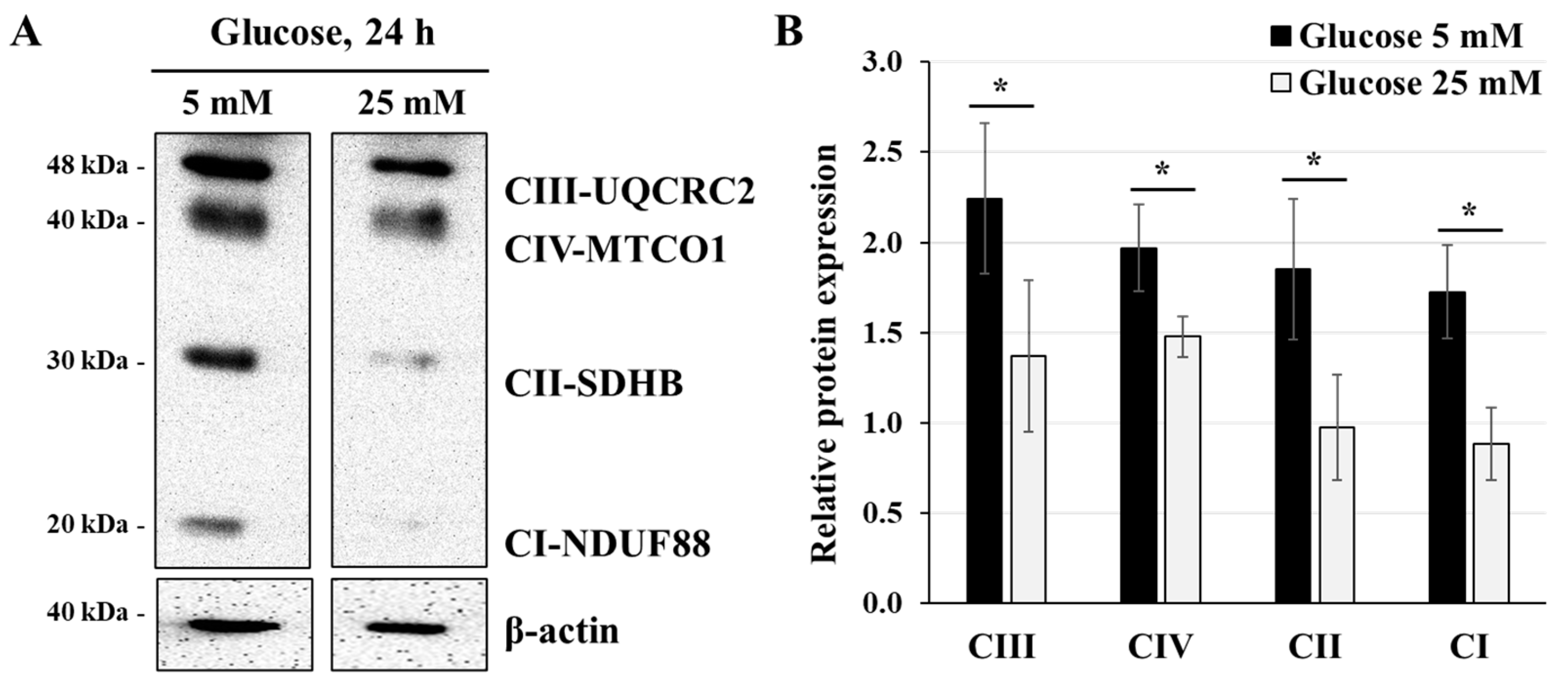
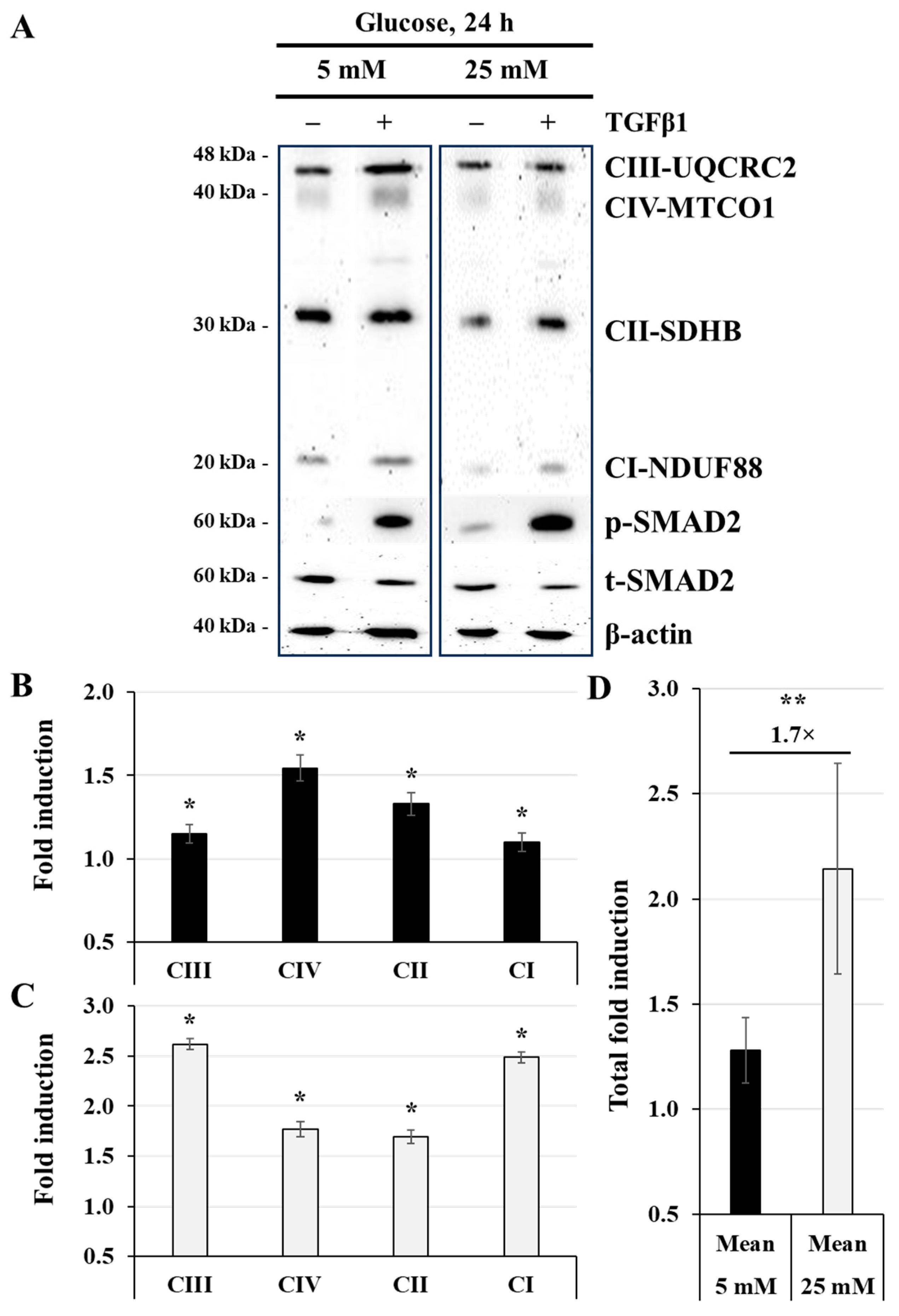
3.2. Effect of High-Glucose Concentration on Mitochondrial Respiratory Chain Protein Expression
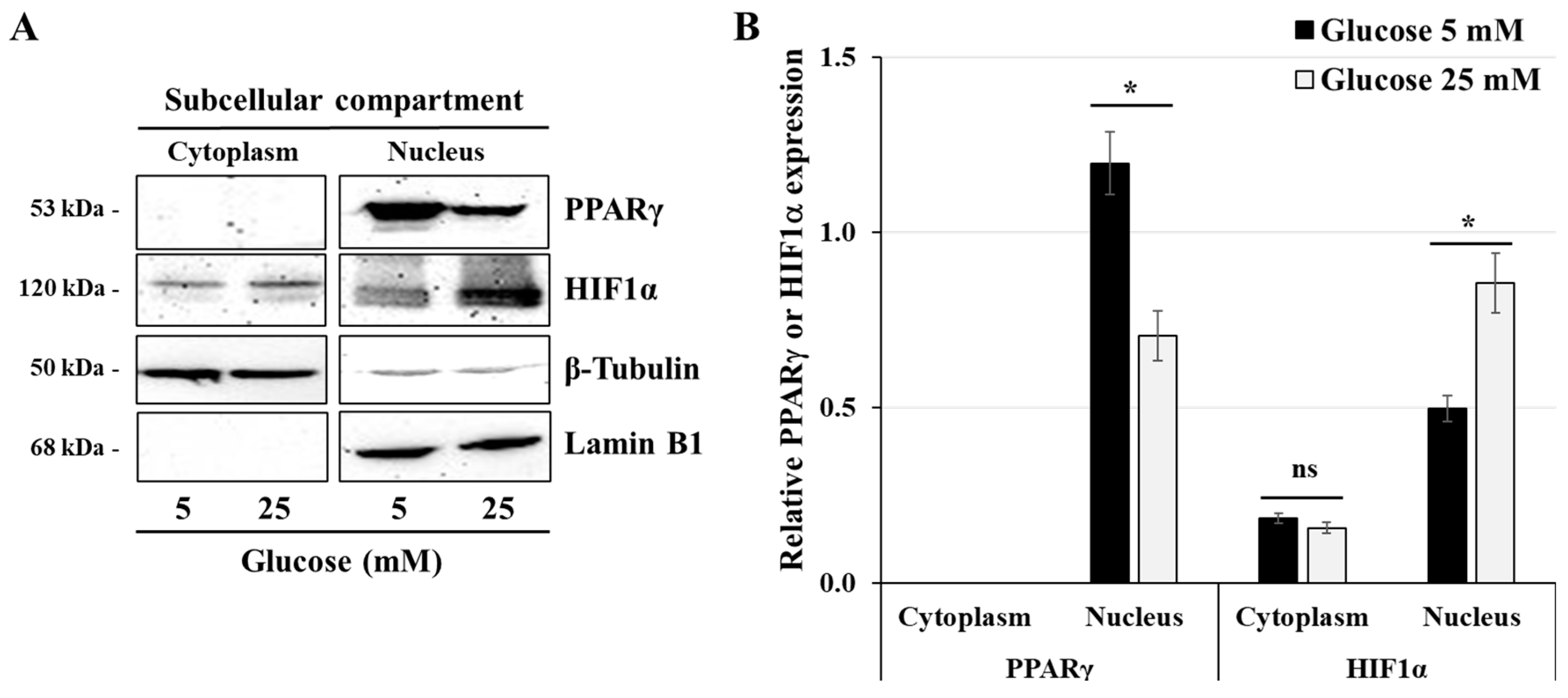
3.3. Effect of High-Glucose Concentrations on the Expression of HIF1α and PPARγ
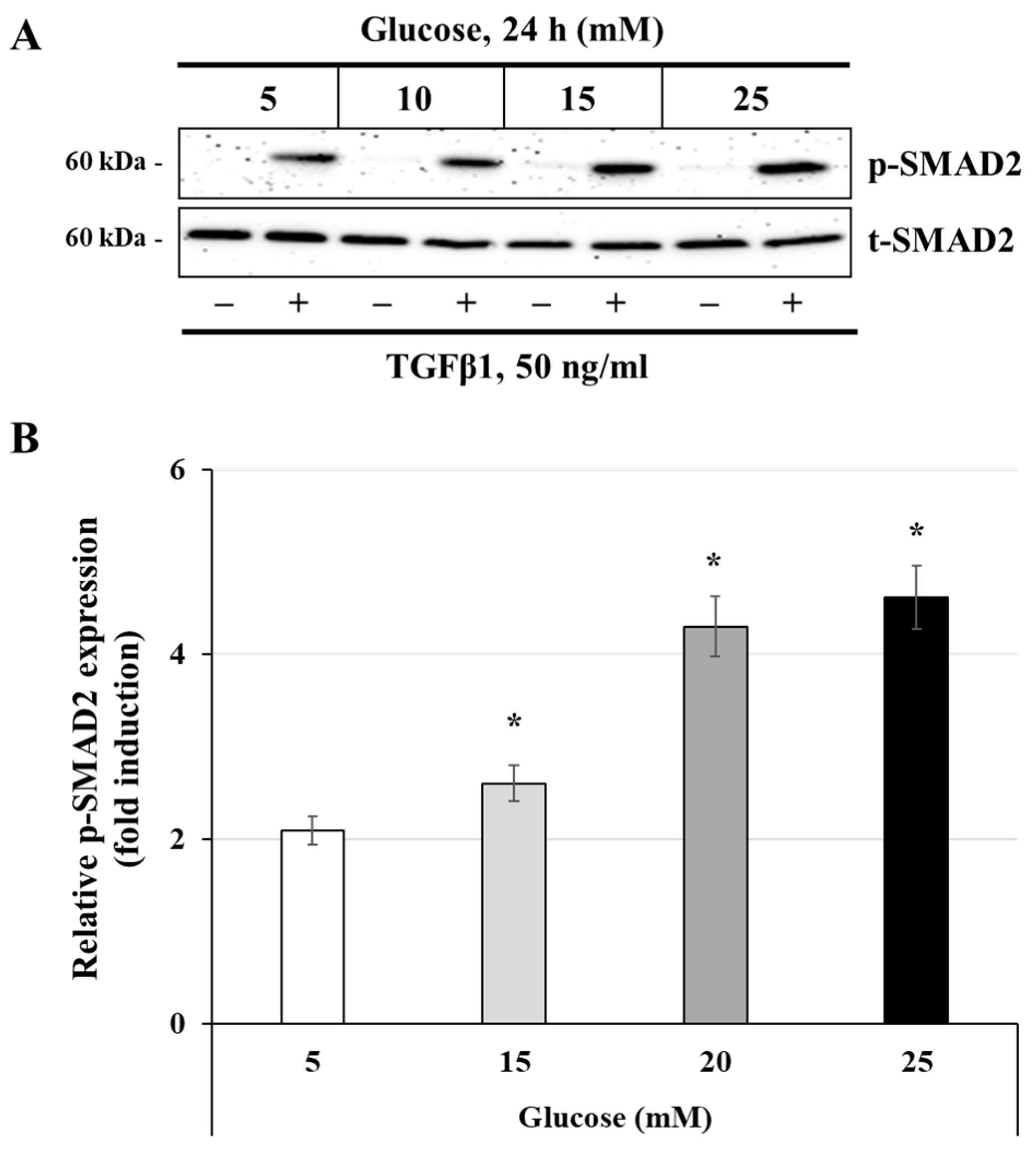
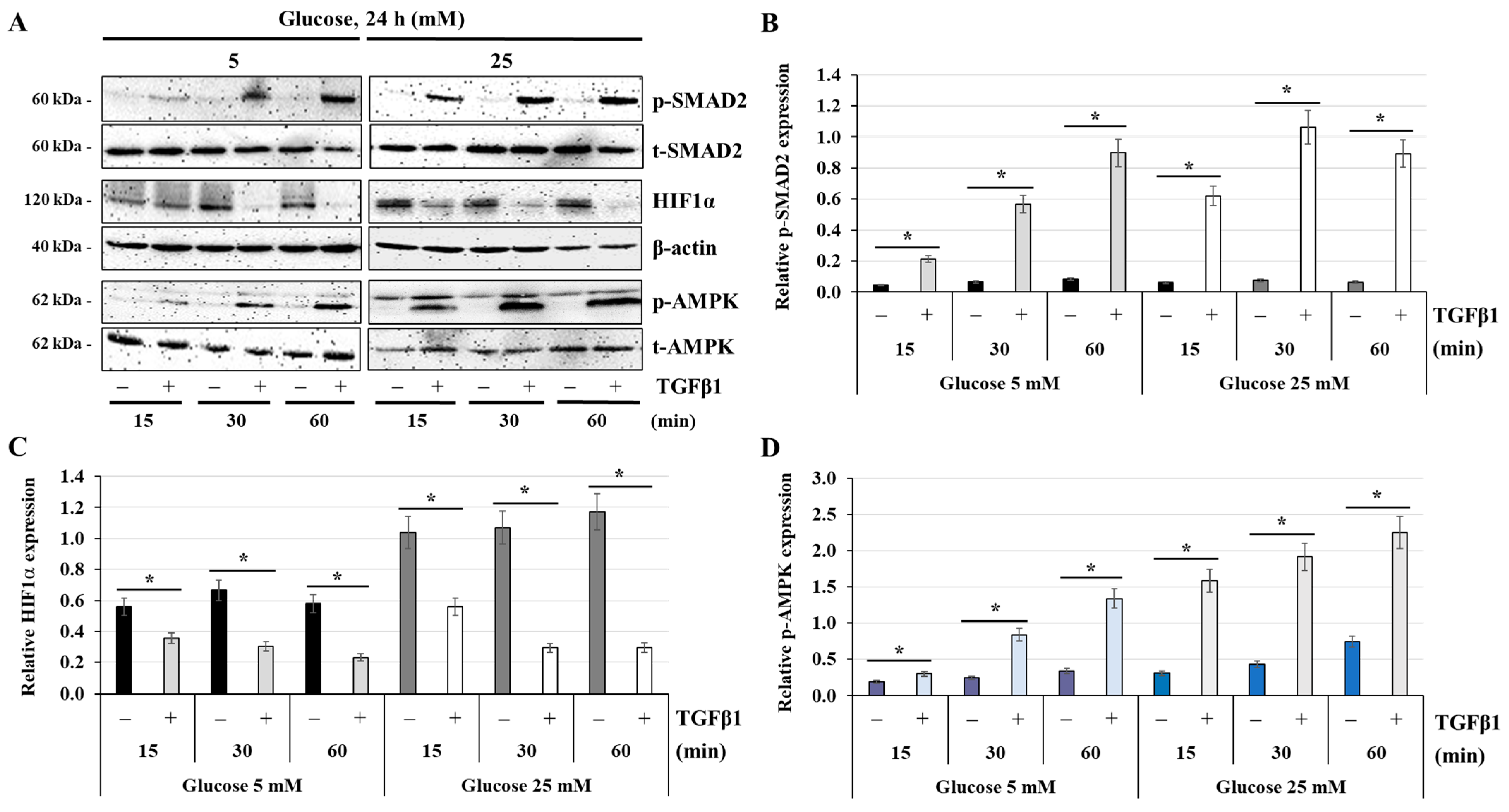
3.4. Effect of Glucose Concentrations on the Activation of the TGFβ1 Signaling Pathway
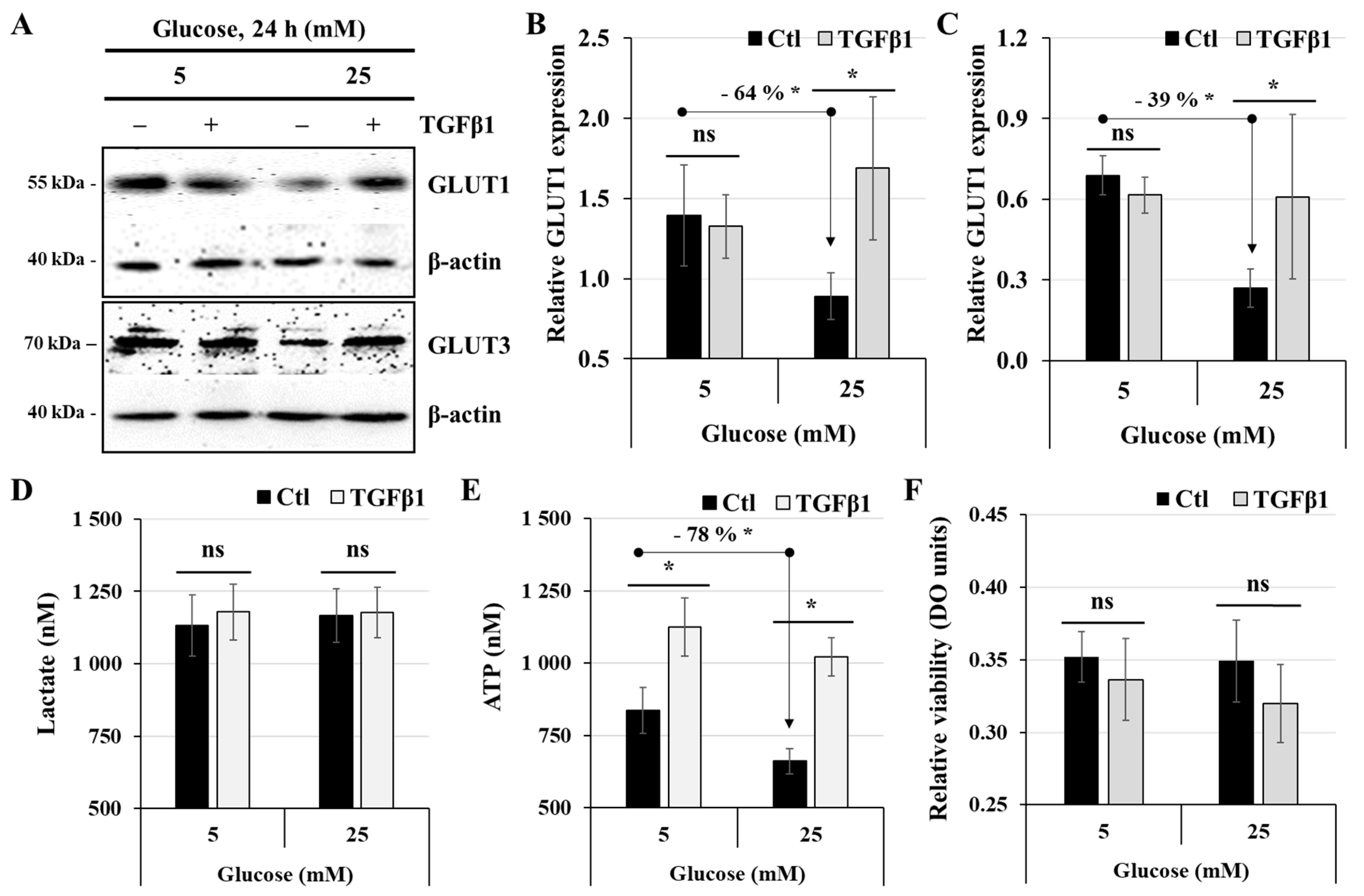
3.5. Effect of TGFβ1 on the Process of Glucose and Energy Metabolism
3.6. Effect of TGFβ1 on Mitochondrial Respiratory Chain Protein Expression
3.7. Effect of TGFβ1 on the Expression of PPARγ
3.8. Effect of TGFβ1 on the Expression of HIF1α and the Activation of AMPK
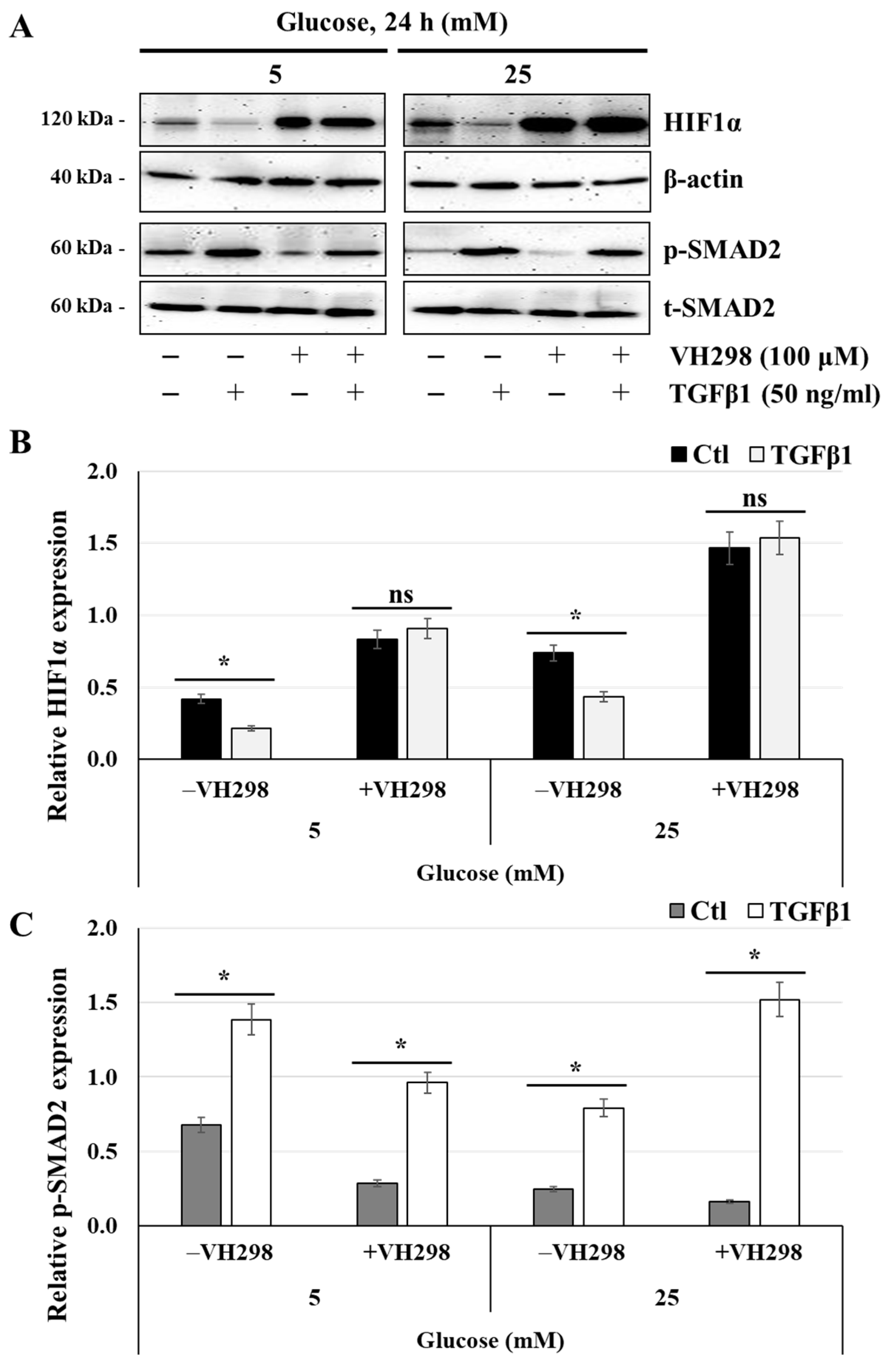
3.9. The Von Hippel–Lindau Protein Antagonist VH298 Blocks TGFβ1-Induced HIF1α Degradation
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, C.; Schwarz, S.; Moser, G.; Cvitic, S.; Jantscher-Krenn, E.; Gauster, M.; Hiden, U. Placental Endocrine Activity: Adaptation and Disruption of Maternal Glucose Metabolism in Pregnancy and the Influence of Fetal Sex. Int. J. Mol. Sci. 2021, 22, 12722. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Xu, Y.; Lin, Y. Hyperglycemia disturbs trophoblast functions and subsequently leads to failure of uterine spiral artery remodeling. Front. Endocrinol. 2023, 14, 1060253. [Google Scholar] [CrossRef] [PubMed]
- Valent, A.M.; Choi, H.; Kolahi, K.S.; Thornburg, K.L. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J. 2021, 35, e21423. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D. Hyperglycemia in pregnancy: Prevalence, impact, and management challenges. Int. J. Womens Health 2016, 8, 519–527. [Google Scholar] [CrossRef]
- Aye, I.; Aiken, C.E.; Charnock-Jones, D.S.; Smith, G.C.S. Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S928–S944. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Karamouti, M.; Drakakis, P.; Loutradis, D.; Antsaklis, A. Fetomaternal immunotolerance. Am. J. Reprod. Immunol. 2008, 60, 482–496. [Google Scholar] [CrossRef]
- Ravelojaona, M.; Girouard, J.; Kana Tsapi, E.S.; Chambers, M.; Vaillancourt, C.; Van Themsche, C.; Thornton, C.A.; Reyes-Moreno, C. Oncostatin M and STAT3 Signaling Pathways Support Human Trophoblast Differentiation by Inhibiting Inflammatory Stress in Response to IFNγ and GM-CSF. Cells 2024, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Dallagi, A.; Girouard, J.; Hamelin-Morrissette, J.; Dadzie, R.; Laurent, L.; Vaillancourt, C.; Lafond, J.; Carrier, C.; Reyes-Moreno, C. The activating effect of IFN-γ on monocytes/macrophages is regulated by the LIF-trophoblast-IL-10 axis via Stat1 inhibition and Stat3 activation. Cell Mol. Immunol. 2015, 12, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Hamelin-Morrissette, J.; Dallagi, A.; Girouard, J.; Ravelojaona, M.; Oufqir, Y.; Vaillancourt, C.; Van Themsche, C.; Carrier, C.; Reyes-Moreno, C. Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol. Immunol. 2020, 120, 32–42. [Google Scholar] [CrossRef]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martín, S. Placental structure in gestational diabetes mellitus. Biochimica Biophysica Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef] [PubMed]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.G. The Interplay Between TGF-β Signaling and Cell Metabolism. Front. Cell Dev. Biol. 2022, 10, 846723. [Google Scholar] [CrossRef]
- Li, S.; Huang, Q.; Mao, J.; Li, Q. TGFβ-dependent mitochondrial biogenesis is activated during definitive endoderm differentiation. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Liao, H.; Lin, W.; Li, Z.; Ma, X.; Xu, Q.; Yu, F. The Role of TGF-β during Pregnancy and Pregnancy Complications. Int. J. Mol. Sci. 2023, 24, 16882. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Presnell, J.S. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE 2017, 12, e0179545. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: Possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cen, H.; Mao, D.; Feng, R. Placental homing peptide guides HIF1α-silenced exosomes conjugates for targeted enhancement of invasion of trophoblast cells. Mol. Med. Rep. 2023, 28, 135. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Wu, Y.; Fu, H.; Xu, P.; Zheng, Y.; Wen, L.; Yang, X.; Zhang, F.; Hu, M.; et al. Gestational Diabetes Mellitus-Associated Hyperglycemia Impairs Glucose Transporter 3 Trafficking in Trophoblasts Through the Downregulation of AMP-Activated Protein Kinase. Front. Cell Dev. Biol. 2021, 9, 722024. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, F.P.; Delany, A.C.; Kenny, L.C.; Walsh, S.K. PPAR-gamma -- a possible drug target for complicated pregnancies. Br. J. Pharmacol. 2013, 168, 1074–1085. [Google Scholar] [CrossRef]
- Díaz, M.; Bassols, J.; López-Bermejo, A.; Gómez-Roig, M.D.; de Zegher, F.; Ibáñez, L. Placental Expression of Peroxisome Proliferator-Activated Receptor γ (PPARγ): Relation to Placental and Fetal Growth. J. Clin. Endocrinol. Metab. 2012, 97, E1468–E1472. [Google Scholar] [CrossRef]
- Xiao, H.; Gu, Z.; Wang, G.; Zhao, T. The Possible Mechanisms Underlying the Impairment of HIF-1α Pathway Signaling in Hyperglycemia and the Beneficial Effects of Certain Therapies. Int. J. Med. Sci. 2013, 10, 1412–1421. [Google Scholar] [CrossRef]
- Gokina, N.I.; Chan, S.L.; Chapman, A.C.; Oppenheimer, K.; Jetton, T.L.; Cipolla, M.J. Inhibition of PPARgamma during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Front. Physiol. 2013, 4, 184. [Google Scholar] [CrossRef]
- McIlvenna, L.C.; Patten, R.K.; McAinch, A.J.; Rodgers, R.J.; Stepto, N.K.; Moreno-Asso, A. Transforming Growth Factor Beta 1 Alters Glucose Uptake but Not Insulin Signalling in Human Primary Myotubes From Women With and Without Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 732338. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitaoka, Y.; Hatta, H. Effects of endurance training under calorie restriction on energy substrate metabolism in mouse skeletal muscle and liver. J. Physiol. Sci. 2024, 74, 32. [Google Scholar] [CrossRef]
- Chang, E.I.; Stremming, J.; Knaub, L.A.; Wesolowski, S.R.; Rozance, P.J.; Sucharov, C.C.; Reusch, J.E.B.; Brown, L.D. Mitochondrial respiration is lower in the intrauterine growth-restricted fetal sheep heart. J. Physiol. 2024, 602, 2697–2715. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free. Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef]
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y.; Liu, S.; Deng, Z.; Tan, W.; Chen, L.; Zhang, Q.; Zhao, X.; et al. Role of Transforming Growth Factor-β1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy. Front. Immunol. 2021, 12, 689181. [Google Scholar] [CrossRef] [PubMed]
- Slattery, K.; Gardiner, C.M. NK Cell Metabolism and TGFβ–Implications for Immunotherapy. Front. Immunol. 2019, 10, 2915. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, C.; Qian, J.; Cui, L.; Wang, S. Energy metabolism and maternal-fetal tolerance working in decidualization. Front. Immunol. 2023, 14, 1203719. [Google Scholar] [CrossRef]
- Hay, W.W. Glucose Metabolism in the Fetal-Placental Unit. In Principles of Perinatal-Neonatal Metabolism; Cowett, R.M., Ed.; Springer: New York, NY, USA, 1991; pp. 250–275. [Google Scholar]
- McIntyre, H.D.; Fuglsang, J.; Kampmann, U.; Knorr, S.; Ovesen, P. Hyperglycemia in Pregnancy and Women’s Health in the 21st Century. Int. J. Environ. Res. Public Health 2022, 19, 16827. [Google Scholar] [CrossRef] [PubMed]
- Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes 2009, 58, 453–459. [CrossRef]
- Visiedo, F.; Bugatto, F.; Sánchez, V.; Cózar-Castellano, I.; Bartha, J.L.; Perdomo, G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E205–E212. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Lepercq, J.; Varastehpour, A.; Basu, S.; Catalano, P.M.; Hauguel-De Mouzon, S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 2009, 201, e1–e209. [Google Scholar] [CrossRef]
- Hulme, C.H.; Stevens, A.; Dunn, W.; Heazell, A.E.P.; Hollywood, K.; Begley, P.; Westwood, M.; Myers, J.E. Identification of the functional pathways altered by placental cell exposure to high glucose: Lessons from the transcript and metabolite interactome. Sci. Rep. 2018, 8, 5270. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Szablewski, L. Diabetes mellitus: Influences on cancer risk. Diabetes/Metab. Res. Rev. 2014, 30, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Huo, H.Y.; Ao, S.; Liu, T.; Yang, L.; Fei, Z.Y.; Zhang, Z.Q.; Ding, L.; Cui, Q.H.; Lin, J.; et al. TGF-β1-induced epithelial-mesenchymal transition increases fatty acid oxidation and OXPHOS activity via the p-AMPK pathway in breast cancer cells. Oncol. Rep. 2020, 44, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Devraj, G.; Beerlage, C.; Brüne, B.; Kempf, V.A.J. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017, 19, 144–156. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Thakur, S.; Viswanadhapalli, S.; Kopp, J.B.; Shi, Q.; Barnes, J.L.; Block, K.; Gorin, Y.; Abboud, H.E. Activation of AMP-activated protein kinase prevents TGF-β1-induced epithelial-mesenchymal transition and myofibroblast activation. Am. J. Pathol. 2015, 185, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Kim, J.-J.; Lee, H.-M.; Jin, H.S.; Lee, S.-H.; Park, J.-H.; Kim, S.J.; Kim, J.-M.; Han, Y.-M.; Lee, M.-S.; et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 2014, 10, 785–802. [Google Scholar] [CrossRef]
- Kemmerer, M.; Finkernagel, F.; Cavalcante, M.F.; Abdalla, D.S.P.; Müller, R.; Brüne, B.; Namgaladze, D. AMP-Activated Protein Kinase Interacts with the Peroxisome Proliferator-Activated Receptor Delta to Induce Genes Affecting Fatty Acid Oxidation in Human Macrophages. PLoS ONE 2015, 10, e0130893. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Galbraith, L.C.A.; Mui, E.; Nixon, C.; Hedley, A.; Strachan, D.; MacKay, G.; Sumpton, D.; Sansom, O.J.; Leung, H.Y.; Ahmad, I. PPAR-gamma induced AKT3 expression increases levels of mitochondrial biogenesis driving prostate cancer. Oncogene 2021, 40, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, F.; Kiani, A.; Darzi, S.; Motavalli, R.; Noori Dolama, F.; Yousefzadeh, Y.; Aghebati-Maleki, L.; Pia, H.; Abdollahi-Fard, S.; Mardi, A.; et al. The evaluation of CD39, CD73, and HIF-1 α expression besides their related miRNAs in PBMCs of women with recurrent pregnancy loss. J. Reprod. Immunol. 2023, 156, 103820. [Google Scholar] [CrossRef]
- Zhu, J.; Song, G.; Zhou, X.; Han, T.-L.; Yu, X.; Chen, H.; Mansell, T.; Novakovic, B.; Baker, P.N.; Cannon, R.D.; et al. CD39/CD73 Dysregulation of Adenosine Metabolism Increases Decidual Natural Killer Cell Cytotoxicity: Implications in Unexplained Recurrent Spontaneous Abortion. Front. Immunol. 2022, 13, 813218. [Google Scholar] [CrossRef] [PubMed]
- Tagoma, A.; Haller-Kikkatalo, K.; Oras, A.; Roos, K.; Kirss, A.; Uibo, R. Plasma cytokines during pregnancy provide insight into the risk of diabetes in the gestational diabetes risk group. J. Diabetes Investig. 2022, 13, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Cao, L.; Jin, R.; Li, R. Exploration of the Shared Genes and Molecular Pathways between Pre-Eclampsia and Type 2 Diabetes Mellitus via Co-Expression Networks Analysis. Clin. Exp. Obstet. Gynecol. 2023, 50, 73. [Google Scholar] [CrossRef]
- Prossler, J.; Chen, Q.; Chamley, L.; James, J.L. The relationship between TGFβ, low oxygen and the outgrowth of extravillous trophoblasts from anchoring villi during the first trimester of pregnancy. Cytokine 2014, 68, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Q.; Gardner, L.; Turco, M.; Zhao, N.; Murray, M.J.; Coleman, N.; Rossant, J.; Hemberger, M.; Moffett, A. What Is Trophoblast? A Combination of Criteria Define Human First-Trimester Trophoblast. Stem Cell Rep. 2016, 6, 257–272. [Google Scholar]
- Weiss, U.; Cervar, M.; Puerstner, P.; Schmut, O.; Haas, J.; Mauschitz, R.; Arikan, G.; Desoye, G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 2001, 44, 209–219. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiat, N.; Girouard, J.; Kana Tsapi, E.S.; Vaillancourt, C.; Van Themsche, C.; Reyes-Moreno, C. TGFβ1 Restores Energy Homeostasis of Human Trophoblast Cells Under Hyperglycemia In Vitro by Inducing PPARγ Expression, AMPK Activation, and HIF1α Degradation. Cells 2025, 14, 45. https://doi.org/10.3390/cells14010045
Khiat N, Girouard J, Kana Tsapi ES, Vaillancourt C, Van Themsche C, Reyes-Moreno C. TGFβ1 Restores Energy Homeostasis of Human Trophoblast Cells Under Hyperglycemia In Vitro by Inducing PPARγ Expression, AMPK Activation, and HIF1α Degradation. Cells. 2025; 14(1):45. https://doi.org/10.3390/cells14010045
Chicago/Turabian StyleKhiat, Nihad, Julie Girouard, Emmanuelle Stella Kana Tsapi, Cathy Vaillancourt, Céline Van Themsche, and Carlos Reyes-Moreno. 2025. "TGFβ1 Restores Energy Homeostasis of Human Trophoblast Cells Under Hyperglycemia In Vitro by Inducing PPARγ Expression, AMPK Activation, and HIF1α Degradation" Cells 14, no. 1: 45. https://doi.org/10.3390/cells14010045
APA StyleKhiat, N., Girouard, J., Kana Tsapi, E. S., Vaillancourt, C., Van Themsche, C., & Reyes-Moreno, C. (2025). TGFβ1 Restores Energy Homeostasis of Human Trophoblast Cells Under Hyperglycemia In Vitro by Inducing PPARγ Expression, AMPK Activation, and HIF1α Degradation. Cells, 14(1), 45. https://doi.org/10.3390/cells14010045







