Beyond Spherical: Unveiling the Significance of Oval Blastocyst Morphology on Euploidy and Implantation Success
Abstract
1. Introduction
2. Materials and Methods
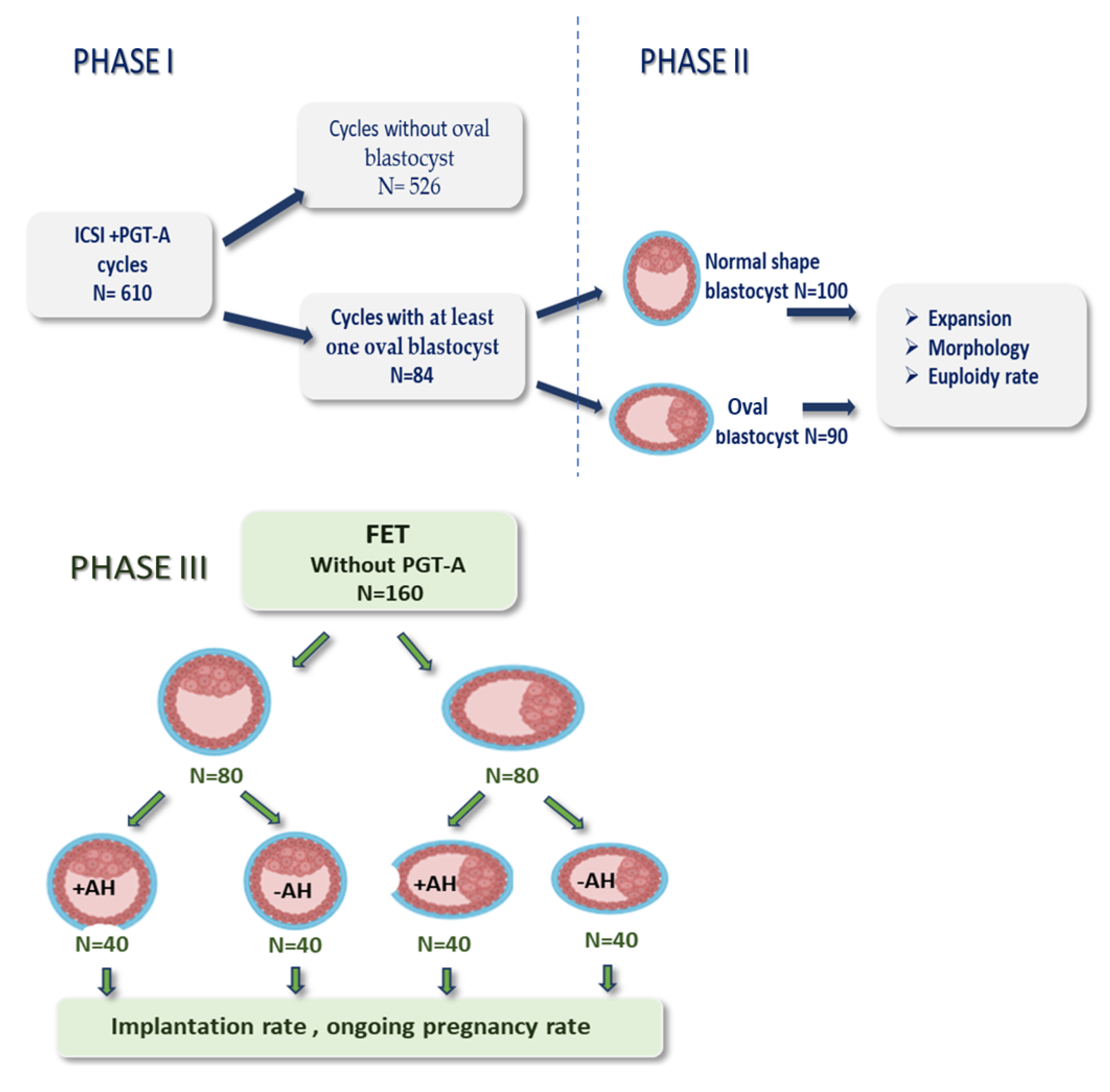
2.1. Study Design
2.2. Clinical Protocols
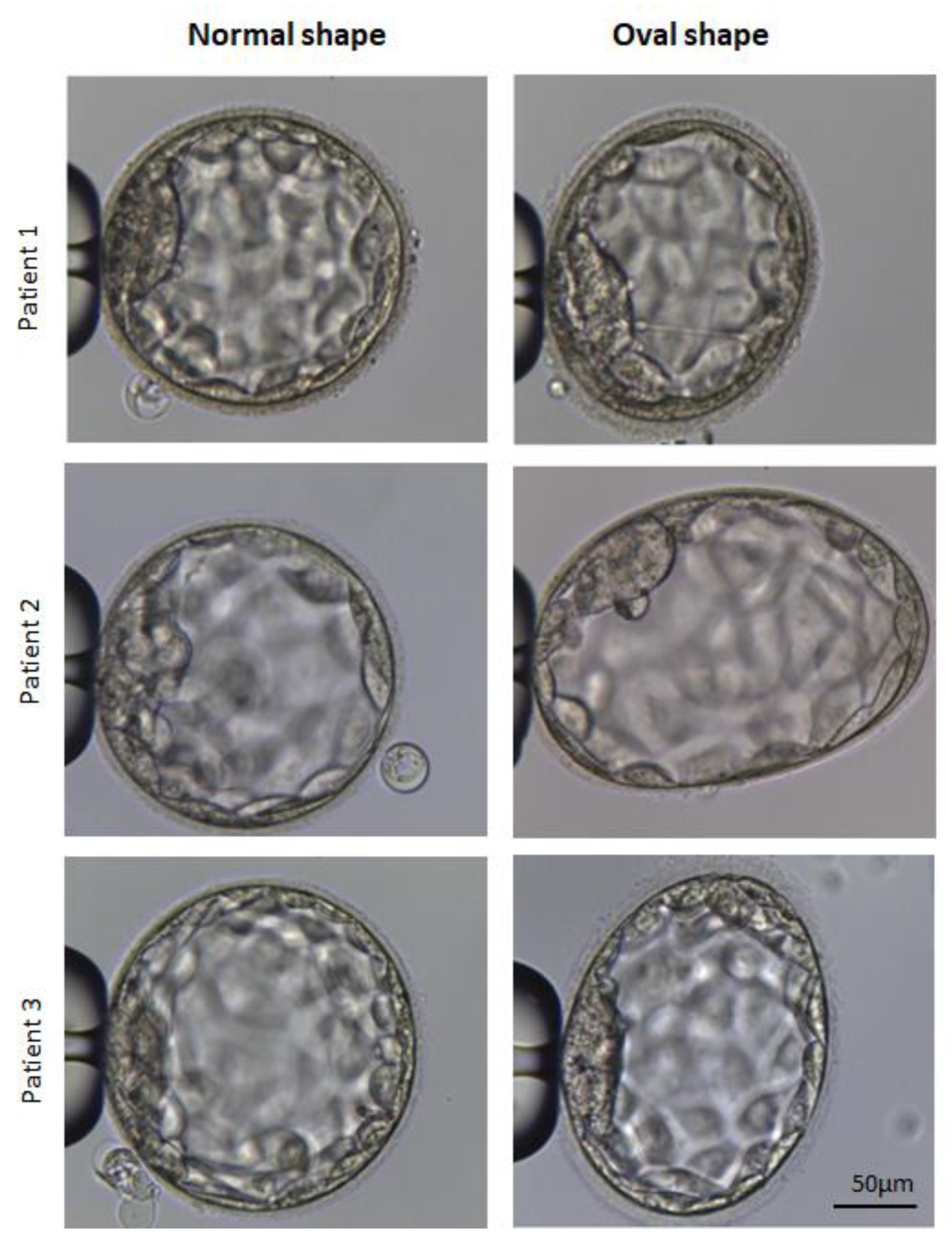
2.3. Laboratory Protocols
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Kirkegaard, K.; Agerholm, I.E.; Ingerslev, H.J. Time-lapse monitoring as a tool for clinical embryo assessment. Hum. Reprod. 2012, 27, 1277–1285. [Google Scholar] [CrossRef]
- Yang, H.; DeWan, A.T.; Desai, M.M.; Vermount, S.H. Preimplantation genetic testing for aneuploidy: Challenges in clinical practice. Hum. Genom. 2011, 16, 69. [Google Scholar] [CrossRef]
- Shear, M.A.; Vaughan, D.A.; Modest, A.M.; Seidler, E.A.; Leung, A.Q.; Hacker, M.R.; Sakkas, D.; Penzias, A.S. Blasts from the past: Is morphology useful in PGT-A tested and untested frozen embryo transfers? Reprod. Biomed. Online 2020, 41, 981–989. [Google Scholar] [CrossRef]
- Huang, T.F.; Huang, D.H.; Ahn, H.J.; Arnett, C.; Huan, C.T. Early blastocyst expansion in euploid and aneuploid human embryos: Evidence for a non-invasive and quantitative marker for embryo selection. Reprod. Biomed. Online 2019, 39, 27–39. [Google Scholar] [CrossRef]
- Chimote, N.M.; Chimote, N.N.; Nath, N.; Mehta, B. Transfer of spontaneously hatching or hatched blastocyst yields better pregnancy rates than expanded blastocyst transfer. J. Hum. Reprod. Sci. 2013, 6, 183–188. [Google Scholar] [CrossRef]
- Du, Q.Y.; Wang, E.Y.; Huang, Y.; Guo, X.Y.; Xiong, Y.J.; Yu, Y.P.; Yao, G.D.; Shi, S.L.; Sun, Y.P. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil. Steril. 2016, 105, 910–919. [Google Scholar] [CrossRef]
- Richter, K.S.; Harris, D.C.; Daneshmand, S.T.; Shapiro, B.S. Quantitative grading of a humanblastocyst: Optimal inner cell mass size and shape. Fertil. Steril. 2001, 76, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Lagalla, C.; Barberi, M.; Orlando, G.; Sciajno, R.; Bonu, M.A.; Borini, A. A quantitative approach to blastocyst quality evaluation: Morphometric analysis and related IVFoutcomes. J. Assist. Reprod. Genet. 2015, 32, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Almagor, M.; Harir, Y.; Fieldust, S.; Or, Y.; Shoham, Z. Ratio between inner cell massdiameter and blastocyst diameter is correlated with successful pregnancy outcomes ofsingle blastocyst transfers. Fertil. Steril. 2016, 106, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.V.; Ginsburg, E.S.; Hornstein, M.D.; Rein, M.S.; Clarke, R.N. Multinucleation in normally fertilized embryos is associated with an accelerated ovulatio inductionresponse and lower implantation and pregnancy rates in in vitro fertilization-embryotransfer cycles. Fertil. Steril. 1998, 70, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Alikani, M.; Cohen, J.; Tomkin, G.; Garrisi, G.J.; Mack, C.; Scott, R.T. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil. Steril. 1999, 71, 836–842. [Google Scholar] [CrossRef]
- Fancsovits, P.; Murber, A.; Gilan, Z.; Rigo, J.; Urbancsek, J. Human oocytes containing large cytoplasmic vacuoles can result in pregnancy and viable offspring. Reprod. Biomed. Online 2011, 23, 513–516. [Google Scholar] [CrossRef]
- Yakin, K.; Balaban, B.; Isiklar, A.; Urman, B. Oocyte dysmorphism is not associated with aneuploidy in the developing embryo. Fertil. Steril. 2007, 88, 811–816. [Google Scholar] [CrossRef]
- Ebner, T.; Shebl, O.; Moser, M.; Sommergruber, M.; Tews, G. Developmental fate of ovoid oocytes. Hum. Reprod. 2008, 23, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stalf, T.; Mehnert, C.; Eichenlaub-Ritter, U.; Tinneberg, H.R. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum. Reprod. 2005, 20, 1596–1606. [Google Scholar] [CrossRef]
- Moghadam, A.; Moghadam, M.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Serhal, P.F.; Ranieri, D.M.; Kinis, A.; Marchant, S.; Davies, M.; Khadum, I.M. Oocyte morphologypredictsoutcomeof intracytoplasmicsperminjection. Hum. Reprod. 1997, 12, 1267–1270. [Google Scholar] [CrossRef]
- Xia, P. Intracytoplasmic sperm injection correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusionswith fertilization rate and embryoquality. Hum. Reprod. 1997, 12, 1750–1755. [Google Scholar] [CrossRef]
- Sauerbrun-Cutler, M.; Vega, M.; Breborowicz, A.; Gonzales, E.; Stein, D.; Lederman, M.; Keltz, M. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J. Ovarian Res. 2015, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Ryan, E.A.J.; Gotlieb, L.; Casper, R.F. Successful pregnancy following transfer of embryos from oocytes with abnormal zona pellucida and cytoplasm morphology. Reprod. Biomed. Online 2005, 5, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Halim, B.; Lubis, H.P.; Novia, D.; Thaharuddin, M. Does oval oocyte have an impact on embryo development in in vitro fertilization? JBRA Assist. Reprod. 2017, 21, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Dolgushina, N.V.; Syrkasheva, A.G.; Makarova, N.P.; Koval skaya, E.V.; Kalinina, E.A.; Sukhikh, G.T. Correlation between oocyte morphology and the embryo aneuploidy rate in IVF cycles. Gynecol. Endocrinol. 2015, 31, 61. [Google Scholar] [CrossRef]
- Watson, A.J.; Natale, D.R.; Barcroft, L.C. Molecular regulation of blastocyst formation. Anim. Reprod. Sci. 2004, 82–83, 583–592. [Google Scholar] [CrossRef]
- Seshagiri, P.B.; Sen Roy, S.; Sireesha, G.; Rao, R.P. Cellular and molecular regulation of mammalian blastocyst hatching. J. Reprod. Immunol. 2009, 83, 79–84. [Google Scholar] [CrossRef]
- Leonavicius, K.; Royer, C.; Preece, C.; Davies, B.; Biggins, J.S.; Srinivas, S. Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay. Proc. Natl. Acad. Sci. USA 2018, 115, 10375–10380. [Google Scholar] [CrossRef]


| Parameters | Normal Shape Blastocysts * | Oval Shape Blastocysts ** | Total | |
|---|---|---|---|---|
| No of patients (cycle), n (%) | 526 (86.3%) | 84 (13.7%) | 610 (100%) | |
| Age (years), range, mean ± SD | 34.9 ± 4.7 | 35.2 ± 4.4 | 35.1 ± 4.5 | |
| BMI (kg/m2), range, mean ± SD | 22.4 ± 2.4 | 22.1 ± 2.5 | 22.3 ± 2.4 | |
| AMH (ng/mL), range, mean ± SD | 3.3 ± 1.98 | 3.1 ± 2.26 | 3.17 ± 2.12 | |
| No of blastocysts, n (%) | 1233 (92.7%) | 95 (7.3%) | 1328 (100%) | |
| No of blastocysts/cycle, mean ± SD | 2.3 ± 0.3 | 1.13 ± 0.2 | 2.17 ± 0.3 | |
| Cause of infertility | Female factor, n (%) | 267 (51%) | 41 (49%) | 308 (50%) |
| Male factor, n (%) | 47 (9%) | 9 (11%) | 56 (10%) | |
| Combined, n (%) | 212 (40%) | 34 (40%) | 246 (40%) | |
| Parameters | Blastocysts | |
|---|---|---|
| Oval Shape | Normal Shape | |
| No of blastocysts, n | 90 | 100 |
| No of expanding (BL 4) blastocysts, n (%) | 79/90 (88%) a | 69/100 (69%) c |
| No of hatching (BL 5) blastocysts, n (%) | 11/90 (12%) a | 31/100 (31%) c |
| No of excellent quality, n (%) | 33/90 (37%) a | 41/100 (41%) a |
| No of good quality, n (%) | 34/90 (38%) a | 33/100 (33%) a |
| No of medium quality, n (%) | 23/73 (25%) a | 26/100 (26%) a |
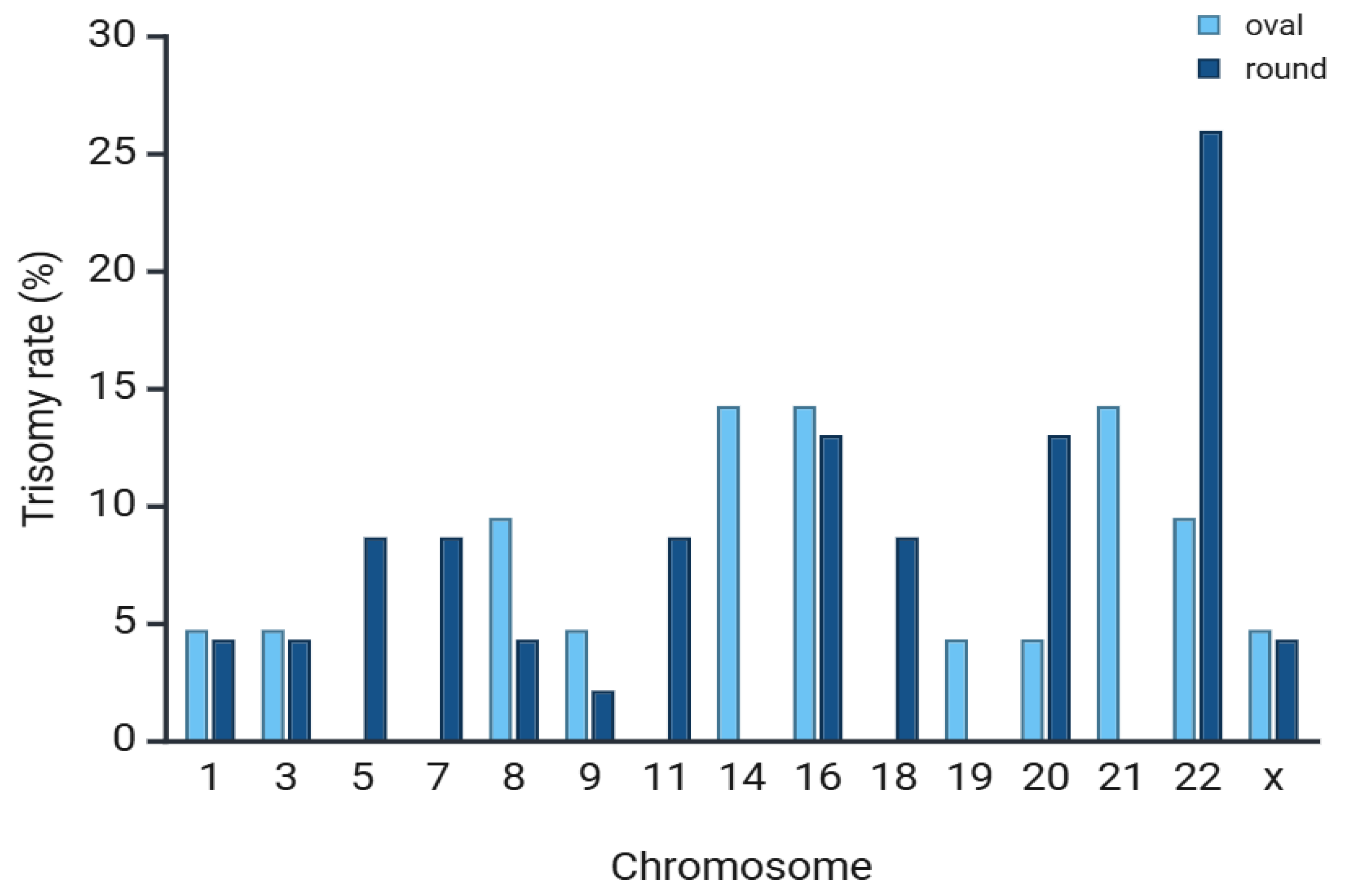
| Euploid rate, n (%) | 46/90 (51%) a | 53/100 (53%) a |
| Trisomy, n (%) | 26/46 (56%) a | 28/53 (53%) a |
| Monosomy, n (%) | 20/46 (44%) a | 25/53 (47%) a |
| Parameters | FET | |
|---|---|---|
| Normal Shape | Oval Shape | |
| No of FET | 80 | 80 |
| Patient age (years), mean ± SD | 32.1 ± 2.3 | 32.3 ± 2.6 |
| Patient BMI (kg/m2), mean ± SD | 21.9 ± 2.2 | 22.1 ± 2.1 |
| Implantation rate without AH, n (%) | 25/40 (63%) a | 20/40 (50%) b |
| Implantation rate with AH, n (%) | 24/40 (61%) a | 25/40 (62%) a |
| Total implantation rate | 49/80 (62%) a | 45/80 (56%) a |
| Ongoing pregnancy rate, n (%) | 46/80 (57%) a | 42/80 (52%) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyroba, J.; Kuczyńska, A.; Kasperkowicz, K.; Kostarczyk, K.; Kordowitzki, P.; Kochan, J. Beyond Spherical: Unveiling the Significance of Oval Blastocyst Morphology on Euploidy and Implantation Success. Cells 2025, 14, 1468. https://doi.org/10.3390/cells14181468
Wyroba J, Kuczyńska A, Kasperkowicz K, Kostarczyk K, Kordowitzki P, Kochan J. Beyond Spherical: Unveiling the Significance of Oval Blastocyst Morphology on Euploidy and Implantation Success. Cells. 2025; 14(18):1468. https://doi.org/10.3390/cells14181468
Chicago/Turabian StyleWyroba, Jakub, Agnieszka Kuczyńska, Klaudia Kasperkowicz, Katarzyna Kostarczyk, Pawel Kordowitzki, and Joanna Kochan. 2025. "Beyond Spherical: Unveiling the Significance of Oval Blastocyst Morphology on Euploidy and Implantation Success" Cells 14, no. 18: 1468. https://doi.org/10.3390/cells14181468
APA StyleWyroba, J., Kuczyńska, A., Kasperkowicz, K., Kostarczyk, K., Kordowitzki, P., & Kochan, J. (2025). Beyond Spherical: Unveiling the Significance of Oval Blastocyst Morphology on Euploidy and Implantation Success. Cells, 14(18), 1468. https://doi.org/10.3390/cells14181468







