Diverse Biological Processes Contribute to Transforming Growth Factor β-Mediated Cancer Drug Resistance
Abstract
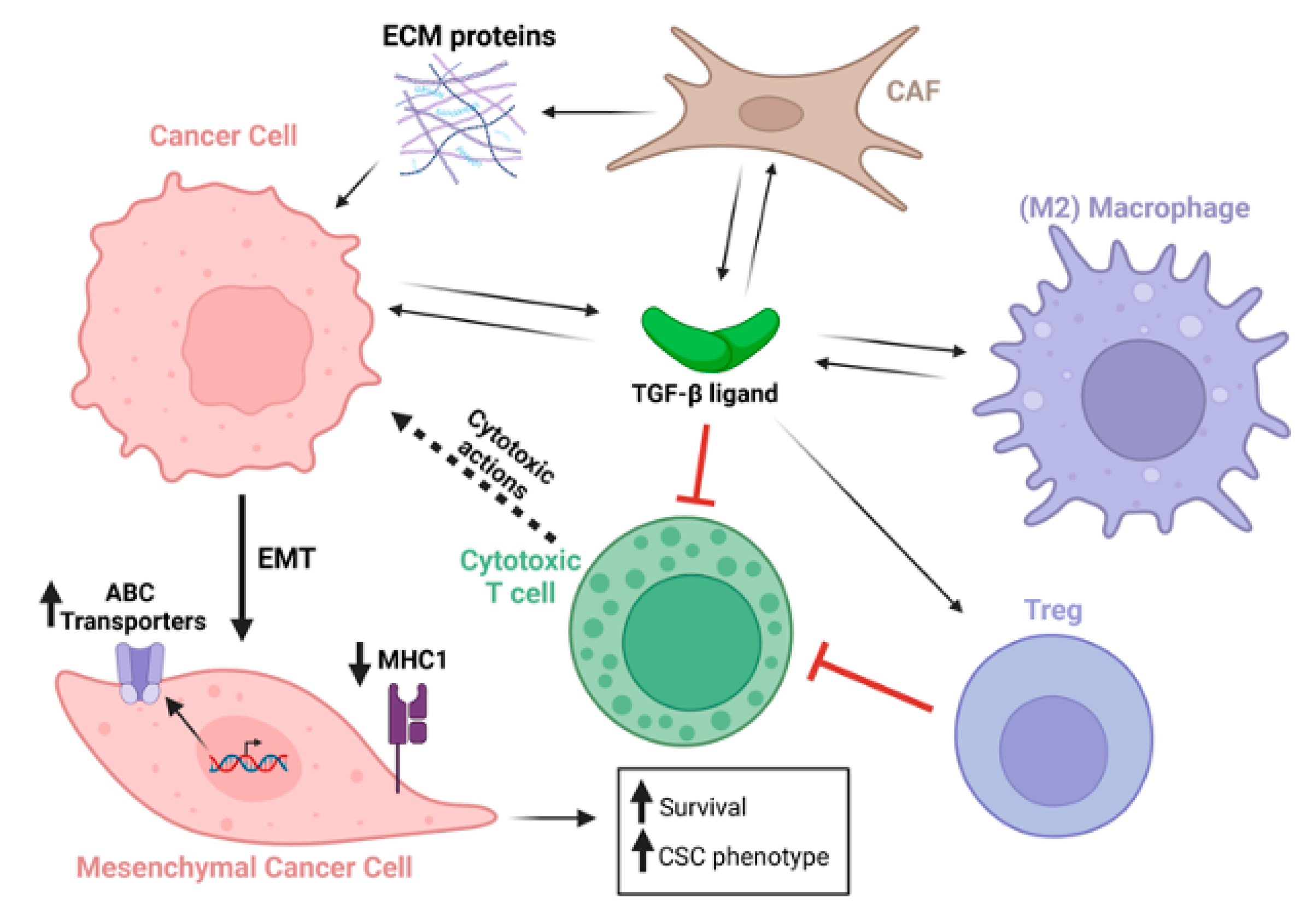
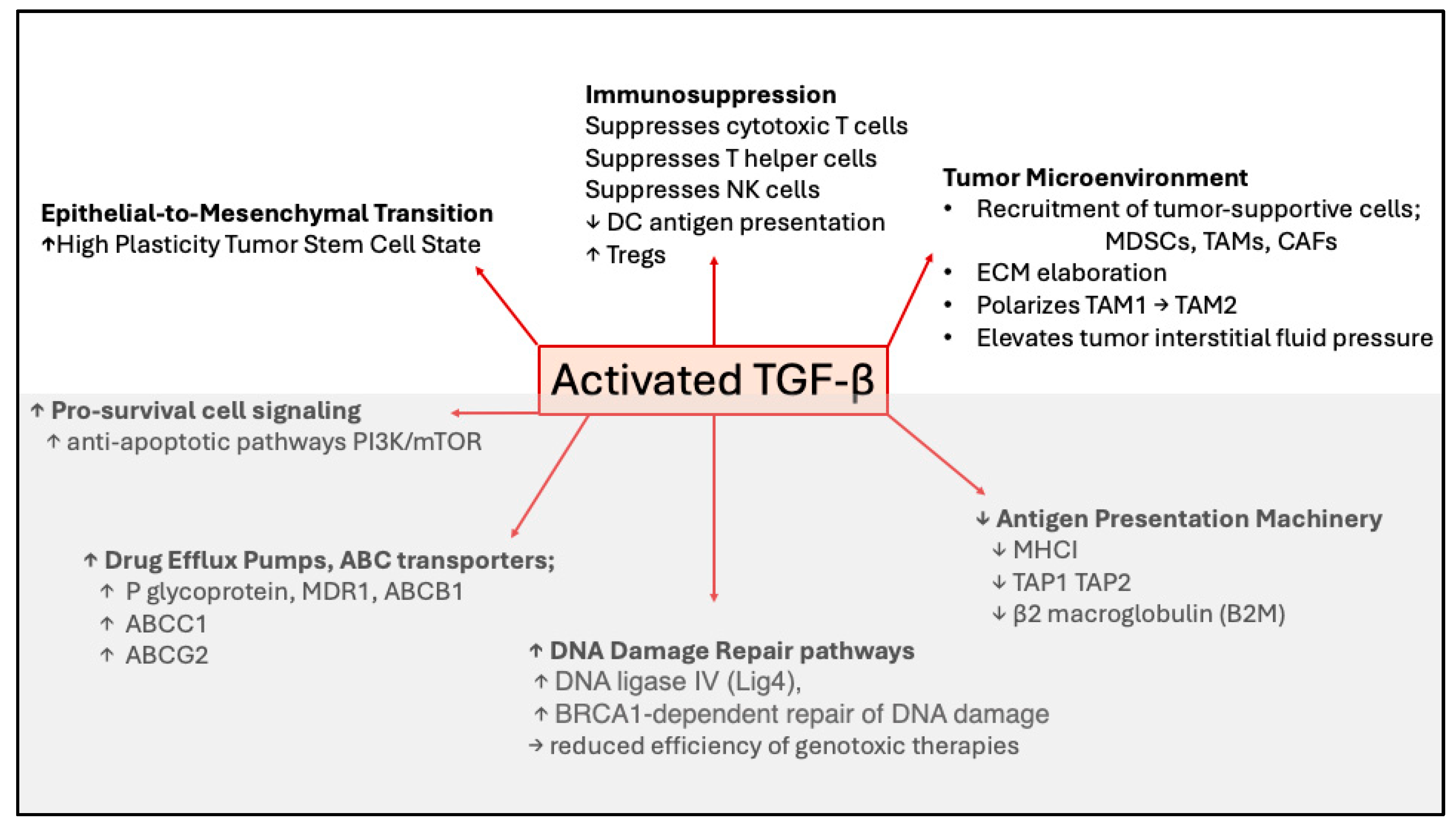
1. Perspectives
2. Introduction
3. TGF-β-Stimulated Epithelial-to-Mesenchymal Transformation Drives Chemotherapy Resistance Through Multiple Mechanisms
4. Induction of Drug-Resistant Cancer Stem Cells by TGF-β
5. TGF-β-Induced Pro-Survival Signaling in Tumor Cells Is Elicited Through SMAD and Non-SMAD Pathways
6. TGF-β Signaling Blockade, an Achilles Heel for Genotoxic Therapies Through Suppression of DNA Damage Repair Pathways
7. TGF-β and Tumor Immunity
8. Contribution of TGF-β to Tumor Microenvironment (TME)-Driven Therapy Resistance
9. Conclusions
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Adenosine triphosphate (ATP)-binding cassette |
| CAF | Cancer-associated fibroblast |
| CSC | Cancer stem cells |
| DDR | DNA damage response |
| DTP | Drug tolerant persister cell |
| ECM | Extracellular Matrix |
| EMT | Epithelial–Mesenchymal Transformation |
| ICB | Immune Checkpoint Blockade |
| MDSC | Myeloid-derived suppressor cell |
| NET | Neutrophil extracellular trap |
| NK | Natural Killer |
| NSCLC | Non-small cell carcinoma |
| PD-1 | Programmed Death -1 |
| PDAC | Pancreatic ductal adenocarcinoma |
| SCC | Squamous Cell Carcinoma |
| TGF-β | Transforming growth factor β |
References
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, F.; Guida, T.; Anaganti, S.; Provitera, L.; Kjaer, S.; McDonald, N.Q.; Ryan, A.J.; Santoro, M. Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. Endocrine-Related Cancer 2009, 16, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Ahronian, L.G.; Sennott, E.M.; Van Allen, E.M.; Wagle, N.; Kwak, E.L.; Faris, J.E.; Godfrey, J.T.; Nishimura, K.; Lynch, K.D.; Mermel, C.H.; et al. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov. 2015, 5, 358–367. [Google Scholar] [CrossRef]
- Thompson, A.; Kerr, D.; Steel, C. Transforming growth factor beta 1 is implicated in the failure of tamoxifen therapy in human breast cancer. Br. J. Cancer 1991, 63, 609–614. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Carty-Dugger, T.; Moses, H.L.; Hurd, S.D.; Pietenpol, J.A. Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth Differ. 1993, 4, 193–201. [Google Scholar]
- Arteaga, C.L.; Koli, K.M.; Dugger, T.C.; Clarke, R. Reversal of tamoxifen resistance of human breast carcinomas in vivo by neu-tralizing antibodies to transforming growth factor-beta. J Natl Cancer Inst. 1999, 91, 46–53. [Google Scholar] [CrossRef]
- Huang, S.; Hölzel, M.; Knijnenburg, T.; Schlicker, A.; Roepman, P.; McDermott, U.; Garnett, M.; Grernrum, W.; Sun, C.; Prahallad, A.; et al. MED12 Controls the Response to Multiple Cancer Drugs through Regulation of TGF-beta Receptor Signaling. Cell 2012, 151, 937–950. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, M.; Gehling, V.S.; Gustafson, A.; Arora, S.; Tindell, C.A.; Wilson, C.; Williamson, K.E.; Guler, G.D.; Gangurde, P.; Manieri, W.; et al. An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat. Chem. Biol. 2016, 12, 531–538. [Google Scholar] [CrossRef]
- Guler, G.D.; Tindell, C.A.; Pitti, R.; Wilson, C.; Nichols, K.; Cheung, T.K.; Kim, H.-J.; Wongchenko, M.; Yan, Y.; Haley, B.; et al. Repression of Stress-Induced LINE-1 Expression Protects Cancer Cell Subpopulations from Lethal Drug Exposure. Cancer Cell 2017, 32, 221–237.e13. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef]
- Sorrentino, A.; Thakur, N.; Grimsby, S.; Marcusson, A.; von Bulow, V.; Schuster, N.; Zhang, S.; Heldin, C.-H.; Landström, M. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 2008, 10, 1199–1207. [Google Scholar] [CrossRef]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.-H.; Landström, M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal. 2017, 10, eaal4186. [Google Scholar] [CrossRef]
- Lee, M.K.; Pardoux, C.; Hall, M.C.; Lee, P.S.; Warburton, D.; Qing, J.; Smith, S.M.; Derynck, R. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007, 26, 3957–3967. [Google Scholar] [CrossRef]
- Yakymovych, I.; Yakymovych, M.; Hamidi, A.; Landström, M.; Heldin, C.-H. The type II TGF-β receptor phosphorylates Tyr 182 in the type I receptor to activate downstream Src signaling. Sci. Signal. 2022, 15, eabp9521. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Fowlis, D.J.; Bryson, S.; Duffie, E.; Ireland, H.; Balmain, A.; Akhurst, R.J. TGFbeta1 Inhibits the Formation of Benign Skin Tumors, but Enhances Progression to Invasive Spindle Carcinomas in Transgenic Mice. Cell 1996, 86, 531–542. [Google Scholar] [CrossRef]
- Portella, G.; Cumming, S.A.; Liddell, J.; Cui, W.; Ireland, H.; Akhurst, R.J.; Balmain, A. Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: Implications for tumor invasion. Cell Growth Differ. 1998, 9, 393–404. [Google Scholar]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Oshimori, N.; Oristian, D.; Fuchs, E. TGF-β Promotes Heterogeneity and Drug Resistance in Squamous Cell Carcinoma. Cell 2015, 160, 963–976. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Webb, B.M.; Bryson, B.L.; Williams-Medina, E.; Bobbitt, J.R.; Seachrist, D.D.; Anstine, L.J.; Keri, R.A. TGF-β/activin signaling promotes CDK7 inhibitor resistance in triple-negative breast cancer cells through upregulation of multidrug transporters. J. Biol. Chem. 2021, 297, 101162. [Google Scholar] [CrossRef]
- Ghosh, S.; Tanbir, S.E.; Mitra, T.; Roy, S.S. Unveiling stem-like traits and chemoresistance mechanisms in ovarian cancer cells through the TGFβ1-PITX2A/B signaling axis. Biochem. Cell Biol. 2024, 102, 394–409. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.M.; Freire-De-Lima, L.; da Fonseca, L.M.; Previato, J.O.; Mendonça-Previato, L.; Valente, R.D.C. ABCB1 and ABCC1 Function during TGF-β-Induced Epithelial-Mesenchymal Transition: Relationship between Multidrug Resistance and Tumor Progression. Int. J. Mol. Sci. 2023, 24, 6046. [Google Scholar] [CrossRef]
- Wei, L.; Lin, Q.; Lu, Y.; Li, G.; Huang, L.; Fu, Z.; Chen, R.; Zhou, Q. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-β1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 2021, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.; Schmoellerl, J.; Piontek, M.; Manhart, G.; Troester, S.; Carter, B.Z.; Neubauer, H.; Moriggl, R.; Szakács, G.; Zuber, J.; et al. ABCC1 and glutathione metabolism limit the efficacy of BCL-2 inhibitors in acute myeloid leukemia. Nat. Commun. 2023, 14, 5709. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yin, T.; Xiang, H.; Wang, L.; Mudgal, P.; Chen, J.; Ding, Y.; Wang, G.; Lim, B.J.W.; Huang, Y.; et al. Aberrant cytoplasmic expression of UHRF1 restrains the MHC-I-mediated anti-tumor immune response. Nat. Commun. 2024, 15, 8569. [Google Scholar] [CrossRef]
- Taylor, M.A.; Kandyba, E.; Halliwill, K.; Delrosario, R.; Khoroshkin, M.; Goodarzi, H.; Quigley, D.; Li, Y.R.; Wu, D.; Bollam, S.R.; et al. Stem-cell states converge in multistage cutaneous squamous cell carcinoma development. Science 2024, 384, eadi7453. [Google Scholar] [CrossRef]
- Katsuno, Y.; Meyer, D.S.; Zhang, Z.; Shokat, K.M.; Akhurst, R.J.; Miyazono, K.; Derynck, R. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 2019, 12, eaau8544. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Fan, Z.; Chen, Z.; Liang, J.; Zeng, G.; Liu, L.; Liu, W.; Yang, T.; Cao, X.; et al. Targeting hyperactive TGFBR2 for treating MYOCD deficient lung cancer. Theranostics 2021, 11, 6592–6606. [Google Scholar] [CrossRef]
- Liu, K.; Tian, F.; Chen, X.; Liu, B.; Tian, S.; Hou, Y.; Wang, L.; Han, M.; Peng, S.; Tan, Y.; et al. Stabilization of TGF-β Receptor 1 by a Receptor-Associated Adaptor Dictates Feedback Activation of the TGF-β Signaling Pathway to Maintain Liver Cancer Stemness and Drug Resistance. Adv. Sci. 2024, 11, e2402327. [Google Scholar] [CrossRef] [PubMed]
- Wildey, G.M.; Patil, S.; Howe, P.H. Smad3 Potentiates Transforming Growth Factor β (TGFβ)-induced Apoptosis and Expression of the BH3-only Protein Bim in WEHI 231 B Lymphocytes. J. Biol. Chem. 2003, 278, 18069–18077. [Google Scholar] [CrossRef]
- Ohgushi, M.; Kuroki, S.; Fukamachi, H.; O’Reilly, L.A.; Kuida, K.; Strasser, A.; Yonehara, S. Transforming Growth Factor β-Dependent Sequential Activation of Smad, Bim, and Caspase-9 Mediates Physiological Apoptosis in Gastric Epithelial Cells. Mol. Cell. Biol. 2005, 25, 10017–10028. [Google Scholar] [CrossRef]
- Ramjaun, A.R.; Tomlinson, S.; Eddaoudi, A.; Downward, J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGFβ-induced apoptosis. Oncogene 2007, 26, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Gautron, A.; Bachelot, L.; Aubry, M.; Leclerc, D.; Quéméner, A.M.; Corre, S.; Rambow, F.; Paris, A.; Tardif, N.; Leclair, H.M.; et al. CRISPR screens identify tumor-promoting genes conferring melanoma cell plasticity and resistance. EMBO Mol. Med. 2021, 13, e13466. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; Salas-Bastos, A.; Nordin, A.; Debbache, J.; Stierli, S.; Cheng, P.F.; Rufli, S.; Wyss, C.; Levesque, M.P.; Dummer, R.; et al. TGFβ signaling sensitizes MEKi-resistant human melanoma to targeted therapy-induced apoptosis. Cell Death Dis. 2024, 15, 925. [Google Scholar] [CrossRef] [PubMed]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer 2021, 2, 587–597. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Ma, M.-J.; Shi, Y.-H.; Liu, Z.-D.; Zhu, Y.-Q.; Zhao, G.-Y.; Ye, J.-Y.; Li, F.-X.; Huang, X.-T.; Wang, X.-Y.; Wang, J.-Q.; et al. N6-methyladenosine modified TGFB2 triggers lipid metabolism reprogramming to confer pancreatic ductal adenocarcinoma gemcitabine resistance. Oncogene 2024, 43, 2405–2420. [Google Scholar] [CrossRef]
- Xu, W.W.; Huang, Z.; Liao, L.; Zhang, Q.; Li, J.; Zheng, C.; He, Y.; Luo, T.; Wang, Y.; Hu, H.; et al. Direct Targeting of CREB1 with Imperatorin Inhibits TGFβ2-ERK Signaling to Suppress Esophageal Cancer Metastasis. Adv. Sci. 2020, 7, 2000925. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Wu, Q.; Xie, L.; Barwick, B.; Fu, C.; Li, X.; Wu, D.; Xia, S.; Chen, J.; et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat. Commun. 2021, 12, 1714. [Google Scholar] [CrossRef]
- Lliakis, G. The role of DNA double strand breaks in lonizing radiation-induced killing of eukaryotic cells. BioEssays 1991, 13, 641–648. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H. Radiation-induced transforming growth factor beta and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res. 1993, 53, 3880–3886. [Google Scholar] [PubMed]
- Barcellos-Hoff, M.H.; Dix, T.A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 1996, 10, 1077–1083. [Google Scholar] [PubMed]
- Wilkinson, B.; Hill, M.A.; Parsons, J.L. The Cellular Response to Complex DNA Damage Induced by Ionising Radiation. Int. J. Mol. Sci. 2023, 24, 4920. [Google Scholar] [CrossRef]
- Ewan, K.B.; Henshall-Powell, R.L.; Ravani, S.A.; Pajares, M.J.; Arteaga, C.; Warters, R.; Akhurst, R.J.; Barcellos-Hoff, M.H. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res. 2002, 62, 5627–5631. [Google Scholar] [PubMed]
- Kirshner, J.; Jobling, M.F.; Pajares, M.J.; Ravani, S.A.; Glick, A.B.; Lavin, M.J.; Koslov, S.; Shiloh, Y. Barcellos-Hoff MH. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006, 66, 10861–10869. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Akhurst, R.J. Transforming growth factor-β in breast cancer: Too much, too late. Breast Cancer Res. 2009, 11, 202. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, L.; Jones, T.; Palomero, L.; Pujana, M.A.; Martinez-Ruiz, H.; Ha, P.K.; Murnane, J.; Cuartas, I.; Seoane, J.; et al. Subjugation of TGFβ Signaling by Human Papilloma Virus in Head and Neck Squamous Cell Carcinoma Shifts DNA Repair from Homologous Recombination to Alternative End Joining. Clin. Cancer Res. 2018, 24, 6001–6014. [Google Scholar] [CrossRef]
- Kim, M.-R.; Lee, J.; An, Y.S.; Jin, Y.B.; Park, I.-C.; Chung, E.; Shin, I.; Barcellos-Hoff, M.H.; Yi, J.Y. TGFβ1 Protects Cells from γ-IR by Enhancing the Activity of the NHEJ Repair Pathway. Mol. Cancer Res. 2015, 13, 319–329. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-R.; Kim, H.-J.; An, Y.S.; Yi, J.Y. TGF-β1 accelerates the DNA damage response in epithelial cells via Smad signaling. Biochem. Biophys. Res. Commun. 2016, 476, 420–425. [Google Scholar] [CrossRef]
- Bouquet, F.; Pal, A.; Pilones, K.A.; Demaria, S.; Hann, B.; Akhurst, R.J.; Babb, J.S.; Lonning, S.M.; DeWyngaert, J.K.; Formenti, S.C.; et al. TGFβ1 Inhibition Increases the Radiosensitivity of Breast Cancer Cells In Vitro and Promotes Tumor Control by Radiation In Vivo. Clin. Cancer Res. 2011, 17, 6754–6765. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Hurd, S.D.; Winnier, A.R.; Johnson, M.D.; Fendly, B.M.; Forbes, J.T. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J. Clin. Investig. 1993, 92, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Ma, H.-Y.; Liang, B.; Li, J.; Meyer, D.S.; Chen, S.-Y.; Sun, K.-H.; Ren, X.; Zivak, B.; Rosenblum, M.D.; et al. Integrin αvβ8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep. 2021, 36, 109309. [Google Scholar] [CrossRef]
- Ni, Y.; Soliman, A.; Joehlin-Price, A.; Rose, P.G.; Vlad, A.; Edwards, R.P.; Mahdi, H. High TGF-β signature predicts immunotherapy resistance in gynecologic cancer patients treated with immune checkpoint inhibition. npj Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Mamai, O.; Akhurst, R.J. TGFβ: Signaling Blockade for Cancer Immunotherapy. Annu. Rev. Cancer Biol. 2022, 6, 123–146. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Strauss, J.; Gulley, J.L.; Schlom, J. Preclinical and clinical studies of bintrafusp alfa, a novel bifunctional anti-PD-L1/TGFβRII agent: Current status. Exp. Biol. Med. 2022, 247, 1124–1134. [Google Scholar] [CrossRef]
- Gulley, J.L.; Schlom, J.; Barcellos-Hoff, M.H.; Wang, X.; Seoane, J.; Audhuy, F.; Lan, Y.; Dussault, I.; Moustakas, A. Dual inhibition of TGF-β and PD-L1: A novel approach to cancer treatment. Mol. Oncol. 2022, 16, 2117–2134. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 2022, 22, 25–44. [Google Scholar] [CrossRef]
- Salnikov, A.V.; Roswall, P.; Sundberg, C.; Gardner, H.; Heldin, N.-E.; Rubin, K. Inhibition of TGF-β modulates macrophages and vessel maturation in parallel to a lowering of interstitial fluid pressure in experimental carcinoma. Lab. Investig. 2005, 85, 512–521. [Google Scholar] [CrossRef]
- Mazzocca, A.; Fransvea, E.; Lavezzari, G.; Antonaci, S.; Giannelli, G. Inhibition of Transforming Growth Factor β Receptor I Kinase Blocks Hepatocellular Carcinoma Growth Through Neo-angiogenesis Regulation. Hepatology 2009, 50, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.C.; Saunier, E.F.; Quigley, D.; Luu, M.T.; De Sapio, A.; Hann, B.; Yingling, J.M.; Akhurst, R.J. Outgrowth of Drug-Resistant Carcinomas Expressing Markers of Tumor Aggression after Long-term TβRI/II Kinase Inhibition with LY2109761. Cancer Res. 2011, 71, 2339–2349. [Google Scholar] [CrossRef]
- Zhang, M.; Kleber, S.; Röhrich, M.; Timke, C.; Han, N.; Tuettenberg, J.; Martin-Villalba, A.; Debus, J.; Peschke, P.; Wirkner, U.; et al. Blockade of TGF-β Signaling by the TGFβR-I Kinase Inhibitor LY2109761 Enhances Radiation Response and Prolongs Survival in Glioblastoma. Cancer Res. 2011, 71, 7155–7167. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Mohanty, S.; Ansari, C.; Lenkov, O.; Shaw, A.; Ito, K.; Hong, S.H.; Hoffmann, M.; Pisani, L.; Boudreau, N.; et al. Alk5 inhibition increases delivery of macromolecular and protein-bound contrast agents to tumors. J. Clin. Investig. 2016, 1, e85608. [Google Scholar] [CrossRef]
- Yoon, H.; Tang, C.-M.; Banerjee, S.; Delgado, A.L.; Yebra, M.; Davis, J.; Sicklick, J.K. TGF-β1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis 2021, 10, 13. [Google Scholar] [CrossRef]
- Al-Bzour, N.N.; Al-Bzour, A.N.; Ababneh, O.E.; Al-Jezawi, M.M.; Saeed, A.; Saeed, A. Cancer-Associated Fibroblasts in Gastrointestinal Cancers: Unveiling Their Dynamic Roles in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 16505. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Do, M.H.; Chou, C.; Stamatiades, E.G.; Nixon, B.G.; Shi, W.; Zhang, X.; Li, P.; Gao, S.; et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature 2020, 587, 121–125. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Tong, Q.; Zhang, S.; Fang, J.; Wu, A.; Wei, G.; Zhang, C.; Yu, S.; Zheng, B.; et al. CD133+PD-L1+ cancer cells confer resistance to adoptively transferred engineered macrophage-based therapy in melanoma. Nat. Commun. 2025, 16, 895. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yan, M.; Zhang, J.; Wang, X.; Shen, Z.; Lv, Z.; Li, Z.; Wei, W.; Chen, W. TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci. Rep. 2016, 6, 20587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Evans, K.S.; Xiao, C.; DeVito, N.; Theivanthiran, B.; Holtzhausen, A.; Siska, P.J.; Blobe, G.C.; Hanks, B.A. Stromal Fibroblasts Mediate Anti–PD-1 Resistance via MMP-9 and Dictate TGFβ Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018, 6, 1459–1471. [Google Scholar] [CrossRef]
- Cremasco, V.; Astarita, J.L.; Grauel, A.L.; Keerthivasan, S.; MacIsaac, K.D.; Woodruff, M.C.; Wu, M.; Spel, L.; Santoro, S.; Amoozgar, Z.; et al. FAP Delineates Heterogeneous and Functionally Divergent Stromal Cells in Immune-Excluded Breast Tumors. Cancer Immunol. Res. 2018, 6, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Chen, J.; Song, E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer 2022, 8, 527–555. [Google Scholar] [CrossRef]
- Chandra Jena, B.; Sarkar, S.; Rout, L.; Mandal, M. The transformation of cancer-associated fibroblasts: Current perspectives on the role of TGF-β in CAF mediated tumor progression and therapeutic resistance. Cancer Lett. 2021, 520, 222–232. [Google Scholar] [CrossRef]
- Forsthuber, A.; Aschenbrenner, B.; Korosec, A.; Jacob, T.; Annusver, K.; Krajic, N.; Kholodniuk, D.; Frech, S.; Zhu, S.; Purkhauser, K.; et al. Cancer-associated fibroblast subtypes modulate the tumor-immune microenvironment and are associated with skin cancer malignancy. Nat. Commun. 2024, 15, 9678. [Google Scholar] [CrossRef]
- Nakamura, A.; Mashima, T.; Lee, J.; Inaba, S.; Kawata, N.; Morino, S.; Kumagai, K.; Yamaguchi, K.; Seimiya, H. Intratumor transforming growth factor-β signaling with extracellular matrix-related gene regulation marks chemotherapy-resistant gastric cancer. Biochem. Biophys. Res. Commun. 2024, 721, 150108. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.X.; Müller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurty, A.T.; Shyer, J.A.; Thai, M.; Gandham, V.; Buechler, M.B.; Yang, Y.A.; Pradhan, R.N.; Wang, A.W.; Sanchez, P.L.; Qu, Y.; et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 2022, 611, 148–154. [Google Scholar] [CrossRef]
- Kawasaki, K.; Noma, K.; Kato, T.; Ohara, T.; Tanabe, S.; Takeda, Y.; Matsumoto, H.; Nishimura, S.; Kunitomo, T.; Akai, M.; et al. PD-L1-expressing cancer-associated fibroblasts induce tumor immunosuppression and contribute to poor clinical outcome in esophageal cancer. Cancer Immunol. Immunother. 2023, 72, 3787–3802. [Google Scholar] [CrossRef]
- Louault, K.; Porras, T.; Lee, M.-H.; Muthugounder, S.; Kennedy, R.J.; Blavier, L.; Sarte, E.; Fernandez, G.E.; Yang, F.; Pawel, B.R.; et al. Fibroblasts and macrophages cooperate to create a pro-tumorigenic and immune resistant environment via activation of TGF-β/IL-6 pathway in neuroblastoma. OncoImmunology 2022, 11, 2146860. [Google Scholar] [CrossRef]
- Yang, K.; Xie, Y.; Xue, L.; Li, F.; Luo, C.; Liang, W.; Zhang, H.; Li, Y.; Ren, Y.; Zhao, M.; et al. M2 tumor-associated macrophage mediates the maintenance of stemness to promote cisplatin resistance by secreting TGF-β1 in esophageal squamous cell carcinoma. J. Transl. Med. 2023, 21, 26. [Google Scholar] [CrossRef]
- Mousset, A.; Lecorgne, E.; Bourget, I.; Lopez, P.; Jenovai, K.; Cherfils-Vicini, J.; Dominici, C.; Rios, G.; Girard-Riboulleau, C.; Liu, B.; et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation. Cancer Cell 2023, 41, 757–775.e10. [Google Scholar] [CrossRef]
- Signore, M.; Alfonsi, R.; Federici, G.; Nanni, S.; Addario, A.; Bertuccini, L.; Aiello, A.; Di Pace, A.L.; Sperduti, I.; Muto, G.; et al. Diagnostic and prognostic potential of the proteomic profiling of serum-derived extracellular vesicles in prostate cancer. Cell Death Dis. 2021, 12, 636. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Russo, A.; Gunasekaran, M.; Buemi, F.; Hester, L.; Fan, X.; Carter-Cooper, B.A.; Lapidus, R.G.; Peleg, A.; Arroyo-Hernández, M.; et al. Baseline extracellular vesicle TGF-β is a predictive biomarker for response to immune checkpoint inhibitors and survival in non–small cell lung cancer. Cancer 2023, 129, 521–530. [Google Scholar] [CrossRef]
- Hosseini, R.; Hosseinzadeh, N.; Asef-Kabiri, L.; Akbari, A.; Ghezelbash, B.; Sarvnaz, H.; Akbari, M.E. Small extracellular vesicle TGF-β in cancer progression and immune evasion. Cancer Gene Ther. 2023, 30, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, X.; Su, P.; Li, H.; Tu, Y.; Du, J.; Pan, C.; Wei, X.; Zheng, M.; Jin, K.; et al. Breast cancer cell-derived extracellular vesicles promote CD8+ T cell exhaustion via TGF-β type II receptor signaling. Nat. Commun. 2022, 13, 4461. [Google Scholar] [CrossRef]
- Lainé, A.; Labiad, O.; Hernandez-Vargas, H.; This, S.; Sanlaville, A.; Léon, S.; Dalle, S.; Sheppard, D.; Travis, M.A.; Paidassi, H.; et al. Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat. Commun. 2021, 12, 6228. [Google Scholar] [CrossRef] [PubMed]
- Seed, R.I.; Kobayashi, K.; Ito, S.; Takasaka, N.; Cormier, A.; Jespersen, J.M.; Publicover, J.; Trilok, S.; Combes, A.J.; Chew, N.W.; et al. A tumor-specific mechanism of Treg enrichment mediated by the integrin αvβ8. Sci. Immunol. 2021, 6, eabf0558. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Begum, H.; Liu, T.; Zhang, J.; Zhang, Q.; Chu, T.-Y.; Li, H.; Lemenze, A.; Hoque, M.; Soteropoulos, P.; et al. NFAT5 governs cellular plasticity-driven resistance to KRAS-targeted therapy in pancreatic cancer. J. Exp. Med. 2024, 221, e20240766. [Google Scholar] [CrossRef] [PubMed]
- Angel, C.Z.; Beattie, S.; Hanif, E.A.M.; Ryan, M.P.; Liberal, F.D.C.G.; Zhang, S.-D.; Monteith, S.; Buckley, N.E.; Parker, E.; Haynes, S.; et al. A SRC-slug-TGFβ2 signaling axis drives poor outcomes in triple-negative breast cancers. Cell Commun. Signal. 2024, 22, 454. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiserman, J.P.; Akhurst, R.J. Diverse Biological Processes Contribute to Transforming Growth Factor β-Mediated Cancer Drug Resistance. Cells 2025, 14, 1518. https://doi.org/10.3390/cells14191518
Heiserman JP, Akhurst RJ. Diverse Biological Processes Contribute to Transforming Growth Factor β-Mediated Cancer Drug Resistance. Cells. 2025; 14(19):1518. https://doi.org/10.3390/cells14191518
Chicago/Turabian StyleHeiserman, James P., and Rosemary J. Akhurst. 2025. "Diverse Biological Processes Contribute to Transforming Growth Factor β-Mediated Cancer Drug Resistance" Cells 14, no. 19: 1518. https://doi.org/10.3390/cells14191518
APA StyleHeiserman, J. P., & Akhurst, R. J. (2025). Diverse Biological Processes Contribute to Transforming Growth Factor β-Mediated Cancer Drug Resistance. Cells, 14(19), 1518. https://doi.org/10.3390/cells14191518






