The Role of Growth Factors and Signaling Pathways in Ovarian Angiogenesis
Abstract
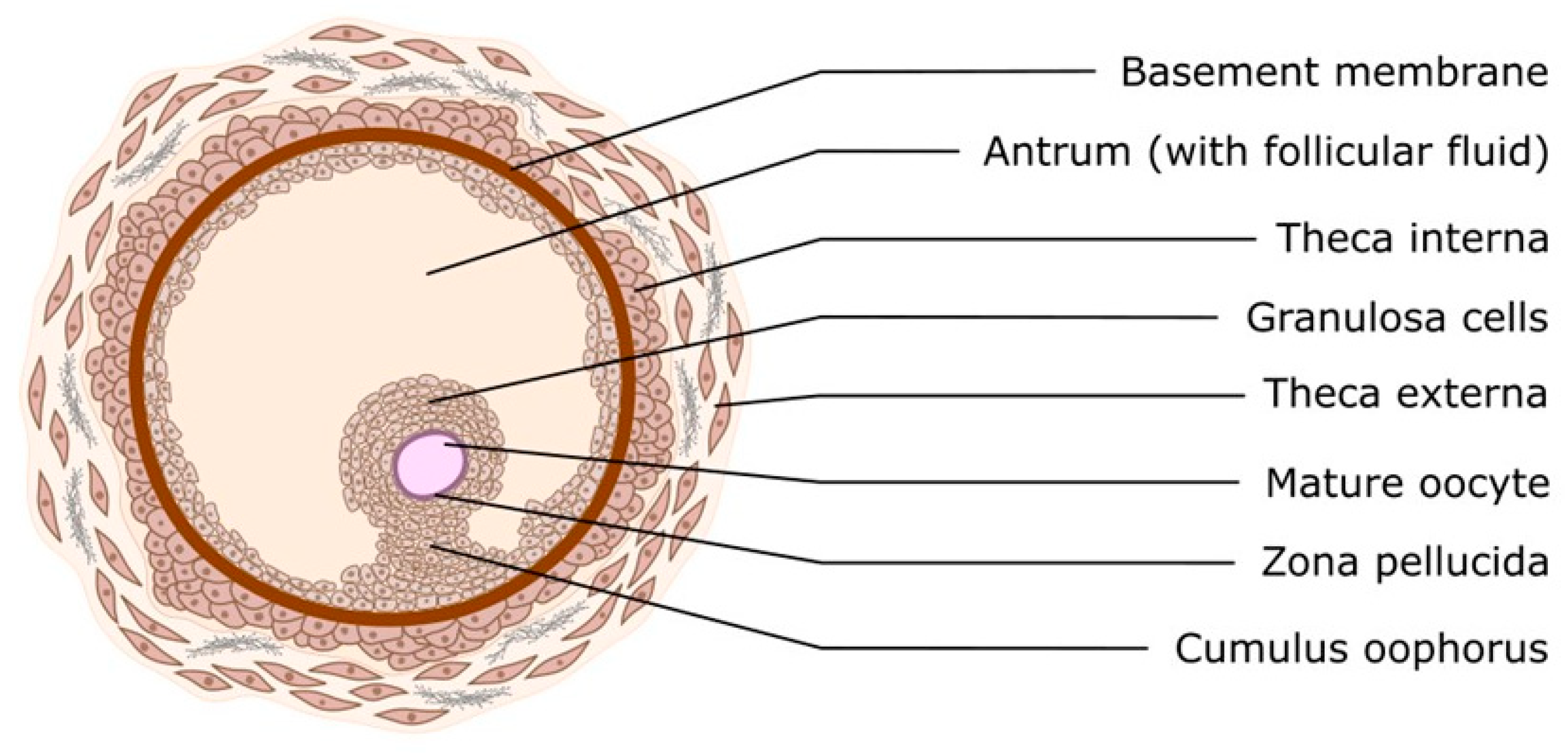
1. Introduction
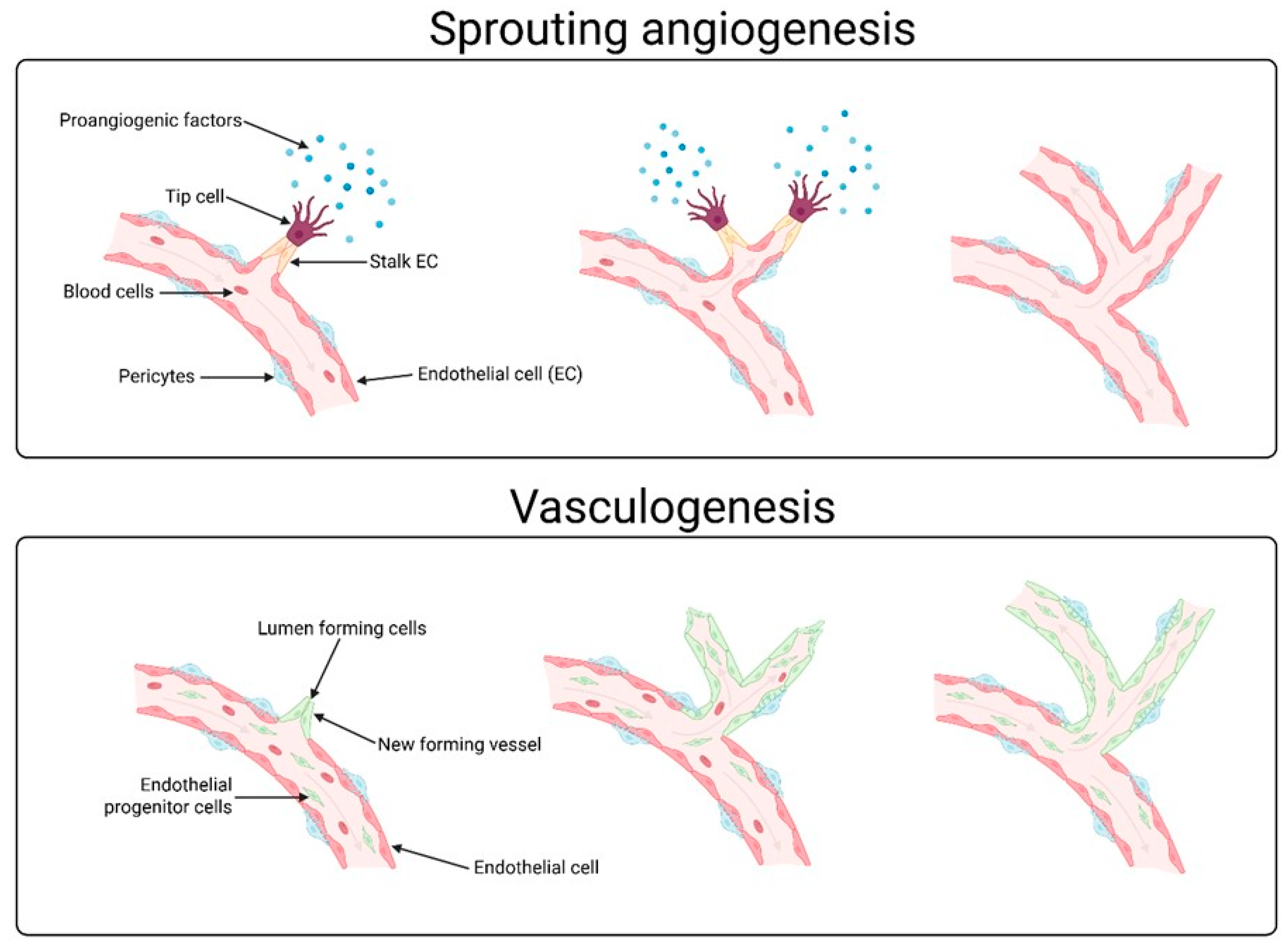
2. Angiogenesis-Promoting Growth Factors
2.1. HIF (Hypoxia-Inducible Factor)
2.2. Transforming Growth Factor-Beta (TGF-β)
2.3. Hepatocyte Growth Factor (HGF)
2.4. Platelet-Derived Growth Factor (PDGF)
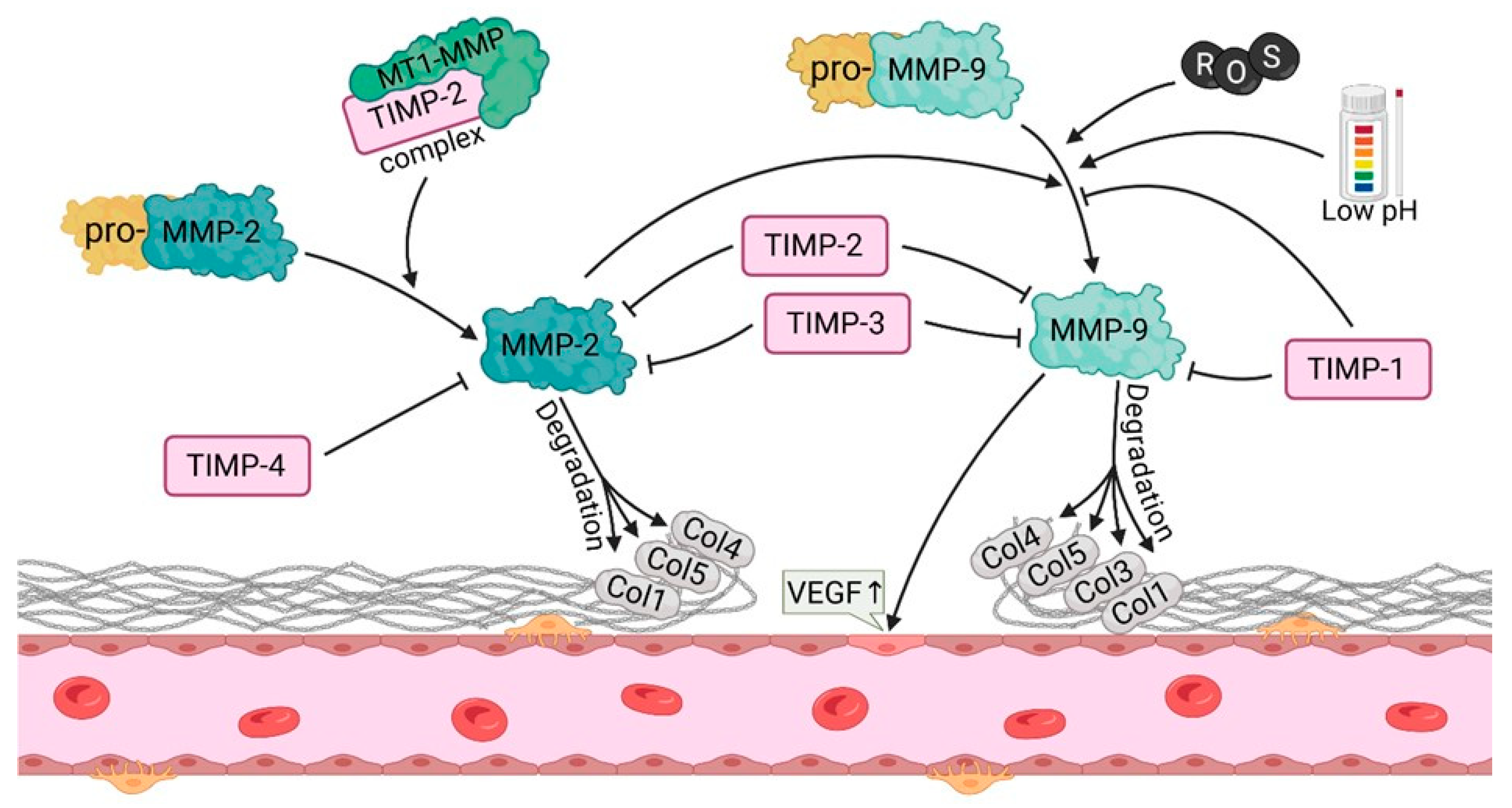
2.5. Matrix Metalloproteinases (MMP-2, MMP-9)
3. Signaling Pathways Involved in Angiogenesis
3.1. FGFR
3.2. VEGFR
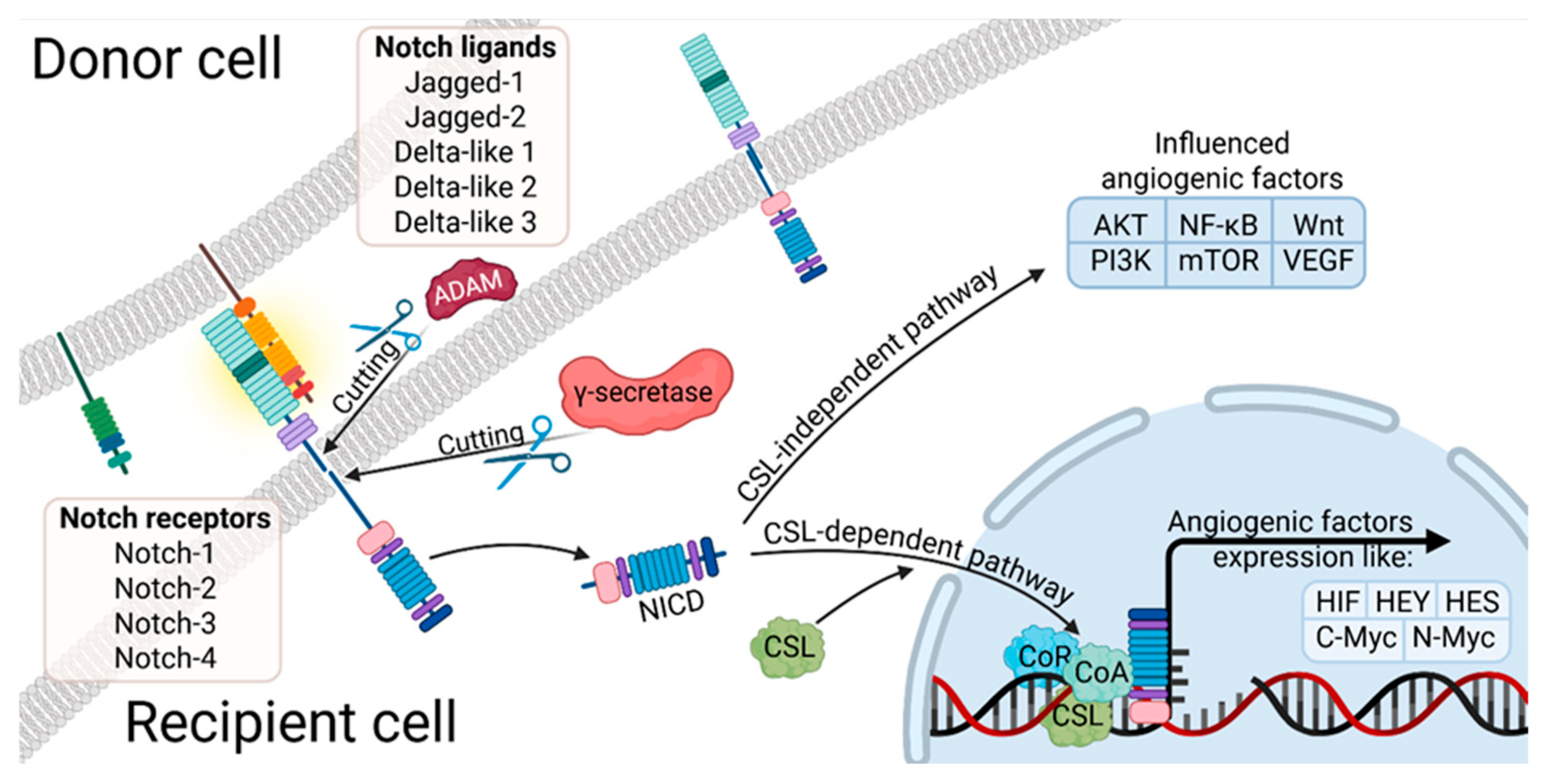
3.3. Notch
3.4. The PI3K/Akt
3.5. HIF Pathway
4. Signaling Pathways—Applications in Medicine
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARNT | Aryl Hydrocarbon Receptor Nuclear Translocator |
| DF | Dominant Follicle |
| EPO | Erythropoietin |
| E2 | Estradiol |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| hCG | Human chorionic gonadotropin |
| HGF | Hepatocyte Growth Factor |
| HIF | Hypoxia-inducible factor |
| HRE | Hypoxia response element |
| IGF | insulin-like growth factor |
| MAPK | Mitogen-activated protein kinase |
| MMPs | Matrix Metalloproteinases |
| LH | Luteinizing hormone |
| PCOS | Polycystic ovary syndrome |
| PDGF | Platelet-Derived Growth Factor |
| PGF2α | Prostaglandin F2α |
| P4 | Progesterone |
| TGF-β | Transforming Growth Factor-beta |
| TSP-1 | Thrombospondin-1 |
| VEGF | Vascular endothelial growth factor |
| ZP | Zona pellucida |
References
- Forde, N.; Beltman, M.E.; Lonergan, P.; Diskin, M.; Roche, J.F.; Crowe, M.A. Oestrous cycles in Bos taurus cattle. Anim. Reprod. Sci. 2011, 124, 163–169. [Google Scholar] [CrossRef]
- Harlow, S.D. Menstruation and Menstrual Disorders in Woman and Health; Academic Press: Cambridge, MA, USA, 2000; pp. 99–113. [Google Scholar] [CrossRef]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Craig, J. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628. [Google Scholar] [CrossRef]
- McNatty, K.P.; Reader, K.; Smith, P.; Heath, D.A.; Juengel, J.L. Control of ovarian follicular development to the gonadotrophin-dependent phase: A 2006 perspective. Soc. Reprod. Fertil. Suppl. 2007, 64, 55–68. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J.W. Initial and Cyclic Recruitment of Ovarian Follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.E. The early stages of follicular development: Activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci. 2003, 78, 135–163. [Google Scholar] [CrossRef]
- Richards, J.A.S. Perspective: The ovarian follicle—A perspective in 2001. Endocrinology 2001, 142, 2184–2193. [Google Scholar] [CrossRef]
- Ebner, T.; Shebl, O.; Moser, M.; Sommergruber, M.; Tews, G. Developmental fate of ovoid oocytes. Hum. Reprod. 2007, 23, 62–66. [Google Scholar] [CrossRef]
- Foster, J.G.; Wong, S.C.K.; Sharp, T.V. The hypoxic tumor microenvironment: Driving the tumorigenesis of non-small-cell lung cancer. Future Oncol. 2014, 10, 2659–2674. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basichelix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Dunning, S.P.; Bunn, H.F. Regulation of the erythropoietin gene: Evidence that the oxygen sensor is a heme protein. Science 1988, 242, 1412–1418. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3 to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Moran, S.M.; Hogenesch, J.B.; Wartman, L.; Bradfield, C.A. Molecular characterization and chromosomal localization of a third—Class hypoxia inducible factor subunit, HIF3α. Gene Expr. 1998, 7, 205–213. [Google Scholar]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial cell–specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019, 33, 7929. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1): Its protein stability and biological functions. Exp. Mol. Med. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- Highet, A.R.; Khoda, S.M.; Buckberry, S.; Leemaqz, S.; Bianco-Miotto, T.; Harrington, E.; Ricciardelli, C.; Roberts, C.T. Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcripttional regulation and promotes invasion. Eur. J. Cell Biol. 2015, 94, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Kallio, P.J.; Pongratz, I.; Gradin, K.; McGuire, J.; Poellinger, L. Activation of hypoxia-inducible factor 1: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl. Acad. Sci. USA 1997, 94, 5667–5672. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Weck, J.; Maizels, E.; Park, Y.; Lee, E.J.; Ashcroft, M.; Hunzicker-Dunn, M. Role of the Phosphatidylinositol-3-Kinase and Extracellular Regulated Kinase Pathways in the Induction of Hypoxia-Inducible Factor (HIF)-1 Activity and the HIF-1 Target Vascular Endothelial Growth Factor in Ovarian Granulosa Cells in Response to Follicle. Endocrinology 2009, 150, 915–928. [Google Scholar] [CrossRef]
- Roberts, A.B.; Anzano, M.A.; Wakefield, L.M.; Roche, N.S.; Stern, D.F.; Sporn, M.B. Type transforming growth factor: A bifunctional regulator of cellular growth. Proc. Natl. Acad. Sci. USA 1985, 82, 119–123. [Google Scholar] [CrossRef]
- Lin, S.Y.; Morrison, J.R.; Phillips, D.J.; de Kretser, D.M. Regulation of ovarian function by the TGF-β superfamily and follistatin. Reproduction 2003, 126, 616–630. [Google Scholar] [CrossRef]
- Massagué, J. TGF signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef] [PubMed]
- Hazzard, T.M. Changes in expression of vascular endothelial growth factor and angiopoietin-1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol. Hum. Reprod. 2000, 6, 993–998. [Google Scholar] [CrossRef]
- Data, K.; Kulus, M.; Ziemak, H.; Chwarzynski, M.; Piotrowska-Kempisty, H.; Bukowska, D.; Antosik, P.; Mozdziak, P.; Kempisty, B. Decellularization of Dense Regular Connective Tissue—Cellular and Molecular Modification with Applications in Regenerative Medicine. Cells 2023, 12, 201648. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Nakagawy, T.; Li, J.H.; Garcia, G.; Mu, W.; Piek, E.; Böttinger, E.P.; Chen, Y.; Zhu, H.J.; Kang, D.H.; Schreiner, G.F.; et al. TGF-β Induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004, 66, 605–613. [Google Scholar] [CrossRef]
- Lai, T.-H.; Chen, H.-T.; Wu, W.-B. TGF1 induces in-vitro and ex-vivo angiogenesis through VEGF production in human ovarian follicular fluid-derived granulosa cells during in-vitro fertilization cycle. J. Reprod. Immunol. 2021, 145, 103311. [Google Scholar] [CrossRef]
- Raja-Khan, N.; Urbanek, M.; Rodgers, R.J.; Legro, R.S. The Role of TGF- in Polycystic Ovary Syndrome. Reprod. Sci. 2014, 21, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Sala, V.; Crepaldi, T. Novel therapy for myocardial infarction: Can HGF/Met be beneficial? Cell. Mol. Life Sci. 2011, 68, 1703–1717. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Woude, G.F.V. HGF/SF-Met signaling in the control of branching morphogenesis and invasion. J. Cell. Biochem. 2003, 88, 408–417. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Seungjoon, M.; Park, M.N.; Kim, B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 2023, 161, 114491. [Google Scholar] [CrossRef]
- Pinkas, H.; Fisch, B.; Rozansky, G.; Felz, C.; Kessler-Icekson, G.; Krissi, H.; Nitke, S.; Ao, A.; Abir, R. Platelet-derived growth factors (PDGF-A and -B) and their receptors in human fetal and adult ovaries. Mol. Hum. Reprod. 2008, 14, 199–206. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Kim, K.-H.; Chung, H.-M.; Choi, D.-H.; Lee, W.-S.; Cha, K.-Y.; Lee, K.-A. Gene expression profiling of early follicular development in primordial, primary, and secondary follicles. Fertil. Steril. 2006, 85, 193–203. [Google Scholar] [CrossRef]
- Sleer, L.S.; Taylor, C.C. Cell-Type Localization of Platelet-Derived Growth Factors and Receptors in the Postnatal Rat Ovary and Follicle. Biol. Reprod. 2007, 76, 379–390. [Google Scholar] [CrossRef]
- Pietras, K.; Sjöblom, T.; Rubin, K.; Heldin, C.-H.; Östman, A. PDGF receptors as cancer drug targets. Cancer Cell 2003, 3, 439–443. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 647 activation by MT1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef]
- Toth, M.; Chvyrkova, I.; Bernardo, M.M.; Hernandez-Barrantes, S.; Fridman, R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: Role of TIMP-2 and plasma membranes. Biochem. Biophys. Res. Commun. 2003, 308, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Acad. Press Inc. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Riley, S.; Gibson, A.; Leask, R.; Mauchline, D.; Pedersen, H.; Watson, E. Secretion of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases into follicular fluid during follicle development in equine ovaries. Reproduction 2001, 121, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Jagarlamudi, K.; Liu, L.; Adhikari, D.; Reddy, P.; Idahl, A.; Ottander, U.; Lundin, E.; Liu, K. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS ONE 2008, 4, e6186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Kuo, C.T.; Lin, C.C.; Hsieh, H.L.; Yang, C.M. IL-1 induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 2010, 160, 1595. [Google Scholar] [CrossRef]
- Sylus, A.M.; Nandeesha, H.; Chitra, T. Matrix metalloproteinase-9 increases and Interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2008, 4, 215–266. [Google Scholar] [CrossRef]
- Lundin, L.; Larsson, H.; Kreuger, J.; Lindah, U.; Salmivirta, M.; Claesson-Welsh, L. Selectively Desulfated Heparin Inhibits Fibroblast Growth Factor-induced Mitogenicity and Angiogenesis. J. Biol. Chem. 2008, 275, 24653–24660. [Google Scholar] [CrossRef] [PubMed]
- Parrott, A.; Skinner, M.K. Developmental and hormonal regulation of keratinocyte growth factor expression and action in the ovarian follicle. Endocrinology 1998, 139, 228–235. [Google Scholar] [CrossRef]
- Price, C.A. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. Endocrinol 2016, 228, R31–R43. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Jeong, M.S.; Ha, K.T.; Jang, S.B. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018, 51, 73–78. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Ruch, C.; Skiniotis, G.; Steinmetz, M.O.; Walz, T.; Ballmer-Hofer, K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat. Struct. Mol. Biol. 2007, 14, 249–250. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, P.; Opatowsky, Y.; Schlessinger, J. Direct contacts between extracellular membrane-proximaldomains are required for VEGF receptor activation and cell signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 1906–1911. [Google Scholar] [CrossRef]
- Chandler, K.B.; Leon, D.R.; Meyer, R.D.; Rahimi, N.; Costello, C.E. Site-Specific N—Glycosylation of Endothelial Cell Receptor Tyrosine Kinase VEGFR-2. J. Proteome Res. 2017, 16, 677–688. [Google Scholar] [CrossRef]
- Chandler, K.B.; Leon, D.R.; Kuang, J.; Meyer, R.D.; Rahimi, N.; Costello, C.E. N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J. Biol. Chem. 2019, 294, 13117–13130. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef]
- Matsumoto, T.; Bohman, S.; Dixelius, J.; Berge, T.; Dimberg, A.; Magnusson, P.; Wang, L.; Wikner, C.; Qi, J.H.; Wernstedt, C.; et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005, 24, 2342–2353. [Google Scholar] [CrossRef]
- Ma, B.; Xiang, Y.; An, L. Structural bases of physiological functions and roles of the vacuolar H+-ATPase. Cell. Signal. 2011, 23, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Ehebauer, M.; Hayward, P.; Martinez-Arias, A. Notch Signaling Pathway. Sci. STKE 2006, 2006, cm7. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Cheng, Z.; Chen, X.; Lobe, C.G.; Liu, J. The role of Notch signalling in ovarian angiogenesis. J. Ovarian Res. 2017, 10, 13. [Google Scholar] [CrossRef]
- Jarriault, S.; Brou, C.; Logeat, F.; Schroeter, E.H.; Kopan, R.; Israel, A. Signalling down-stream of activated mammalian Notch. Nature 1995, 377, 355–358. [Google Scholar] [CrossRef]
- Johnson, J.; Espinoza, T.; McGaughey, R.W.; Rawls, A.; Wilson-Rawls, J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech. Dev. 2008, 109, 355–361. [Google Scholar] [CrossRef]
- Maillard, I.; Adler, S.H.; Pear, W.S. Notch and the Immune System. Immunity 2003, 19, 781–791. [Google Scholar] [CrossRef]
- Leong, K.G.; Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis. Blood 2006, 107, 2223–2233. [Google Scholar] [CrossRef]
- Penton, A.L.; Leonard, L.D.; Spinner, N.B. Notch signaling in human development and disease. Semin. Cell Dev. Biol. 2012, 23, 450–457. [Google Scholar] [CrossRef]
- Mirdamadi, Y.; Bommhardt, U.; Goihl, A.; Guttek, K.; Zouboulis, C.C.; Quist, S.; Gollnick, H. Insulin and Insulin-like growth factor-1 can activate the phosphoinositide-3-kinase /Akt/FoxO1 pathway in T cells in vitro. Dermato-Endocrinology 2017, 9, e1356518. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2008, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Byzova, T.V.; Goldman, C.K.; Pampori, N.; Thomas, K.A.; Bett, A.; Shattil, S.J.; Plow, E.F. A Mechanism for Modulation of Cellular Responses to VEGF. Mol. Cell 2000, 6, 851–860. [Google Scholar] [CrossRef]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papa-Petropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef]
- Carnero, A. The PKB/AKT Pathway in Cancer. Curr. Pharm. Des. 2009, 16, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Testa, J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005, 124, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Lohela, M.; Morisada, T.; Tornberg, J.; Norrmen, C.; Oike, Y.; Pajusola, K.; Thurston, G.; Suda, T.; et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005, 105, 4642–4648. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, S.; Nakada, K.; Kuge, Y.; Tamaki, N.; Okada, F.; Wang, J.; Shindo, M.; Higashino, F.; Takeda, K.; et al. Dominant-Negative Hypoxia-Inducible Factor-1 Reduces Tumorigenicity of Pancreatic Cancer Cells through the Suppression of Glucose Metabolism. Am. J. Pathol. 2003, 162, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Kung, A.L.; Wang, S.; Klco, J.M.; Kaelin, W.G.; Livingston, D.M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat. Med. 2000, 6, 1335–1340. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska-Ziemak, H.; Kulus, M.; Partynska, A.; Kulus, J.; Data, K.P.; Domagala, D.; Niebora, J.; Gorska, A.; Podralska, M.; Podhorska-Okolow, M.; et al. The Role of Growth Factors and Signaling Pathways in Ovarian Angiogenesis. Cells 2025, 14, 1555. https://doi.org/10.3390/cells14191555
Jankowska-Ziemak H, Kulus M, Partynska A, Kulus J, Data KP, Domagala D, Niebora J, Gorska A, Podralska M, Podhorska-Okolow M, et al. The Role of Growth Factors and Signaling Pathways in Ovarian Angiogenesis. Cells. 2025; 14(19):1555. https://doi.org/10.3390/cells14191555
Chicago/Turabian StyleJankowska-Ziemak, Hanna, Magdalena Kulus, Aleksandra Partynska, Jakub Kulus, Krzysztof Piotr Data, Dominika Domagala, Julia Niebora, Aleksandra Gorska, Marta Podralska, Marzenna Podhorska-Okolow, and et al. 2025. "The Role of Growth Factors and Signaling Pathways in Ovarian Angiogenesis" Cells 14, no. 19: 1555. https://doi.org/10.3390/cells14191555
APA StyleJankowska-Ziemak, H., Kulus, M., Partynska, A., Kulus, J., Data, K. P., Domagala, D., Niebora, J., Gorska, A., Podralska, M., Podhorska-Okolow, M., Chmielewski, P., Antosik, P., Bukowska, D., Kaminski, A., Piotrowska-Kempisty, H., Zabel, M., Mozdziak, P., Dziegiel, P., & Kempisty, B. (2025). The Role of Growth Factors and Signaling Pathways in Ovarian Angiogenesis. Cells, 14(19), 1555. https://doi.org/10.3390/cells14191555





