Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review
Abstract
:1. Introduction
1.1. Beneficial Effects of VPA
1.2. Toxic Effects of VPA
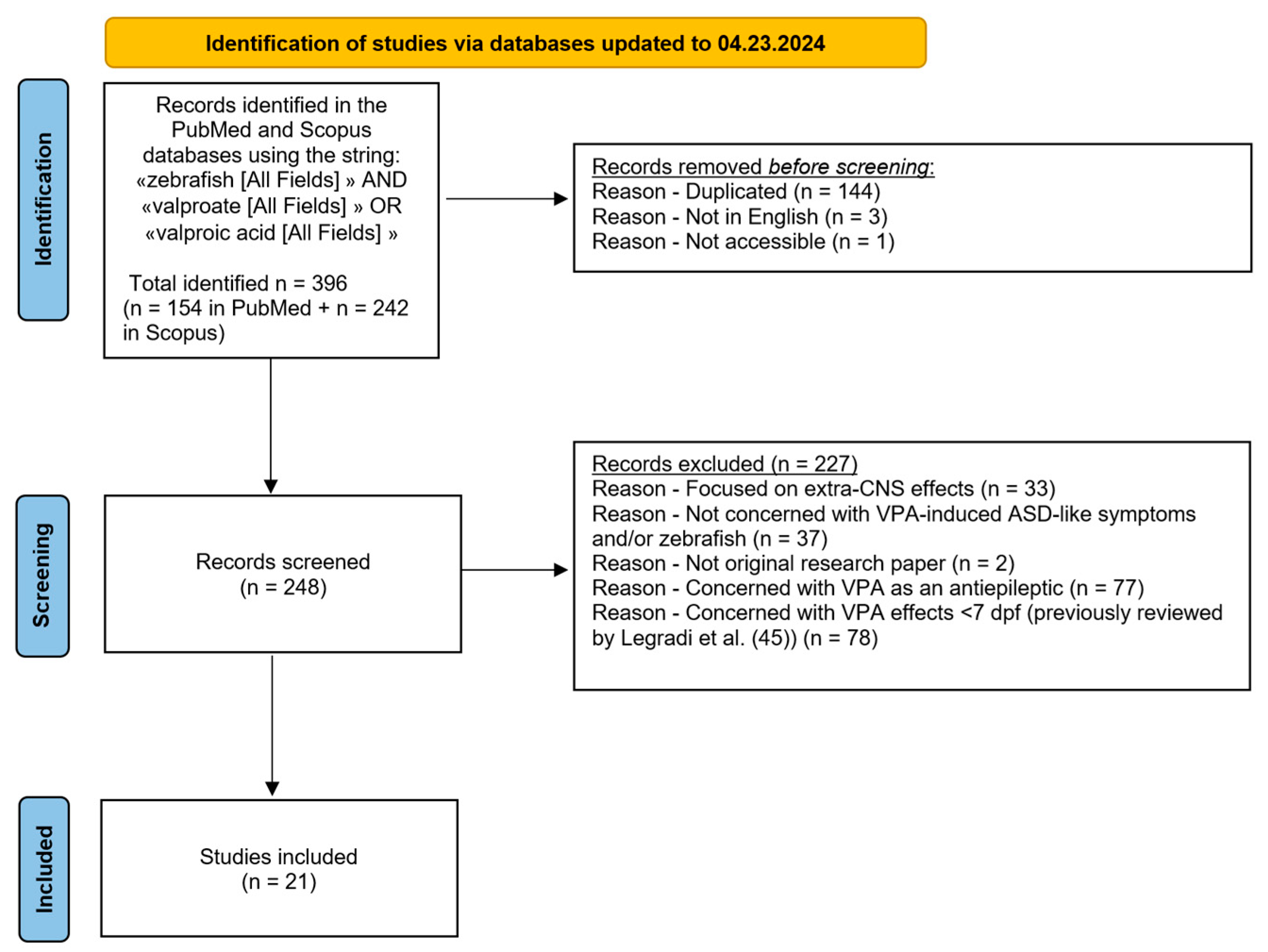
2. Materials and Methods
3. Results
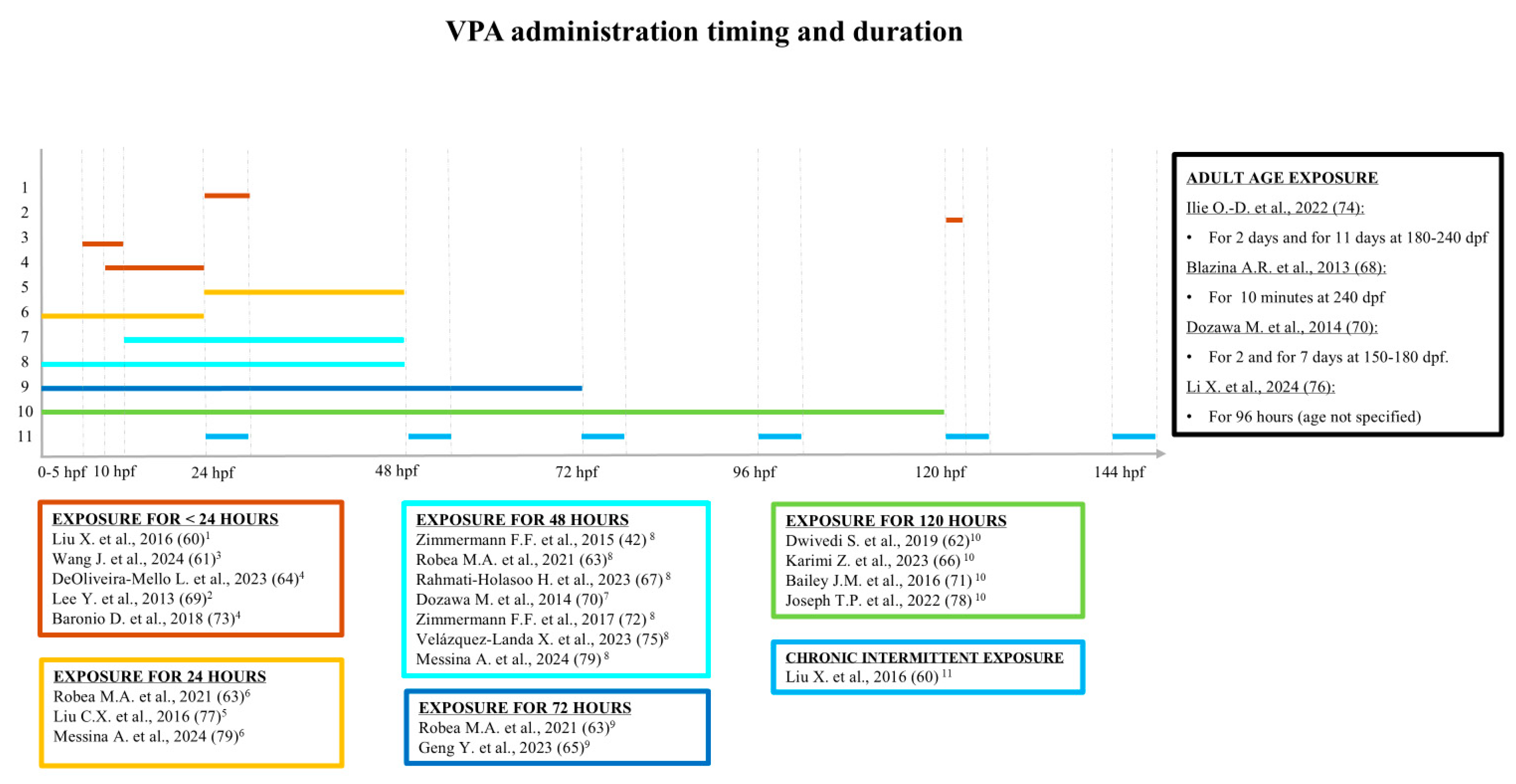
3.1. Studies Analyzing Juvenile Specimens (Younger than 90 dpf)
- -
- Liu et al. [60] exposed 24 hpf zebrafish larvae to either chronic intermittent treatment (VPA 20 µM for 7 h/day for 6 days) or acute treatment (VPA 100 µM for 7 h). At 30 dpf, they observed a deficit in locomotion only in the acutely treated zebrafish and a social behavior impairment only in the chronically exposed zebrafish. No difference between the two groups was observed in thigmotaxis or responses to sudden changes in light, which means that no anxiety-like behavior was observed.
- -
- Wang et al. [43,61] exposed larvae to increasing concentrations of VPA (5–500 µM) for 8–120 hpf and observed ASD-like behavior at 10–13 dpf, even with just 5 µM VPA. Impairment of social behavior was assessed by evaluating shoaling, shoaling L/D background exploration, social contact, and the mirror biting test.
- -
- Dwivedi et al. [62] exposed larvae to 75 µM VPA for 4–120 hpf. At 7 dpf, molecular (qRT-PCR and Western blot), behavioral (social preference and thigmotaxis, inattentive behavior), and non-behavioral (circling) tests were performed. The social preference test, as previously performed by Dreosti et al. [62], was conducted at 21 dpf, and the authors observed a severe social impairment in the treated zebrafish. They also administered drugs approved for the treatment of ASD-related symptoms (including aripiprazole and risperidone) and achieved their goal of reducing the abnormal behaviors and further confirming the phenotype.
- -
- Robea et al. [63] exposed zebrafish larvae to 48 µM VPA for 24, 48, or 72 hpf. They studied the impact of early administration of VPA on zebrafish sleep patterns and on autistic-like social phenotype through social preference and the mirror biting test. Exposure to 48 µM VPA, even just for 24 h, was found to be sufficient to induce a significant social impairment persisting until at least 42 dpf. Instead, no difference in sleep pattern was observed at 6 dpf.
- -
- DeOliveira-Mello et al. [64] explored the relationship between VPA-induced ASD-like behavioral alterations and visual impairment by exposing Turku strain zebrafish to 25 µM VPA for periods ranging from 10 to 24 hpf. They performed immunostaining and gene analysis and observed a delay in the early development of the retina and optic nerve at ≃7 dpf, while no significant difference in visual function, assessed through optomotor behavior and color preference test, was detected at 30 dpf. In contrast with Robea et al. [63], they observed abnormal sleep-like behavior at 7 dpf.
- -
- Geng et al. [65] exposed zebrafish larvae to 1 µM VPA for up to 72 hpf, and then, at 21 dpf, they treated some zebrafish for 1–3 h with 237 neuroactive compounds, known to interfere with social behavior. Right after the treatment, they performed the “ZeChat”, a social contact assay exploring unsupervised deep learning, to characterize sociality. They observed a significant social impairment in the group treated with VPA alone and increased sociality in the zebrafish exposed to VPA and D3 receptor agonists.
- -
- Karimi et al. [66] exposed zebrafish larvae to VPA concentrations of 1–75 µM for up to 120 hpf. Taking into account survival rate and teratogenic side effects, they chose a concentration of 1 µM to perform molecular Wingless/Integrated (WNT)-related pathway analysis at 7 dpf. Also, an inattentive behavior test was performed at 7 dpf, which highlighted a lack of response to aversive stimuli in treated zebrafish embryos compared with controls, which could be taken as a sign of learning impairment. The social preference test was performed at 21 dpf, revealing a social interaction impairment in the treated zebrafish. To investigate the long-lasting impact of 1 µM VPA, they conducted further tests at 42 dpf without, however, observing significant differences between the exposed fish and the controls.
- -
- Rahmati-Holasoo et al. [67] treated zebrafish larvae with 48 µM VPA for up to 48 h and, similarly to Wang et al. [61], evaluated shoaling, social contact, mirror biting, and shoaling L/D background exploration at 10–13 dpf. They also administered oxytocin, a nonapeptide known to be crucial in modulating social behavior [81]. Interestingly, despite not using isotocin, the bony fish analog of mammalian oxytocin [82], they observed a recovery of social and aggressive behavior.
3.2. Studies Analyzing Adult Specimens (90 dpf and Older)
- -
- Blazina et al. [68], exposing 240 dpf adult zebrafish to high doses of VPA (88, 265, 884 µM) for 10 min, found impaired swimming behavior, used as a measure of social behavior, at these high concentrations.
- -
- Lee et al. [69] explored the link between altered cell proliferation in telencephalic regions and ASD-like phenotype in adult fish. They acutely exposed fish embryos to high-dose (2000 µM) VPA for 3 h at 5 dpf. Despite confirming the molecular phenotype through qRT-PCR at 95 dpf, they found no difference in passive avoidance learning or on the bottom dwelling anxiety test. Their findings suggest that the VPA effect is time- and timing-dependent, regardless of the administration of a high dose of the drug.
- -
- Dozawa et al. [70] explored the effect of VPA as an HDAC inhibitor. They exposed adult zebrafish to 500 µM VPA (for 48 or 168 h, depending on the outcome being sought) and performed a cell linage analysis, immunostaining, and in situ hybridization, confirming findings previously reported in mouse models [83]. VPA inhibits HDAC activity and upregulates Notch signaling, which in turn reduces cell proliferation in the optic tectum of adult zebrafish. The HDAC–Notch pathway is critical for proper neurodevelopment [84] and is known to be altered in multiple neurological and psychiatric disorders [85]. Given VPA’s inhibitory function on HDAC, it could be hypothesized that a disruption (particularly an early disruption) of the precise spatial and temporal regulation of this pathway could be linked to the development of molecular and behavioral ASD-associated traits, particularly social behavior-related ones, in both humans and zebrafish.
- -
- Bailey et al. [71] exposed zebrafish larvae to increasing concentrations of VPA (0.5, 5, or 50 µM) for ≃120 hpf. They evaluated adult zebrafish (age not specified) using a neurobehavioral test battery (including startle reflex habituation, novel tank, shoaling behavior, and predator avoidance). The VPA-treated fish showed no significant difference in startle reflex habituation or predator avoidance but, in contrast, displayed hyperactivity in the novel tank exploration assay. Moreover, even just 5 µM VPA negatively affected shoaling behavior. Based on their observations, the authors concluded that possible motor or visual impairments were not likely to be the cause of the observed behavioral differences. These data are consistent with results obtained in juvenile animals by DeOliveira-Mello et al., who, using 25 µM VPA, did not find long-lasting retinal impairment [64].
- -
- Zimmermann et al. [72] conducted qRT-PCR, high-performance liquid chromatography (HPLC), and enzymatic activity assays on their previously characterized zebrafish model of autism [42]. They found a purinergic system dysfunction at 120 dpf, confirming their previous findings, and proposed a possible pathway underlying social behavior impairment.
- -
- Baronio et al. [73] performed qRT-PCR and HPLC to investigate VPA-induced alterations of the histaminergic system in both larvae and adult zebrafish. They exposed Turku strain zebrafish larvae to 25 µM VPA from 10 to 24 hpf and performed, at 180 dpf, hyperactivity and social preference tests. No significant difference was found in locomotion between the treated and control groups. On the contrary, a significant link between social impairment and histaminergic system disruption was detected, supporting the hypothesis that the histaminergic system contributes to the development of ASD-like symptoms [86].
- -
- Ilie et al. [74] used 18 µM VPA for both acute (2-day-long) and chronic (11-day-long) treatment in adult zebrafish (180–240 dpf). Immediately after the treatment, they performed social preference and mirror biting tests, finding disrupted social behavior and increased aggressivity in both the acutely and chronically treated fish.
- -
- Velázquez-Landa et al. [75], on the basis of human evidence regarding sexual behavior-related problems in social interaction in ASD [87], endeavored to “contribute to the knowledge related to sexual behavior in this disorder” using a zebrafish model of autism. They adopted a previously described protocol [42], exposing zebrafish larvae to 48 µM VPA for 48 hpf and, at 180 dpf, observed a disruption of sexual behavioral stages in VPA-treated fish, with the females displaying reduced oviposition.
- -
- Li et al. [76], like Ilie and colleagues [74], did not perform early-stage VPA administration but directly exposed adult fish (age not specified) to a high dose (500 µM) of VPA for 4 days. They performed social preference and mirror biting tests, finding social impairment but, unlike Ilie et al., a decrease in aggression. Moreover, they found anxiety-like behavior, assessed through a bottom dwelling test and a hyperactivity test.
3.3. Studies Analyzing Both Juvenile and Adult Specimens
- -
- The first study in both juvenile and adult zebrafish was conducted by Zimmerman and her group [42], who demonstrated effects on ASD core symptoms of early VPA exposure. They exposed zebrafish larvae to 48 µM VPA for 48 hpf and then performed analyses of both anxiety-related behavior (hyperactivity and bottom dwelling tests at 6, 30, 70, and 120 dpf) and social behavior (social preference and mirror biting tests at 70 and 120 dpf). They found hyperactivity at 6 dpf but not at 30, 70, or 120 dpf. They also found an increase in anxiety up to 70 dpf, while an anxiolytic effect was observed at 120 dpf. A social behavior impairment was detected at both 70 and 120 dpf, while no difference in aggressive behavior was detected.
- -
- Liu et al. [77] focused on the characterization of the expression of shank3, a gene strongly implicated in the pathogenesis of ASD and related syndromes [88]. These recent findings demonstrated a critical role for shank3 in synaptic function and in social interaction and communication, providing a functional link between shank3 and ASD behavioral features. To assess the viability of a zebrafish model of autism for elucidating shank3 involvement in the etiopathogenesis of ASD, Liu et al. exposed zebrafish embryos to 500 or 1000 µM VPA at 24–48 hpf. They observed altered differential expression of shank3 isoforms at different time points (1 to 60 dpf) [77].
- -
- Joseph et al. [78] utilized a VPA-induced ASD zebrafish model to verify the protective function of duloxetine (DLX, a serotonin and noradrenaline reuptake inhibitor) on hyperactivity, anxiety-like behavior, and social deficit. They formed two groups of specimens aged from 8 hpf to 108 hpf; one group was treated with 10 µM VPA, and the other also with increasing doses of DLX. After the treatment, they performed an L/D response and a social contact assay in juvenile fish (5–7/13 dpf). They then performed social preference, hyperactivity, and bottom dwelling tests in adult fish (90–120 dpf). Hyperactivity, increased anxiety-like behavior, and social deficits were found to persist up to 120 dpf, while 20 µM DLX was found to rescue ASD-like features.
- -
- Messina et al. [79] focused on the potential link between loss of brain asymmetry and social cognition impairment on the basis of the consideration that the right hemisphere seems to be mainly linked to emotional and social processing and the left one to attention and categorization [89,90]. A lack of left visual bias (i.e., of the predominant use of the left eye) has been shown to be related to impaired facial expression recognition [91] and seems to characterize some neuropsychiatric disorders, such as ASD [92]. Building on their previous study [93], the authors exposed zebrafish larvae to 1 µM VPA for 24–48 h and then performed the visual mirror test (21 dpf) to assess possible reduction of left visual bias, a social preference test (28 dpf), and a molecular test to explore asymmetric lateralization gene expression (90 dpf). Both exposed groups exhibited decreased social preference and a reduction of left visual bias. Moreover, the molecular analysis (qRT-PCR) showed a neutralization of the asymmetric distribution of genes responsible for brain lateralization [79].
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diederich, M.; Chateauvieux, S.; Morceau, F.; Dicato, M. Molecular and Therapeutic Potential and Toxicity of Valproic Acid. J. Biomed. Biotechnol. 2010, 2010, 479364. [Google Scholar] [CrossRef]
- Romoli, M.; Costa, C.; Siliquini, S.; Corbelli, I.; Eusebi, P.; Bedetti, C.; Caproni, S.; Cupini, L.M.; Calabresi, P.; Sarchielli, P. Antiepileptic Drugs in Migraine and Epilepsy: Who Is at Increased Risk of Adverse Events? Cephalalgia 2018, 38, 274–282. [Google Scholar] [CrossRef]
- Johannessen, C.U. Mechanisms of Action of Valproate: A Commentatory. Neurochem. Int. 2000, 37, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic Acid Pathway: Pharmacokinetics and Pharmacodynamics. Pharmacogenet. Genom. 2013, 23, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.A.; Anderson, L.L.; Gertler, T.S.; Laux, L.; George, A.L.; Kearney, J.A. Screening of Conventional Anticonvulsants in a Genetic Mouse Model of Epilepsy. Ann. Clin. Transl. Neurol. 2017, 4, 326–339. [Google Scholar] [CrossRef]
- Zhang, Y.; Heylen, L.; Partoens, M.; Mills, J.D.; Kaminski, R.M.; Godard, P.; Gillard, M.; de Witte, P.A.M.; Siekierska, A. Connectivity Mapping Using a Novel Sv2a Loss-of-Function Zebrafish Epilepsy Model as a Powerful Strategy for Anti-Epileptic Drug Discovery. Front. Mol. Neurosci. 2022, 15, 881933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Yan, T.; Zhang, Y.; Xu, H.J.; Yu, H.P.; Tu, Z.; Guo, X.; Jiang, Y.H.; Li, X.J.; et al. Maternal Valproic Acid Exposure Leads to Neurogenesis Defects and Autism-like Behaviors in Non-Human Primates. Transl. Psychiatr. 2019, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Pannangrong, W.; Sirichoat, A.; Wongsiri, T.; Wigmore, P.; Welbat, J.U. Valproic Acid Withdrawal Ameliorates Impairments of Hippocampal-Spatial Working Memory and Neurogenesis. J. Zhejiang Univ. Sci. B 2019, 20, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Zhou, H.; Lu, L.; Kong, X.; Wang, T.; Pan, B.; Feng, S. Valproic Acid-Mediated Neuroprotection and Neurogenesis after Spinal Cord Injury: From Mechanism to Clinical Potential. Regen. Med. 2015, 10, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Tuz-Sasik, M.U.; Boije, H.; Manuel, R. Characterization of Locomotor Phenotypes in Zebrafish Larvae Requires Testing under Both Light and Dark Conditions. PLoS ONE 2022, 17, e0266491. [Google Scholar] [CrossRef]
- Nguyen, M.; Stewart, A.M.; Kalueff, A.V. Aquatic Blues: Modeling Depression and Antidepressant Action in Zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2014, 55, 26–39. [Google Scholar] [CrossRef]
- Hortopan, G.A.; Dinday, M.T.; Baraban, S.C. Zebrafish as a Model for Studying Genetic Aspects of Epilepsy. DMM Dis. Model. Mech. 2010, 3, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.S.; Kotova, M.M.; Kolesnikova, T.O.; Ilyin, N.P.; Galstyan, D.S.; Vyunova, T.V.; Petersen, E.V.; Kalueff, A.V. Experimental Zebrafish Models of Synaptopathies. J. Evol. Biochem. Physiol. 2023, 59, 2101–2113. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In Vivo Drug Discovery in the Zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the Moment: Zebrafish Epilepsy Models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef]
- Beker van Woudenberg, A.; Snel, C.; Rijkmans, E.; De Groot, D.; Bouma, M.; Hermsen, S.; Piersma, A.; Menke, A.; Wolterbeek, A. Zebrafish Embryotoxicity Test for Developmental (Neuro)Toxicity: Demo Case of an Integrated Screening Approach System Using Anti-Epileptic Drugs. Reprod. Toxicol. 2014, 49, 101–116. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish Models of Autism Spectrum Disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Galgani, A.; Marchese, M.; Tantussi, F.; Faraguna, U.; De Angelis, F.; Giorgi, F.S. Zebrafish as an Innovative Tool for Epilepsy Modeling: State of the Art and Potential Future Directions. Int. J. Mol. Sci. 2023, 24, 7702. [Google Scholar] [CrossRef]
- Gupta, P.; Khobragade, S.B.; Shingatgeri, V.M. Effect of Various Antiepileptic Drugs in Zebrafish PTZ-Seizure Model. Indian J. Pharm. Sci. 2014, 76, 157–163. [Google Scholar] [PubMed]
- Lee, Y.; Kim, D.; Kim, Y.H.; Lee, H.; Lee, C.J. Improvement of Pentylenetetrazol-Induced Learning Deficits by Valproic Acid in the Adult Zebrafish. Eur. J. Pharmacol. 2010, 643, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Singh, D. Dietary Flavonoids Interaction with CREB-BDNF Pathway: An Unconventional Approach for Comprehensive Management of Epilepsy. Curr. Neuropharmacol. 2019, 17, 1158–1175. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sharma, P.; Mazumder, A.G.; Rana, A.K.; Sharma, S.; Singh, D. Development and Validation of Chemical Kindling in Adult Zebrafish: A Simple and Improved Chronic Model for Screening of Antiepileptic Agents. J. Neurosci. Methods 2020, 346, 108916. [Google Scholar] [CrossRef] [PubMed]
- Chitolina, R.; Gallas-Lopes, M.; Reis, C.G.; Benvenutti, R.; Stahlhofer-Buss, T.; Calcagnotto, M.E.; Herrmann, A.P.; Piato, A. Chemically-Induced Epileptic Seizures in Zebrafish: A Systematic Review. Epilepsy Res. 2023, 197, 107236. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Löscher, W. What New Modeling Approaches Will Help Us Identify Promising Drug Treatments? Springer: Dordrecht, The Netherlands, 2014; Volume 813, pp. 283–294. [Google Scholar]
- Griffin, A.; Hamling, K.R.; Hong, S.G.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef]
- Baraban, S.C.; Dinday, M.T.; Hortopan, G.A. Drug Screening in Scn1a Zebrafish Mutant Identifies Clemizole as a Potential Dravet Syndrome Treatment. Nat. Commun. 2013, 4, 2410. [Google Scholar] [CrossRef] [PubMed]
- Grone, B.P.; Qu, T.; Baraban, S.C. Behavioral Comorbidities and Drug Treatments in a Zebrafish Scn1lab Model of Dravet Syndrome. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Hong, S.G.; Lee, P.; Baraban, S.C.; Lee, L.P. A Novel Long-Term, Multi-Channel and Non-Invasive Electrophysiology Platform for Zebrafish. Sci. Rep. 2016, 6, 28248. [Google Scholar] [CrossRef]
- Della Vecchia, S.; Ogi, A.; Licitra, R.; Abramo, F.; Nardi, G.; Mero, S.; Landi, S.; Battini, R.; Sicca, F.; Ratto, G.M.; et al. Trehalose Treatment in Zebrafish Model of Lafora Disease. Int. J. Mol. Sci. 2022, 23, 6874. [Google Scholar] [CrossRef]
- Liao, G.; Li, R.; Chen, X.; Zhang, W.; Du, S.; Yuan, Y. Sodium Valproate Prevents Radiation-Induced Injury in Hippocampal Neurons via Activation of the Nrf2/HO-1 Pathway. Neuroscience 2016, 331, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Watchon, M.; Luu, L.; Robinson, K.J.; Yuan, K.C.; De Luca, A.; Suddull, H.J.; Tym, M.C.; Guillemin, G.J.; Cole, N.J.; Nicholson, G.A.; et al. Sodium Valproate Increases Activity of the Sirtuin Pathway Resulting in Beneficial Effects for Spinocerebellar Ataxia-3 in Vivo. Mol. Brain 2021, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F. A Comprehensive Review on Pharmacological Applications and Drug-Induced Toxicity of Valproic Acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Chopp, M.; Kassis, H.; Jia, L.F.; Hozeska-Solgot, A.; Zhang, R.L.; Chen, C.; Cui, Y.S.; Zhang, Z.G. Valproic Acid Increases White Matter Repair and Neurogenesis after Stroke. Neuroscience 2012, 220, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Derry, S.; Wiffen, P.J.; Moore, R.A. Valproic Acid and Sodium Valproate for Neuropathic Pain and Fibromyalgia in Adults. Cochrane Database Syst. Rev. 2011, 2011. [Google Scholar] [CrossRef]
- Driessen, M.; Kienhuis, A.S.; Vitins, A.P.; Pennings, J.L.; Pronk, T.E.; van den Brandhof, E.J.; Roodbergen, M.; van de Water, B.; van der Ven, L.T. Gene Expression Markers in the Zebrafish Embryo Reflect a Hepatotoxic Response in Animal Models and Humans. Toxicol. Lett. 2014, 230, 48–56. [Google Scholar] [CrossRef]
- Fietz, A.K.; Onken, M.; Padberg, S.; Schaefer, C.; Dathe, K. Impact of Maternal First Trimester Treatment Regimen on the Outcome of Valproate Exposed Pregnancies: An Observational Embryotox Cohort Study. Sci. Rep. 2024, 14, 674. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic Acid in Pregnancy: How Much Are We Endangering the Embryo and Fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; pp. 50–59. [Google Scholar]
- Tropepe, V.; Sive, H.L. Can Zebrafish Be Used as a Model to Study the Neurodevelopmental Causes of Autism? Genes Brain Behav. 2003, 2, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; De Paula Cognato, G.; Bonan, C.D. Embryological Exposure to Valproic Acid Induces Social Interaction Deficits in Zebrafish (Danio rerio): A Developmental Behavior Analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lei, L.; Tian, L.; Hou, F.; Roper, C.; Ge, X.; Zhao, Y.; Chen, Y.; Dong, Q.; Tanguay, R.L.; et al. Developmental and Behavioral Alterations in Zebrafish Embryonically Exposed to Valproic Acid (VPA): An Aquatic Model for Autism. Neurotoxicol. Teratol. 2018, 66, 8–16. [Google Scholar] [CrossRef]
- Saleh Hodin, N.A.; Chong, S.G.; Bakar, N.A.; Fahmi, M.S.A.M.; Ramlan, N.F.; Hamid, N.N.A.Z.Z.; Fadzar, M.S.I.M.; Zulkifli, A.R.; Norazhar, A.I.; Mastuki, S.N.; et al. Toxicity and Teratogenicity Effects of Valproic Acid on Zebrafish (Danio rerio) Embryos in Relation to Autism Spectrum Disorder. Birth Defects Res. 2023, 115, 1475–1485. [Google Scholar] [CrossRef]
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of Behavioural Assays Using Zebrafish Larvae to Assess Neurotoxicity. Environ. Sci. Pollut. Res. 2014, 22, 16277–16289. [Google Scholar] [CrossRef] [PubMed]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments-A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Sant, K.E.; Navarrete, J.; George, U.Z. Mathematical Modeling of the Interaction between Yolk Utilization and Fish Growth in Zebrafish, Danio rerio. Development 2021, 148, dev193508. [Google Scholar] [CrossRef]
- Wilson, C. Aspects of Larval Rearing. ILAR J. 2012, 53, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, B.; Wang, Z.; Li, Y.; Li, H.; Wu, S.; Li, Z. Quantitative Characterization of Zebrafish Development Based on Multiple Classifications Using Mueller Matrix OCT. Biomed. Opt. Express 2023, 14, 2889. [Google Scholar] [CrossRef] [PubMed]
- Rombough, P. Gills Are Needed for Ionoregulation before They Are Needed for O2 Uptake in Developing Zebrafish, Danio rerio. J. Exp. Biol. 2002, 205, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.C.; Bill, B.R.; Glanzman, D.L. Learning and Memory in Zebrafish Larvae. Front. Neural Circuits 2013, 7, 126. [Google Scholar] [CrossRef]
- Nelson, J.C.; Granato, M. Zebrafish Behavior as a Gateway to Nervous System Assembly and Plasticity. Development 2022, 149, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Lopes, G.; Kampff, A.R.; Wilson, S.W. Development of Social Behavior in Young Zebrafish. Front. Neural Circuits 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, L. AI for Drug Discovery. Nat. Rev. Urol. 2024, 21, 517. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Sugiyama, D. Zebrafish Erythropoiesis and the Utility of Fish as Models of Anemia. Stem Cell Res. Ther. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, T.; Mizoguchi, T.; Tonoki, A.; Itoh, M. Modeling of Age-Related Neurological Disease: Utility of Zebrafish. Front. Aging Neurosci. 2024, 16, 1399098. [Google Scholar] [CrossRef] [PubMed]
- Pluimer, B.R.; Harrison, D.L.; Boonyavairoje, C.; Prinssen, E.P.; Rogers-Evans, M.; Peterson, R.T.; Thyme, S.B.; Nath, A.K. Behavioral Analysis through the Lifespan of Disc1 Mutant Zebrafish Identifies Defects in Sensorimotor Transformation. iScience 2023, 26, 107099. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Singleman, C.; Holtzman, N.G. Growth and Maturation in the Zebrafish, Danio rerio: A Staging Tool for Teaching and Research. Zebrafish 2014, 11, 396–406. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Lin, J.; Xia, Q.; Guo, N.; Li, Q. Social Preference Deficits in Juvenile Zebrafish Induced by Early Chronic Exposure to Sodium Valproate. Front. Behav. Neurosci. 2016, 10, 201. [Google Scholar] [CrossRef]
- Wang, J.; Zou, L.; Jiang, P.; Yao, M.; Xu, Q.; Hong, Q.; Zhu, J.; Chi, X. Vitamin A Ameliorates Valproic Acid-Induced Autism-like Symptoms in Developing Zebrafish Larvae by Attenuating Oxidative Stress and Apoptosis. Neurotoxicology 2024, 101, 93–101. [Google Scholar] [CrossRef]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval Zebrafish Model for Studying the Effects of Valproic Acid on Neurodevelopment: An Approach towards Modeling Autism. J. Pharmacol. Toxicol. Methods 2019, 95, 56–65. [Google Scholar] [CrossRef]
- Robea, M.A.; Ciobica, A.; Curpan, A.S.; Plavan, G.; Strungaru, S.; Lefter, R.; Nicoara, M. Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder. Brain Sci. 2021, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira-Mello, L.; Baronio, D.; Panula, P. Zebrafish Embryonically Exposed to Valproic Acid Present Impaired Retinal Development and Sleep Behavior. Autism Res. 2023, 16, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Yates, C.; Peterson, R.T. Social Behavioral Profiling by Unsupervised Deep Learning Reveals a Stimulative Effect of Dopamine D3 Agonists on Zebrafish Sociality. Cell Reports Methods 2023, 3, 100381. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Zarifkar, A.; Dianatpour, M.; Mirzaei, E.; Dara, M.; Aligholi, H. Finding a Proper Valproic Acid-Based Autism Spectrum Disorder Model in Zebrafish: Early and Long-Term Neurobehavioral Studies. Iran. J. Psychiatr. Behav. Sci. 2023, 17, e137118. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Maghsoudi, A.S.; Akbarzade, M.; Gholami, M.; Shadboorestan, A.; Vakhshiteh, F.; Armandeh, M.; Hassani, S. Oxytocin Protective Effects on Zebrafish Larvae Models of Autism-like Spectrum Disorder. Iran. J. Basic Med. Sci. 2023, 26, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Blazina, A.R.; Vianna, M.R.; Lara, D.R. The Spinning Task: A New Protocol to Easily Assess Motor Coordination and Resistance in Zebrafish. Zebrafish 2013, 10, 480–485. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.H.; Yun, J.S.; Lee, C.J. Valproic Acid Decreases the Proliferation of Telencephalic Cells in Zebrafish Larvae. Neurotoxicol. Teratol. 2013, 39, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dozawa, M.; Kono, H.; Sato, Y.; Ito, Y.; Tanaka, H.; Ohshima, T. Valproic Acid, a Histone Deacetylase Inhibitor, Regulates Cell Proliferation in the Adult Zebrafish Optic Tectum. Dev. Dyn. 2014, 243, 1401–1415. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Karbhari, N.; Brooks, R.A.J.; De La Rocha, A.J.; Janardhan, S.; Levin, E.D. Persistent Behavioral Effects Following Early Life Exposure to Retinoic Acid or Valproic Acid in Zebrafish. Neurotoxicology 2016, 52, 23–33. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Siebel, A.M.; Leite, C.E.; Kist, L.W.; Bogo, M.R.; Bonan, C.D. Analysis of Extracellular Nucleotide Metabolism in Adult Zebrafish After Embryological Exposure to Valproic Acid. Mol. Neurobiol. 2017, 54, 3542–3553. [Google Scholar] [CrossRef]
- Baronio, D.; Puttonen, H.A.J.; Sundvik, M.; Semenova, S.; Lehtonen, E.; Panula, P. Embryonic Exposure to Valproic Acid Affects the Histaminergic System and the Social Behaviour of Adult Zebrafish (Danio rerio). Br. J. Pharmacol. 2018, 175, 797–809. [Google Scholar] [CrossRef]
- Ilie, O.D.; Duta, R.; Jijie, R.; Nita, I.B.; Nicoara, M.; Faggio, C.; Dobrin, R.; Mavroudis, I.; Ciobica, A.; Doroftei, B. Assessing Anti-Social and Aggressive Behavior in a Zebrafish (Danio rerio) Model of Parkinson’s Disease Chronically Exposed to Rotenone. Brain Sci. 2022, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Landa, X.; Carrillo, P.; Coria-Avila, G.A.; Herrera-Covarrubias, D.; García, L.I.; Toledo-Cárdenas, M.R.; Hernández-Aguilar, M.E.; Manzo, J. Zebrafish Sexual Behavior in Plain and Enriched Environments: Parameters in the Valproate Model of Autism. Fishes 2023, 8, 156. [Google Scholar] [CrossRef]
- Li, X.; Feng, T.; Lu, W. The Effects of Valproic Acid Neurotoxicity on Aggressive Behavior in Zebrafish Autism Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 275, 109783. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Peng, X.L.; Hu, C.C.; Li, C.Y.; Li, Q.; Xu, X. Developmental Profiling of ASD-Related Shank3 Transcripts and Their Differential Regulation by Valproic Acid in Zebrafish. Dev. Genes Evol. 2016, 226, 389–400. [Google Scholar] [CrossRef]
- Joseph, T.P.; Zhou, F.; Sai, L.Y.; Chen, H.; Lin, S.L.; Schachner, M. Duloxetine Ameliorates Valproic Acid-Induced Hyperactivity, Anxiety-like Behavior, and Social Interaction Deficits in Zebrafish. Autism Res. 2022, 15, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Sovrano, V.A.; Baratti, G.; Musa, A.; Gobbo, A.; Adiletta, A.; Sgadò, P. Valproic Acid Exposure Affects Social Visual Lateralization and Asymmetric Gene Expression in Zebrafish Larvae. Sci. Rep. 2024, 14, 4474. [Google Scholar] [CrossRef]
- Swaney, W.T.; Ellwood, C.; Davis, J.P.; Reddon, A.R. Familiarity Preferences in Zebrafish (Danio rerio) Depend on Shoal Proximity. J. Fish Biol. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Mariti, C.; Pirrone, F.; Baragli, P.; Gazzano, A. The Influence of Oxytocin on Maternal Care in Lactating Dogs. Animals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Ogi, A.; Naef, V.; Santorelli, F.M.F.M.; Mariti, C.; Gazzano, A. Oxytocin Receptor Gene Polymorphism in Lactating Dogs. Animals 2021, 11, 3099. [Google Scholar] [CrossRef]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone Deacetylase Inhibition-Mediated Neuronal Differentiation of Multipotent Adult Neural Progenitor Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef]
- Jaworska, J.; Ziemka-Nalecz, M.; Zalewska, T. Histone Deacetylases 1 and 2 Are Required for Brain Development. Int. J. Dev. Biol. 2015, 59, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Engler, A.; Taylor, V. Notch: An Interactive Player in Neurogenesis and Disease. Cell Tissue Res. 2018, 371, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Nuutinen, S. The Histaminergic Network in the Brain: Basic Organization and Role in Disease. Nat. Rev. Neurosci. 2013, 14, 472–487. [Google Scholar] [CrossRef]
- Kellaher, D.C. Sexual Behavior and Autism Spectrum Disorders: An Update and Discussion. Curr. Psychiatr. Rep. 2015, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Lauri, M.; Sarasua, S.M.; Skinner, C.D.; Buccella, D.; Dwivedi, A.; Orteschi, D.; Collins, J.S.; Zollino, M.; Visconti, P.; et al. Prevalence of SHANK3 Variants in Patients with Different Subtypes of Autism Spectrum Disorders. Eur. J. Hum. Genet. 2013, 21, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The Role of Asymmetric Frontal Cortical Activity in Emotion-Related Phenomena: A Review and Update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef]
- Rutherford, H.J.V.; Lindell, A.K. Thriving and Surviving: Approach and Avoidance Motivation and Lateralization. Emot. Rev. 2011, 3, 333–343. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Andrew, R.J. Eye Use during Viewing a Reflection: Behavioural Lateralisation in Zebrafish Larvae. Behav. Brain Res. 2006, 167, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Gotts, S.J.; Jo, H.J.; Wallace, G.L.; Saad, Z.S.; Cox, R.W.; Martin, A. Two Distinct Forms of Functional Lateralization in the Human Brain. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3444. [Google Scholar] [CrossRef]
- Messina, A.; Boiti, A.; Sovrano, V.A.; Sgadò, P. Micromolar Valproic Acid Doses Preserve Survival and Induce Molecular Alterations in Neurodevelopmental Genes in Two Strains of Zebrafish Larvae. Biomolecules 2020, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Facciol, A.; Gerlai, R. Zebrafish Shoaling, Its Behavioral and Neurobiological Mechanisms, and Its Alteration by Embryonic Alcohol Exposure: A Review. Front. Behav. Neurosci. 2020, 14, 572175. [Google Scholar] [CrossRef]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social Preference Tests in Zebrafish: A Systematic Review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.E.; Srivorakiat, L.; Wink, L.K.; Pedapati, E.V.; Erickson, C.A. Aggression in Autism Spectrum Disorder: Presentation and Treatment Options. Neuropsychiatr. Dis. Treat. 2016, 12, 1525–1538. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Liu, F.C. Pathophysiological Studies of Monoaminergic Neurotransmission Systems in Valproic Acid-Induced Model of Autism Spectrum Disorder. Biomedicines 2022, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Ago, Y.; Taruta, A.; Hasebe, S.; Kawase, H.; Tanabe, W.; Tsukada, S.; Nakazawa, T.; Hashimoto, H.; Matsuda, T.; et al. Risperidone and Aripiprazole Alleviate Prenatal Valproic Acid-Induced Abnormalities in Behaviors and Dendritic Spine Density in Mice. Psychopharmacology 2017, 234, 3217–3228. [Google Scholar] [CrossRef] [PubMed]
- Oblak, A.; Gibbs, T.T.; Blatt, G.J. Neocortical Region in Autism. Autism Res. 2013, 6, 1–23. [Google Scholar] [CrossRef]
- Veenstra-VanderWeele, J.; Muller, C.L.; Iwamoto, H.; Sauer, J.E.; Owens, W.A.; Shah, C.R.; Cohen, J.; Mannangatti, P.; Jessen, T.; Thompson, B.J.; et al. Autism Gene Variant Causes Hyperserotonemia, Serotonin Receptor Hypersensitivity, Social Impairment and Repetitive Behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 5469–5474. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, B.M.; Sharma, B. Benefits of Agomelatine in Behavioral, Neurochemical and Blood Brain Barrier Alterations in Prenatal Valproic Acid Induced Autism Spectrum Disorder. Neurochem. Int. 2015, 91, 34–45. [Google Scholar] [CrossRef]
- Jacob, J.; Ribes, V.; Moore, S.; Constable, S.C.; Sasai, N.; Gerety, S.S.; Martin, D.J.; Sergeant, C.P.; Wilkinson, D.G.; Briscoe, J. Valproic Acid Silencing of Ascl1b/Ascl1 Results in the Failure of Serotonergic Differentiation in a Zebrafish Model of Fetal Valproate Syndrome. DMM (Dis. Models Mech.) 2014, 7, 107–117. [Google Scholar] [CrossRef]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Höcht, C.; Depino, A.M. Sociability Deficits after Prenatal Exposure to Valproic Acid Are Rescued by Early Social Enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Deisseroth, K. Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 221–227. [Google Scholar] [CrossRef]
- Dichter, G.S.; Damiano, C.A.; Allen, J.A. Reward Circuitry Dysfunction in Psychiatric and Neurodevelopmental Disorders and Genetic Syndromes: Animal Models and Clinical Findings. J. Neurodev. Disord. 2012, 4, 19. [Google Scholar] [CrossRef]
- Hara, Y.; Takuma, K.; Takano, E.; Katashiba, K.; Taruta, A.; Higashino, K.; Hashimoto, H.; Ago, Y.; Matsuda, T. Reduced Prefrontal Dopaminergic Activity in Valproic Acid-Treated Mouse Autism Model. Behav. Brain Res. 2015, 289, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zieminska, E.; Ruszczynska, A.; Augustyniak, J.; Toczylowska, B.; Lazarewicz, J.W. Zinc and Copper Brain Levels and Expression of Neurotransmitter Receptors in Two Rat ASD Models. Front. Mol. Neurosci. 2021, 14, 656740. [Google Scholar] [CrossRef]
- Keehn, B.; Kadlaskar, G.; Bergmann, S.; McNally Keehn, R.; Francis, A. Attentional Disengagement and the Locus Coeruleus—Norepinephrine System in Children With Autism Spectrum Disorder. Front. Integr. Neurosci. 2021, 15, 716447. [Google Scholar] [CrossRef]
- Zhou, J.; Cattoglio, C.; Shao, Y.; Tirumala, H.P.; Vetralla, C.; Bajikar, S.S.; Li, Y.; Chen, H.; Wang, Q.; Wu, Z.; et al. A Novel Pathogenic Mutation of MeCP2 Impairs Chromatin Association Independent of Protein Levels. Genes Dev. 2023, 37, 883–900. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Hong, M.; Kim, K.C.; Kim, J.W.; Yang, S.M.; Seung, H.; Ko, M.J.; Choi, D.H.; You, J.S.; Shin, C.Y.; et al. Effects of Atomoxetine on Hyper-Locomotive Activity of the Prenatally Valproate-Exposed Rat Offspring. Biomol. Ther. 2014, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; de Campos, L.J.; Conda-Sheridan, M.; de Melo, E.B. Pharmacophore Modeling, Molecular Docking, and Molecular Dynamics Studies to Identify New 5-HT2AR Antagonists with the Potential for Design of New Atypical Antipsychotics. Mol. Divers. 2023, 27, 2217–2238. [Google Scholar] [CrossRef]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
- Molina-Hernández, A.; Díaz, N.F.; Arias-Montaño, J.A. Histamine in Brain Development. J. Neurochem. 2012, 122, 872–882. [Google Scholar] [CrossRef]
- Wright, C.; Shin, J.H.; Rajpurohit, A.; Deep-Soboslay, A.; Collado-Torres, L.; Brandon, N.J.; Hyde, T.M.; Kleinman, J.E.; Jaffe, A.E.; Cross, A.J.; et al. Altered Expression of Histamine Signaling Genes in Autism Spectrum Disorder. Transl. Psychiatr. 2017, 7, e1126–e1128. [Google Scholar] [CrossRef]
- Stewart, A.M.; Nguyen, M.; Wong, K.; Poudel, M.K.; Kalueff, A.V. Developing Zebrafish Models of Autism Spectrum Disorder (ASD). Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2014, 50, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V. The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer: Cham, Switzerland, 2017; ISBN 9783319337746. [Google Scholar]
- Spinello, C.; Yang, Y.; Macrì, S.; Porfiri, M. Zebrafish Adjust Their Behavior in Response to an Interactive Robotic Predator. Front. Robot. AI 2019, 6, 38. [Google Scholar] [CrossRef]
- Gerlai, R. Zebrafish Antipredatory Responses: A Future for Translational Research? Behav. Brain Res. 2010, 207, 223–231. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.T.; Lai, Y.H.; Hsiao, C. Der A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Tan, J.X.M.; Ang, R.J.W.; Wee, C.L. Larval Zebrafish as a Model for Mechanistic Discovery in Mental Health. Front. Mol. Neurosci. 2022, 15, 900213. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring Anxiety in Zebrafish: A Critical Review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Mariti, C.; Falaschi, C.; Zilocchi, M.; Fatjó, J.; Sighieri, C.; Ogi, A.; Gazzano, A. Analysis of the Intraspecific Visual Communication in the Domestic Dog (Canis familiaris): A Pilot Study on the Case of Calming Signals. J. Vet. Behav. 2017, 18, 49–55. [Google Scholar] [CrossRef]
- George, A.; Luz, R.F.; De Tychey, C.; Thilly, N.; Spitz, E. Anxiety Symptoms and Coping Strategies in the Perinatal Period. BMC Pregnancy Childbirth 2013, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Todd, B.K. A comparative perspective on lateral biases and social behavior. Prog. Brain Res. 2018, 238, 377–403. [Google Scholar] [PubMed]
- Cortese, S.; Wang, F.; Angriman, M.; Masi, G.; Bruni, O. Sleep Disorders in Children and Adolescents with Autism Spectrum Disorder: Diagnosis, Epidemiology, and Management. CNS Drugs 2020, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V. Sleep and Its Regulation in Zebrafish. Rev. Neurosci. 2011, 22, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, A.; Ogi, A.; Torracca, B.; Mariti, C.; Casini, L. Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level. Animals 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]

| Authors | Strain * | Administration—Timing and Duration | Administered Dose (µM) | Test Timing | Molecular Analysis ** | Social Behavioral Analysis ** | Non-Social Behavioral Analysis ** | Non-Strictly Behavioral Analysis ** | Undesired Effects |
|---|---|---|---|---|---|---|---|---|---|
| Studies conducted in juvenile specimens (younger than 90 dpf) (n = 8) | |||||||||
| Liu X. et al., 2016 [60] | AB | Chronic exposure: from 24 hpf for 7 h/day for 6 days OR Acute exposure: from 24 hpf for 7 h | 20 (chronic), 100 (acute) | 30 dpf | / | Social preference | L/D response | / | / |
| Wang J. et al., 2024 [61] | AB | 8–120 hpf | 5, 50, 500 | 10–13 dpf | Immunostaining | Shoaling, social contact, mirror biting, shoaling L/D background exploration | L/D response | Morphology, startle response | / |
| Dwivedi S. et al., 2019 [62] | AB | 4–120 hpf | 75 | 7 dpf, 21 dpf | qRT-PCR, Western blot (7 dpf) | Social preference (21 dpf) | Thigmotaxis (7 dpf) | Circling behavior, inattentive behavior test (7 dpf) | / |
| Robea M.A. et al., 2021 [63] | AB | 24 hpf, 48 hpf, 72 hpf | 48 | 6 dpf, 42 dpf | / | Social preference, mirror biting (42 dpf) | Hyperactivity, L/D activity (sleep analysis (6 dpf) | / | / |
| DeOliveira-Mello L. et al., 2023 [64] | Turku | 10–24 hpf | 25 | ≃7 dpf, 30 dpf | Serotoninergic immunostaining, qRT-PCR (≃7 dpf) | / | L/D activity (sleep-like behavior) (≃7 dpf) | Optomotor behavior, color preference (30 dpf), length measurement of optic nerve zones (≃7 dpf) | Delay in visual system development (no visual impairment in adult age) |
| Geng Y. et al., 2023 [65] | EKW | 0–72 hpf | 1 | 21 dpf | / | Social contact (ZeChat) | / | / | / |
| Karimi Z. et al., 2023 [66] | AB | 4–120 hpf | 1 | 7 dpf, 21 dpf, 42 dpf | qRT-PCR (7 dpf) | Social preference (21, 42 dpf) | Thigmotaxis (7 dpf), inattentive behavior test (7 dpf) | / | / |
| Rahmati-Holasoo H. et al., 2023 [67] | AB | 0–48 hpf | 48 | 10–13 dpf | qRT-PCR | Shoaling, social contact, mirror biting, shoaling L/D background exploration | / | / | / |
| Studies conducted in adult specimens (90 dpf and older) (n = 9) | |||||||||
| Blazina A.R. et al., 2013 [68] | AB/TU | 10 min | 88, 265, 884 | 240 dpf | / | Shoaling L/D background exploration | Bottom dwelling | Motor coordination | Altered motor coordination for very high-dose VPA |
| Lee Y. et al., 2013 [69] | n.s. | 3 h/day at 120 hpf | 2000 | 5–15 dpf, 95 dpf | qRT-PCR, immunostaining (5–15 dpf) | / | Learning (passive avoidance), bottom dwelling (95 dpf) | / | / |
| Dozawa M. et al., 2014 [70] | RW, gfap:GFP, elavl3:GFP | 10–48 hpf, 2 or 7 days at 150–180 dpf | 1000, 1500, 2000 (embryo), 500 (adult) | 150–180 dpf | Cell lineage analysis, immunostaining, ISH, Western blot, qRT-PCR | / | / | / | / |
| Bailey J.M. et al., 2016 [71] | AB | 4–120 hpf | 0.5, 5, 50 | Adult | / | Shoaling | Hyperactivity, bottom dwelling, predator avoidance, novel tank | Startle reflex habituation | Possible visual or motor impairment for higher doses of VPA or RA |
| Zimmermann F.F. et al., 2017 [72] | AB | 0–48 hpf | 48 | 120 dpf | qRT-PCR, HPLC, enzymatic activity assays | / | / | / | / |
| Baronio D. et al., 2018 [73] | Turku | 10–24 hpf | 25 | 5 dpf, 180 dpf | HPLC, qRT-PCR | Social preference (180 dpf) | L/D response (5 dpf), hyperactivity (180 dpf) | / | / |
| Ilie O.-D. et al., 2022 [74] | AB | For 2 days and for 11 days at 180–240 dpf | 18 | 180–240 dpf | / | Social preference, mirror biting | / | / | / |
| Velázquez-Landa X. et al., 2023 [75] | AB | 0–48 hpf | 48 | 180 dpf | / | Sexual behavior recording | / | / | / |
| Li X. et al., 2024 [76] | AB | 4 days | 500 | Adult | qRT-PCR, body cortisol extraction | Social preference, mirror biting | Bottom dwelling, hyperactivity | / | / |
| Studies conducted in both juvenile and adult specimens (n = 4) | |||||||||
| Zimmermann F.F. et al., 2015 [42] | n.s. | 0–48 hpf | 48 | 6, 30, 70, 120 dpf | / | Social preference, mirror biting (70 and 120 dpf) | Hyperactivity, bottom dwelling (6, 30, 70, 120 dpf) | / | / |
| Liu C.X. et al., 2016 [77] | TU | 24–48 hpf | 500, 1000 | 1, 3, 7, 10, 13, 15, 30, 60 dpf | qRT-PCR | / | / | / | / |
| Joseph T.P. et al., 2022 [78] | AB | 8–108 hpf | 5, 10, 20, 30, 40 | 5–7, 13, 90–120 dpf | AChE activity, Western blot (90–120 dpf) | Social contact (13 dpf) social preference (90–120 dpf) | Hyperactivity, bottom dwelling (90–120 dpf) L/D response (5–7 dpf) | / | / |
| Messina A. et al., 2024 [79] | AB | 5–29 hpf, 5–53 hpf | 1 | 21, 28, 90 dpf | qRT-PCR (90 dpf) | Visual mirror (21 dpf) social preference (28 dpf) | / | / | / |
| Authors | Objective(s) | Key Findings |
|---|---|---|
| Studies conducted in juvenile specimens (younger than 90 dpf) (n = 8) | ||
|
| Impairment of social preference by chronic exposure to VPA 20 µM, while no effect on other simple behaviors (locomotor activity/anxiety/behavioral responses to light change). Impairment of locomotor activity by acute exposure to VPA 100 µM, but no effect on other behaviors. |
|
| Induction of autism-like behavior and social behavior impairment in zebrafish larvae exposed to either VPA 25 or 50 μM. Vitamin A-induced amelioration of VPA-induced social impairment and neurotoxicity, through oxidative damage and apoptosis attenuation. |
|
| Non-social behavioral impairment, and neurodevelopmental-related gene dysregulation induced by VPA 75 μM, assessed at 7 dpf. Social behavioral impairment assessed at 21 dpf. Effect of positive and negative control drugs on VPA induced behavioral despair. |
|
| Hyperactivity induced in VPA 48 μM exposed zebrafish larvae for 24 h. Social behavioral impairment induced by VPA 48 μM exposed zebrafish larvae for 24–48–72 h. No significant effects on sleep nor aggression in VPA 48 μM exposed zebrafish larvae for 24–48–72 h. |
|
| Larval retinal abnormalities after embryonic exposure to VPA 25 μM for 24 h, with normalization at 5 dpf and no visual impairment. Abnormal sleep-like behavior at assessed at 7 dpf after VPA 25 μM exposure for 24 h. |
|
| Impairment of social behavior, assessed at 21 dpf in VPA 1 μM exposed larvae for 72 hpf. Rescue of social impairment by D3-agonist neuroactive compounds acutely administered for 1 h before social preference test at 21 dpf. |
|
| Significant reduction in survival rate in 5–15–25–48–75 μM exposed zebrafish larvae for 120 h. Induction of ASD-like phenotype and impairment in social interaction persisting at 42 dpf in zebrafish larvae exposed to VPA 1 μM for 120 h. |
|
| Behavioral and molecular improvement in VPA 48 μM exposed zebrafish larvae for 48 h, by the administration of oxytocin 50 μM both for 24 and 48 h. |
| Studies conducted in adult specimens (90 dpf and older) (n = 9) | ||
|
| Reduction in swimming time against water current in the spinning task in 240 dpf adult zebrafish exposed to VPA 884 μM for 10 min. No difference in total distance traveled for VPA 88–265–884 μM exposed zebrafish larvae. |
|
| Transient decrease in cell proliferation in the telencephalon of VPA 2000 μM treated zebrafish larvae at 5 dpf for 3 h, with restoration of cell proliferation rate 10 days after VPA treatment. No development of severe deficits in bottom dwelling or passive avoidance responses in 95 dpf adult fish after VPA treatment. |
|
| Inhibition of HDAC activity and upregulation of Notch signaling, with consequent reduction of cell proliferation in the optic tectum of adult zebrafish exposed to VPA 500 µM for 48 or 168 h. |
|
| Reduction in the dark phase of larval activity in VPA 30–50 μM exposed larvae for 120 h, but hyperactivity in VPA 15 μM exposed larvae for 120 h. Decreased shoaling behavior in VPA 5 μM treated fish. |
|
| Alteration of biochemical and molecular parameters related to purinergic system in 120 dpf adult zebrafish exposed to 48 μM during the first 48 hpf. |
|
| High mortality/malformation rate in zebrafish embryos exposed to VPA 50–35 μM for 120 h, and identification of the optimal dosage as VPA 25 μM for 120 h. Molecular and neurochemical changes in histaminergic, noradrenergic, and dopaminergic systems induced by VPA 25 μM treatment for 120 h. |
|
| Social and aggressive behavior disruption in VPA 18 µM acutely (2-day-long) and chronically (11-day-long) exposed adult zebrafish (180–240 dpf). |
|
| Alteration of sexual behaviors and oviposition in adult zebrafish (180 dpf) exposed in the first 48 h of life to VPA 48 µM. |
|
| Social and aggressive behavior deficit in adult zebrafish exposed to VPA 500 µM for days. Increased anxiety in adult zebrafish exposed to VPA 500 µM for days. |
| Studies conducted in juvenile and adult specimens (n = 4) | ||
|
| Increase in hyperactive behavior at 6 dpf (but not at 30–70–120 dpf) zebrafish exposed to VPA 48 µM for 48 hpf. Increase in anxiety up to 70 dpf. Decrease in anxiety-related behavior at 120 dpf. Social behavior impairment at 70 and 120 dpf. No difference in aggressive behavior. |
|
| Altered differential expression of shank3 isoforms at different time points (1 to 60 dpf) in zebrafish embryos exposed to 500 and 1000 µM VPA at 24–48 hpf. |
|
| Attenuation hyperactivity, anxiety-like behavior, and social deficit by duloxetine 20 µM, in juvenile zebrafish (5–7,13,90,120 dpf) treated with VPA 10 µM from 8 to 108 hpf. Persistence up to 120 dpf of hyperactivity, increased anxiety-like behavior, and social deficits in VPA-only treated group. |
|
| Decrease in social preference and reduction of left visual bias in 28 dpf zebrafish exposed to VPA 1 µM for 24 and 48 hpf. Neutralization of the asymmetric distribution of genes responsible for brain lateralization assessed at 90 dpf in zebrafish exposed to VPA 1 µM for 24 and 48 hpf. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camussi, D.; Marchese, M.; Nicoletti, F.; Santorelli, F.M.; Ogi, A. Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review. Cells 2025, 14, 109. https://doi.org/10.3390/cells14020109
Camussi D, Marchese M, Nicoletti F, Santorelli FM, Ogi A. Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review. Cells. 2025; 14(2):109. https://doi.org/10.3390/cells14020109
Chicago/Turabian StyleCamussi, Diletta, Maria Marchese, Ferdinando Nicoletti, Filippo Maria Santorelli, and Asahi Ogi. 2025. "Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review" Cells 14, no. 2: 109. https://doi.org/10.3390/cells14020109
APA StyleCamussi, D., Marchese, M., Nicoletti, F., Santorelli, F. M., & Ogi, A. (2025). Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review. Cells, 14(2), 109. https://doi.org/10.3390/cells14020109







