Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives
Abstract
1. Introduction
2. Morphological Changes in Response to Cold Stress in Plants
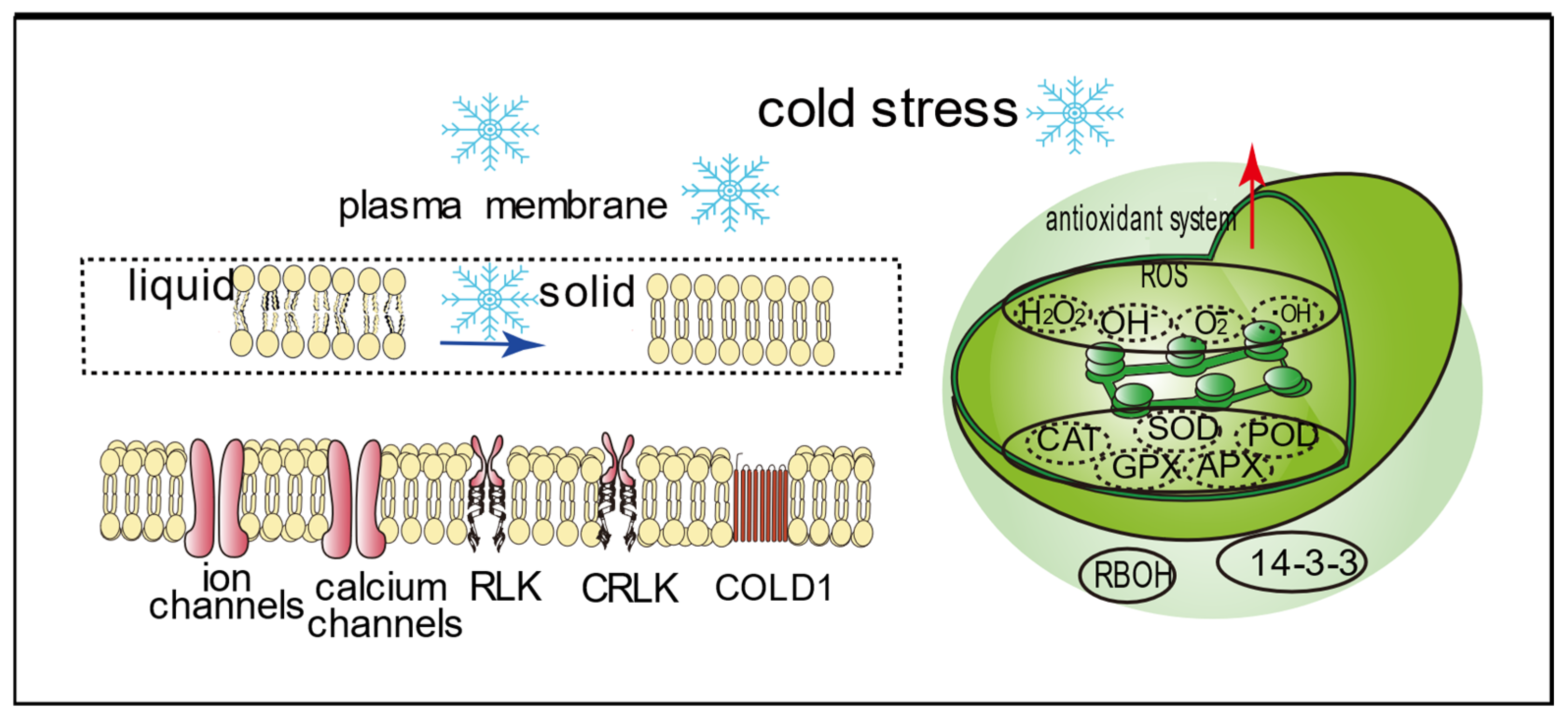
2.1. Plant Cell Membrane
2.2. Chloroplast
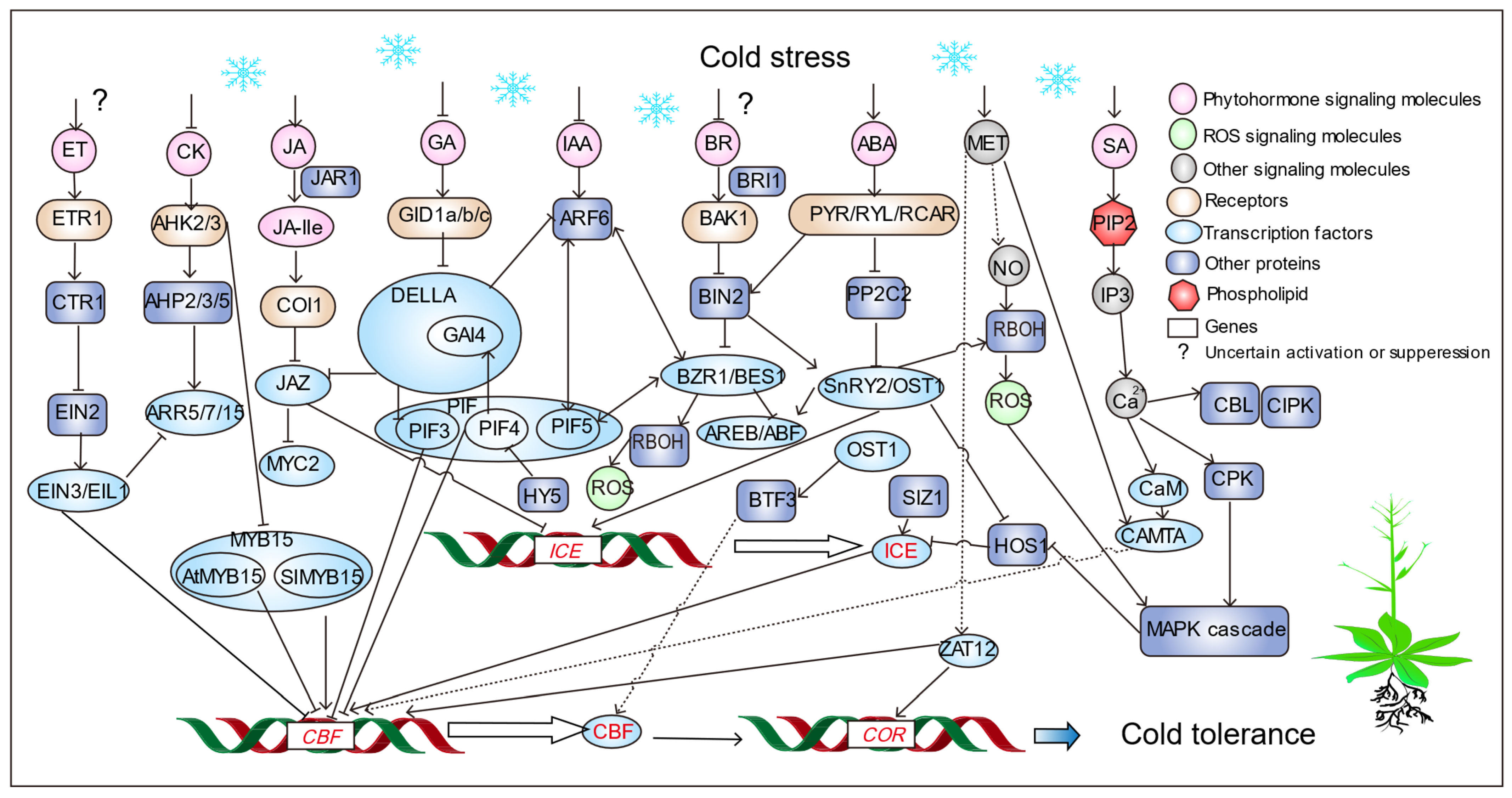
3. Mechanism of Cold Tolerance in Plants
3.1. Phytohormones and Plant Growth Regulators
3.1.1. Abscisic Acid (ABA)
3.1.2. Jasmonic Acid (JA)
3.1.3. Gibberellin (GA)
3.1.4. Auxin (IAA)
3.1.5. Salicylic Acid (SA)
3.1.6. Brassinosteroids (BRs)
3.1.7. Ethylene (ET)
3.1.8. Cytokinin (CTK)
3.1.9. Melatonin (MET)
3.2. Signalling Compounds
3.2.1. Reactive Oxygen Species (ROS)
3.2.2. Protein Kinases
3.2.3. Ca2+
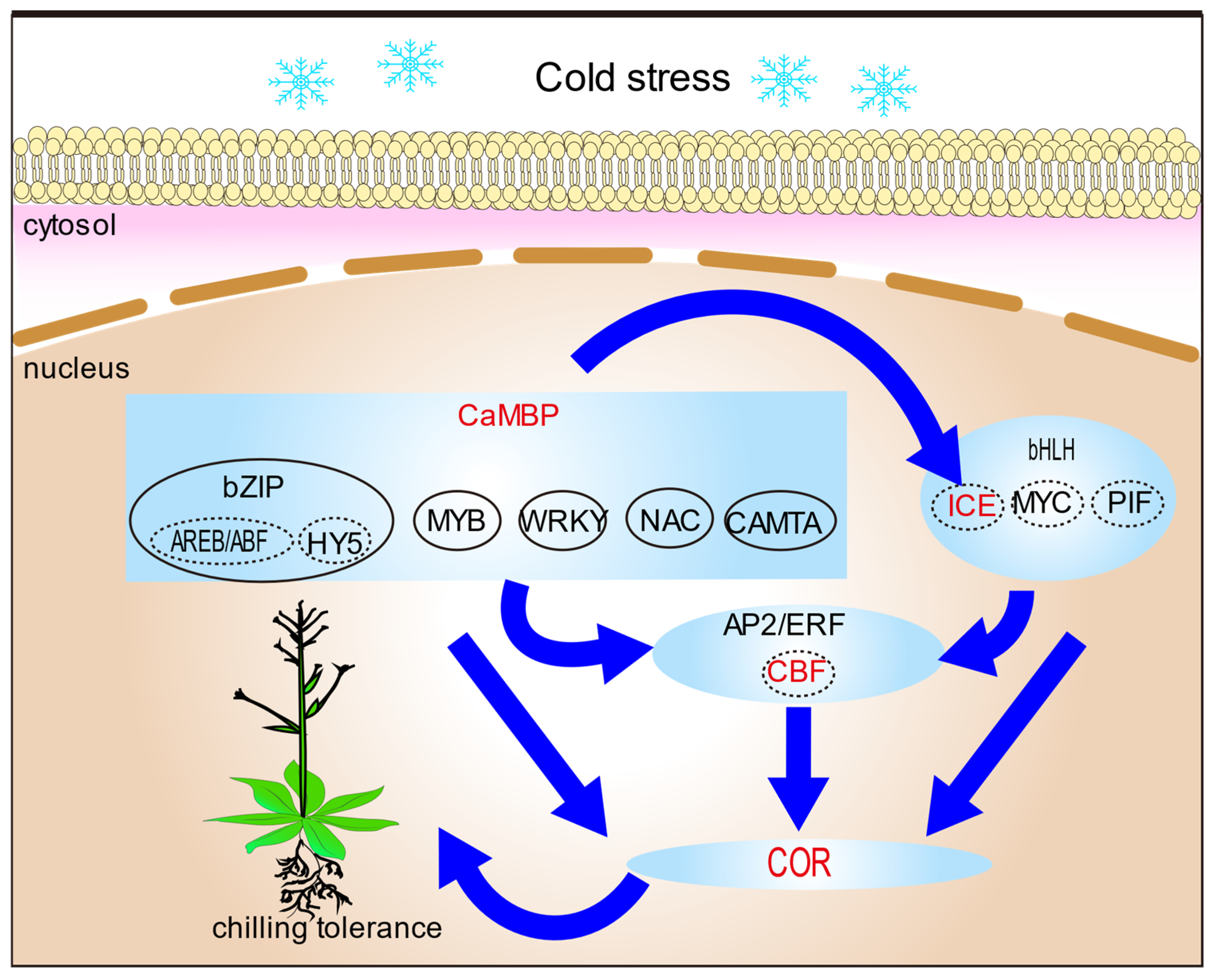
4. Downstream Mechanisms and Regulation for Cold Signaling in Plants
4.1. Transcription Factors in Plant Cold Signaling
4.1.1. AP2/ERF
4.1.2. MYB
4.1.3. WRKY
4.1.4. bZIP
4.1.5. NAC
4.1.6. bHLH
| Families | Genes | Species | Cold Stress Regulation | References |
|---|---|---|---|---|
| AP2/ERF | AtCBF1/DREB1B | A. thaliana (L.) Heynh. | Positive | [137] |
| AtCBF2/DREB1C | A. thaliana (L.) Heynh. | Negative | [138] | |
| AtCBF3/DREB1A | A. thaliana (L.) Heynh. | Positive | [137] | |
| MYB | AtMYB15 | A. thaliana (L.) Heynh. | Negative | [139,140] |
| SlMYB15 | S. lycopersicum L. | Positive | [145] | |
| AtMYB96 | A. thaliana (L.) Heynh. | Positive | [188] | |
| MpMYBS3 | M. nana Lour. | Positive | [141] | |
| OsMYB30 | O. sativa L. | Negative | [189] | |
| CaMYB306 | C. annuum L. | Negative | [147] | |
| WRKY | AtWRKY34 | A. thaliana (L.) Heynh. | Negative | [150] |
| AtWRKY22 | A. thaliana (L.) Heynh. | Positive | [151] | |
| AtWRKY40 | A. thaliana (L.) Heynh. | Negative | [155] | |
| SlWRKY50 | S. lycopersicum L. | Positive | [156] | |
| CdWRKY2 | C. dactylon (L.) Pers. | Positive | [157] | |
| CsWRKY46 | C. sativus L. | Positive | [158] | |
| bZIP | CbABF1 | C. bungeana Fisch. et Mey. | Positive | [161] |
| AcePosF21 | A. eriantha Benth. | Positive | [162] | |
| ZmbZIP68 | Z. mays L. | Negative | [163] | |
| CsbZIP18 | C. sinensis (L.) O. Kuntze | Negative | [164] | |
| OsbZIP38/LIP19 | O. sativa L. | Positive | [165] | |
| SlHY5 | S. lycopersicum L. | Positive | [145,167] | |
| NAC | AtJUB1 | A. thaliana (L.) Heynh. | Negative | [170] |
| MaNAC25 | M. nana Lour. | Negative | [172] | |
| MaNAC28 | M. nana Lour. | Negative | [172] | |
| CaNAC1 | C. annuum L. | Negative | [173] | |
| CaNAC064 | C. annuum L. | Positive | [174] | |
| MbNAC25 | M. baccata Borkh. | Positive | [175] | |
| bHLH | HbICE2 | H. brasiliensis (Willd. ex A. Juss.) Müll. Arg. | Positive | [178] |
| SiICE1 | S. involucrata (Kar. & Kir.) Sch. Bip. | Positive | [180] | |
| ZjICE2 | Z. japonica Steud. | Positive | [181] | |
| IbbHLH116 | I. batatas (L.) Poir. | Positive | [182] | |
| MdbHLH4 | M. pumila Mill. | Negative | [183] | |
| SlPIF4 | S. lycopersicum L. | Positive | [185] | |
| PtrMYC2 | P. trifoliata (L.) Raf. | Positive | [186] | |
| SlMYC2 | S. lycopersicum L. | Positive | [187] |
4.2. Post-Translational Modification in Plant Cold Signaling
4.2.1. Sumoylation (SUMO)
4.2.2. Protein Phosphorylation
4.2.3. Mitogen-Activated Protein Kinase (MAPK/MPK) Cascade
4.3. Multi-Omics Analysis Facilitates the Identification of Cold Resistance Genes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Karkute, S.G.; Krishna, R.; Ansari, W.A.; Singh, B.; Singh, P.M.; Singh, M.; Singh, A.K. Heterologous expression of the AtDREB1A gene in tomato confers tolerance to chilling stress. Biol. Plant. 2019, 63, 268–277. [Google Scholar] [CrossRef]
- Qin, X.H.; Li, W.K.; Duan, Y.S. Research progress of plant cold stress signal. Mol. Plant Breed. 2018, 16, 7187–7194. [Google Scholar] [CrossRef]
- Liu, L.L.; Ji, H.T.; An, J.P.; Shi, K.J.; Ma, J.F.; Liu, B.; Tang, L.; Cao, W.X.; Zhu, Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, R.Y.; Xiang, C.G.; Zhang, R.Y.; Wang, Q.; Wang, T.; Li, X.J.; Lu, X.H.; Gao, S.L.; Liu, Z.X. Transcriptomic and physiological analysis reveal that α-linolenic acid biosynthesis responds to early chilling tolerance in pumpkin rootstock varieties. Front. Plant Sci. 2021, 12, 669565. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.T.; Wang, F.; Shao, Y.L.; Zhang, A.M.; Chang, W. The effects of chilling stress on antioxidant enzymes activities and proline, malondialdehyde, soluble sugar contents in three Paphiopedilum species. Russ. J. Plant Physiol. 2023, 70, 61. [Google Scholar] [CrossRef]
- Angmo, D.; Sharma, S.P.; Kalia, A.; Brar, N.S.; Bhardwaj, V. Effect of cold stress on field performance, chlorophyll fluorescence, electrolyte leakage and leaf gas exchange parameters of potato (Solanum tuberosum L.) genotypes. Potato Res. 2023, 66, 641–661. [Google Scholar] [CrossRef]
- Penfield, S.; Warner, S.; Wilkinson, L. Molecular responses to chilling in a warming climate and their impacts on plant reproductive development and yield. J. Exp. Bot. 2021, 72, 7374–7383. [Google Scholar] [CrossRef]
- Guo, J.H.; Beemster, G.T.S.; Liu, F.L.; Wang, Z.M.; Li, X.N. Abscisic Acid Regulates Carbohydrate Metabolism, Redox Homeostasis and Hormonal Regulation to Enhance Cold Tolerance in Spring Barley. Int. J. Mol. Sci. 2023, 24, 11348. [Google Scholar] [CrossRef]
- Liu, L.; Gai, Z.J.; Qiu, X.; Liu, T.H.; Li, S.X.; Ye, F.; Jian, S.L.; Shen, Y.H.; Li, X.N. Salt stress improves the low-temperature tolerance in sugar beet in which carbohydrate metabolism and signal transduction are involved. Environ. Exp. Bot. 2023, 208, 105239. [Google Scholar] [CrossRef]
- Awan, A. Calcium and boron effect on production and quality of autumn potato crop under chilling temperature. Commun. Soil Sci. Plant Anal. 2021, 52, 375–388. [Google Scholar] [CrossRef]
- Vaitkevičiūtė, G.; Aleliūnas, A.; Gibon, Y.; Armonienė, R. The effect of cold acclimation, deacclimation and reacclimation on metabolite profiles and freezing tolerance in winter wheat. Front. Plant Sci. 2022, 13, 959118. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Zhou, Y.D.; Shen, L.; Zhao, E.P.; Yang, X. Effect of grafting on cold tolerance of eggplant. J. Anhui Agric. Sci. 2022, 50, 52–55. [Google Scholar] [CrossRef]
- Ratajczak, K.; Sulewska, H.; Panasiewicz, K.; Faligowska, A.; Szymańska, G. Phytostimulator application after cold stress for better maize (Zea mays L.) plant recovery. Agriculture 2023, 13, 569. [Google Scholar] [CrossRef]
- Min, X.Y.; Liu, Z.P.; Wang, Y.R.; Liu, W.X. Comparative transcriptomic analysis provides insights into the coordinated mechanisms of leaves and roots response to cold stress in common vetch. Ind. Crop. Prod. 2020, 158, 112949. [Google Scholar] [CrossRef]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening corn hybrids for cold tolerance using morphological traits for early-season seeding. Crop Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef]
- Rymen, B.; Fiorani, F.; Kartal, F.; Vandepoele, K.; Inzé, D.; Beemster, G.T.S. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 2007, 143, 1429–1438. [Google Scholar] [CrossRef]
- Juurakko, C.L.; Walker, V.K. Cold acclimation and prospects for cold-resilient crops. Plant Stress 2021, 2, 100028. [Google Scholar] [CrossRef]
- Fan, S.H.; Liu, H.F.; Liu, J.; Hua, W.; Li, J. BnGF14-2c positively regulates rlowering via the vernalization pathway in semi-winter rapeseed. Plants 2022, 11, 2312. [Google Scholar] [CrossRef]
- Khatun, L.; Karim, M.R.; Talukder, F.U.; Rahman, M.S. Combined effects of vernalization and gibberellic acid on quality seed production of summer onion (Allium cepa L.). Agric. Sci. 2020, 2, 148. [Google Scholar] [CrossRef]
- Lainé, C.M.S.; AbdElgawad, H.; Beemster, G.T.S. Cellular dynamics in the maize leaf growth zone during recovery from chilling depends on the leaf developmental stage. Plant Cell Rep. 2024, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Matoša Kočar, M.; Jambrović, A.; Sudarić, A.; Volenik, M.; Duvnjak, T.; Zdunić, Z. Crop-specific responses to cold stress and priming: Insights from chlorophyll fluorescence and spectral reflectance analysis in maize and soybean. Plants 2024, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Driever, S.M.; Mossink, L.; Ocaña, D.N.; Kaiser, E. A simple system for phenotyping of plant transpiration and stomatal conductance response to drought. Plant Sci. 2023, 329, 111626. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Gabarain, V.B.; Lopez, L.E.; Lehuedé, T.U.; Ocaranza, D.; Estevez, J.M. Understanding signaling pathways governing the polar development of root hairs in low-temperature, nutrient-deficient environments. Curr. Opin. Plant Biol. 2023, 75, 102386. [Google Scholar] [CrossRef]
- Tiwari, M.; Kumar, R.; Subramanian, S.; Doherty, C.J.; Jagadish, S.V.K. Auxin–cytokinin interplay shapes root functionality under low-temperature stress. Trends Plant Sci. 2023, 28, 447–459. [Google Scholar] [CrossRef]
- Zhou, Y.; Sommer, M.L.; Hochholdinger, F. Cold response and tolerance in cereal roots. J. Exp. Bot. 2021, 72, 7474–7481. [Google Scholar] [CrossRef]
- Lin, F.F.; Li, C.; Xu, B.; Chen, J.; Chen, A.H.; Hassan, M.A.; Liu, B.B.; Xu, H.; Chen, X.; Sun, J.Q. Late spring cold reduces grain number at various spike positions by regulating spike growth and assimilate distribution in winter wheat. Crop J. 2023, 11, 1272–1278. [Google Scholar] [CrossRef]
- Wang, R.J.; Bai, Y.G.; Low, N.H.; Tanino, K. Seed size variation in cold and freezing tolerance during seed germination of winterfat (Krascheninnikovia lanata) (Chenopodiaceae). Botany 2006, 84, 49–59. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, H.; Li, X.; Ji, H. Enhancement of plant cold tolerance by soybean RCC1 family gene GmTCF1a. BMC Plant Biol. 2021, 21, 369. [Google Scholar] [CrossRef]
- Wei, Y.L.; Chen, H.Z.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T.E. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef]
- Martini, L.F.; Nilda, R.B.; Tseng, T.M.; Fipke, M.V.; Noldin, J.A.; Avila, L.A.D. Acclimation to cold stress reduces injury from low temperature and bispyribac-sodium on rice. Pest Manag. Sci. 2021, 77, 4016–4025. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.K.; O’Connor, K.; Alahmad, S.; Lee, J.H.; Dinglasan, E.; Park, H.; Lee, S.M.; Hirsz, D.; Kwon, S.W.; Kwon, Y. Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant 2022, 15, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.H.; Zhang, M.Y.; Xia, G.W.; Xiong, C. Effect of cold stratification on the temperature range for germination of Pinus koraiensis. J. For. Res. 2023, 34, 221–231. [Google Scholar] [CrossRef]
- Auge, G.A.; Blair, L.K.; Neville, H.; Donohue, K. Maternal vernalization and vernalization-pathway genes influence progeny seed germination. New Phytol. 2017, 216, 388–400. [Google Scholar] [CrossRef]
- Zheng, D.X.; Yang, X.G.; Mínguez, M.I.; Connor, D.J.; Mu, C.Y.; Guo, E.J.; Chen, X. Tolerance of different winter wheat cultivars to prolonged freezing injury at their critical temperatures. Crop Sci. 2018, 58, 1740–1750. [Google Scholar] [CrossRef]
- Bredow, M.; Walker, V.K. Ice-binding proteins in plants. Front. Plant Sci. 2017, 8, 325466. [Google Scholar] [CrossRef]
- Welling, A.; Rinne, P.; Aarnio, A.V.; Soppela, S.K.; Heino, P.; Palva, E.T. Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J. Exp. Bot. 2004, 55, 507–516. [Google Scholar] [CrossRef]
- Widrlechner, M.P.; Daly, C.; Keller, M.; Kaplan, K. Horticultural applications of a newly revised USDA Plant Hardiness Zone Map. HortTechnology 2012, 22, 6–19. [Google Scholar] [CrossRef]
- Gorb, S.N.; Gorb, E.V. Anti-icing strategies of plant surfaces: The ice formation on leaves visualized by Cryo-SEM experiments. Sci. Nat. 2022, 109, 24. [Google Scholar] [CrossRef]
- Cho, S.M.; Kim, S.H.; Cho, H.J.; Lee, H.S.; Lee, J.H.; Lee, H.; Park, H.; Kang, S.H.; Choi, H.G.; Lee, J.G. Type II ice-binding proteins isolated from an arctic microalga are similar to adhesin-like proteins and increase freezing tolerance in transgenic plants. Plant Cell Physiol. 2019, 60, 2744–2757. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef] [PubMed]
- Aaliya, K.; Nasir, I.A.; Khan, A.; Toufiq, N.; Yousaf, I.; Adeyinka, O.S.; Iftikhar, S.; Farooq, A.M.; Tabassum, B. Expression of ice recrystallization inhibition protein in transgenic potato lines associated with reduced electrolyte leakage and efficient recovery post freezing injury. J. Biotechnol. 2021, 327, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Liu, R.M.; Huang, X.H.; Du, Z.H.; Heng, S.P.; Zeng, W. Characterization of low temperature-induced plasma membrane lipidome remodeling combined with gene expression analysis reveals mechanisms that regulate membrane lipid desaturation in Carica papaya. Sci. Hortic. 2020, 272, 109505. [Google Scholar] [CrossRef]
- Hu, D.; Yao, Y.l.; Lv, Y.; You, J.; Zhang, Y.; Lv, Q.Y.; Li, J.W.; Hutin, S.; Xiong, H.Y.; Zubieta, C. The OsSRO1c-OsDREB2B complex undergoes protein phase transition to enhance cold tolerance in rice. Mol. Plant 2024, 17, 1520–1538. [Google Scholar] [CrossRef]
- Lunn, D.; Smith, G.A.; Wallis, J.G.; Browse, J. Overexpression mutants reveal a role for a chloroplast MPD protein in regulation of reactive oxygen species during chilling in Arabidopsis. J. Exp. Bot. 2022, 73, 2666–2681. [Google Scholar] [CrossRef]
- Gao, Y.; Thiele, W.; Saleh, O.; Scossa, F.; Arabi, F.; Zhang, H.M.; Sampathkumar, A.; Kühn, K.; Fernie, A.; Bock, R. Chloroplast translational regulation uncovers nonessential photosynthesis genes as key players in plant cold acclimation. Plant Cell 2022, 34, 2056–2079. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.T.; Yang, H.D.; Tang, Y.Q.; Liu, B.C.; Hu, X.L.; Hu, Z.D. Cold acclimation alleviates photosynthetic inhibition and oxidative damage induced by cold stress in citrus seedlings. Plant Signal. Behav. 2023, 18, 2285169. [Google Scholar] [CrossRef]
- Ovsyannikov, A.Y.; Koteyeva, N.K. Seasonal movement of chloroplasts in mesophyll cells of two Picea species. Protoplasma 2020, 257, 183–195. [Google Scholar] [CrossRef]
- Herrmann, H.A.; Dyson, B.C.; Miller, M.A.E.; Schwartz, J.M.; Johnson, G.N. Metabolic flux from the chloroplast provides signals controlling photosynthetic acclimation to cold in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 171–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, P.G. The DREB transcription factor, a biomacromolecule, responds to abiotic stress by regulating the expression of stress-related genes. Int. J. Biol. Macromol. 2023, 243, 125231. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Li, J.Z.; Yang, Q.o.; Jamil, W.; Teng, Y.W.; Bai, S.L. Phylogenetic, molecular, and functional characterization of PpyCBF proteins in Asian pears (Pyrus pyrifolia). Int. J. Mol. Sci. 2019, 20, 2074. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, S.C. ABA-dependent and ABA-independent functions of RCAR5/PYL11 in response to cold stress. Front. Plant Sci. 2020, 11, 587620. [Google Scholar] [CrossRef]
- Ding, Y.L.; Jia, Y.X.; Shi, Y.T.; Zhang, X.Y.; Song, C.P.; Gong, Z.Z.; Yang, S.H. OST 1-mediated BTF 3L phosphorylation positively regulates CBF s during plant cold responses. EMBO J. 2018, 37, e98228. [Google Scholar] [CrossRef]
- Nai, G.J.; Liang, G.P.; Ma, W.F.; Lu, S.X.; Li, Y.M.; Gou, H.M.; Guo, L.L.; Chen, B.H.; Mao, J. Overexpression VaPYL9 improves cold tolerance in tomato by regulating key genes in hormone signaling and antioxidant enzyme. BMC Plant Biol. 2022, 22, 344. [Google Scholar] [CrossRef]
- Yu, M.M.; Wang, R.; Xia, J.Q.; Li, C.; Xu, Q.H.; Cang, J.; Wang, Y.Y.; Zhang, D. JA-induced TaMPK6 enhanced the freeze tolerance of Arabidopsis thaliana through regulation of ICE-CBF-COR module and antioxidant enzyme system. Plant Sci. 2023, 329, 111621. [Google Scholar] [CrossRef]
- Hu, Y.R.; Jiang, L.Q.; Wang, F.; Yu, D.Q. Jasmonate regulates the inducer of CBF expression–c-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef]
- Fu, A.Z.; Zheng, Y.Y.; Lv, Y.H.; Watkins, C.B.; Bai, C.M.; Ma, L.; Yuan, S.Z.; Zheng, S.F.; Jia, L.E.; Gao, L.P. Multi-omics analysis reveals specific modifications associated with reduced chilling injury in bell pepper fruit by methyl jamonate. Postharvest Biol. Technol. 2022, 185, 111799. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Singh, I.K. Recent insights into the molecular mechanism of jasmonate signaling during insect-plant interaction. Australas. Plant Pathol. 2016, 45, 123–133. [Google Scholar] [CrossRef]
- Che, L.L.; Lu, S.X.; Gou, H.M.; Li, M.; Guo, L.L.; Yang, J.B.; Mao, J. VvJAZ13 positively regulates cold tolerance in Arabidopsis and grape. Int. J. Mol. Sci. 2024, 25, 4458. [Google Scholar] [CrossRef]
- Luo, R.; Wang, R.; Cao, L.; Li, L.L.; Li, X.; Yuan, Y.; Yan, J.R.; Hou, J.; Hu, J.B. Effects of plant growth regulators on physiological characteristics and related gene expression in melon seedlings under cold stress. J. Henan Agric. Univ. 2022, 56, 411–419+428. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Caccialupi, G.; Milc, J.; Caradonia, F.; Nasar, M.F.; Francia, E. The Triticeae CBF gene cluster—To frost resistance and beyond. Cells 2023, 12, 2606. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.H.; Li, C.Q.; Liu, L.; Han, G.Y.; Zhang, Y.L.; Wang, C.M. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Albertos, P.; Wlk, T.; Griffiths, J.; Pimenta Lange, M.J.; Unterholzner, S.J.; Rozhon, W.; Lange, T.; Jones, A.M.; Poppenberger, B. Brassinosteroid-regulated bHLH transcription factor CESTA induces the gibberellin 2-oxidase GA2ox7. Plant Physiol. 2022, 188, 2012–2025. [Google Scholar] [CrossRef]
- Zhou, M.Q.; Chen, H.; Wei, D.H.; Ma, H.; Lin, J. Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci. Rep. 2017, 7, 39819. [Google Scholar] [CrossRef]
- Rapacz, M.; Waligórski, P.; Janowiak, F. ABA and gibberellin-like substances during prehardening, cold acclimation, de-and reacclimation of oilseed rape. Acta Physiol. Plant. 2003, 25, 151–161. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.X.; Wang, W.S.; Gong, W.; Liu, W.C.; Chen, H.G.; Xu, H.H.; Lu, Y.T. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Fu, J.; Wang, S.P.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Liu, D.D.; He, Y.R.; Wang, Y.J.; Chen, W.W.; Yang, J.L.; Zhang, Y.Z.; Feng, Y.Y.; Zhao, Y.X.; Lin, S.; Huang, L. Tetrad stage transient cold stress skews auxin-mediated energy metabolism balance in Chinese cabbage pollen. Plant Physiol. 2024, 195, 1312–1332. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, P.Y.; Liang, W.W.; Cheng, Q.; Mu, B.B.; Niu, F.F.; Yan, J.L.; Liu, C.L.; Xie, H.; Kav, N.N.V. A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes. J. Agric. Food Chem. 2020, 68, 7348–7359. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted role of salicylic acid in combating cold stress in plants: A review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Taşgín, E.; Atící, Ö.; Nalbantoğlu, B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003, 41, 231–236. [Google Scholar] [CrossRef]
- Taşgın, E.; Atıcı, Ö.; Nalbantoğlu, B.; Popova, L.P. Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry 2006, 67, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Li, S.H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Wu, Z.J.; Han, S.M.; Zhou, H.D.; Tuang, Z.K.; Wang, Y.Z.; Jin, Y.; Shi, H.Z.; Yang, W.N. Cold stress activates disease resistance in Arabidopsis thaliana through a salicylic acid dependent pathway. Plant Cell Environ. 2019, 42, 2645–2663. [Google Scholar] [CrossRef]
- Wang, Y.T.; Jiang, Q.Q.; Wang, X.F.; Xi, Z.M. Brassinosteroid stimulates hydrogen peroxide biosynthesis and reduces the effect of cold stress. J. Plant Growth Regul. 2023, 42, 3757–3769. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.Y.; Shi, Y.T.; Cheng, J.K.; Zhang, X.Y.; Yang, S.H. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef]
- Wang, L.M.; Yang, R.Z.; Sun, J.Q. Regulation of crop agronomic traits and abiotic stress responses by brassinosteroids: A review. Chin. J. Biotechnol. 2022, 38, 34–49. [Google Scholar] [CrossRef]
- Pociecha, E.; Dziurka, M.; Oklestkova, J.; Janeczko, A. Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. Plant Growth Regul. 2016, 80, 127–135. [Google Scholar] [CrossRef]
- Arfan, M.; Zhang, D.W.; Zou, L.J.; Luo, S.S.; Tan, W.R.; Zhu, T.; Lin, H.H. Hydrogen peroxide and nitric oxide crosstalk mediates brassinosteroids induced cold stress tolerance in Medicago truncatula. Int. J. Mol. Sci. 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Robison, J.D.; Yamasaki, Y.; Randall, S.K. The ethylene signaling pathway negatively impacts CBF/DREB-regulated cold response in soybean (Glycine max). Front. Plant Sci. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, X.R.; Li, H.; Xu, M.; Zhang, M.X.; Li, S.J.; Liu, X.F.; Shi, Y.N.; Grierson, D.; Chen, K.S. ETHYLENE RESPONSE FACTOR39–MYB8 complex regulates low-temperature-induced lignification of loquat fruit. J. Exp. Bot. 2020, 71, 3172–3184. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Shen, L.; Fan, B.; Yu, M.M.; Zheng, Y.; Lv, S.N.; Sheng, J.P. Ethylene and cold participate in the regulation of LeCBF1 gene expression in postharvest tomato fruits. FEBS Lett. 2009, 583, 3329–3334. [Google Scholar] [CrossRef]
- Zhao, M.G.; Liu, W.J.; Xia, X.Z.; Wang, T.Z.; Zhang, W.H. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef]
- Wang, Y.C.; Jiang, H.Y.; Mao, Z.L.; Liu, W.J.; Jiang, S.H.; Xu, H.F.; Su, M.Y.; Zhang, J.; Wang, N.; Zhang, Z.Y. Ethylene increases the cold tolerance of apple via the MdERF1B–MdCIbHLH1 regulatory module. Plant J. 2021, 106, 379–393. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Ervin, E.H.; Waltz, C.; Murphy, T. Metabolic changes during cold acclimation and deacclimation in five bermudagrass varieties: II. cytokinin and abscisic acid metabolism. Crop Sci. 2011, 51, 847–853. [Google Scholar] [CrossRef]
- Chun, J.; Wan, M.; Guo, H.W.; Zhang, Q.P.; Feng, Y.; Tang, Y.C.; Zhu, B.; Sang, Y.S.; Jing, S.L.; Chen, T. Cytokinin-mediated enhancement of potato growth and yield by Verticillium Dahliae effector VDAL under low temperature stress. BMC Plant Biol. 2024, 24, 1115. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, N.Y.; Kim, S.; Kang, N.Y.; Novák, O.; Ku, S.J.; Cho, C.; Lee, D.J.; Lee, E.J.; Strnad, M. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 2010, 285, 23371–23386. [Google Scholar] [CrossRef]
- Shi, Y.T.; Tian, S.W.; Hou, L.Y.; Huang, X.Z.; Zhang, X.Y.; Guo, H.W.; Yang, S.H. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Xi, Q.Q.; Wei, X.Y.; Xu, L.; Wang, Q.Q.; Fu, J.Y.; Ling, C.; Zuo, Y.; Zhao, Y.; He, H.Y. Rhythmical redox homeostasis can be restored by exogenous melatonin in hulless barley (Hordeum vulgare L. var. nudum) under cold stress. Environ. Exp. Bot. 2022, 194, 104756. [Google Scholar] [CrossRef]
- Zhao, H.L.; Ye, L.; Wang, Y.P.; Zhou, X.T.; Yang, J.W.; Wang, J.W.; Cao, K.; Zou, Z.R. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Zhao, H.L.; Zhang, K.; Zhou, X.T.; Xi, L.J.; Wang, Y.P.; Xu, H.J.; Pan, T.H.; Zou, Z.R. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017, 7, 4998. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Pramanick, K. Melatonin mediated activation of MAP kinase pathway may reduce DNA damage stress in plants: A review. BioFactors 2022, 48, 965–971. [Google Scholar] [CrossRef]
- Li, X.N.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.C.; Liu, S.Q.; Song, F.B.; Reiter, R.J.; Liu, F.L. Melatonin alleviates low PS I-limited carbon assimilation under elevated CO 2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Wang, S.M.; Wang, Y.S.; Su, B.Y.; Zhou, Y.Y.; Chang, L.F.; Ma, X.Y.; Li, X.M. Ecophysiological responses of five mangrove species (Bruguiera gymnorrhiza, Rhizophora stylosa, Aegiceras corniculatum, Avicennia marina, and Kandelia obovata) to chilling stress. Front. Mar. Sci. 2022, 9, 846566. [Google Scholar] [CrossRef]
- Buer, J.V.; Cvetkovic, J.; Baier, M. Cold regulation of plastid ascorbate peroxidases serves as a priming hub controlling ROS signaling in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 1–20. [Google Scholar] [CrossRef]
- Shi, H.T.; Ye, T.T.; Zhong, B.; Liu, X.; Jin, R.; Chan, Z.L. At HAP 5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 2014, 203, 554–567. [Google Scholar] [CrossRef]
- Vogel, J.T.; Zarka, D.G.; The Plant JournalBuskirk, H.A.V.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Fu, C.C. Cold-inducible MaC2H2s are associated with cold stress response of banana fruit via regulating MaICE1. Plant Cell Rep. 2019, 38, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.J.; Zhu, Y.F.; Zhang, L.N.; Chen, T.Y.; Zhou, F.; Chen, H.; Lin, Y.J. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Sun, X.M.; Kou, S.; Liu, T.T.; Dong, J.K.; Tu, W.; Zhang, Y.L.; Song, B.T. The mitogen-activated protein kinase kinase MKK2 positively regulates constitutive cold resistance in the potato. Environ. Exp. Bot. 2022, 194, 104702. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, H.T.; Li, N.N.; Wei, N.N.; Tian, Y.; Peng, J.F.; Chen, X.C.; Zhang, L.Y.; Zhang, M.X.; Dong, H.S. Aquaporin OsPIP2; 2 links the H2O2 signal and a membrane-anchored transcription factor to promote plant defense. Plant Physiol. 2022, 188, 2325–2341. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Doczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Ding, Y.L.; Yang, S.H. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Geng, B.H.; Wang, Q.; Huang, R.S.; Liu, Y.J.; Guo, Z.F.; Lu, S.Y. A novel LRR-RLK (CTLK) confers cold tolerance through regulation on the C-repeat-binding factor pathway, antioxidants, and proline accumulation. Plant J. 2021, 108, 1679–1689. [Google Scholar] [CrossRef]
- Constable, G.A. Temperature effects on the early field development of cotton. Aust. J. Exp. Agric. 1976, 16, 905–910. [Google Scholar] [CrossRef]
- Cui, Y.M.; Lu, S.; Li, Z.; Cheng, J.W.; Hu, P.; Zhu, T.Q.; Wang, X.; Jin, M.; Wang, X.X.; Li, L.Q. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Wang, J.C.; Ren, Y.L.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.B.; Zhu, S.S.; Wan, H.; Yang, Y. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Barnham, E.J.; Wang, L.M.; Ning, Y.Z.; Davies, J.M. The complex story of plant cyclic nucleotide-gated channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Hou, C.C.; Tian, W.; Li, L.G.; Zhu, H.F. Electrophysiological studies revealed CaM1-mediated regulation of the Arabidopsis calcium channel CNGC12. Front. Plant Sci. 2019, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Ding, S.; Zhang, H.; Du, H.; An, L.Z. CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci. 2011, 181, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Feng, Q.N.; Li, C.L.; Li, E.; Liu, Q.; Kang, H.; Zhang, W.; Zhang, Y.; Li, S. A tonoplast-associated calcium-signaling module dampens ABA signaling during stomatal movement. Plant Physiol. 2018, 177, 1666–1678. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Bethke, P.C.; Cheong, Y.H.; Chang, H.S.; Zhu, T.; Jones, R.L. A gibberellin-regulated calcineurin B in rice localizes to the tonoplast and is implicated in vacuole function. Plant Physiol. 2005, 138, 1347–1358. [Google Scholar] [CrossRef]
- Washington, E.J.; Mukhtar, M.S.; Finkel, O.M.; Wan, L.; Banfield, M.J.; Kieber, J.J.; Dangl, J.L. Pseudomonas syringae type III effector HopAF1 suppresses plant immunity by targeting methionine recycling to block ethylene induction. Proc. Natl. Acad. Sci. USA 2016, 113, E3577–E3586. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, H.; Xie, F.; Pan, Z.Y.; Qiu, W.M.; Tong, Z.; Wang, Z.Q.; He, X.J.; Xu, Y.H.; Sun, Z.H. Evolution, gene expression, and protein–protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biol. 2022, 22, 420. [Google Scholar] [CrossRef]
- Cai, J.; Chang, T.L.; Zhao, Y.; He, H.Y.; Li, J.X.; Xi, Q.Q.; Fu, J.Y.; Zhao, Y.W. Effects of varying temperature on rhythmic expression of abiotic stress-responding genes in Tibetan hulless barley. Acta Physiol. Plant. 2022, 44, 38. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Bae, H.H. Genome-wide identification of calcium dependent protein kinase gene family in plant lineage shows presence of novel DxD and DEL motifs in EF-hand domain. Front. Plant Sci. 2015, 6, 1146. [Google Scholar] [CrossRef]
- Hetherington, A.; Trewavas, A. Calcium-dependent protein kinase in pea shoot membranes. FEBS Lett. 1982, 145, 67–71. [Google Scholar] [CrossRef]
- Boudsocq, M.; Droillard, M.J.; Regad, L.; Laurière, C. Characterization of Arabidopsis calcium-dependent protein kinases: Activated or not by calcium? Biochem. J. 2012, 447, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.F.; Dhindsa, R.S. Low-temperature signal transduction: Induction of cold acclimation-specific genes of alfalfa by calcium at 25 degrees C. Plant Cell 1995, 7, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, P.; Ehlert, B.; Romeis, T. ZmCPK1, a calcium-independent kinase member of the Zea mays CDPK gene family, functions as a negative regulator in cold stress signalling. Plant Cell Environ. 2015, 38, 544–558. [Google Scholar] [CrossRef]
- Szczegielniak, J.; Borkiewicz, L.; Szurmak, B.; Gnatowska, E.L.; Statkiewicz, M.; Klimecka, M.; Cieśla, J.; Muszyńska, G. Maize calcium-dependent protein kinase (ZmCPK11): Local and systemic response to wounding, regulation by touch and components of jasmonate signaling. Physiol. Plant. 2012, 146, 1–14. [Google Scholar] [CrossRef]
- Ding, Y.F.; Cao, J.M.; Ni, L.; Zhu, Y.; Zhang, A.Y.; Tan, M.P.; Jiang, M.Y. ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize. J. Exp. Bot. 2013, 64, 871–884. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK21, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., is involved in grape response to salt stress. J. Plant Physiol. 2016, 124, 137–150. [Google Scholar] [CrossRef]
- Xu, X.J.; Liang, C.Z.; Liu, H.Y. Joint multi-generations genetic analysis on chilling tolerance of melon (Cucumis melo) seedlings. J. Agric. Biotechnol. 2020, 28, 420–428. [Google Scholar] [CrossRef]
- Xie, Y.P.; Chen, P.X.; Yan, Y.; Bao, C.N.; Li, X.W.; Wang, L.P.; Shen, X.X.; Li, H.Y.; Liu, X.F.; Niu, C.D. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018, 218, 201–218. [Google Scholar] [CrossRef]
- Abdullah, S.N.A.; Azzeme, A.M.; Yousefi, K. Fine-tuning cold stress response through regulated cellular abundance and mechanistic actions of transcription factors. Front. Plant Sci. 2022, 13, 850216. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takahashi, Y.; Fukatsu, K.; Tada, Y.; Takahashi, K.; Kuwata, K.; Suzuki, T.; Kinoshita, T. Identification of abscisic acid-dependent phosphorylated basic helix-Loop-Helix transcription factors in guard cells of Vicia faba by mass spectrometry. Front. Plant Sci. 2021, 12, 735271. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Gao, T.; Chen, J.F.; Yang, J.K.; Huang, H.Y.; Yu, Y.B. The late embryogenesis abundant gene family in tea plant (Camellia sinensis): Genome-wide characterization and expression analysis in response to cold and dehydration stress. Plant Physiol. Biochem. 2019, 135, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.W.; Li, J.; Zhao, K.; Chen, S.; Nie, J.; Zhang, W.L.; Liu, G.F.; Wei, H.R. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020, 292, 110375. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.L.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.D.; Lu, Y.; Chen, J.H.; Yang, L.M. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.Y.; Li, M.Z.; Yang, H.; Fu, D.Y.; Lv, J.; Ding, Y.L.; Gong, Z.Z.; Shi, Y.T.; Yang, S.H. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021, 63, 1874–1887. [Google Scholar] [CrossRef]
- Shan, X.Z.; Yang, Y.; Wei, S.Q.; Wang, C.; Shen, W.S.; Chen, H.B.; Shen, J.Y. Involvement of CBF in the fine-tuning of litchi flowering time and cold and drought stresses. Front. Plant Sci. 2023, 14, 1167458. [Google Scholar] [CrossRef]
- Novillo, F.; Medina, J.; Salinas, J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. USA 2007, 104, 21002–21007. [Google Scholar] [CrossRef]
- Novillo, F.; Alonso, J.M.; Ecker, J.R.; Salinas, J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 3985–3990. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S.; Bahk, S.; An, J.; Yoo, Y.; Kim, J.Y.; Chung, W.S. Phosphorylation of the transcriptional repressor MYB15 by mitogen-activated protein kinase 6 is required for freezing tolerance in Arabidopsis. Nucleic Acids Res. 2017, 45, 6613–6627. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.L.; Li, Z.Y.; Shi, Y.T.; Wang, J.L.; Hua, J.; Gong, Z.Z.; Zhou, J.M.; Yang, S.H. PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev. Cell 2019, 51, 222–235. [Google Scholar] [CrossRef]
- Dou, T.X.; Hu, C.H.; Sun, X.X.; Shao, X.H.; Wu, J.H.; Ding, L.J.; Gao, J.; He, W.D.; Biswas, M.K.; Yang, Q.s. MpMYBS3 as a crucial transcription factor of cold signaling confers the cold tolerance of banana. Plant Cell Tissue Organ. Cult. 2016, 125, 93–106. [Google Scholar] [CrossRef]
- Su, C.F.; Wang, Y.C.; Hsieh, T.H.; Lu, C.A.; Tseng, T.H.; Yu, S.M. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010, 153, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.Y.; Yang, Y.; Guo, Y.D.; Zhang, X.C. Functional analysis of SlMYB96 gene in tomato under cold stress. Biotechnol. Bull. 2023, 39, 236. [Google Scholar] [CrossRef]
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Jiang, X.C.; Liu, Q.Y.; Ahammed, G.J.; Lin, R.; Wang, L.Y.; Shao, S.J.; Yu, J.Q.; Zhou, Y.H. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Teng, Z.N.; Liu, B.H.; Lv, J.H.; Chen, Y.K.; Qin, Z.E.; Peng, Y.; Meng, S.; He, Y.C.; Duan, M.J. Transcription factor OsMYB30 increases trehalose content to inhibit α-amylase and seed germination at low temperature. Plant Physiol. 2024, 194, 1815–1833. [Google Scholar] [CrossRef]
- Ma, X.; Yu, Y.N.; Jia, J.H.; Li, Q.H.; Gong, Z.H. The pepper MYB transcription factor CaMYB306 accelerates fruit coloration and negatively regulates cold resistance. Sci. Hortic. 2022, 295, 110892. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhao, H.Q.; Chen, L.; Lin, J.C.; Wang, Z.L.; Pan, J.Q.; Yang, F.; Ni, X.L.; Wang, Y.A.; Wang, Y.H. Multifaceted roles of WRKY transcription factors in abiotic stress and flavonoid biosynthesis. Front. Plant Sci. 2023, 14, 1303667. [Google Scholar] [CrossRef]
- Zou, C.S.; Jiang, W.B.; Yu, D.Q. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef]
- Stock, J.; Bräutigam, A.; Melzer, M.; Bienert, G.P.; Bunk, B.; Nagel, M.; Overmann, J.; Keller, E.R.J.; Mock, H.P. The transcription factor WRKY22 is required during cryo-stress acclimation in Arabidopsis shoot tips. J. Exp. Bot. 2020, 71, 4993–5009. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Das, A.; Khandagale, P.; Maiti, I.B.; Chattopadhyay, S.; Dey, N. Interaction of Arabidopsis TGA3 and WRKY53 transcription factors on Cestrum yellow leaf curling virus (CmYLCV) promoter mediates salicylic acid-dependent gene expression in planta. Planta 2018, 247, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Khurana, P. A salicylic acid inducible mulberry WRKY transcription factor, MiWRKY53 is involved in plant defence response. Plant Cell Rep. 2021, 40, 2151–2171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Boro, P.; Sultana, A.; Mandal, K.; Chattopadhyay, S. Interplay between glutathione and mitogen-activated protein kinase 3 via transcription factor WRKY40 under combined osmotic and cold stress in Arabidopsis. J. Plant Physiol. 2022, 271, 153664. [Google Scholar] [CrossRef]
- Wang, L.H.; Chen, H.; Chen, G.Y.; Luo, G.B.; Shen, X.Y.; Ouyang, B.; Bie, Z.L. Transcription factor SlWRKY50 enhances cold tolerance in tomato by activating the jasmonic acid signaling. Plant Physiol. 2024, 194, 1075–1090. [Google Scholar] [CrossRef]
- Huang, X.B.; Cao, L.W.; Fan, J.B.; Ma, G.J.; Chen, L. CdWRKY2-mediated sucrose biosynthesis and CBF-signalling pathways coordinately contribute to cold tolerance in bermudagrass. Plant Biotechnol. J. 2022, 20, 660–675. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.J.; Yang, X.Y.; Li, Q.; Ling, J.; Wang, H.; Gu, X.F.; Huang, S.W.; Jiang, W.J. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016, 108, 478–487. [Google Scholar] [CrossRef]
- Foster, R.; Izawa, T.; Chua, N.H. Plant bZIP proteins gather at ACGT elements. FASEB J. 1994, 8, 192–200. [Google Scholar] [CrossRef]
- Wang, X.J.; Guo, C.; Peng, J.; Li, C.; Wan, F.F.; Zhang, S.M.; Zhou, Y.Y.; Yan, Y.; Qi, L.J.; Sun, K.W. ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol. 2019, 221, 341–355. [Google Scholar] [CrossRef]
- Yue, X.L.; Zhang, G.Y.; Zhao, Z.; Yue, J.L.; Pu, X.H.; Sui, M.J.; Zhan, Y.; Shi, Y.L.; Wang, Z.Y.; Meng, G.H. A cryophyte transcription factor, CbABF1, confers freezing, and drought tolerance in tobacco. Front. Plant Sci. 2019, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Bulley, S.M.; Gasic, E.V.; Zhong, C.H.; Li, D.W. Kiwifruit bZIP transcription factor AcePosF21 elicits ascorbic acid biosynthesis during cold stress. Plant Physiol. 2023, 192, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Fu, D.Y.; Wang, X.; Zeng, R.; Zhang, X.; Tian, J.; Zhang, S.S.; Yang, X.H.; Tian, F.; Lai, J.S. The transcription factor bZIP68 negatively regulates cold tolerance in maize. Plant Cell 2022, 34, 2833–2851. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.N.; Hao, X.Y.; Cao, H.L.; Ding, C.Q.; Yang, Y.J.; Wang, L.; Wang, X.C. ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep. 2020, 39, 553–565. [Google Scholar] [CrossRef]
- Shimizu, H.; Sato, K.; Berberich, T.; Miyazaki, A.; Ozaki, R.; Imai, R.; Kusano, T. LIP19, a basic region leucine zipper protein, is a Fos-like molecular switch in the cold signaling of rice plants. Plant Cell Physiol. 2005, 46, 1623–1634. [Google Scholar] [CrossRef]
- Liao, Y.; Zou, H.F.; Wei, W.; Hao, Y.J.; Tian, A.G.; Huang, J.; Liu, Y.F.; Zhang, J.S.; Chen, S.Y. Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 2008, 228, 225–240. [Google Scholar] [CrossRef]
- Wang, F.; Wu, N.; Zhang, L.Y.; Ahammed, G.J.; Chen, X.X.; Xiang, X.; Zhou, J.; Xia, X.J.; Shi, K.; Yu, J.Q. Light signaling-dependent regulation of photoinhibition and photoprotection in tomato. Plant Physiol. 2018, 176, 1311–1326. [Google Scholar] [CrossRef]
- Li, Y.P.; Shi, Y.T.; Li, M.Z.; Fu, D.Y.; Wu, S.F.; Li, J.G.; Gong, Z.Z.; Liu, H.T.; Yang, S.H. The CRY2–COP1–HY5–BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef]
- Su, H.Y.; Zhang, S.Z.; Yuan, X.W.; Chen, C.T.; Wang, X.F.; Hao, Y.J. Genome-wide analysis and identification of stress-responsive genes of the NAM–ATAF1, 2–CUC2 transcription factor family in apple. Plant Physiol. Biochem. 2013, 71, 11–21. [Google Scholar] [CrossRef]
- Pooam, M.; El-Ballat, E.M.; Jourdan, N.; Ali, H.M.; Hano, C.; Ahmad, M.; El-Esawi, M.A. SNAC3 transcription factor enhances arsenic stress tolerance and grain yield in rice (Oryza sativa L.) through regulating physio-biochemical mechanisms, stress-responsive genes, and cryptochrome 1b. Plants 2023, 12, 2731. [Google Scholar] [CrossRef]
- Matschi, S.; Werner, S.; Schulze, W.X.; Legen, J.; Hilger, H.H.; Romeis, T. Function of calcium-dependent protein kinase CPK 28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J. 2013, 73, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Song, C.B.; Wu, M.B.; Zhou, Y.; Gong, Z.H.; Yu, W.W.; Zhang, Y.; Yang, Z.F. NAC-mediated membrane lipid remodeling negatively regulates fruit cold tolerance. Hortic. Res. 2022, 9, uhac039. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.M.; Zhou, Q.; Zhou, X.; Wei, B.D.; Ji, S.J. Transcription factor CaNAC1 regulates low-temperature-induced phospholipid degradation in green bell pepper. J. Exp. Bot. 2020, 71, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.M.; Zhang, H.F.; Liu, S.Y.; Wang, X.K.; Zhang, Y.M.; Meng, Y.C.; Luo, D.; Chen, R.G. The NAC transcription factor CaNAC064 is a regulator of cold stress tolerance in peppers. Plant Sci. 2020, 291, 110346. [Google Scholar] [CrossRef]
- Han, D.G.; Du, M.; Zhou, Z.Y.; Wang, S.; Li, T.M.; Han, J.X.; Xu, T.L.; Yang, G.H. Overexpression of a Malus baccata NAC transcription factor gene MbNAC25 increases cold and salinity tolerance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1198. [Google Scholar] [CrossRef]
- Hu, X.L.; Xie, F.F.; Liang, W.W.; Liang, Y.H.; Zhang, Z.K.; Zhao, J.T.; Hu, G.B.; Qin, Y.H. HuNAC20 and HuNAC25, two novel NAC genes from pitaya, confer cold tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2189. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Sui, N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 397–401. [Google Scholar] [CrossRef]
- Chen, W.J.; Wang, X.; Yan, S.; Huang, X.; Yuan, H.M. The ICE-like transcription factor HbICE2 is involved in jasmonate-regulated cold tolerance in the rubber tree (Hevea brasiliensis). Plant Cell Rep. 2019, 38, 699–714. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Wu, C.L.; Lin, L.F.; Hsu, H.C.; Huang, L.F.; Hsiao, C.D.; Chou, M.L. Saussurea involucrata (Snow Lotus) ICE1 and ICE2 orthologues involved in regulating cold stress tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10850. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Kang, H.G.; Hong, Q.C.; Park, M.Y.; Sun, H.J.; Kim, J.; Song, P.S.; Lee, H.Y. A novel basic helix-loop-helix transcription factor, ZjICE2 from Zoysia japonica confers abiotic stress tolerance to transgenic plants via activating the DREB/CBF regulon and enhancing ROS scavenging. Plant Mol. Biol. 2020, 102, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Buitrago, S.; Peng, Y.; Elwafa, S.F.A.; Wan, K.; Liu, Y.; Wang, R.; Yang, X.S.; Zhang, W.Y. Genome-wide identification of cold-tolerance genes and functional analysis of IbbHLH116 gene in sweet potato. Gene 2022, 837, 146690. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, X.; Mei, Q.L.; Qiu, L.; Chen, P.H.; Li, W.H.; Mao, K.; Ma, F.W. MdbHLH4 negatively regulates apple cold tolerance by inhibiting MdCBF1/3 expression and promoting MdCAX3L-2 expression. Plant Physiol. 2023, 191, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 1998, 95, 657–667. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.X.; Dong, S.J.; Jiang, X.C.; Wang, L.Y.; Yu, J.Q.; Zhou, Y.H. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 2020, 18, 1041–1055. [Google Scholar] [CrossRef]
- Ming, R.H.; Zhang, Y.; Wang, Y.; Khan, M.; Dahro, B.; Liu, J.H. The JA-responsive MYC2-BADH-like transcriptional regulatory module in Poncirus trifoliata contributes to cold tolerance by modulation of glycine betaine biosynthesis. New Phytol. 2021, 229, 2730–2750. [Google Scholar] [CrossRef]
- Min, D.D.; Li, F.J.; Zhang, X.H.; Cui, X.X.; Shu, P.; Dong, L.L.; Ren, C.T. SlMYC2 involved in methyl jasmonate-induced tomato fruit chilling tolerance. J. Agric. Food Chem. 2018, 66, 3110–3117. [Google Scholar] [CrossRef]
- Lee, H.G.; Seo, P.J. The MYB 96–HHP module integrates cold and abscisic acid signaling to activate the CBF–COR pathway in Arabidopsis. Plant J. 2015, 82, 962–977. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, M.; Hu, D.; Yang, Z.Y.; Ma, S.Q.; Li, X.H.; Xiong, L.Z. The OsMYB30 transcription factor suppresses cold tolerance by interacting with a JAZ protein and suppressing β-amylase expression. Plant Physiol. 2017, 173, 1475–1491. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cheng, L.; Zhang, W.H.; Hu, J.L.; Liu, Y.; Lin, Y.Z. Characterization of transcription activation domain of EcaICE1 and its interaction with EcaSIZ1 in Eucalyptus camaldulensis. Trees 2020, 34, 1243–1253. [Google Scholar] [CrossRef]
- Lv, J.; Liu, J.Y.; Ming, Y.H.; Shi, Y.T.; Song, C.P.; Gong, Z.Z.; Yang, S.H.; Ding, Y.L. Reciprocal regulation between the negative regulator PP2CG1 phosphatase and the positive regulator OST1 kinase confers cold response in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1568–1587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, W.H.; Hu, J.L.; Zhang, Z.Y.; Liu, Y.; Lin, Y.Z. Characterization of the key region and putative phosphorylation sites of EcaICE1 in its molecular interaction with the EcaHOS1 protein in Eucalyptus camaldulensis. Plant Biol. 2021, 23, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.Y.; Li, H.; Ding, Y.L.; Shi, Y.T.; Song, C.P.; Gong, Z.Z.; Yang, S.H. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar] [CrossRef] [PubMed]
- Bange, M.P.; Milroy, S.P. Impact of short-term exposure to cold night temperatures on early development of cotton (Gossypium hirsutum L.). Aust. J. Agric. Res. 2004, 55, 655–664. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhou, X.J.; Chang, S.; Chu, Z.L.; Wang, H.M.; Han, S.C.; Wang, Y.D. Calcium-dependent protein kinase 21 phosphorylates 14-3-3 proteins in response to ABA signaling and salt stress in rice. Biochem. Biophys. Res. Commun. 2017, 493, 1450–1456. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Banana fruit NAC transcription factor MaNAC 1 is a direct target of MaICE 1 and involved in cold stress through interacting with MaCBF 1. Plant Cell Environ. 2014, 37, 2116–2127. [Google Scholar] [CrossRef]
- Kovtun, Y.; Chiu, W.L.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef]
- Uchida, K.; Yamaguchi, M.; Kanamori, K.; Ariga, H.; Isono, K.; Kajino, T.; Tanaka, K.; Saijo, Y.; Yotsui, I.; Sakata, Y. MAP KINASE PHOSPHATASE1 promotes osmotolerance by suppressing PHYTOALEXIN DEFICIENT4-independent immunity. Plant Physiol. 2022, 189, 1128–1138. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Wang, P.C.; Si, T.; Hsu, C.C.; Wang, L.; Zayed, O.; Yu, Z.P.; Zhu, Y.F.; Dong, J.; Tao, W.A. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 2017, 43, 618–629. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Hoehenwarter, W.; Scheel, D.; Lee, J. Phosphorylation of the CAMTA3 transcription factor triggers its destabilization and nuclear export. Plant Physiol. 2020, 184, 1056–1071. [Google Scholar] [CrossRef]
- Kameniarová, M.; Černý, M.; Novák, J.; Ondrisková, V.; Hrušková, L.; Berka, M.; Vankova, R.; Brzobohatý, B. Light quality modulates plant cold response and freezing tolerance. Front. Plant Sci. 2022, 13, 887103. [Google Scholar] [CrossRef]
- Long, J.L.; Xing, W.; Wang, Y.G.; Wu, Z.D.; Li, W.J.; Zou, Y.; Sun, J.P.; Zhang, F.S.; Pi, Z. Comparative proteomic analysis on chloroplast proteins provides new insights into the effects of low temperature in sugar beet. Bot. Stud. 2022, 63, 18. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.J.; Xie, L.; Wang, Y.H.; Cao, Y.R.; Wan, M.Y.; Lv, D.Q.; Li, J.N.; Lu, K.; Xu, X.F.; Liu, L.Z. Characterization of cold stress responses in different rapeseed ecotypes based on metabolomics and transcriptomics analyses. PeerJ 2020, 8, e8704. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, S.S.; Ma, Q.Y.; Yan, K.Y.; Ren, J.; Chen, Z.; Wen, J.; Li, Q.Z. An Acer palmatum R2R3-MYB gene, ApMYB77, confers freezing and drought tolerance in Arabidopsis thaliana. J. Plant Growth Regul. 2023, 42, 1017–1030. [Google Scholar] [CrossRef]
- Yao, C.Y.; Li, X.G.; Li, Y.M.; Yang, G.H.; Liu, W.D.; Shao, B.T.; Zhong, J.L.; Huang, P.F.; Han, D.G. Overexpression of a Malus baccata MYB transcription factor gene MbMYB4 increases cold and drought tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 1794. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhao, C.D.; Gao, Y.Q.; Xu, Y.; Wang, S.J.; Li, C.S.; Xie, Y.P.; Chen, P.X.; Yang, P.Z.; Yuan, L. A multifaceted module of BRI1 ETHYLMETHANE SULFONATE SUPRESSOR1 (BES1)-MYB88 in growth and stress tolerance of apple. Plant Physiol. 2021, 185, 1903–1923. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, X.Q.; Zhang, Y.J.; Li, W.J.; Zhang, D.M.; Dai, J.L.; Fang, S.; Chu, J.F.; Dong, H.Z. Leaf-derived jasmonate mediates water uptake from hydrated cotton roots under partial root-zone irrigation. Plant Physiol. 2019, 180, 1660–1676. [Google Scholar] [CrossRef]

| Impacts | Class | Content | References |
|---|---|---|---|
| Positive | Cold acclimation | Improving plant cold tolerance | [18] |
| Vernalization | Improvement of flowering | [19] | |
| Improvement of seed yield | [20] | ||
| Negative | Insufficient leaf development | Reducing leaf elongation | [21] |
| Leaf chlorosis (wilting, even necrosis) | [22] | ||
| Reducing stomatal conductance | [23] | ||
| Insufficient root development | Swelling root tips | [24] | |
| Thicker root axis | [16] | ||
| Less lateral and more seminal roots | [25] | ||
| Reducing root length | [26] | ||
| Growth retardation | Spikelet sterility | [27] | |
| Limiting seed size | [28] | ||
| Lower survival rate | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives. Cells 2025, 14, 110. https://doi.org/10.3390/cells14020110
Feng Y, Li Z, Kong X, Khan A, Ullah N, Zhang X. Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives. Cells. 2025; 14(2):110. https://doi.org/10.3390/cells14020110
Chicago/Turabian StyleFeng, Yan, Zengqiang Li, Xiangjun Kong, Aziz Khan, Najeeb Ullah, and Xin Zhang. 2025. "Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives" Cells 14, no. 2: 110. https://doi.org/10.3390/cells14020110
APA StyleFeng, Y., Li, Z., Kong, X., Khan, A., Ullah, N., & Zhang, X. (2025). Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives. Cells, 14(2), 110. https://doi.org/10.3390/cells14020110






