The MCPH7 Gene Product STIL Is Essential for Dendritic Spine Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Antibodies
2.4. Quantitative RT-PCR
2.5. Plasmid and Viral Vector Construction
2.6. In Utero Electroporation (IUE)
2.7. Immunocytochemistry and Immunohistochemistry
2.8. Chemical Long-Term Potentiation (cLTP) Induction in Cultured Neurons
2.9. Spine Density Analysis
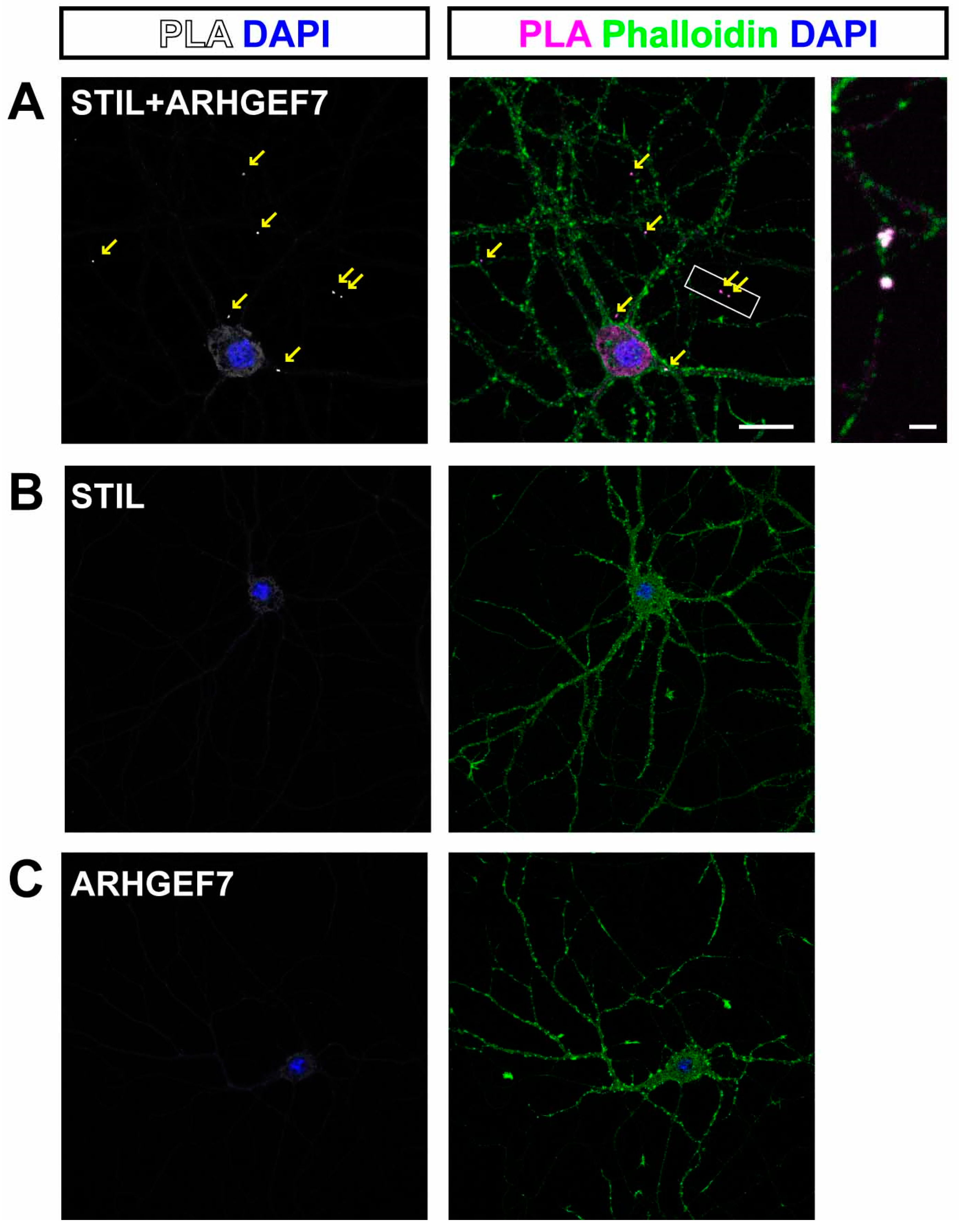
2.10. Proximity Ligation Assay (PLA)
2.11. Fluorescence Resonance Energy Transfer (FRET) Analysis
2.12. Statistical Analysis
3. Results
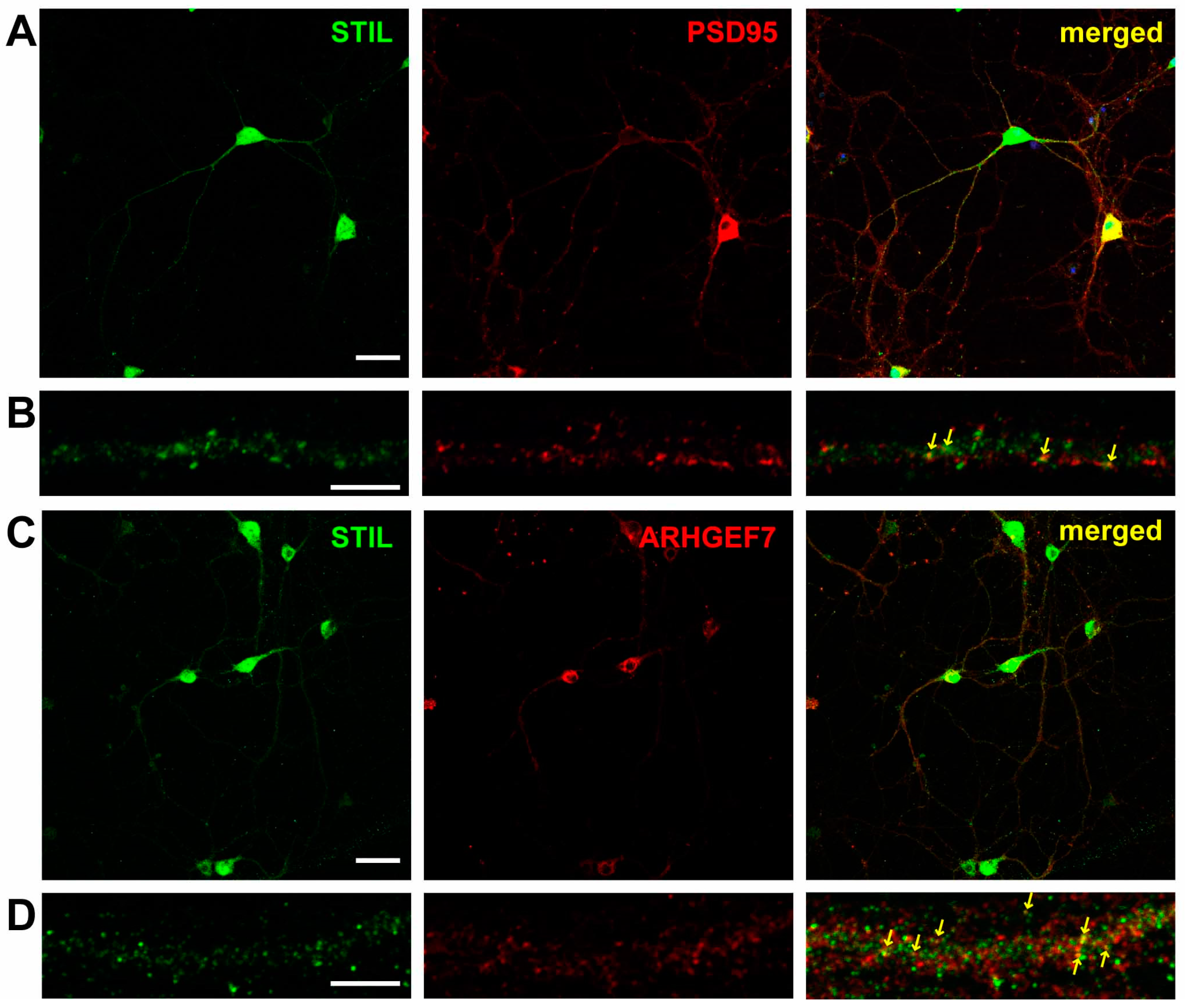
3.1. STIL Is in the Dendritic Spine and Is Closely Associated with ARHGEF7
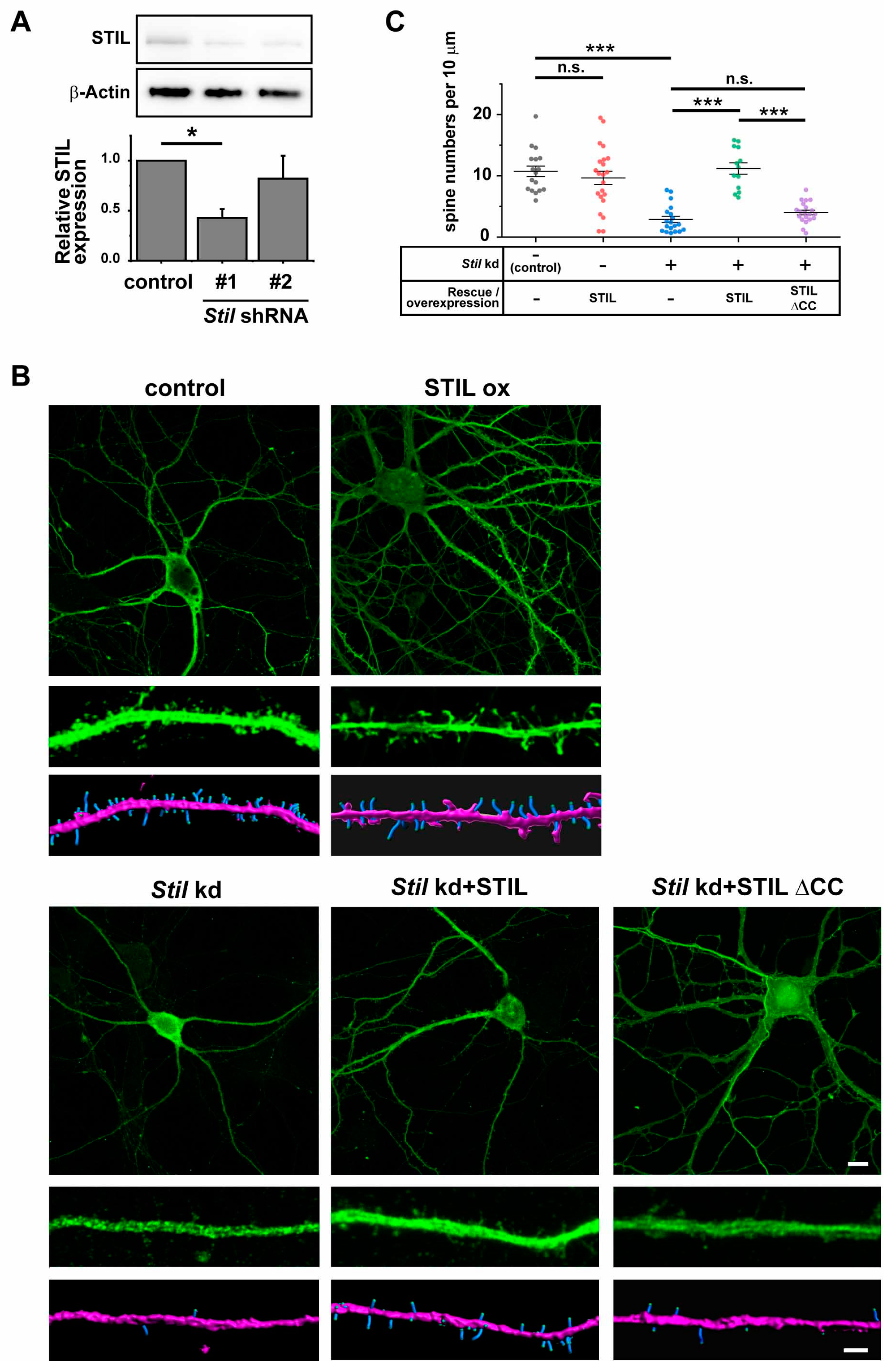
3.2. Stil Knockdown Results in the Reduction in Dendritic Spine Density In Vitro
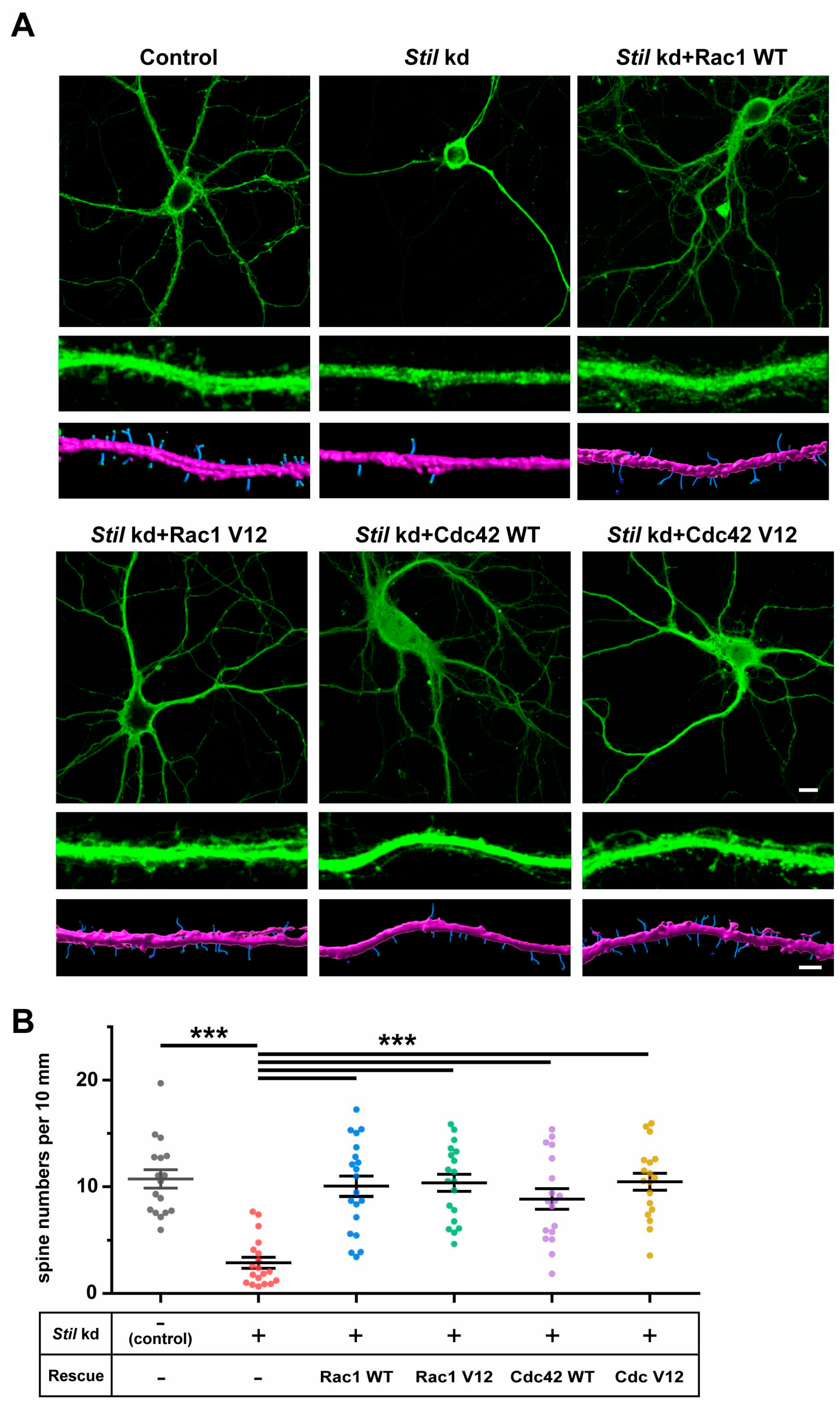
3.3. Rac1 and Cdc42 Induction Can Compensate for the Reduction in Dendritic Spine Caused by Stil Knockdown
3.4. STIL Is Required for Rac Activation in Dendrites
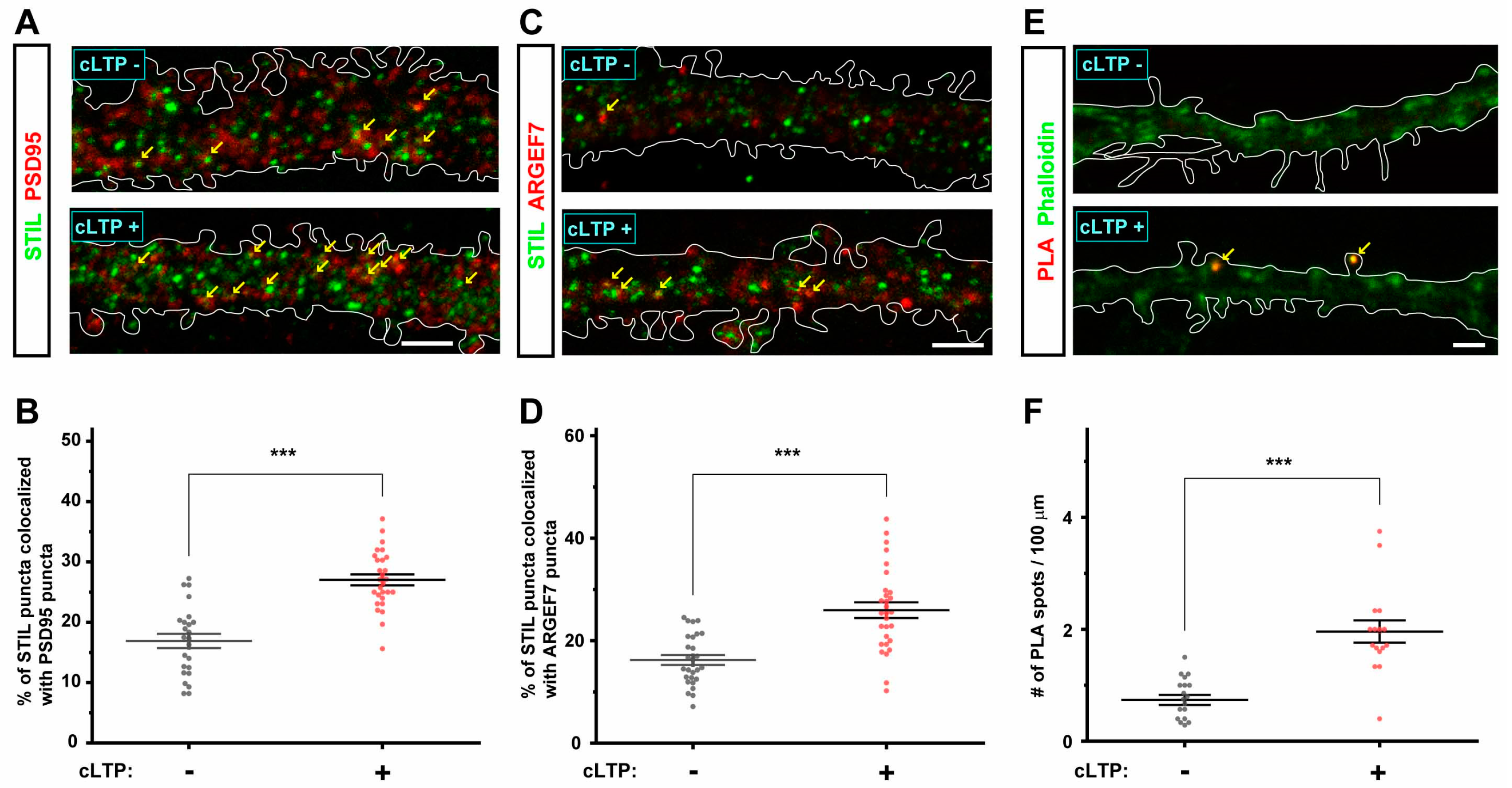
3.5. cLTP Induces STIL Accumulation in Dendritic Spines and Promotes Its Association with ARHGEF7
3.6. Stil Knock Down Results in the Reduction in Dendritic Spine Density In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, K.P.; Nedivi, E. Spine Dynamics: Are They All the Same? Neuron 2017, 96, 43–55. [Google Scholar] [CrossRef]
- Ramón y Cajal, S. Neue darstellung vom histologischen bau des centralnervensystem. Arch. Anat. Entwick 1893, 3, 319–428. [Google Scholar]
- Ridley, A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006, 16, 522–529. [Google Scholar] [CrossRef]
- Costa, J.F.; Dines, M.; Lamprecht, R. The Role of Rac GTPase in Dendritic Spine Morphogenesis and Memory. Front. Synaptic Neurosci. 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Woolfrey, K.M.; Srivastava, D.P. Control of Dendritic Spine Morphological and Functional Plasticity by Small GTPases. Neural Plast. 2016, 2016, 3025948. [Google Scholar] [CrossRef]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; Srivastava, D.P. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Saneyoshi, T.; Wayman, G.; Fortin, D.; Davare, M.; Hoshi, N.; Nozaki, N.; Natsume, T.; Soderling, T.R. Activity-dependent synaptogenesis: Regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron 2008, 57, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Webb, D.J.; Asmussen, H.; Horwitz, A.F. Synapse formation is regulated by the signaling adaptor GIT1. J. Cell Biol. 2003, 161, 131–142. [Google Scholar] [CrossRef]
- Itoh, R.E.; Kurokawa, K.; Ohba, Y.; Yoshizaki, H.; Mochizuki, N.; Matsuda, M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 2002, 22, 6582–6591. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Inaguma, S.; Yoneyama, A.; Yoshikawa, K.; Ikeda, H. SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res. 2008, 68, 7723–7729. [Google Scholar] [CrossRef]
- Matsuki, T.; Iio, A.; Ueda, M.; Tsuneura, Y.; Howell, B.W.; Nakayama, A. STK25 and MST3 Have Overlapping Roles to Regulate Rho GTPases during Cortical Development. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 8887–8903. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, R.S.; Cameron, D.A.; Zipfel, W.R.; Olson, E.C. Reelin Prevents Apical Neurite Retraction during Terminal Translocation and Dendrite Initiation. J. Neurosci. 2015, 35, 10659–10674. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Castro, J.; Saneyoshi, T.; Matsuno, H.; Sur, M.; Hayashi, Y. Structural and Molecular Remodeling of Dendritic Spine Substructures during Long-Term Potentiation. Neuron 2014, 82, 444–459. [Google Scholar] [CrossRef]
- Ito, H.; Tsunoda, T.; Riku, M.; Inaguma, S.; Inoko, A.; Murakami, H.; Ikeda, H.; Matsuda, M.; Kasai, K. Indispensable role of STIL in the regulation of cancer cell motility through the lamellipodial accumulation of ARHGEF7-PAK1 complex. Oncogene 2020, 39, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, O.; Gullberg, M.; Jarvius, M.; Ridderstråle, K.; Leuchowius, K.J.; Jarvius, J.; Wester, K.; Hydbring, P.; Bahram, F.; Larsson, L.G.; et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 2006, 3, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Webb, D.J.; Asmussen, H.; Niu, S.; Horwitz, A.F. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 3379–3388. [Google Scholar] [CrossRef]
- Fortin, D.A.; Davare, M.A.; Srivastava, T.; Brady, J.D.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 11565–11575. [Google Scholar] [CrossRef]
- Park, M.; Salgado, J.M.; Ostroff, L.; Helton, T.D.; Robinson, C.G.; Harris, K.M.; Ehlers, M.D. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 2006, 52, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Arquint, C.; Nigg, E.A. The PLK4-STIL-SAS-6 module at the core of centriole duplication. Biochem. Soc. Trans. 2016, 44, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Gönczy, P.; Hatzopoulos, G.N. Centriole assembly at a glance. J. Cell Sci. 2019, 132, jcs228833. [Google Scholar] [CrossRef]
- Ohta, M.; Ashikawa, T.; Nozaki, Y.; Kozuka-Hata, H.; Goto, H.; Inagaki, M.; Oyama, M.; Kitagawa, D. Direct interaction of Plk4 with STIL ensures formation of a single procentriole per parental centriole. Nat. Commun. 2014, 5, 5267. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Holland, A.J. Once and only once: Mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Siskos, N.; Stylianopoulou, E.; Skavdis, G.; Grigoriou, M.E. Molecular Genetics of Microcephaly Primary Hereditary: An Overview. Brain Sci. 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, D.; Bae, B.I.; Walsh, C.A. The Genetics of Primary Microcephaly. Annu. Rev. Genom. Hum. Genet. 2018, 19, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Kazmi, S.K.; Amin, M.; Asif, Z.; Islam, U.; Shahid, K.; Tehreem, S. Comprehensive review on the molecular genetics of autosomal recessive primary microcephaly (MCPH). Genet. Res. 2018, 100, e7. [Google Scholar] [CrossRef]
- Martin, C.A.; Ahmad, I.; Klingseisen, A.; Hussain, M.S.; Bicknell, L.S.; Leitch, A.; Nürnberg, G.; Toliat, M.R.; Murray, J.E.; Hunt, D.; et al. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat. Genet. 2014, 46, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Yokoi, S.; Miya, F.; Miyata, M.; Kato, M.; Okamoto, N.; Tsunoda, T.; Yamasaki, M.; Kanemura, Y.; Kosaki, K.; et al. Novel compound heterozygous variants in PLK4 identified in a patient with autosomal recessive microcephaly and chorioretinopathy. Eur. J. Hum. Genet. EJHG 2016, 24, 1702–1706. [Google Scholar] [CrossRef]
- Insolera, R.; Bazzi, H.; Shao, W.; Anderson, K.V.; Shi, S.-H. Cortical neurogenesis in the absence of centrioles. Nat. Neurosci. 2014, 17, 1528–1535. [Google Scholar] [CrossRef]
- Izraeli, S.; Lowe, L.A.; Bertness, V.L.; Good, D.J.; Dorward, D.W.; Kirsch, I.R.; Kuehn, M.R. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature 1999, 399, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 2009, 139, 663–678. [Google Scholar] [CrossRef]
- David, A.; Liu, F.; Tibelius, A.; Vulprecht, J.; Wald, D.; Rothermel, U.; Ohana, R.; Seitel, A.; Metzger, J.; Ashery-Padan, R.; et al. Lack of centrioles and primary cilia in STIL(-/-) mouse embryos. Cell Cycle 2014, 13, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Carr, A.L.; Li, P.; Lee, J.; McGregor, M.; Li, L. Characterization of the human oncogene SCL/TAL1 interrupting locus (Stil) mediated Sonic hedgehog (Shh) signaling transduction in proliferating mammalian dopaminergic neurons. Biochem. Biophys. Res. Commun. 2014, 449, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Aplan, P.D.; Lombardi, D.P.; Kirsch, I.R. Structural characterization of SIL, a gene frequently disrupted in T-cell acute lymphoblastic leukemia. Mol. Cell. Biol. 1991, 11, 5462–5469. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.; Sun, L.; Lee, E.; Li, P.; Antonacci, C.; Gorbea, E.; Finlay, C.; Li, L. The human oncogene SCL/TAL1 interrupting locus is required for mammalian dopaminergic cell proliferation through the Sonic hedgehog pathway. Cell. Signal. 2014, 26, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Cottee, M.A.; Muschalik, N.; Johnson, S.; Leveson, J.; Raff, J.W.; Lea, S.M. The homo-oligomerisation of both Sas-6 and Ana2 is required for efficient centriole assembly in flies. eLife 2015, 4, e07236. [Google Scholar] [CrossRef]
- Gartenmann, L.; Vicente, C.C.; Wainman, A.; Novak, Z.A.; Sieber, B.; Richens, J.H.; Raff, J.W. Drosophila Sas-6, Ana2 and Sas-4 self-organise into macromolecular structures that can be used to probe centriole and centrosome assembly. J. Cell Sci. 2020, 133, jcs244574. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, D.; Mani, S.; Passemard, S.; Gressens, P.; El Ghouzzi, V. STIL balancing primary microcephaly and cancer. Cell Death Dis. 2018, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Amartely, H.; Rabinowicz, N.; Shamir, M.; Friedler, A.; Izraeli, S. Molecular basis of the STIL coiled coil oligomerization explains its requirement for de-novo formation of centrosomes in mammalian cells. Sci. Rep. 2016, 6, 24296. [Google Scholar] [CrossRef]
- Manser, E.; Loo, T.H.; Koh, C.G.; Zhao, Z.S.; Chen, X.Q.; Tan, L.; Tan, I.; Leung, T.; Lim, L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1998, 1, 183–192. [Google Scholar] [CrossRef]
- Govek, E.E.; Newey, S.E.; Akerman, C.J.; Cross, J.R.; Van der Veken, L.; Van Aelst, L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat. Neurosci. 2004, 7, 364–372. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, C.M.; Chang, S. SNX26, a GTPase-activating protein for Cdc42, interacts with PSD-95 protein and is involved in activity-dependent dendritic spine formation in mature neurons. J. Biol. Chem. 2013, 288, 29453–29466. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Yuste, R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: Evidence for two forms of spine motility. Mol. Cell. Neurosci. 2004, 26, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.Y.; Harms, M.B.; Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.M.; Nebhan, C.A.; Hu, L.; Majumdar, D.; Meier, K.M.; Weaver, A.M.; Webb, D.J. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 2008, 283, 15912–15920. [Google Scholar] [CrossRef]
- Jaudon, F.; Raynaud, F.; Wehrlé, R.; Bellanger, J.M.; Doulazmi, M.; Vodjdani, G.; Gasman, S.; Fagni, L.; Dusart, I.; Debant, A.; et al. The RhoGEF DOCK10 is essential for dendritic spine morphogenesis. Mol. Biol. Cell 2015, 26, 2112–2127. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Minden, A.; Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: Antagonistic roles of Rac and Rho. Cereb. Cortex 2000, 10, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, N.; Gu, Y.; Wang, J.; Janoschka, S.; Takemaru, K.; Levine, J.; Ge, S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 2012, 15, 399–405. [Google Scholar] [CrossRef]
- Node-Langlois, R.; Muller, D.; Boda, B. Sequential implication of the mental retardation proteins ARHGEF6 and PAK3 in spine morphogenesis. J. Cell Sci. 2006, 119, 4986–4993. [Google Scholar] [CrossRef]
- Ramakers, G.J.; Wolfer, D.; Rosenberger, G.; Kuchenbecker, K.; Kreienkamp, H.J.; Prange-Kiel, J.; Rune, G.; Richter, K.; Langnaese, K.; Masneuf, S.; et al. Dysregulation of Rho GTPases in the alphaPix/Arhgef6 mouse model of X-linked intellectual disability is paralleled by impaired structural and synaptic plasticity and cognitive deficits. Hum. Mol. Genet. 2012, 21, 268–286. [Google Scholar] [CrossRef] [PubMed]


| Application | Nucleotide Sequence (5′—3′) |
|---|---|
| Forward qPCR primer for Actb | ACCTTCTACAATGAGCTGBCG |
| Reverse qPCR primer for Actb | CTGGATGGCTACGTACATGG |
| Forward qPCR primer for Stil | AGGTATGGGCTTGCTGCTTGAGAT |
| Reverse qPCR primer for Stil | GTAACTGAAACTGAAGGTCGGGCG |
| Forward PCR primer for STIL cloning | ATGTACCCATACGATGTTCCAGATTACGCTAATACCAGGTTTCCTTCAAGCAAGATGG |
| Reverse PCR primer for STIL cloning | GAACCCTTAAACATGTTAAAATAACTTAGGTAACTGTCTGAGACGCTTTACG |
| Stil knockdown shRNA #1 | GCCGCAGCTAATCAAGTTCA |
| Stil knockdown shRNA #2 | CAAGTTCAGGGAACCTATAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuki, T.; Tabata, H.; Ueda, M.; Ito, H.; Nagata, K.-i.; Tsuneura, Y.; Eda, S.; Kasai, K.; Nakayama, A. The MCPH7 Gene Product STIL Is Essential for Dendritic Spine Formation. Cells 2025, 14, 62. https://doi.org/10.3390/cells14020062
Matsuki T, Tabata H, Ueda M, Ito H, Nagata K-i, Tsuneura Y, Eda S, Kasai K, Nakayama A. The MCPH7 Gene Product STIL Is Essential for Dendritic Spine Formation. Cells. 2025; 14(2):62. https://doi.org/10.3390/cells14020062
Chicago/Turabian StyleMatsuki, Tohru, Hidenori Tabata, Masashi Ueda, Hideaki Ito, Koh-ichi Nagata, Yumi Tsuneura, Shima Eda, Kenji Kasai, and Atsuo Nakayama. 2025. "The MCPH7 Gene Product STIL Is Essential for Dendritic Spine Formation" Cells 14, no. 2: 62. https://doi.org/10.3390/cells14020062
APA StyleMatsuki, T., Tabata, H., Ueda, M., Ito, H., Nagata, K.-i., Tsuneura, Y., Eda, S., Kasai, K., & Nakayama, A. (2025). The MCPH7 Gene Product STIL Is Essential for Dendritic Spine Formation. Cells, 14(2), 62. https://doi.org/10.3390/cells14020062







