Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Spinal Cord Harvest and Primary NSPC Culture
2.3. Skin Harvest and Primary Fibroblast Culture
2.4. iPSC Reprogramming, Culture, and Differentiation
2.5. iPSC Primary Culture, Propagation and Freezing
2.6. Generation and Differentiation of iPSC-Derived Forebrain and Spinal Cord NSPCs
2.7. Proliferation and Differentiation of iPSC-Derived NSPCs
2.8. RNA Sequencing and Data Analysis
3. Results
3.1. iPSC-Br NSPCs Exhibit Closer Morphological and Marker Resemblance to Bona Fide NSPCs
3.2. IPSC-Br NSPCs Exhibit Increased Neurogenic Differentiation Potential and Proliferation Rates
3.3. iPSC-Br NSPCs Exhibit Transcriptomic Parity with Bona Fide NSPCs
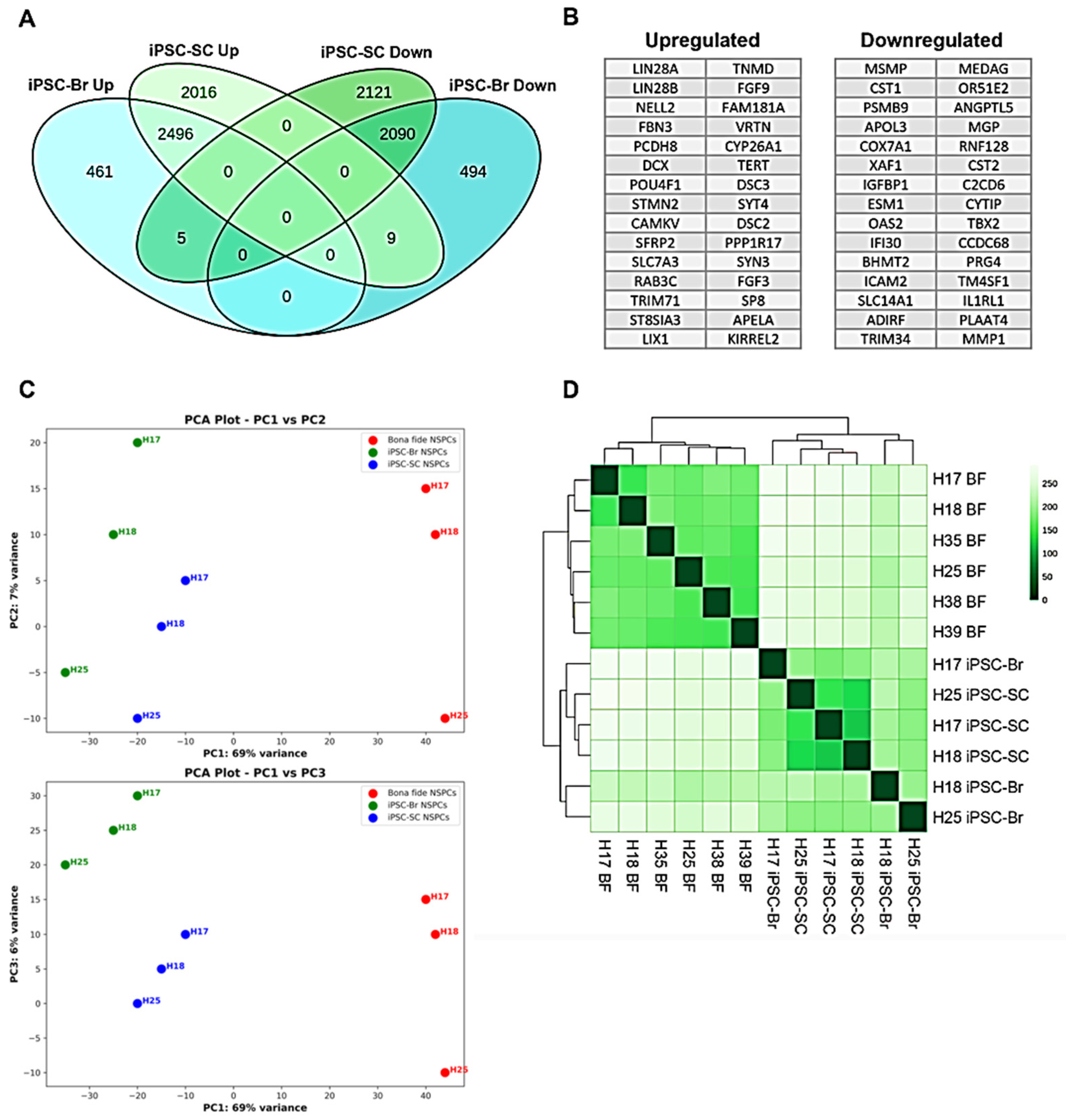
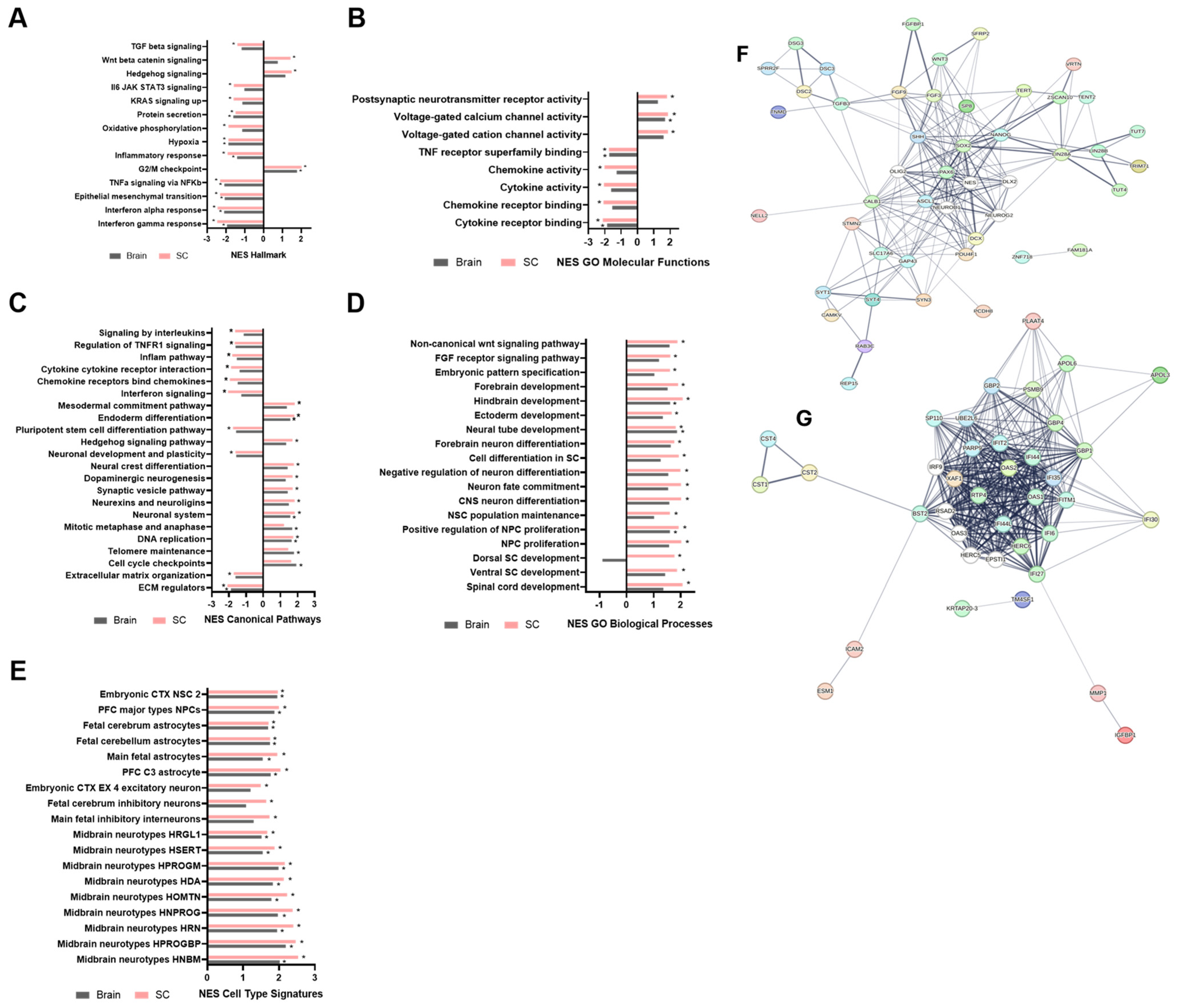
3.4. Transcriptomic Divergence and Pluripotency Retention in iPSC-Derived NSPCs Relative to Bona Fide NSPCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Saremi, J.; Mahmoodi, N.; Rasouli, M.; Ranjbar, F.E.; Mazaheri, E.L.; Akbari, M.; Hasanzadeh, E.; Azami, M. Advanced approaches to regenerate spinal cord injury: The development of cell and tissue engineering therapy and combinational treatments. Biomed. Pharmacother. 2022, 146, 112529. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 3994. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Xu, B.; Yin, M.; Yang, Y.; Zou, Y.; Liu, W.; Qiao, L.; Zhang, J.; Wang, Z.; Wu, Y.; Shen, H.; et al. Transplantation of neural stem progenitor cells from different sources for severe spinal cord injury repair in rat. Bioact. Mater. 2023, 23, 300–313. [Google Scholar] [CrossRef]
- Finkel, Z.; Esteban, F.; Rodriguez, B.; Fu, T.; Ai, X.; Cai, L. Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease. Cells 2021, 10, 2045. [Google Scholar] [CrossRef]
- Bonnamain, V.; Neveu, I.; Naveilhan, P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front. Cell Neurosci. 2012, 6, 17. [Google Scholar] [CrossRef]
- Poiana, G.; Gioia, R.; Sineri, S.; Cardarelli, S.; Lupo, G.; Cacci, E. Transcriptional regulation of adult neural stem/progenitor cells: Tales from the subventricular zone. Neural. Regen. Res. 2020, 15, 1773. [Google Scholar]
- Deleyrolle, L.P.; Reynolds, B.A. Isolation, Expansion, and Differentiation of Adult Mammalian Neural Stem and Progenitor Cells Using the Neurosphere Assay. In Neural Cell Transplantation; Humana Press: Totowa, NJ, USA, 2009; pp. 91–101. [Google Scholar]
- Zheng, Y.; Zhou, J.; Wang, Y.; Fan, F.; Liu, S.; Wang, Y. Neural Stem/Progenitor Cell Transplantation in Parkinson’s Rodent Animals: A Meta-Analysis and Systematic Review. Stem Cells Transl. Med. 2022, 11, 383–393. [Google Scholar] [CrossRef]
- Kibschull, M.; Nguyen, T.T.N.; Chow, T.; Alarab, M.; Lye, S.J.; Rogers, I.; Shynlova, O. Differentiation of patient-specific void urine-derived human induced pluripotent stem cells to fibroblasts and skeletal muscle myocytes. Sci. Rep. 2023, 13, 4746. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease–Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, K.; Imaizumi, K.; Shinozaki, M.; Shibata, S.; Shindo, T.; Kitagawa, T.; Shibata, R.; Kamata, Y.; Kojima, K.; Nagoshi, N.; et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol. Brain 2020, 13, 120. [Google Scholar] [CrossRef]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.M.; Carelli, S.; Cereda, C. From Neuronal Differentiation of iPSCs to 3D Neuro-Organoids: Modelling and Therapy of Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef]
- Malik, N.; Rao, M.S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar]
- Son, D.; Zheng, J.; Kim, I.Y.; Kang, P.J.; Park, K.; Priscilla, L.; Hong, W.; Yoon, B.S.; Park, G.; Yoo, J.E.; et al. Human induced neural stem cells support functional recovery in spinal cord injury models. Exp. Mol. Med. 2023, 55, 1182–1192. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, B.; Chen, J.; Liu, D.; Ma, J.; Li, B.; Hao, J.; Zhou, X. Epigenetic regulation and factors that influence the effect of iPSCs-derived neural stem/progenitor cells (NS/PCs) in the treatment of spinal cord injury. Clin. Epigenet. 2024, 16, 30. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Chooi, W.H.; Jeon, H.; Chen, J.; Tan, J.; Roxby, D.N.; Lee, C.Y.; Ng, S.Y.; Chew, S.Y.; Han, J. Label-Free and High-Throughput Removal of Residual Undifferentiated Cells From iPSC-Derived Spinal Cord Progenitor Cells. Stem Cells Transl. Med. 2024, 13, 387–398. [Google Scholar] [CrossRef]
- Volpato, V.; Webber, C. Addressing variability in iPSC-derived models of human disease: Guidelines to promote reproducibility. Dis. Model Mech. 2020, 13, dmm042317. [Google Scholar] [CrossRef]
- Edri, S.; Hayward, P.; Jawaid, W.; Martinez Arias, A. Neuro-mesodermal progenitors (NMPs): A comparative study between pluripotent stem cells and embryo-derived populations. Development 2019, 146, dev180190. [Google Scholar] [CrossRef]
- Kuruş, M.; Akbari, S.; Eskier, D.; Bursalı, A.; Ergin, K.; Erdal, E.; Karakülah, G. Transcriptome Dynamics of Human Neuronal Differentiation From iPSC. Front. Cell Dev. Biol. 2021, 9, 727747. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Sanosaka, T.; Lundin, A.; Imaizumi, K.; Etal, D.; Karlsson, F.H.; Clausen, M.; Cairns, J.; Hicks, R.; Kohyama, J.; et al. Single-cell study of neural stem cells derived from human iPSCs reveals distinct progenitor populations with neurogenic and gliogenic potential. Genes Cells 2019, 24, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Galiakberova, A.A.; Brovkina, O.I.; Kondratyev, N.V.; Artyuhov, A.S.; Momotyuk, E.D.; Kulmukhametova, O.N.; Lagunin, A.A.; Shilov, B.V.; Zadorozhny, A.D.; Zakharov, I.S.; et al. Different iPSC-derived neural stem cells shows various spectrums of spontaneous differentiation during long term cultivation. Front. Mol. Neurosci. 2023, 16, 1037902. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research. Natural Sciences and Research Council of Canada and Social Sciences and Humanities Research Council. In Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans; Canadian Institutes of Health Research: Ottawa, ON, Canada, 2022. [Google Scholar]
- Yadav, A.; Matson, K.J.E.; Li, L.; Hua, I.; Petrescu, J.; Kang, K.; Alkaslasi, M.R.; Lee, D.I.; Hasan, S.; Galuta, A.; et al. A cellular taxonomy of the adult human spinal cord. Neuron 2023, 111, 328–344.e7. [Google Scholar] [CrossRef]
- Galuta, A.; Sandarage, R.; Ghinda, D.; Auriat, A.M.; Chen, S.; Kwan, J.C.S.; Tsai, E.C. A Guide to Extract Spinal Cord for Translational Stem Cell Biology Research: Comparative Analysis of Adult Human, Porcine, and Rodent Spinal Cord Stem Cells. Front. Neurosci. 2020, 14, 607. [Google Scholar] [CrossRef]
- University of Ottawa. Consent Process and Templates. Available online: https://research.uottawa.ca/ethics/guidelines/consent-process (accessed on 2 November 2024).
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15, 723–731. [Google Scholar] [CrossRef]
- Balzeau, J.; Menezes, M.R.; Cao, S.; Hagan, J.P. The LIN28/let-7 Pathway in Cancer. Front. Genet. 2017, 8, 31. [Google Scholar] [CrossRef]
- Tsialikas, J.; Romer-Seibert, J. LIN28: Roles and regulation in development and beyond. Development 2015, 142, 2397–2404. [Google Scholar] [CrossRef]
- Dykes, I.M.; Lanier, J.; Raisa Eng, S.; Turner, E.E. Brn3a regulates neuronal subtype specification in the trigeminal ganglion by promoting Runx expression during sensory differentiation. Neural Dev. 2010, 5, 3. [Google Scholar] [CrossRef]
- Pruszak, J.; Ludwig, W.; Blak, A.; Alavian, K.; Isacson, O. CD15, CD24, and CD29 Define a Surface Biomarker Code for Neural Lineage Differentiation of Stem Cells. Stem Cells 2009, 27, 2928–2940. [Google Scholar] [CrossRef]
- Forouzanfar, M.; Lachinani, L.; Dormiani, K.; Nasr-Esfahani, M.H.; Gure, A.O.; Ghaedi, K. Intracellular functions of RNA-binding protein, Musashi1, in stem and cancer cells. Stem Cell Res. Ther. 2020, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Facchino, S.; Abdouh, M.; Chatoo, W.; Bernier, G. BMI1 Confers Radioresistance to Normal and Cancerous Neural Stem Cells through Recruitment of the DNA Damage Response Machinery. J. Neurosci. 2010, 30, 10096–10111. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G. Epidermal Growth Factor in the CNS: A Beguiling Journey from Integrated Cell Biology to Multiple Sclerosis. An Extensive Translational Overview. Cell Mol. Neurobiol. 2022, 42, 891–916. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Zhao, C.; Su, L.; Jiao, J. BCAT1 Controls Embryonic Neural Stem Cells Proliferation and Differentiation in the Upper Layer Neurons. Mol. Brain. 2023, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed Res. Int. 2015, 2015, 727542. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; He, J.Q.; Sinha, R.; Eastman, A.E.; Toland, A.M.; Morri, M.; Neff, N.F.; Vogel, H.; Uchida, N.; Weissman, I.L. Purification and characterization of human neural stem and progenitor cells. Cell 2023, 186, 1179–1194.e15. [Google Scholar] [CrossRef]
- Bidlingmaier, S.; Zhu, X.; Cao, B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. PLoS ONE 2014, 9, e106694. [Google Scholar] [CrossRef]
- Alizadeh, J.; Glogowska, A.; Smith, S.; Dreolini, L.; Holt, R.A.; Karimi-Busheri, F.; Parameswaran, K.; Maddika, S.; Wiechec, E.; Hitt, M.; et al. CD133: Molecular features and potential therapeutic implications. Int. J. Mol. Sci. 2023, 24, 10910. [Google Scholar]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.-S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Kreuser, U.; Buchert, J.; Haase, A.; Richter, W.; Diederichs, S. Initial WNT/β-Catenin Activation Enhanced Mesoderm Commitment, Extracellular Matrix Expression, Cell Aggregation and Cartilage Tissue Yield From Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2020, 8, 581331. [Google Scholar] [CrossRef]
- Togo, K.; Fukusumi, H.; Shofuda, T.; Ohnishi, H.; Yamazaki, H.; Hayashi, M.K.; Kawasaki, N.; Takei, N.; Nakazawa, T.; Saito, Y.; et al. Postsynaptic structure formation of human iPS cell-derived neurons takes longer than presynaptic formation during neural differentiation in vitro. Mol. Brain 2021, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.; Davis-Anderson, K.; Hovde, B.; Micheva-Viteva, S.; Harris, J.F.; Twary, S.; Iyer, R. Global transcriptome profile of the developmental principles of in vitro iPSC-to-motor neuron differentiation. BMC Mol. Cell Biol. 2021, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Sagner, A.; Gaber, Z.B.; Delile, J.; Kong, J.H.; Rousso, D.L.; Pearson, C.A.; Weicksel, S.E.; Melchionda, M.; Mousavy Gharavy, S.N.; Briscoe, J.; et al. Olig2 and Hes regulatory dynamics during motor neuron differentiation revealed by single cell transcriptomics. PLoS Biol. 2018, 16, e2003127. [Google Scholar] [CrossRef]
- Grochowski, C.; Radzikowska, E.; Maciejewski, R. Neural stem cell therapy-Brief review. Clin. Neurol. Neurosurg. 2018, 173, 8–14. [Google Scholar] [CrossRef]
- Stenudd, M.; Sabelström, H.; Frisén, J. Role of Endogenous Neural Stem Cells in Spinal Cord Injury and Repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef]
- Xue, W.; Fan, C.; Chen, B.; Zhao, Y.; Xiao, Z.; Dai, J. Direct Neuronal Differentiation of Neural Stem Cells for Spinal Cord Injury Repair. Stem Cells 2021, 39, 1025–1032. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Xie, X.; Guo, L.; Yu, D.; Zhuo, J.; Lin, J.; Kol, L.; Gan, L. ISL1 and POU4F1 directly interact to regulate the differentiation and survival of inner ear sensory neurons. J. Neurosci. 2024, 44, e1718232024. [Google Scholar] [CrossRef]
- Rust, R.; Weber, R.Z.; Generali, M.; Kehl, D.; Bodenmann, C.; Uhr, D.; Wanner, D.; Zürcher, K.J.; Saito, H.; Hoerstrup, S.P.; et al. Xeno-free induced pluripotent stem cell-derived neural progenitor cells for in vivo applications. J. Transl. Med. 2022, 20, 421. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagadeesan, S.K.; Galuta, A.; Sandarage, R.V.; Tsai, E.C. Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells. Cells 2025, 14, 64. https://doi.org/10.3390/cells14020064
Jagadeesan SK, Galuta A, Sandarage RV, Tsai EC. Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells. Cells. 2025; 14(2):64. https://doi.org/10.3390/cells14020064
Chicago/Turabian StyleJagadeesan, Sasi Kumar, Ahmad Galuta, Ryan Vimukthi Sandarage, and Eve Chung Tsai. 2025. "Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells" Cells 14, no. 2: 64. https://doi.org/10.3390/cells14020064
APA StyleJagadeesan, S. K., Galuta, A., Sandarage, R. V., & Tsai, E. C. (2025). Transcriptomic and Functional Landscape of Adult Human Spinal Cord NSPCs Compared to iPSC-Derived Neural Progenitor Cells. Cells, 14(2), 64. https://doi.org/10.3390/cells14020064





