Release of Mast Cell Mediators from Cochlear Tissue Following Short Exposure to Compound 48/80 or Cisplatin, and Their Damage to Cochlear Structure
Abstract
Highlights
- The supernatant from degranulated BMMCs induces structural changes to coch-lear tissues.
- Cochlear tissues stimulated with Compound 48/80 or cisplatin release histamine and chymase.
- Mast cell mediators have a potential to remodel cochlear tissues.
- Resident cochlear mast cells can be stimulated during chemotherapy with cispla-tin to contribute to hearing loss by releasing proinflammatory mediators.
Abstract
1. Introduction
2. Materials and Methods
2.1. MC Isolation and Culture
2.2. MC Degranulation Assay
2.3. Cochlear Explant Isolation
2.4. Cochlear Tissue Treatment
2.4.1. Flat Cochlear Tissue Explant Treated with Supernatant from IgE-anti-DNP/DNP Stimulated BMMCS
2.4.2. Stimulation of Floating Cochlear Tissue with Compound 48/80
2.4.3. Exposure of Floating Cochlear Tissue to Cisplatin and Sodium Cromolyn
2.5. Enzyme-Linked Immunosorbent Assay
2.6. HC Quantification
2.7. Statistical Analysis
3. Results
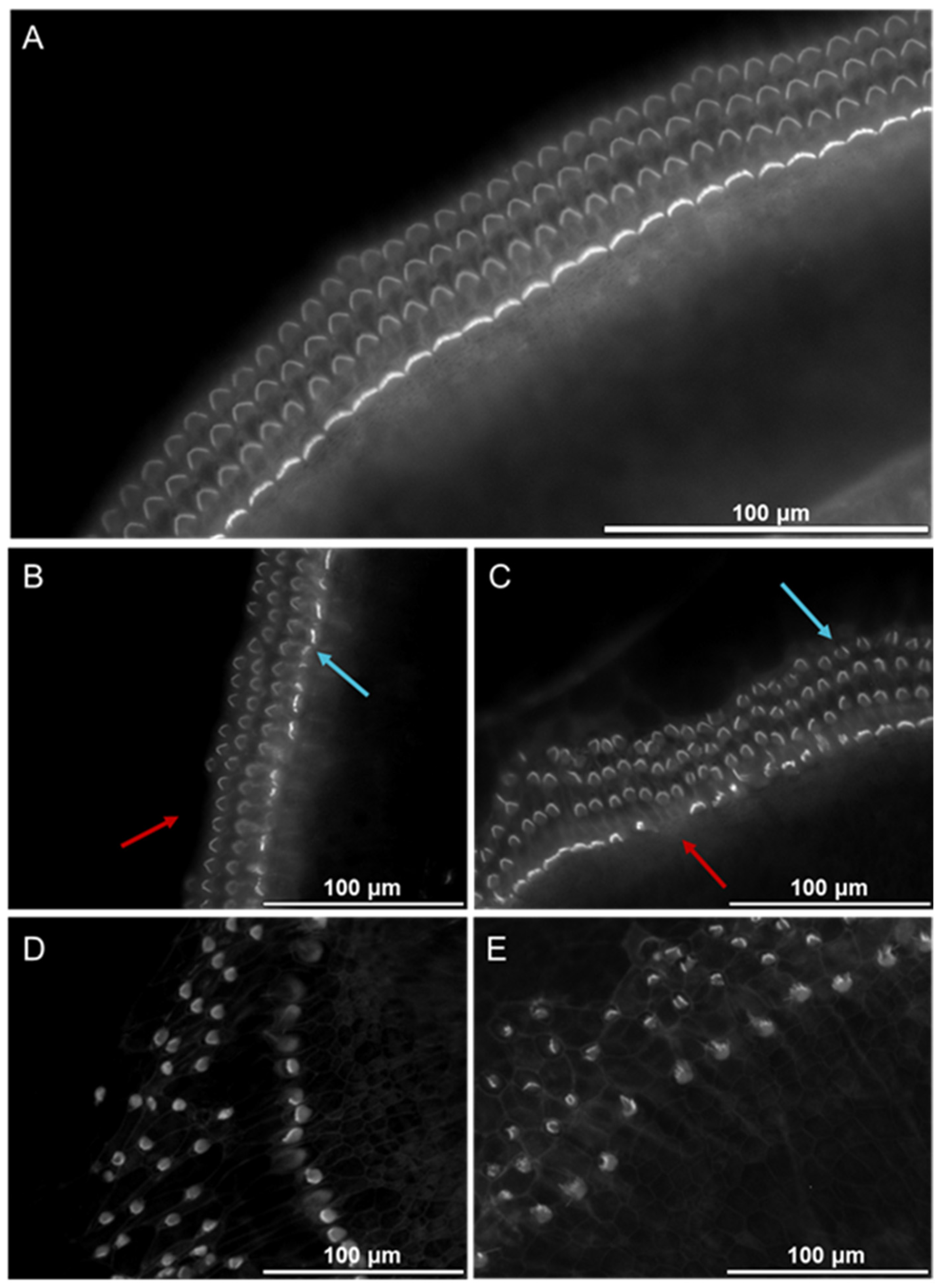
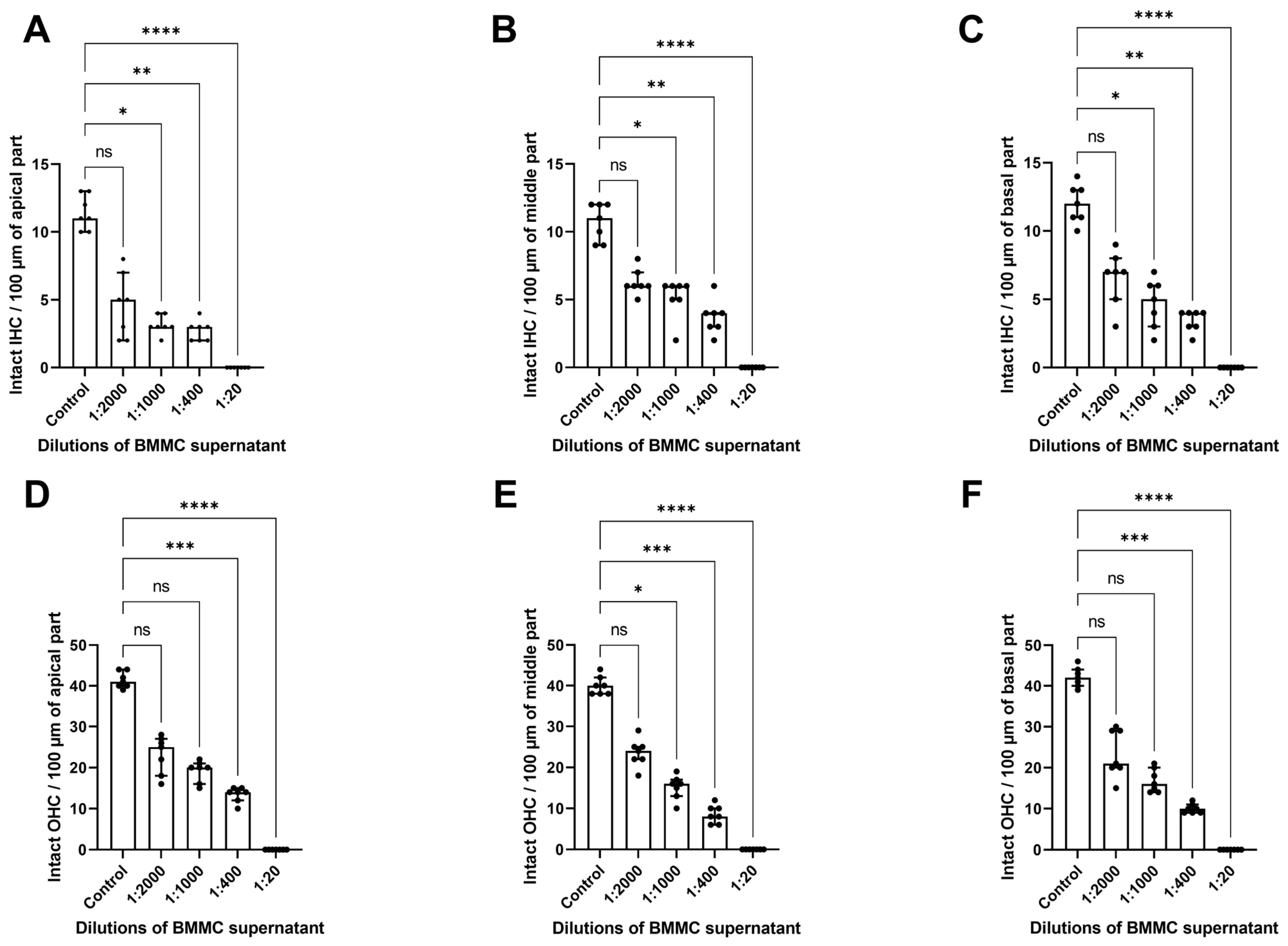
3.1. Cochlear Morphology Alterations Following Exposure to Supernatant from Bone Marrow-Derived MCs Stimulated with Mouse IgE-anti-DNP/DNP
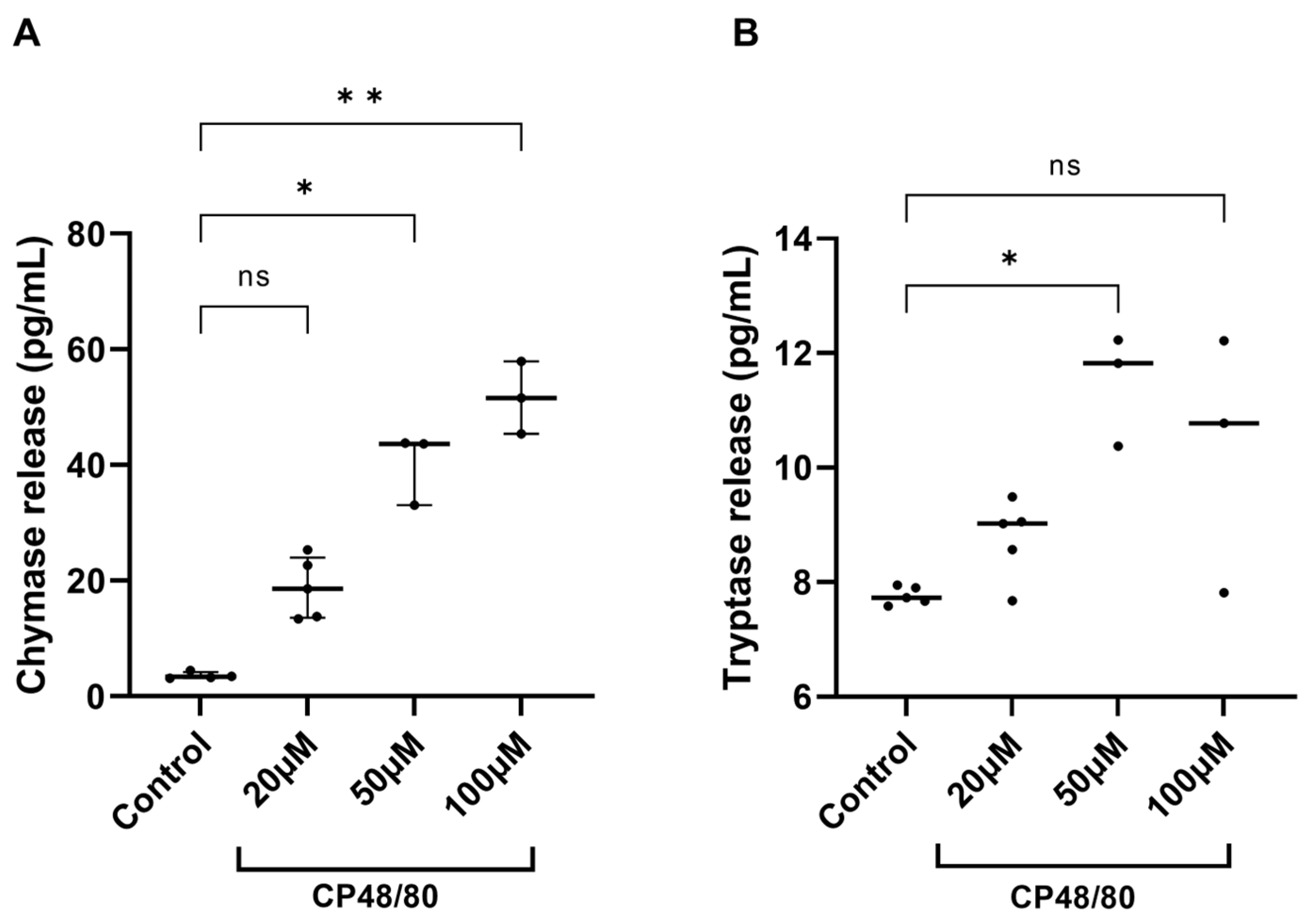
3.2. Compound 48/80 Stimulates the Release of Chymase and Tryptase from Cochlear Explants
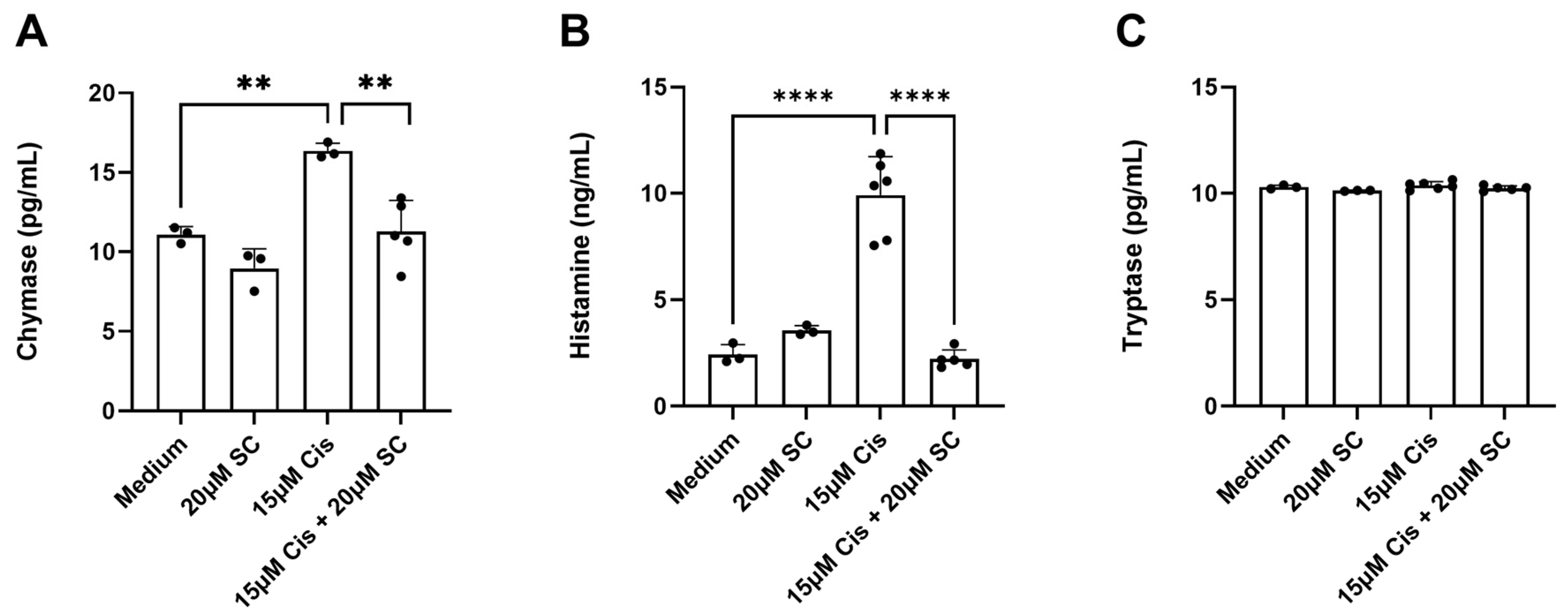
3.3. Chymase and Histamine Release from Cochlear Explants Exposed to 15 µm of Cisplatin
4. Discussion
4.1. Effect of IgE/Antigen-Induced MC Degranulation on Cochlear Morphology
4.2. Effect of Compound 48/80 Stimulation on Cochlear MCs
4.3. The Effect of Cisplatin on Cochlear MCs
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALHL | Acute low-tone hearing loss |
| BMMC | Bone marrow-derived mast cell |
| CIHL | Cisplatin-induced hearing loss |
| CP48/80 | Compound 48/80 |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DNP | Dinitrophenol |
| ELISA | Enzyme-linked immunosorbent assay |
| HC | Hair cell |
| IHC | Inner hair cell |
| ISSHL | Idiopathic sudden sensorineural hearing loss |
| MC | Mast cell |
| MD | Ménière’s disease |
| mMCP-1 | Mouse mast cell protease-1 |
| MRGPRX2, Mrgprb2 | Mas-related G protein-coupled receptor X2 |
| OC | Organ of Corti |
| OHC | Outer hair cell |
| RT | Room temperature |
| SC | Sodium cromolyn |
| SEM | Standard error of the mean |
| SNHL | Sensorineural hearing loss |
References
- Groves, A.K. Developmental, Physiological, and Functional Neurobiology of the Inner Ear; Humana: New York, NY, USA, 2022. [Google Scholar]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Parfitt, R.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J. Toxicol. 2016, 2016, 1809394. [Google Scholar] [CrossRef] [PubMed]
- Callejo, A.; Sedó-Cabezón, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef]
- Karayay, B.; Olze, H.; Szczepek, A.J. Degranulation of Murine Resident Cochlear Mast Cells: A Possible Factor Contributing to Cisplatin-Induced Ototoxicity and Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 4620. [Google Scholar] [CrossRef]
- Yuan, V.G.; Xia, A.; Santa Maria, P.L. Immunological mechanisms in Meniere’s disease. Front. Immunol. 2025, 16, 1639916. [Google Scholar] [CrossRef]
- Szczepek, A.J.; Dudnik, T.; Karayay, B.; Sergeeva, V.; Olze, H.; Smorodchenko, A. Mast Cells in the Auditory Periphery of Rodents. Brain Sci. 2020, 10, 697. [Google Scholar] [CrossRef]
- Derebery, M.J.; Berliner, K.I. Prevalence of allergy in Meniere’s disease. Otolaryngol.-Head Neck Surg. 2000, 123, 69–75. [Google Scholar] [CrossRef]
- Keles, E.; Gödekmerdan, A.; Kalidağ, T.; Kaygusuz, I.; Yalçin, S.; Cengiz Alpay, H.; Aral, M. Meniere’s disease and allergy: Allergens and cytokines. J. Laryngol. Otol. 2004, 118, 688–693. [Google Scholar] [CrossRef]
- Keleş, E.; Sapmaz, E.; Gödekmerdan, A. The role of allergy in the etiopathogenesis of idiopathic sudden sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2013, 270, 1795–1801. [Google Scholar] [CrossRef]
- Derebery, M.J. Allergic management of Meniere’s disease: An outcome study. Otolaryngol.-Head Neck Surg. 2000, 122, 174–182. [Google Scholar] [CrossRef]
- Sen, P.; Georgalas, C.; Papesch, M. Co-morbidity of migraine and Ménière’s disease—Is allergy the link? J. Laryngol. Otol. 2005, 119, 455–460. [Google Scholar] [CrossRef]
- Zeng, B.; Domarecka, E.; Kong, L.; Olze, H.; Scheffel, J.; Moñino-Romero, S.; Siebenhaar, F.; Szczepek, A.J. A systematic review of the clinical evidence for an association between type I hypersensitivity and inner ear disorders. Front. Neurol. 2024, 15, 1378276. [Google Scholar] [CrossRef]
- Frejo, L.; Cara, F.E.; Flook, M.; Robles-Bolivar, P.; Escalera-Balsera, A.; Montilla-Ibañez, M.A.; Dominguez-Duran, E.; Martinez-Martinez, M.; Perez-Carpena, P.; Lopez-Escamez, J.A. Allergy and autoinflammation drive persistent systemic inflammatory response in Meniere Disease: A longitudinal study. Clin. Immunol. 2025, 271, 110413. [Google Scholar] [CrossRef]
- Kothandaraman, K.; Mohindra, S.; Panda, N.K.; Nayak, G.R.; Munjal, S. Unveiling the Allergy-Meniere Connection: Exploring the Impact of Allergen Positivity on Meniere’s Disease Severity. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Schulman, E.S.; MacGlashan, D.W., Jr.; Peters, S.P.; Schleimer, R.P.; Newball, H.H.; Lichtenstein, L.M. Human lung mast cells: Purification and characterization. J. Immunol. 1982, 129, 2662–2667. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B. Function and Regulation of Mast Cells in the Inner Ear and Applications to Mast Cell-Driven Diseases. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2025. [Google Scholar]
- Loewendorf, A.I.; Matynia, A.; Saribekyan, H.; Gross, N.; Csete, M.; Harrington, M. Roads Less Traveled: Sexual Dimorphism and Mast Cell Contributions to Migraine Pathology. Front. Immunol. 2016, 7, 140. [Google Scholar] [CrossRef]
- Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.; Muñoz-Valle, J.F.; Oregon-Romero, E.; Corona-Angeles, J.A.; Hernández-Bello, J. Sex hormones and allergies: Exploring the gender differences in immune responses. Front. Allergy 2024, 5, 1483919. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Bohn, J.W.; Ferry, E.L.; Yamamoto, H.; Molinaro, C.A.; Sherman, L.A.; Klinman, N.R.; Katz, D.H. Monoclonal dinitrophenyl-specific murine IgE antibody: Preparation, isolation, and characterization. J. Immunol. 1980, 124, 2728–2737. [Google Scholar] [CrossRef]
- Eady, R.A.; Cowen, T.; Marshall, T.F.; Plummer, V.; Greaves, M.W. Mast cell population density, blood vessel density and histamine content in normal human skin. Br. J. Dermatol. 1979, 100, 623–633. [Google Scholar] [CrossRef]
- Strobel, S.; Miller, H.R.; Ferguson, A. Human intestinal mucosal mast cells: Evaluation of fixation and staining techniques. J. Clin. Pathol. 1981, 34, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.M.; Butrus, S.I.; Tabbara, K.F.; Schwartz, L.B. Human conjunctival mast cells: Distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J. Allergy Clin. Immunol. 1990, 86, 34–40. [Google Scholar] [CrossRef]
- Sperr, W.R.; Bankl, H.C.; Mundigler, G.; Klappacher, G.; Grossschmidt, K.; Agis, H.; Simon, P.; Laufer, P.; Imhof, M.; Radaszkiewicz, T.; et al. The human cardiac mast cell: Localization, isolation, phenotype, and functional characterization. Blood 1994, 84, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Derebery, M.J.; Berliner, K.I. Allergy and its relation to Meniere’s disease. Otolaryngol. Clin. N. Am. 2010, 43, 1047–1058. [Google Scholar] [CrossRef]
- Uno, K.; Miyamura, K.; Kanzaki, Y.; Fukuda, H.; Masuyama, K.; Ishikawa, T. Type I allergy in the inner ear of the guinea pig. Ann. Otol. Rhinol. Laryngol. 1992, 157, 78–81. [Google Scholar] [CrossRef]
- Uno, K.; Miyamura, K.; Kanzaki, Y.; Fukuda, H.; Masuyama, K.; Ishikawa, T. Audiological study in guinea pigs with type I allergy induced in the inner ear. Ann. Otol. Rhinol. Laryngol. 1993, 102, 537–542. [Google Scholar] [CrossRef]
- Miyamura, K.; Kanzaki, Y.; Nagata, M.; Ishikawa, T. Provocation of nystagmus and deviation by type I allergy in the inner ear of the guinea pig. Ann. Allergy 1987, 58, 36–40. [Google Scholar] [PubMed]
- Takeda, T.; Takeda, S.; Egami, N.; Kakigi, A.; Nishioka, R.; Yamasoba, T. Type 1 allergy-induced endolymphatic hydrops and the suppressive effect of leukotriene receptor antagonist. Otol. Neurotol. 2012, 33, 886–890. [Google Scholar] [CrossRef]
- Egami, N.; Kakigi, A.; Takeda, T.; Takeda, S.; Nishioka, R.; Hyodo, M.; Yamasoba, T. Type 1 allergy-induced endolymphatic hydrops and the suppressive effect of H1-receptor antagonist (olopatadine hydrochloride). Otol. Neurotol. 2014, 35, e104–e109. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells—Key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef]
- Ferry, X.; Brehin, S.; Kamel, R.; Landry, Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 2002, 23, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef]
- Baldo, B.A. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcεRI-mediated events. Br. J. Clin. Pharmacol. 2023, 89, 3232–3246. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J. Leukoc. Biol. 2019, 106, 1069–1077. [Google Scholar] [CrossRef]
- Laurell, G.; Andersson, A.; Engström, B.; Ehrsson, H. Distribution of cisplatin in perilymph and cerebrospinal fluid after intravenous administration in the guinea pig. Cancer Chemother. Pharmacol. 1995, 36, 83–86. [Google Scholar] [CrossRef]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef]
- Maurer, M.; Wedemeyer, J.; Metz, M.; Piliponsky, A.M.; Weller, K.; Chatterjea, D.; Clouthier, D.E.; Yanagisawa, M.M.; Tsai, M.; Galli, S.J. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 2004, 432, 512–516. [Google Scholar] [CrossRef]
- Metz, M.; Piliponsky, A.M.; Chen, C.C.; Lammel, V.; Abrink, M.; Pejler, G.; Tsai, M.; Galli, S.J. Mast cells can enhance resistance to snake and honeybee venoms. Science 2006, 313, 526–530. [Google Scholar] [CrossRef]
- Knight, P.A.; Wright, S.H.; Lawrence, C.E.; Paterson, Y.Y.; Miller, H.R. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000, 192, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.R.; Bartram, R.E.; Knight, P.A.; Miller, H.R.; Garrod, D.R.; Grencis, R.K. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 2003, 100, 7761–7766. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yoshimoto, T.; Maruyama, H.; Tegoshi, T.; Ohta, N.; Arizono, N.; Nakanishi, K. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J. Exp. Med. 2005, 202, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.B.; Fernandes, J.P.S.; Uliassi, E. Tackling Neuroinflammation in Cognitive Disorders with Single-targeted and Multi-targeted Histamine H3 Receptor Modulators. Curr. Top. Med. Chem. 2024, 24, 2421–2430. [Google Scholar] [CrossRef]
- Heiger-Bernays, W.J.; Essigmann, J.M.; Lippard, S.J. Effect of the antitumor drug cis-diamminedichloroplatinum(II) and related platinum complexes on eukaryotic DNA replication. Biochemistry 1990, 29, 8461–8466. [Google Scholar] [CrossRef]
- Jordan, P.; Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell. Mol. Life Sci. CMLS 2000, 57, 1229–1235. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Oronsky, B.; Caroen, S.; Oronsky, A.; Dobalian, V.E.; Oronsky, N.; Lybeck, M.; Reid, T.R.; Carter, C.A. Electrolyte disorders with platinum-based chemotherapy: Mechanisms, manifestations and management. Cancer Chemother. Pharmacol. 2017, 80, 895–907. [Google Scholar] [CrossRef]
- Summers, S.A.; Chan, J.; Gan, P.Y.; Dewage, L.; Nozaki, Y.; Steinmetz, O.M.; Nikolic-Paterson, D.J.; Kitching, A.R.; Holdsworth, S.R. Mast cells mediate acute kidney injury through the production of TNF. J. Am. Soc. Nephrol. 2011, 22, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Wang, D.; Wang, Y.; Zhou, Z.; Ma, X.; Liu, X.; Dong, Y. Cisplatin-induced ototoxicity: From signaling network to therapeutic targets. Biomed. Pharmacother. 2023, 157, 114045. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Frischbutter, S.; Moñino-Romero, S.; Scheffel, J.; Siebenhaar, F.; Olze, H.; Szczepek, A.J. Release of Mast Cell Mediators from Cochlear Tissue Following Short Exposure to Compound 48/80 or Cisplatin, and Their Damage to Cochlear Structure. Cells 2025, 14, 1615. https://doi.org/10.3390/cells14201615
Zeng B, Frischbutter S, Moñino-Romero S, Scheffel J, Siebenhaar F, Olze H, Szczepek AJ. Release of Mast Cell Mediators from Cochlear Tissue Following Short Exposure to Compound 48/80 or Cisplatin, and Their Damage to Cochlear Structure. Cells. 2025; 14(20):1615. https://doi.org/10.3390/cells14201615
Chicago/Turabian StyleZeng, Bin, Stefan Frischbutter, Sherezade Moñino-Romero, Jörg Scheffel, Frank Siebenhaar, Heidi Olze, and Agnieszka J. Szczepek. 2025. "Release of Mast Cell Mediators from Cochlear Tissue Following Short Exposure to Compound 48/80 or Cisplatin, and Their Damage to Cochlear Structure" Cells 14, no. 20: 1615. https://doi.org/10.3390/cells14201615
APA StyleZeng, B., Frischbutter, S., Moñino-Romero, S., Scheffel, J., Siebenhaar, F., Olze, H., & Szczepek, A. J. (2025). Release of Mast Cell Mediators from Cochlear Tissue Following Short Exposure to Compound 48/80 or Cisplatin, and Their Damage to Cochlear Structure. Cells, 14(20), 1615. https://doi.org/10.3390/cells14201615





