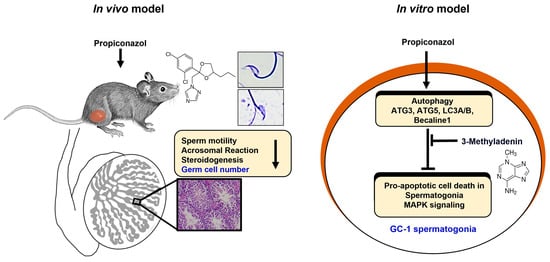
Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells
Abstract
Highlights
- PRO causes testicular toxicity and sperm dysfunction by triggering autophagy-mediated apoptosis in germ cells.
- This is the first report demonstrating PRO’s effects on sperm motility and autophagy in mouse spermatogonia.
- Exposure to propiconazole poses a potential risk to male reproductive health, mediated in part by autophagy-dependent toxicity.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Sperm Collection and Motility Assessment
2.3. Cell Culture and Treatment
2.4. Histological Analysis and Immunostaining
2.5. Western Blotting
2.6. Quantitative PCR Analysis
2.7. Flow Cytometry
2.8. Measurement of Testosterone
2.9. Statistical Analysis
3. Results
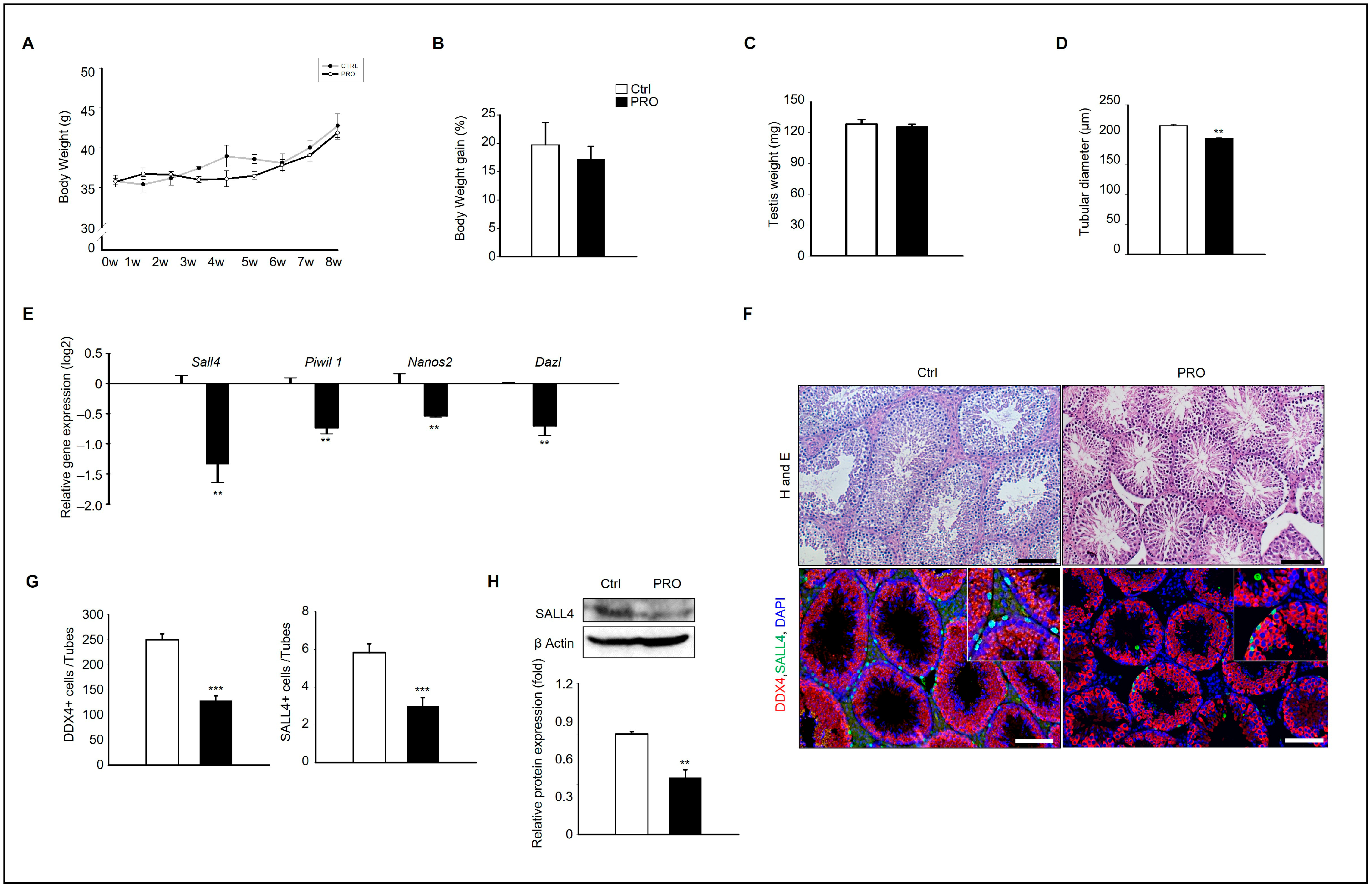
3.1. Effects of PRO on Mice Testis and Germ Cell
3.2. Effects of PRO Steroidogenesis and Sperm Motility in Mice Testis
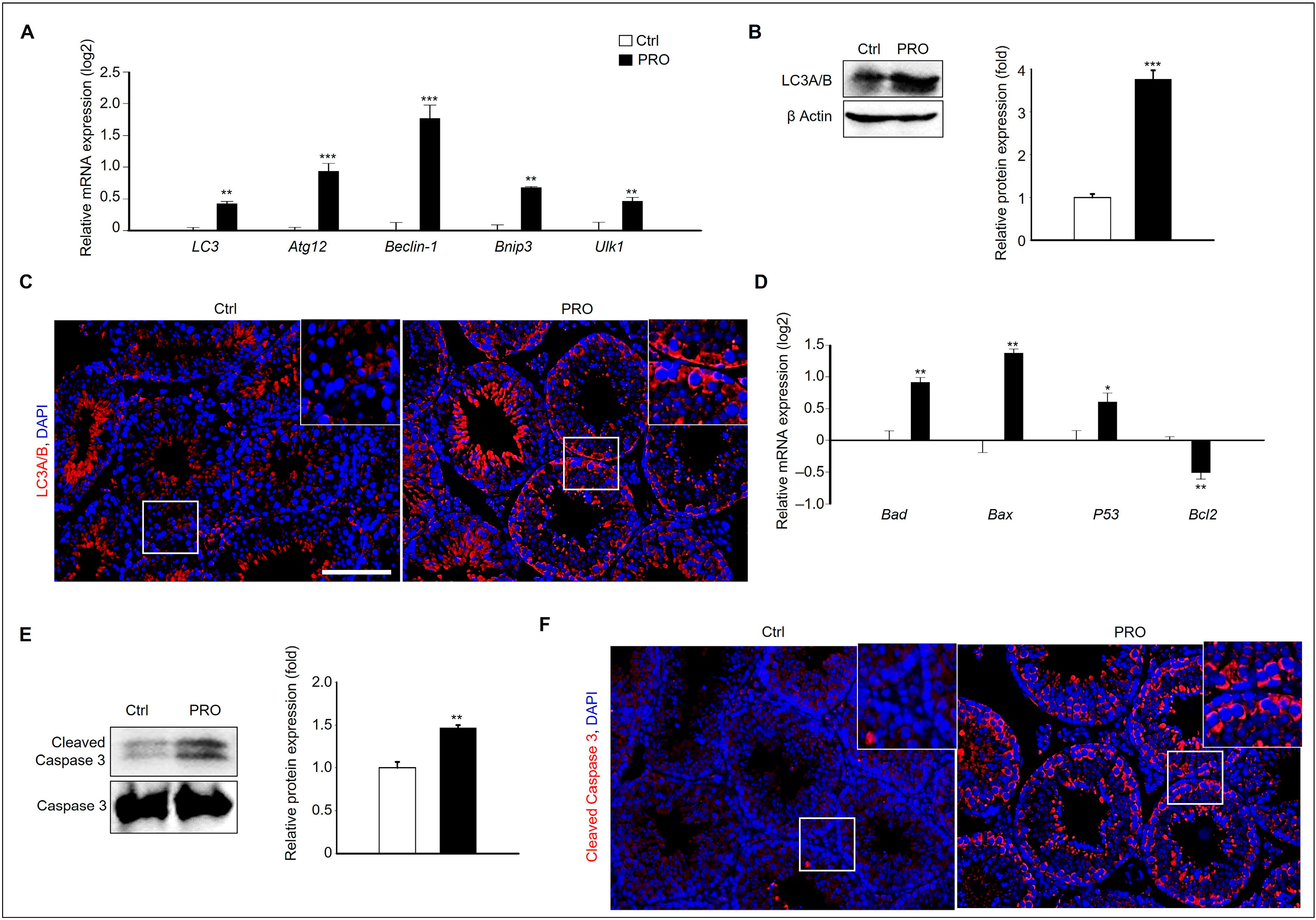
3.3. PRO Induces Germ Cell Damage via Autophagy and Pro-Apoptosis in the Testis
3.4. PRO Induces GC-1 Spermatogonia Cellular Damage via a Pro-Apoptotic Pathway
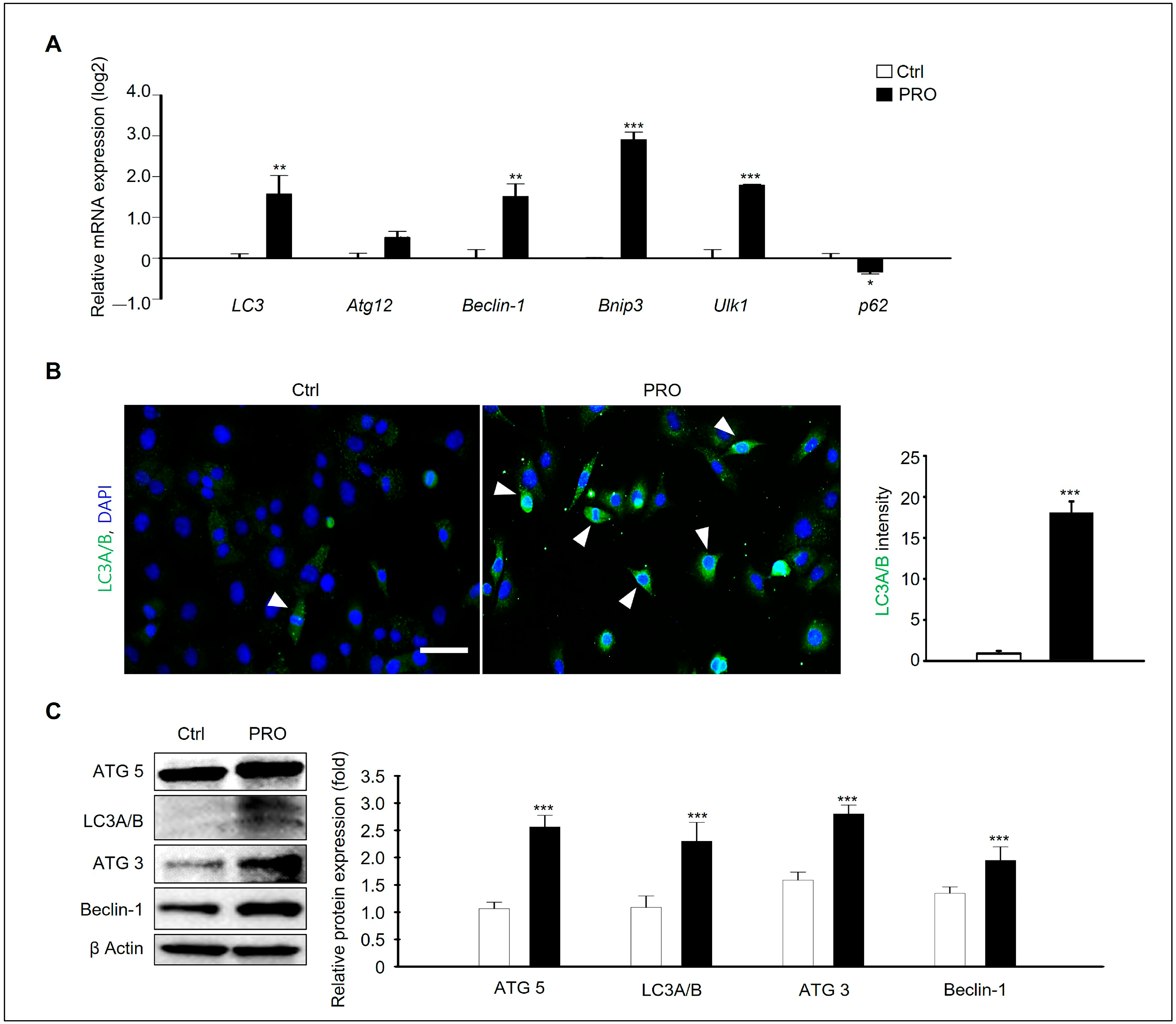
3.5. PRO Induces Autophagic Cell Death in GC-1 Spg Cells
3.6. 3-Methyladenine (3-MA) Protects Against PRO-Induced Autophagy in GC-1 Spg Cells
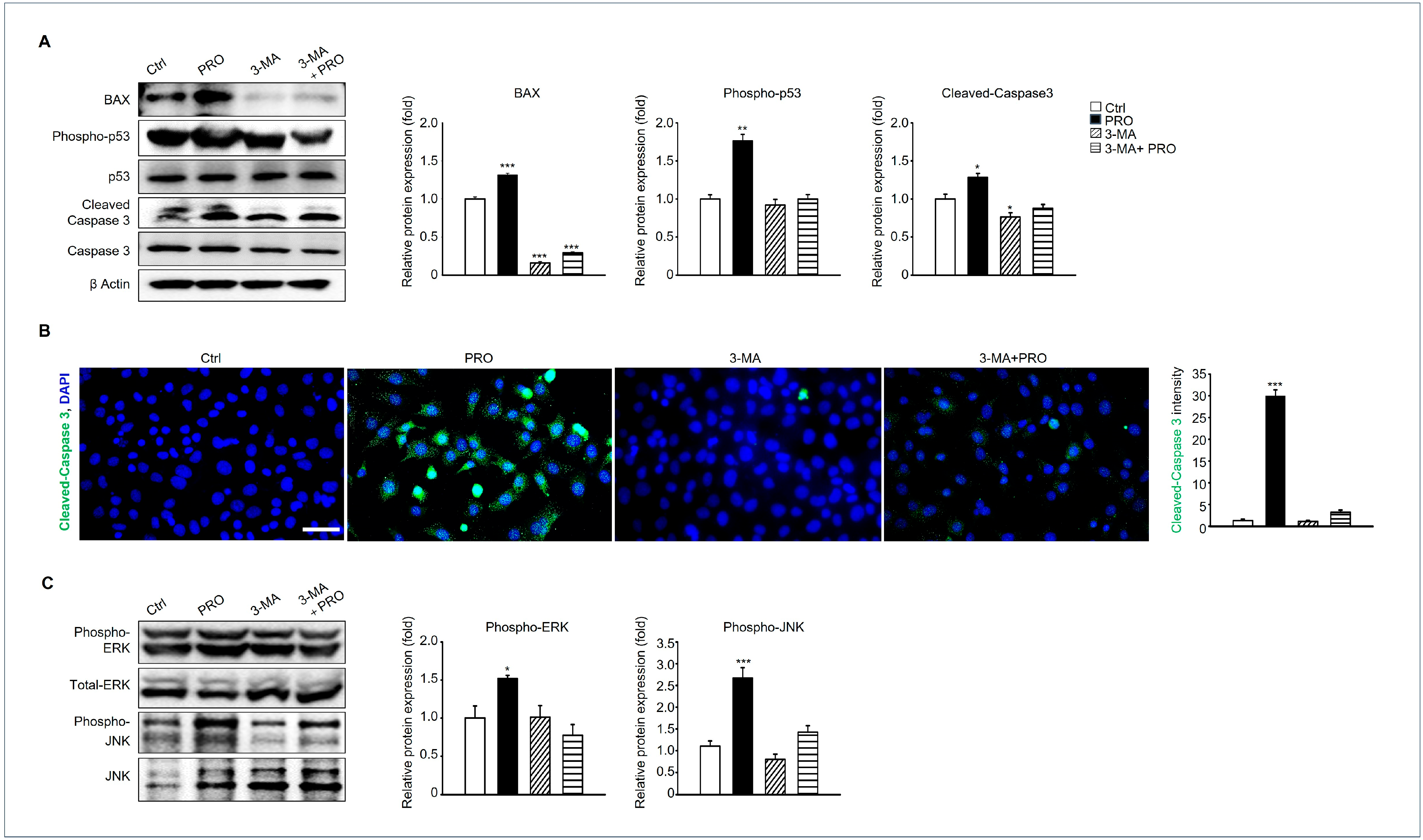
3.7. Inhibition of Autophagy Can Suppress the Pro-Apoptotic Pathway of GC-1 Spg by PRO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, N.T.; Stone, W.W. Estimated Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 2008–2012; Data Series; U.S. Geological Survey: Reston, VA, USA, 2015; p. 18. Available online: https://pubs.er.usgs.gov/publication/ds907 (accessed on 14 January 2015).
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation-Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Chen, P.J.; Moore, T.; Nesnow, S. Cytotoxic effects of propiconazole and its metabolites in mouse and human hepatoma cells and primary mouse hepatocytes. Toxicol. Vitr. Int. J. Publ. Assoc. 2008, 22, 1476–1483. [Google Scholar] [CrossRef]
- Sun, G.; Thai, S.F.; Tully, D.B.; Lambert, G.R.; Goetz, A.K.; Wolf, D.C.; Dix, D.J.; Nesnow, S. Propiconazole-induced cytochrome P450 gene expression and enzymatic activities in rat and mouse liver. Toxicol. Lett. 2005, 155, 277–287. [Google Scholar] [CrossRef]
- Kwon, H.C.; Sohn, H.; Kim, D.H.; Shin, D.M.; Jeong, C.H.; Chang, Y.H.; Yune, J.H.; Kim, Y.J.; Kim, D.W.; Kim, S.H.; et al. In Vitro and In Vivo Study on the Toxic Effects of Propiconazole Fungicide in the Pathogenesis of Liver Fibrosis. J. Agric. Food Chem. 2021, 69, 7399–7408. [Google Scholar] [CrossRef] [PubMed]
- Svanholm, S.; Säfholm, M.; Brande-Lavridsen, N.; Larsson, E.; Berg, C. Developmental reproductive toxicity and endocrine activity of propiconazole in the Xenopus tropicalis model. Sci. Total Environ. 2021, 753, 141940. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Svanholm, S.; Eriksson, A.; Chidiac, J.; Eriksson, J.; Jernerén, F.; Berg, C. Pesticide-induced multigenerational effects on amphibian reproduction and metabolism. Sci. Total Environ. 2021, 775, 145771. [Google Scholar] [CrossRef]
- Peng, F.J.; Hardy, E.M.; Mezzache, S.; Bourokba, N.; Palazzi, P.; Stojiljkovic, N.; Bastien, P.; Li, J.; Soeur, J.; Appenzeller, B.M.R. Exposure to multiclass pesticides among female adult population in two Chinese cities revealed by hair analysis. Environ. Int. 2020, 138, 105633. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.K.; Ren, H.; Schmid, J.E.; Blystone, C.R.; Thillainadarajah, I.; Best, D.S.; Nichols, H.P.; Strader, L.F.; Wolf, D.C.; Narotsky, M.G.; et al. Disruption of testosterone homeostasis as a mode of action for the reproductive toxicity of triazole fungicides in the male rat. Toxicol. Sci. Off. J. Soc. Toxicol. 2007, 95, 227–239. [Google Scholar] [CrossRef]
- Aït-Aïssa, S.; Laskowski, S.; Laville, N.; Porcher, J.M.; Brion, F. Anti-androgenic activities of environmental pesticides in the MDA-kb2 reporter cell line. Toxicol. Vitr. Int. J. Publ. Assoc. 2010, 24, 1979–1985. [Google Scholar] [CrossRef]
- Kjeldsen, L.S.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol. Appl. Pharmacol. 2013, 272, 453–464. [Google Scholar] [CrossRef]
- Kjaerstad, M.B.; Taxvig, C.; Andersen, H.R.; Nellemann, C. A Mixture effects of endocrine disrupting compounds in vitro. Int. J. Androl. 2010, 33, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kjærstad, M.B.; Taxvig, C.; Nellemann, C.; Vinggaard, A.M.; Andersen, H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010, 30, 573–582. [Google Scholar] [CrossRef]
- Vieira, M.L.; Costa, N.O.; Pereira, M.R.F.; de Fátima Paccola Mesquita, S.; Moreira, E.G.; Gerardin, D.C.C. Chronic exposure to the fungicide propiconazole: Behavioral and reproductive evaluation of F1 and F2 generations of male rats. Toxicology 2017, 389, 85–93. [Google Scholar] [CrossRef]
- Rockett, J.C.; Narotsky, M.G.; Thompson, K.E.; Thillainadarajah, I.; Blystone, C.R.; Goetz, A.K.; Ren, H.; Best, D.S.; Murrell, R.N.; Nichols, H.P.; et al. Effect of conazole fungicides on reproductive development in the female rat. Reprod. Toxicol. 2006, 22, 647–658. [Google Scholar] [CrossRef]
- Costa, N.O.; Vieira, M.L.; Sgarioni, V.; Pereira, M.R.; Montagnini, B.G.; Mesquita, S.d.F.; Gerardin, D.C. Evaluation of the reproductive toxicity of fungicide propiconazole in male rats. Toxicology 2015, 335, 55–61. [Google Scholar] [CrossRef]
- Chen, P.J.; Padgett, W.T.; Moore, T.; Winnik, W.; Lambert, G.R.; Thai, S.F.; Hester, S.D.; Nesnow, S. Three conazoles increase hepatic microsomal retinoic acid metabolism and decrease mouse hepatic retinoic acid levels in vivo. Toxicol. Appl. Pharmacol. 2009, 234, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Park, H.J. T-2 mycotoxin Induces male germ cell apoptosis by ROS-mediated JNK/p38 MAPK pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115323. [Google Scholar] [CrossRef]
- Lee, R.; Lee, W.Y.; Kim, D.W.; Park, H.J. Diazinon induces testicular dysfunction and testicular cell damage through increased reactive oxygen species production in mouse. Cell. Death. Discov. 2025, 11, 113. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, R.; Yoo, H.; Hong, K.; Song, H. Nonylphenol Induces Apoptosis through ROS/JNK Signaling in a Spermatogonia Cell Line. Int. J. Mol. Sci. 2020, 22, 307. [Google Scholar] [CrossRef]
- Serra, L.; Bourdon, G.; Estienne, A.; Fréville, M.; Ramé, C.; Chevaleyre, C.; Didier, P.; Chahnamian, M.; Ganier, P.; Pinault, F.; et al. Triazole pesticides exposure impaired steroidogenesis associated to an increase in AHR and CAR expression in testis and altered sperm parameters in chicken. Toxicol. Rep. 2023, 10, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Christin-Maitre, S.; Young, J. Androgens and spermatogenesis. Ann. D’endocrinologie 2022, 83, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Hakoi, K.; Cabral, R.; Hoshiya, T.; Hasegawa, R.; Shirai, T.; Ito, N. Analysis of carcinogenic activity of some pesticides in a medium-term liver bioassay in the rat. Teratog. Carcinog. Mutagenesis. 1992, 12, 269–276. [Google Scholar] [CrossRef]
- Murphy, L.A.; Moore, T.; Nesnow, S. Propiconazole-enhanced hepatic cell proliferation is associated with dysregulation of the cholesterol biosynthesis pathway leading to activation of Erk1/2 through Ras farnesylation. Toxicol. Appl. Pharmacol. 2012, 260, 146–154. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lee, R.; Park, H.J. Tebuconazole Induces Mouse Fetal Testes Damage via ROS Generation in an Organ Culture Method. Int. J. Mol. Sci. 2024, 25, 7050. [Google Scholar] [CrossRef]
- Bae, J.W.; Kwon, W.S. Proteomic analysis of fipronil-induced molecular defects in spermatozoa. Sci. Rep. 2024, 14, 7668. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhang, D.; Zhang, Y.; Li, Y.; Fang, F.; Zhang, Z.; Zhang, Y. Prothioconazole exposure disrupts oocyte maturation and fertilization by inducing mitochondrial dysfunction and apoptosis in mice. Free Radic. Biol. Med. 2024, 213, 274–284. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Zhang, J.; Duan, M.; Xu, H.; Zhao, W.; Yang, Y.; Wang, C.; Xu, Y. Exposure to difenoconazole induces reproductive toxicity in zebrafish by interfering with gamete maturation and reproductive behavior. Sci. Total Environ. 2022, 838 Pt 1, 155610. [Google Scholar] [CrossRef] [PubMed]
- Svanholm, S.; Brouard, V.; Roza, M.; Marini, D.; Karlsson, O.; Berg, C. Impaired spermatogenesis and associated endocrine effects of azole fungicides in peripubertal Xenopus tropicalis. Ecotoxicol. Environ. Saf. 2024, 270, 115876. [Google Scholar] [CrossRef]
- Ruiz-Yance, I.; Siguas, J.; Bardales, B.; Robles-Castañeda, I.; Cordova, K.; Ypushima, A.; Estela-Villar, E.; Quintana-Criollo, C.; Estacio, D.; Rodríguez, J.L. Potential Involvement of Oxidative Stress, Apoptosis and Proinflammation in Ipconazole-Induced Cytotoxicity in Human Endothelial-like Cells. Toxics 2023, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Nesnow, S.; Grindstaff, R.D.; Lambert, G.; Padgett, W.T.; Bruno, M.; Ge, Y.; Chen, P.J.; Wood, C.E.; Murphy, L. Propiconazole increases reactive oxygen species levels in mouse hepatic cells in culture and in mouse liver by a cytochrome P450 enzyme mediated process. Chem.-Biol. Interact. 2011, 194, 79–89. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, M.; Jiang, P.; Wang, J.; Zhu, R.; Liu, C. Myclobutanil induces neurotoxicity by activating autophagy and apoptosis in zebrafish larvae (Danio rerio). Chemosphere 2024, 357, 142027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Liu, F.; Fang, W.; Dong, J.; Zhao, P. Difenoconazole causes cardiotoxicity in common carp (Cyprinus carpio): Involvement of oxidative stress, inflammation, apoptosis and autophagy. Chemosphere 2022, 306, 135562. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.; Zhang, T.; Zou, L.; Chen, Y.; Wang, K.; Lei, Y.; Yuan, K.; Li, Y.; Lan, J.; et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: Involvement of abnormal cholesterol trafficking. Autophagy 2014, 10, 1241–1255. [Google Scholar] [CrossRef]
- Raee, P.; Tan, S.C.; Najafi, S.; Zandsalimi, F.; Low, T.Y.; Aghamiri, S.; Fazeli, E.; Aghapour, M.; Mofarahe, Z.S.; Heidari, M.H.; et al. Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: A therapeutic point of view. Reprod. Biol. Endocrinol. 2023, 21, 88. [Google Scholar] [CrossRef]
- Malmir, M.; Faraji, T.; Ghafarizadeh, A.A.; Khodabandelo, H. Effect of nonylphenol on spermatogenesis: A systematic review. Andrologia 2020, 52, e13748. [Google Scholar] [CrossRef]
- Griswold, M.D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol. Reprod. 2018, 99, 87–100. [Google Scholar] [CrossRef]
- Yu, J.E.; Kim, Y.; Hong, D.E.; Lee, D.W.; Chang, J.Y.; Yoo, S.S.; Kim, M.J.; Son, D.J.; Yun, J.; Han, S.B.; et al. Bee Venom Triggers Autophagy-Induced Apoptosis in Human Lung Cancer Cells via the mTOR Signaling Pathway. J. Oncol. 2022, 2022, 8916464. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Pereira, S.C.; Seco-Rovira, V.; Oliveira, P.F.; Alves, M.G.; Pereira, M.L. Pesticides and Male Fertility: A Dangerous Crosstalk. Metabolites 2021, 11, 799. [Google Scholar] [CrossRef]
- Béranger, R.; Hardy, E.M.; Dexet, C.; Guldner, L.; Zaros, C.; Nougadère, A.; Metten, M.A.; Chevrier, C.; Appenzeller, B.M.R. Multiple pesticide analysis in hair samples of pregnant French women: Results from the ELFE national birth cohort. Environ. Int. 2018, 120, 43–53. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Company | Catalog Number | Dilution, IHC (WB) |
|---|---|---|---|
| DDX4 | Abcam | ab13840 | 1:300 |
| SALL4 | Santa Cruz Biotech | SC-101147 | 1:200 (1:1000) |
| 3β-HSD | Santa Cruz Biotech | SC-30820 | 1:200 (1:1000) |
| β Actin | Santa Cruz Biotech | SC-47778 | 1:200 (1:1000) |
| Ki67 | Abcam | ab15580 | 1:300 |
| Cleaved Caspase 3 | Cell signaling | #12994 | 1:300 (1:1000) |
| Caspase 3 | Cell signaling | #9662 | (1:1000) |
| LC3A/B | Cell signaling | #12741 | 1:300 (1:1000) |
| ATG5 | Cell signaling | #12994 | (1:1000) |
| ATG3 | Cell signaling | #3415 | (1:1000) |
| Beclin-1 | Cell signaling | #3738 | (1:1000) |
| phospho-Erk1/2 | Cell signaling | #4370 | (1:1000) |
| Total-ERK1/2 | Cell signaling | #9102 | (1:1000) |
| phospho-JNK | Cell signaling | #4668 | (1:1000) |
| JNK | Cell signaling | #9252 | (1:1000) |
| BAX | Cell signaling | #2772 | (1:1000) |
| phospho-p53 | Cell signaling | #3556 | (1:1000) |
| p53 | Cell signaling | #2524 | (1:1000) |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Gapdh | 5′-GTCGGTGTGAACGGATTTG-3′ | 5′-CTTGCCGTGGGTAGAGTCAT-3′ |
| Sall4 | 5′-TCTCAGCAAGTGTCCGTGTC-3′ | 5′-GCATGAGGTAGCTTGGCTTG-3 |
| Piwil 1 | 5′-TGGTGATTGGAATGGATGTG-3 | 5′-TGGTGATTGGAATGGATGTG-3 |
| Nanos2 | 5′-ATAATTCAGAGCCGGAAGCA-3′ | 5′-TCTTCAGCTGGTGTGAGGTG-3′ |
| DAZL | 5′-GTCGAAGGGCTATGGATTTG-3′ | 5′-ACGTGGCTGCACATGATAAG-3′ |
| StAR | 5′-TGGGCATACTCAACAACCAG-3 | 5′-GTCTACCACCACCTCCAAGC-3 |
| Cyp11a1 | 5′-GACAATGGTTGGCTAAACCTG-3 | 5′-GGGTCCACGATGTAAACTGAC-3 |
| Cyp17a1 | 5′-TCCAGCATTGGAGAGTTTGC-3 | 5′-ATGAGATGGCTTCCTGTTGG-3 |
| 3β-HSD1 | 5′-AATCTGAAAGGTACCCAGAA-3′ | 5′-TCATCATAGCTTTGGTGAGG-3 |
| Insl3 | 5′-TGCAGTGGCTAGAGCAGAGAC-3′ | 5′-GAGAAGCCTGGAGAGGAAGC-3 |
| Ulk1 | 5′-ACACACCTTCTCCCCAAGTG-3 | 5′-GACGCACAACATGGAAGTCG-3 |
| Bnip3 | 5′-GCTCCTGGGTAGAACTGCAC-3′ | 5′-GCTGGGCATCCAACAGTATT-3′ |
| Beclin-1 | 5′-GCGGGAGTATAGTGAGTT-3ʹ | 5′-GGTGGCATTGAAGACATT-3 |
| Atg12 | 5′-TAAACTGGTGGCCTCGGAAC-3 | 5′-ATCCCCATGCCTGGGATTTG-3′ |
| Atg5 | 5′-ACTTGCTTTACTCTCTATCAG-3 | 5′-CATCTTCTTGTCTCATAACCT-3 |
| LC3 | 5′-CTTCGCCGACCGCTGTAA-3′ | 5′-GCCGGATGATCTTGACCAACT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-Y.; Lee, R.; Sim, H.W.; Park, H.-J. Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells. Cells 2025, 14, 1624. https://doi.org/10.3390/cells14201624
Lee W-Y, Lee R, Sim HW, Park H-J. Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells. Cells. 2025; 14(20):1624. https://doi.org/10.3390/cells14201624
Chicago/Turabian StyleLee, Won-Young, Ran Lee, Hyeon Woo Sim, and Hyun-Jung Park. 2025. "Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells" Cells 14, no. 20: 1624. https://doi.org/10.3390/cells14201624
APA StyleLee, W.-Y., Lee, R., Sim, H. W., & Park, H.-J. (2025). Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells. Cells, 14(20), 1624. https://doi.org/10.3390/cells14201624