Alterations in Blood and Hippocampal mRNA and miRNA Expression, Along with Fat Deposition in Female B6C3F1 Mice Continuously Exposed to Prenatal Low-Dose-Rate Radiation and Their Comparison with Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Irradiation Procedure
2.2. Pathological Examination
2.3. Immunohistochemical Staining of the Hippocampus
2.4. RNA Extraction from Female B6C3F1 Mouse Hippocampus and Whole Blood
2.5. Systematic mRNA Sequencing Analysis and miRNA Sequencing (miRSeq)
2.6. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) Analysis of mRNA
2.7. Real-Time qRT-PCR Analysis for miRNA
2.8. Statistical Analyses
3. Results
3.1. Continuous Prenatal Low-Dose-Rate Irradiation Reduced Organ Weight and Increased Adipose Tissue Deposits in Female B6C3F1 Mice
3.2. Immunohistochemistry Examination
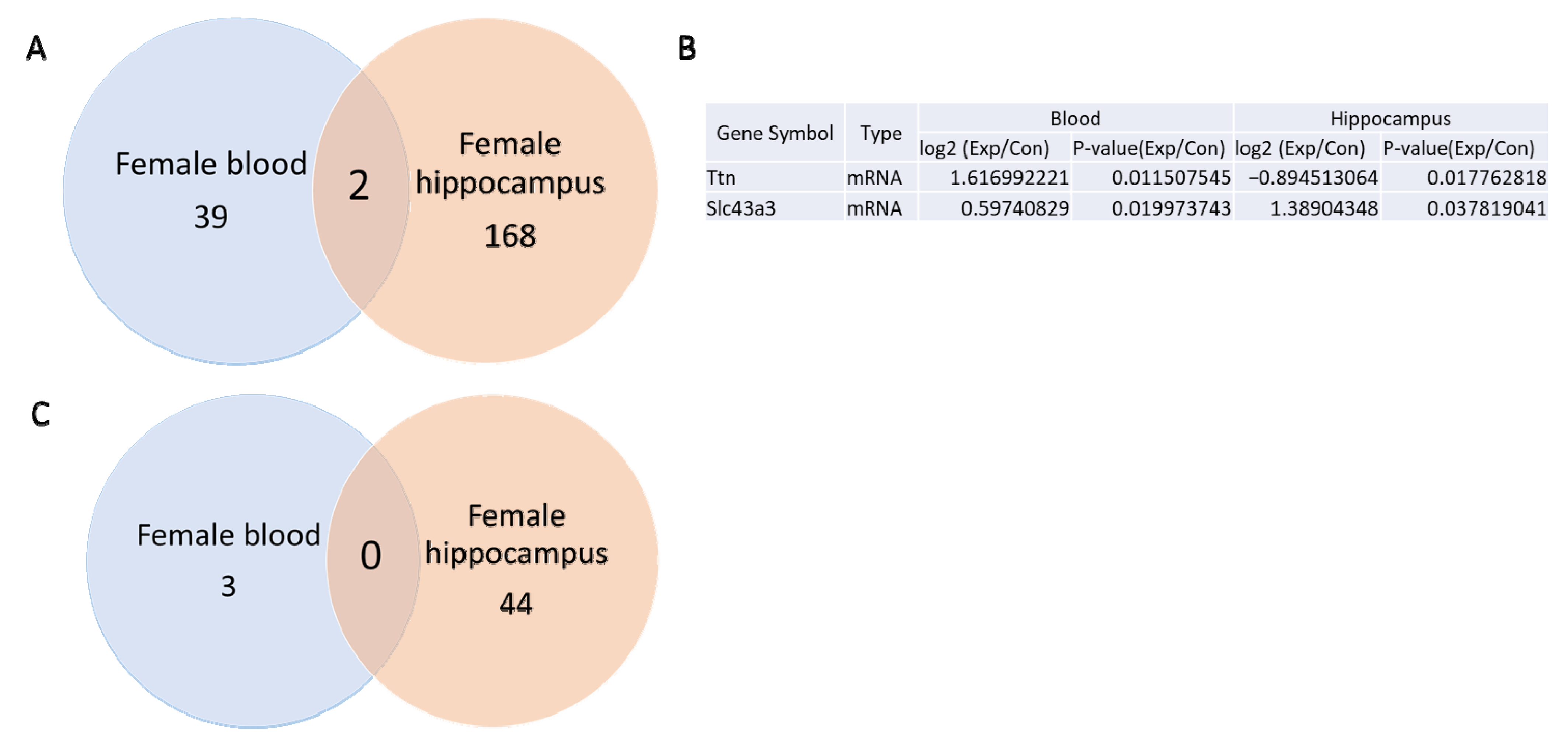
3.3. mRNA and miRNA Sequencing in Female Blood and Hippocampus
3.4. qRT-PCR Validation of mRNA and miRNA Expression in Female Hippocampus and Blood
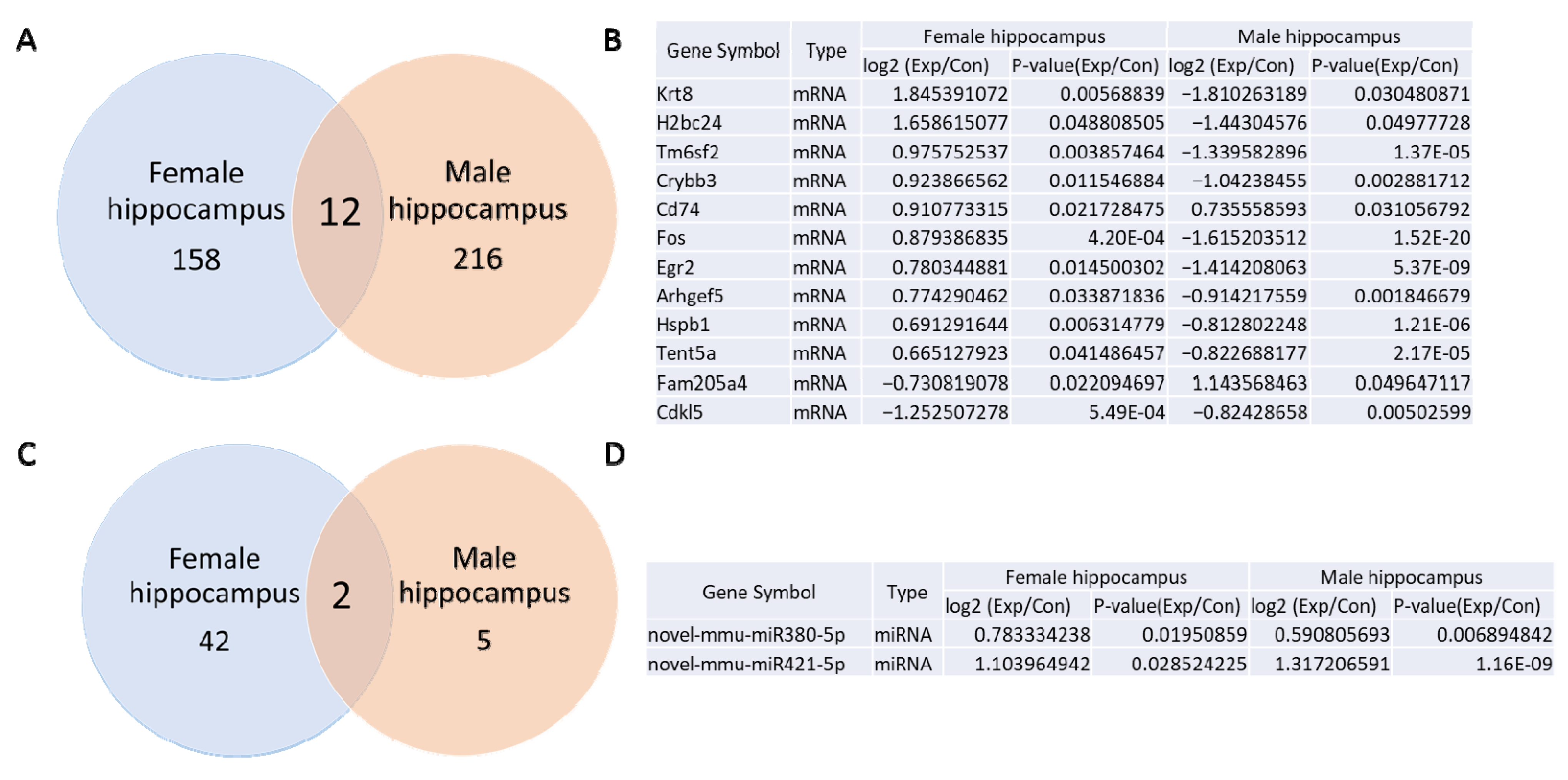
3.5. Comparison of mRNA and miRNA Sequencing of Whole Blood from Prenatally Irradiated Male and Female B6C3F1 Mice
3.6. Comparison Between mRNA and miRNA Sequencing of the Hippocampus from Prenatally Irradiated Male and Female B6C3F1 Mice
4. Discussion
4.1. Prenatal Continuous Low-Dose-Rate Irradiation Induced mRNA and miRNA Changes in Whole Blood and the Hippocampus, with No Cellular Changes in the Dentate Gyrus of Female Offspring
4.2. Sex Differences in Weight Changes, Adipose Tissue Deposit, and Expression of mRNA and miRNA After Continuous Prenatal Low-Dose-Rate Irradiation
4.2.1. Weight Changes and Lipid Metabolism
4.2.2. mRNA and miRNA Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALK7 | Activin receptor-like kinase 7 |
| Arhgef5 | Rho guanine nucleotide exchange factor 5 |
| Ccl4 | Chemokine (C-C motif) ligands 4 |
| Cdkl5 | Cyclin dependent kinase like 5 |
| Cldn5 | Claudin 5 |
| Crybb3 | Crystallin Beta B3 |
| DCX | Doublecortin |
| Egr2 | Early growth response protein 2 |
| Fos | Fos proto-oncogene |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| Gdf3 | Growth differentiation factor 3 |
| GFAP | Glial fibrillary acidic protein |
| H2bc24 | H2B clustered histone 24 |
| Hba-a1 | Hemoglobin Subunit Alpha 1 |
| Hba-a2 | Hemoglobin subunit alpha 2 |
| HDAC | Histone deacetylase |
| Hes1 | Hairy and enhancer of split-1 |
| Hspb1 | Heat shock protein family B (small) member 1 |
| Iba1 | Ionized calcium binding adapter protein 1 |
| Inpp5j | Inositol polyphosphate-5-phosphatase J |
| Klra4 | Killer cell lectin-like receptor, subfamily A, member 4 |
| NeuN | Neuronal nuclear protein |
| PDGFRA | Platelet derived growth factor receptor alpha |
| Rpl37rt | Ribosomal protein L37, retrotransposed |
| Sap25 | Sin3A associated protein 25 |
| Scd1 | Stearoyl-CoA desaturase 1 |
| Sin3A | SIN3 Transcription Regulator Family Member A |
| Slc43a3 | Solute carrier family 43 member 3 |
| Slco4a1 | Solute carrier organic anion transporter family member 4A1 |
| Tent5a | Terminal nucleotidyltransferase 5A |
| Tm6sf2 | Transmembrane 6 superfamily member 2 |
| Traip | TRAF interacting protein |
| Ttn | Titin |
| Vmn1r58 | Vomeronasal 1 receptor 58 |
References
- Kumar, R.; De Jesus, O. Radiation Effects on The Fetus. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Körblein, A. A hypothesis to derive the shape of the dose-response curve for teratogenic radiation effects. Environ. Health 2022, 21, 25. [Google Scholar] [CrossRef]
- Akleyev, A.; Deltour, I.; Krestinina, L.; Sokolnikov, M.; Tsareva, Y.; Tolstykh, E.; Schüz, J. Incidence and Mortality of Solid Cancers in People Exposed in Utero to Ionizing Radiation: Pooled Analyses of Two Cohorts from the Southern Urals, Russia. PLoS ONE 2016, 11, e0160372. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Born, M.; Dolezel, P.; Rutten, B.P.; de Saint-Georges, L.; Hof, P.R.; Korr, H. Prenatal protracted irradiation at very low dose rate induces severe neuronal loss in rat hippocampus and cerebellum. Neuroscience 2005, 130, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Tanaka, I.B., 3rd; Wang, H.; Lau, S.; Tanaka, S.; Tan, A.; Takai, D.; Abe, A. Effects of continuous prenatal low dose rate irradiation on neurobehavior, hippocampal cellularity, messenger RNA and microRNA expression on B6C3F1 mice. Cells 2024, 13, 1423. [Google Scholar] [CrossRef]
- Hamilton, K.F.; Sacher, G.A.; Grahn, D. A sex difference in mouse survival under daily gamma irradiation and its modification by gonadectomy. Radiat. Res. 1963, 18, 12–16. [Google Scholar] [CrossRef]
- Heidenreich, W.F.; Carnes, B.A.; Paretzke, H.G. Lung cancer risk in mice: Analysis of fractionation effects and neutron RBE with a biologically motivated model. Radiat. Res. 2006, 166, 794–801. [Google Scholar] [CrossRef]
- Henderson, M.A.; Valluri, S.; DesRosiers, C.; Lopez, J.T.; Batuello, C.N.; Caperell-Grant, A.; Mendonca, M.S.; Powers, E.M.; Bigsby, R.M.; Dynlacht, J.R. Effect of gender on radiation-induced cataractogenesis. Radiat. Res. 2009, 172, 129–133. [Google Scholar] [CrossRef]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018, 74, 106–120. [Google Scholar] [CrossRef]
- Krukowski, K.; Grue, K.; Becker, M.; Elizarraras, E.; Frias, E.S.; Halvorsen, A.; Koenig-Zanoff, M.; Frattini, V.; Nimmagadda, H.; Feng, X.; et al. The impact of deep space radiation on cognitive performance: From biological sex to biomarkers to countermeasures. Sci. Adv. 2021, 7, eabg6702. [Google Scholar] [CrossRef]
- Schroeder, M.K.; Liu, B.; Hinshaw, R.G.; Park, M.A.; Wang, S.; Dubey, S.; Liu, G.G.; Shi, Q.; Holton, P.; Reiser, V.; et al. Long-Term Sex- and Genotype-Specific Effects of (56)Fe Irradiation on Wild-Type and APPswe/PS1dE9 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 13305. [Google Scholar] [CrossRef]
- Tang, F.R.; Liu, L.; Wang, H.; Ho, K.J.N.; Sethi, G. Spatiotemporal dynamics of γH2AX in the mouse brain after acute irradiation at different postnatal days with special reference to the dentate gyrus of the hippocampus. Aging 2021, 13, 15815–15832. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Z.; Shen, H.; Wu, Z.; Liu, L.; Ren, B.; Wong, P.; Sethi, G.; Tang, F. Early life irradiation-induced hypoplasia and impairment of neurogenesis in the dentate gyrus and adult depression are mediated by microRNA- 34a-5p/T-cell intracytoplasmic antigen-1 pathway. Cells 2021, 10, 2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Z.W.; Ho, F.M.; Sethi, G.; Tang, F.R. Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation. Cells 2023, 12, 649. [Google Scholar] [CrossRef]
- Wang, H.; Lau, S.; Tan, A.; Tang, F.R. Chronic Low-Dose-Rate Radiation-Induced Persistent DNA Damage and miRNA/mRNA Expression Changes in Mouse Hippocampus and Blood. Cells 2024, 13, 1705. [Google Scholar] [CrossRef]
- Gulay, K.C.M.; Tanaka, I.B., 3rd; Komura, J.; Tanaka, S. Effects of Continuous Gamma-Ray Exposure in Utero in B6C3F1 Mice on Gestation Day 18 and at 10 Weeks of Age. Radiat. Res. 2018, 189, 425–440. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, I.B., 3rd; Sasagawa, S.; Ichinohe, K.; Takabatake, T.; Matsushita, S.; Matsumoto, T.; Otsu, H.; Sato, F. No lengthening of life span in mice continuously exposed to gamma rays at very low dose rates. Radiat. Res. 2003, 160, 376–379. [Google Scholar] [CrossRef]
- Tanaka, I.B., 3rd; Tanaka, S.; Ichinohe, K.; Matsushita, S.; Matsumoto, T.; Otsu, H.; Oghiso, Y.; Sato, F. Cause of death and neoplasia in mice continuously exposed to very low dose rates of gamma rays. Radiat. Res. 2007, 167, 417–437. [Google Scholar] [CrossRef]
- Maze, I.; Noh, K.M.; Allis, C.D. Histone regulation in the CNS: Basic principles of epigenetic plasticity. Neuropsychopharmacology 2013, 38, 3–22. [Google Scholar] [CrossRef]
- Hoffmann, S.; Smedegaard, S.; Nakamura, K.; Mortuza, G.B.; Räschle, M.; Ibañez de Opakua, A.; Oka, Y.; Feng, Y.; Blanco, F.J.; Mann, M.; et al. TRAIP is a PCNA-binding ubiquitin ligase that protects genome stability after replication stress. J. Cell Biol. 2016, 212, 63–75. [Google Scholar] [CrossRef]
- Shishkina, G.T.; Kalinina, T.S.; Lanshakov, D.A.; Bulygina, V.V.; Komysheva, N.P.; Bannova, A.V.; Drozd, U.S.; Dygalo, N.N. Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation. Biomedicines 2023, 11, 2595. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, L.; Hu, R.; Gong, D.; Liu, M.; Ding, J. TENT5A Increases Glioma Malignancy and Promotes its Progression. Recent. Pat. Anticancer Drug Discov. 2024, 20, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Banks, C.A.S.; Thornton, J.; Bengs, B.D.; Sardiu, M.E.; Florens, L.; Washburn, M.P. Distinct Regions within SAP25 Recruit O-Linked Glycosylation, DNA Demethylation, and Ubiquitin Ligase and Hydrolase Activities to the Sin3/HDAC Complex. J. Proteome Res. 2024, 23, 5016–5029. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Meng, Y.; Shi, Y. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem. Biophys. Res. Commun. 2004, 321, 1024–1031. [Google Scholar] [CrossRef]
- Zhou, W.; Deng, X.; Liu, L.; Yuan, Y.; Meng, X.; Ma, J. PELI1 overexpression contributes to pancreatic cancer progression through upregulating ubiquitination-mediated INPP5J degradation. Cell Signal 2024, 120, 111194. [Google Scholar] [CrossRef]
- Wong, F.L.; Yamada, M.; Sasaki, H.; Kodama, K.; Hosoda, Y. Effects of radiation on the longitudinal trends of total serum cholesterol levels in the atomic bomb survivors. Radiat. Res. 1999, 151, 736–746. [Google Scholar] [CrossRef]
- Varma, C.; Schroeder, M.K.; Price, B.R.; Khan, K.A.; Curty da Costa, E.; Hochman-Mendez, C.; Caldarone, B.J.; Lemere, C.A. Long-Term, Sex-Specific Effects of GCRsim and Gamma Irradiation on the Brains, Hearts, and Kidneys of Mice with Alzheimer’s Disease Mutations. Int. J. Mol. Sci. 2024, 25, 8948. [Google Scholar] [CrossRef]
- De Guzman, A.E.; Gazdzinski, L.M.; Alsop, R.J.; Stewart, J.M.; Jaffray, D.A.; Wong, C.S.; Nieman, B.J. Treatment age, dose and sex determine neuroanatomical outcome in irradiated juvenile mice. Radiat. Res. 2015, 183, 541–549. [Google Scholar] [CrossRef]
- Koturbash, I.; Zemp, F.; Kolb, B.; Kovalchuk, O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat. Res. 2011, 722, 114–118. [Google Scholar] [CrossRef]
- Minatohara, K.; Akiyoshi, M.; Okuno, H. Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front. Mol. Neurosci. 2015, 8, 78. [Google Scholar] [CrossRef]
- Xu, C.; Tu, Y.; Zhou, J.; Xu, X.; Qin, S.; Wang, L. Dynamic changes in c-Fos and NF-κB gene expression and Ca, Fe, Cu, Zn and Mg content due to brain injury in irradiated rats. Neuroreport 2021, 32, 1241–1247. [Google Scholar] [CrossRef]
- Duan, X.Q.; Wu, S.L.; Li, T.; Liang, J.C.; Qiou, J.Y.; Rao, Z.R.; Ju, G. Expression and significance of three types of Fos-immunoreactive cells after gamma knife irradiation of the forebrain in the rat. Neurosci. Res. 1999, 33, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kempf, S.J.; Casciati, A.; Buratovic, S.; Janik, D.; von Toerne, C.; Ueffing, M.; Neff, F.; Moertl, S.; Stenerlöw, B.; Saran, A.; et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 2014, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.S.; Moon, C.; Son, Y. Profiling of gene expression in the brain associated with anxiety-related behaviors in the chronic phase following cranial irradiation. Sci. Rep. 2022, 12, 13162. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.C.; Wang, F.M.; Cai, J.M. Gene expression changes in residual advanced cervical cancer after radiotherapy: Indicators of poor prognosis and radioresistance? Med. Sci. Monit. 2015, 21, 1276–1287. [Google Scholar]
- Wage, J.; Ma, L.; Peluso, M.; Lamont, C.; Evens, A.M.; Hahnfeldt, P.; Hlatky, L.; Beheshti, A. Proton irradiation impacts age-driven modulations of cancer progression influenced by immune system transcriptome modifications from splenic tissue. J. Radiat. Res. 2015, 56, 792–803. [Google Scholar] [CrossRef]
- Mishra, A.; Iyer, S.; Kesarwani, A.; Baligar, P.; Arya, S.P.; Arindkar, S.; Kumar, M.J.; Upadhyay, P.; Majumdar, S.S.; Nagarajan, P. Role of antigen presenting cell invariant chain in the development of hepatic steatosis in mouse model. Exp. Cell Res. 2016, 346, 188–197. [Google Scholar] [CrossRef]
- Chan, P.C.; Wu, T.N.; Chen, Y.C.; Lu, C.H.; Wabitsch, M.; Tian, Y.F.; Hsieh, P.S. Targetted inhibition of CD74 attenuates adipose COX-2-MIF-mediated M1 macrophage polarization and retards obesity-related adipose tissue inflammation and insulin resistance. Clin. Sci. 2018, 132, 1581–1596. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, Y.; Xin, L.; Kong, S.; Chen, Y.; Yang, S.; Li, K. Global Transcriptomic Profiling of Cardiac Hypertrophy and Fatty Heart Induced by Long-Term High-Energy Diet in Bama Miniature Pigs. PLoS ONE 2015, 10, e0132420. [Google Scholar] [CrossRef]
- Eramo, M.J.; Mitchell, C.A. Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem. Soc. Trans. 2016, 44, 240–252. [Google Scholar] [CrossRef]
- Rodgers, S.J.; Ferguson, D.T.; Mitchell, C.A.; Ooms, L.M. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci. Rep. 2017, 37, BSR20160432. [Google Scholar] [CrossRef]
- Ramos, A.R.; Elong Edimo, W.; Erneux, C. Phosphoinositide 5-phosphatase activities control cell motility in glioblastoma: Two phosphoinositides PI(4,5)P2 and PI(3,4)P2 are involved. Adv. Biol. Regul. 2018, 67, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Ghosh, S.; Suhel, T.; Chevalier, C.; Obeng, E.O.; Fafilek, B.; Krejci, P.; Beck, B.; Erneux, C. Phosphoinositide 5-phosphatases SKIP and SHIP2 in ruffles, the endoplasmic reticulum and the nucleus: An update. Adv. Biol. Regul. 2020, 75, 100660. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Korach-Andre, M.; Reissmann, E.; Ibáñez, C.F.; Bertolino, P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc. Natl. Acad. Sci. USA 2008, 105, 7252–7256. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T. The GDF3-ALK7 signaling axis in adipose tissue: A possible therapeutic target for obesity and associated diabetes? Endocr. J. 2023, 70, 761–770. [Google Scholar] [CrossRef]
- Hasbargen, K.B.; Shen, W.J.; Zhang, Y.; Hou, X.; Wang, W.; Shuo, Q.; Bernlohr, D.A.; Azhar, S.; Kraemer, F.B. Slc43a3 is a regulator of free fatty acid flux. J. Lipid Res. 2020, 61, 734–745. [Google Scholar] [CrossRef]
- Abend, M.; Pfeiffer, R.M.; Ruf, C.; Hatch, M.; Bogdanova, T.I.; Tronko, M.D.; Riecke, A.; Hartmann, J.; Meineke, V.; Boukheris, H.; et al. Iodine-131 dose dependent gene expression in thyroid cancers and corresponding normal tissues following the Chernobyl accident. PLoS ONE 2012, 7, e39103. [Google Scholar] [CrossRef]





| Gene Name | Primer Sequence |
|---|---|
| H2bc24 F | AGAGTTCCAGAGTTCCAGTCTCATC |
| H2bc24 R | GAACTCACTTGGAGCTGGTGT |
| Slc43a3 F | CCTCACGCTGATTTCCCTCA |
| Slc43a3 R | AGGAGACATTGCTCACAGGC |
| Tm6sf2 F | TTCTCACACATGGGTGCCTC |
| Tm6sf2 R | CTTGGTCCTGTGGCGAAGAT |
| Crybb3 F | AAGCAGGTCTCTGCCTCCT |
| Crybb3 R | TACGATCTCCATCTTGCGCC |
| Cd74 F | AGTGCCAGGAAGAAGTCAGC |
| Cd74 R | CCAGCGTCCTCCTTCTGTTC |
| Fos F | AGTCAAGGCCTGGTCTGTGT |
| Fos R | TGGAACACGCTATTGCCAGG |
| Hba-a2 F | GCTGAAGCCCTGGAAAGGAT |
| Hba-a2 R | GGAGCTTGAAGTTGACGGGA |
| Egr2 F | GCCAGGAGTGACGAAAGGAA |
| Egr2 R | GTGAGAAGGTGGGACAGAGC |
| Arhgef5 F | GACTCTGGGTGGTCGTGGAG |
| Arhgef5 R | GGCCTCAGCCAGAAGGATTT |
| Hba-a1 F | GCTGAAGCCCTGGAAAGGAT |
| Hba-a1 R | GGGAGAGAAGAAGGGCATGG |
| Traip F | ACCTTTTGACCCTGTTGGTGT |
| Traip R | GTAAGCAGGCCTCCTGAGTG |
| Hspb1 F | ATAGAGACCTGAAGCACCGC |
| Hspb1 R | CGGTCATGTTCTTGGCTGGT |
| Tent5a F | CTCCAGGACTGACCAAGGC |
| Tent5a R | CGGACACCTATGCCCTTCTC |
| Cldn5 F | GCTCTCAGAGTCCGTTGACC |
| Cldn5 R | TTCTCCAGCTGCCCTTTCAG |
| Hes1 F | GCCGTCTATCCGTATTGCCA |
| Hes1 R | GTTTGTCCGGTGTCGTGTTG |
| Sap25 F | GTTGTGGGCGCTTTCCAAAA |
| Sap25 R | CGAAGTGGCAGTGGAGACAT |
| Cdkl5 F | AACGGCGAGAATCCAAGCAT |
| Cdkl5 R | AAGGCGTTTGTTGGTCACTGT |
| GAPDH F | ACCACAGTCCATGCCATCAC |
| GAPDH R | TCCACCACCCTGTTGCTGTA |
| Gene Name | Primer Sequence |
|---|---|
| Rpl37rt F | CCAAGGCCTACCACCTTCAG |
| Rpl37rt R | AAGAACTGGATGCTGCGACA |
| Scd1 F | GAGTAGCTGAGCTTTGGGCT |
| Scd1 R | ACTTCATCAGCGGGGACTTG |
| Cd59b F | CTGTTGCCTTGGATCAGCCT |
| Cd59b R | TGATACACTTGCCTTCCGGC |
| Vmn1r58 F | GGTCAAAACACGGCCAAACC |
| Vmn1r58 R | AGGAGAAACAGCCTTCTCTCAA |
| Slc43a3 F | CCTCACGCTGATTTCCCTCA |
| Slc43a3 R | AGGAGACATTGCTCACAGGC |
| Slco4a1 F | CTTGGGCGATGAATGAAGCG |
| Slco4a1 R | ACACATACTGCACCTCACGG |
| Klra4 F | CGCCTCAGAGTGTGTTCAGT |
| Klra4 R | TGTCTGAAGGAACCACGAGC |
| Ccl4 F | CTAACCCCGAGCAACACCAT |
| Ccl4 R | TGAACGTGAGGAGCAAGGAC |
| Inpp5j F | ATCTGCCACTCTGTCTTGGC |
| Inpp5j R | TCTGTCACATCTGCAACTGCT |
| Gdf3 F | GTGCCCCTTCTCAATGACCA |
| Gdf3 R | GCTCACCAAGGGGTCCATAG |
| GAPDH F | ACCACAGTCCATGCCATCAC |
| GAPDH R | TCCACCACCCTGTTGCTGTA |
| miRNA | Primer Sequence |
|---|---|
| mmu-miR-182-5p | TTTGGCAATGGTAGAACTCACACCG |
| mmu-miR-183-5p | TATGGCACTGGTAGAATTCACT |
| mmu-miR-148a-3p | TCAGTGCACTACAGAACTTTGT |
| mmu-let-7i-3p | CTGCGCAAGCTACTGCCTTGCT |
| mmu-miR-135b-5p | TATGGCTTTTCATTCCTATGTGA |
| mmu-miR-8112 | ATATCTCCGCCACCTCCACCGCA |
| mmu-miR-206-3p | TGGAATGTAAGGAAGTGTGTGG |
| mmu-miR-68 | GCTGTACTGACTTGATGAAAGTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tanaka, I.B.; Lau, S.; Tanaka, S.; Tan, A.; Tang, F.R. Alterations in Blood and Hippocampal mRNA and miRNA Expression, Along with Fat Deposition in Female B6C3F1 Mice Continuously Exposed to Prenatal Low-Dose-Rate Radiation and Their Comparison with Male Mice. Cells 2025, 14, 173. https://doi.org/10.3390/cells14030173
Wang H, Tanaka IB, Lau S, Tanaka S, Tan A, Tang FR. Alterations in Blood and Hippocampal mRNA and miRNA Expression, Along with Fat Deposition in Female B6C3F1 Mice Continuously Exposed to Prenatal Low-Dose-Rate Radiation and Their Comparison with Male Mice. Cells. 2025; 14(3):173. https://doi.org/10.3390/cells14030173
Chicago/Turabian StyleWang, Hong, Ignacia Braga Tanaka, Salihah Lau, Satoshi Tanaka, Amanda Tan, and Feng Ru Tang. 2025. "Alterations in Blood and Hippocampal mRNA and miRNA Expression, Along with Fat Deposition in Female B6C3F1 Mice Continuously Exposed to Prenatal Low-Dose-Rate Radiation and Their Comparison with Male Mice" Cells 14, no. 3: 173. https://doi.org/10.3390/cells14030173
APA StyleWang, H., Tanaka, I. B., Lau, S., Tanaka, S., Tan, A., & Tang, F. R. (2025). Alterations in Blood and Hippocampal mRNA and miRNA Expression, Along with Fat Deposition in Female B6C3F1 Mice Continuously Exposed to Prenatal Low-Dose-Rate Radiation and Their Comparison with Male Mice. Cells, 14(3), 173. https://doi.org/10.3390/cells14030173







