Bilirubin Triggers Calcium Elevations and Dysregulates Giant Depolarizing Potentials During Rat Hippocampus Maturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acute Hippocampal Slices
2.2. Treatments
2.3. Live Calcium Imaging and Analysis
2.4. Electrophysiological Recordings and Analysis
2.5. Western Blot Analysis
2.6. Statistics
3. Results
3.1. Bilirubin Dose-Dependent Modulation of Neuronal Calcium Dynamics in Wild-Type Hippocampal Slices
3.2. In Wild-Type Hippocampal Slices, Bilirubin Transiently Dysregulates Network Activity Emerging During Early Postnatal Development: GDPs
3.3. In Wild-Type Hippocampal Slices, Bilirubin Dysregulation of Intracellular Calcium Dynamics Depends on the Postnatal Age of the Hippocampus
3.4. Hippocampal Neuron Intracellular Calcium Signaling Is Altered in an Age-Dependent Manner in Hyperbilirubinemic jj Gunn Rats
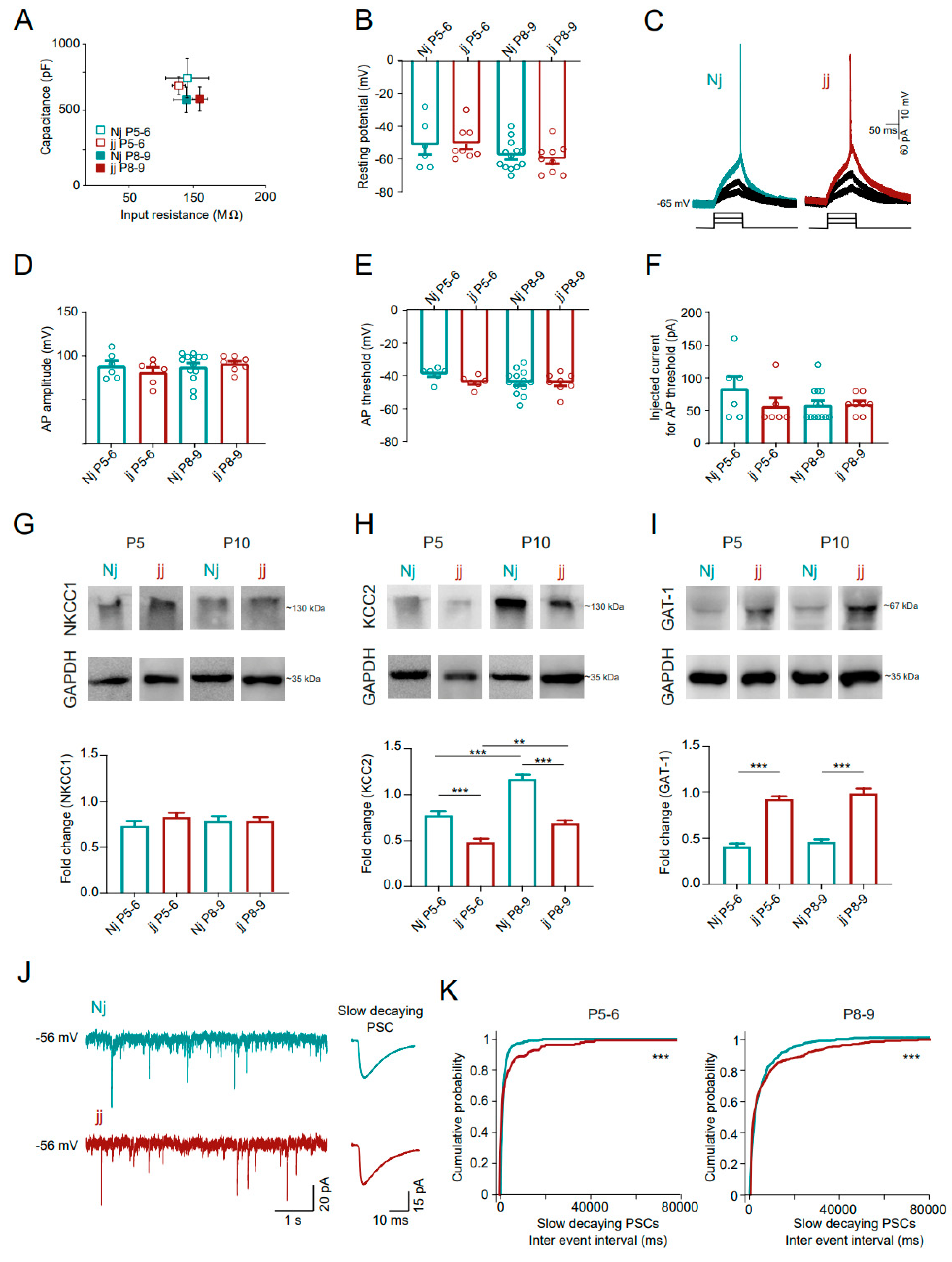
3.5. Hippocampal GDPs Are Altered in the Hyperbilirubinemic jj Gunn Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Zucker, S.D.; Smith, R.; Laposata, M.; Boudinot, F.D.; Bergsneider, M. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J. Biol. Chem. 1999, 274, 10852–10862. [Google Scholar] [CrossRef]
- Gazzin, S.; Strazielle, N.; Tiribelli, C.; Ghersi-Egea, J.F. Transport and metabolism at blood–brain interfaces and in neural cells: Relevance to bilirubin-induced encephalopathy. Front. Pharmacol. 2012, 3, 89. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Johnson, L.H.; Shapiro, S.M. Kernicterus in sick and preterm infants (1999–2002): A need for an effective preventive approach. Semin. Perinatol. 2004, 28, 319–325. [Google Scholar] [CrossRef]
- Wusthoff, C.J.; Loeb, I. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 2015, 20, 52–57. [Google Scholar] [CrossRef]
- Amin, S.B.; Smith, T.; Timler, G. Developmental influence of unconjugated hyperbilirubinemia and neurobehavioral disorders. Pediatr. Res. 2019, 85, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Watchko, J.F.; Tiribelli, C. Bilirubin-induced neurologic damage-mechanisms and management approaches. N. Engl. J. Med. 2013, 369, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Kumar, P.; Testai, F.D. Bilirubin encephalopathy. Curr. Neurol. Neurosci. Rep. 2022, 22, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Vassar, R. Movement disorders due to bilirubin toxicity. Semin. Fetal Neonatal Med. 2015, 20, 20–25. [Google Scholar] [CrossRef]
- Olds, C.; Oghalai, J.S. Audiologic impairment associated with bilirubin-induced neurologic damage. Semin. Fetal Neonatal Med. 2015, 20, 42–46. [Google Scholar] [CrossRef]
- Rauti, R.; Qaisiya, M.; Tiribelli, C.; Ballerini, L.; Bellarosa, C. Bilirubin disrupts calcium homeostasis in neonatal hippocampal neurons: A new pathway of neurotoxicity. Arch. Toxicol. 2020, 94, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Qaisiya, M.; Brischetto, C.; Jašprová, J.; Vitek, L.; Tiribelli, C.; Bellarosa, C. Bilirubin-induced ER stress contributes to the inflammatory response and apoptosis in neuronal cells. Arch. Toxicol. 2017, 91, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Cherubini, E.; Corradetti, R.; Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989, 416, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Griguoli, M.; Cherubini, E. Early correlated network activity in the hippocampus: Its putative role in shaping neuronal circuits. Front. Cell. Neurosci. 2017, 11, 255. [Google Scholar] [CrossRef]
- Lein, P.J.; Barnhart, C.D.; Pessah, I.N. Acute hippocampal slice preparation and hippocampal slice cultures. Methods Mol. Biol. 2011, 758, 115–134. [Google Scholar] [PubMed]
- Gunn, C.H. Hereditary acholuric jaundice in a new mutant strain of rats. J. Hered. 1938, 29, 137–139. [Google Scholar] [CrossRef]
- Chowdhury, J.R.; Kondapalli, R.; Chowdhury, N.R. Gunn rat: A model for inherited deficiency of bilirubin glucuronidation. Adv. Vet. Sci. Comp. Med. 1993, 37, 149–173. [Google Scholar]
- Cellot, G.; Maggi, L.; Di Castro, M.; Catalano, M.; Migliore, R.; Migliore, M.; Scattoni, M.L.; Calamandrei, G.; Cherubini, E. Premature changes in neuronal excitability account for hippocampal network impairment and autistic-like behavior in neonatal BTBR T+tf/J mice. Sci. Rep. 2016, 6, 31696. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Assisi, F. The ready isomerization of bilirubin IX- in aqueous solution. Biochem. J. 1972, 129, 797–800. [Google Scholar] [CrossRef]
- Roca, L.; Ruiz, J.; Munro, C.; Valverde, C. Factors affecting the binding of bilirubin to serum albumins: Validation and application of the peroxidase method. Pediatr. Res. 2006, 60, 724–728. [Google Scholar] [CrossRef]
- Panattoni, G.; Amoriello, R.; Memo, C.; Thalhammer, A.; Ballerini, C.; Ballerini, L. Diverse inflammatory threats modulate astrocytes Ca2+ signaling via connexin43 hemichannels in organotypic spinal slices. Mol. Brain 2021, 14, 159. [Google Scholar] [CrossRef]
- Ostrow, J.D.; Pascolo, L.; Tiribelli, C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr. Res. 2003, 54, 926. [Google Scholar] [CrossRef]
- Bonifazi, P.; Goldin, M.; Picardo, M.A.; Jorquera, I.; Cattani, A.; Bianconi, G.; Represa, A.; Ben-Ari, Y.; Cossart, R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 2009, 326, 1419–1424. [Google Scholar] [CrossRef]
- Carp, J.S. Physiological properties of primate lumbar motoneurons. J. Neurophysiol. 1992, 68, 1121–1132. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Li, Q.; Wang, Y. Differential alterations in the morphology and electrophysiology of layer II pyramidal cells in the primary visual cortex of a mouse model prenatally exposed to LPS. Neurosci. Lett. 2015, 591, 138–143. [Google Scholar] [CrossRef]
- Djuric, U.; Cheung, A.Y.; Zhang, W.; Mok, R.S.; Lai, W.; Piekna, A.; Hendry, J.A.; Ross, P.J.; Pasceri, P.; Kim, D.-S.; et al. MECP2e1 isoform mutation affects the form and function of neurons derived from Rett syndrome patient iPS cells. Neurobiol. Dis. 2015, 76, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-chloride cotransporters and neuronal function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Borden, L.A. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996, 29, 335–356. [Google Scholar] [CrossRef]
- Hollrigel, G.S.; Soltesz, I. Slow Kinetics of Miniature IPSCs during Early Postnatal Development in Granule Cells of the Dentate Gyrus. J. Neurosci. 1997, 17, 5119–5128. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Sciancalepore, M.; Stratta, F.; Martina, M.; Cherubini, E. Developmental changes in spontaneous GABAA-mediated synaptic events in rat hippocampal CA3 neurons. Eur. J. Neurosci. 1994, 6, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Venance, L.; Rozov, A.; Blatow, M.; Monyer, H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 10260–10265. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Silva, R.F.M.; Falcão, A.S.; Brito, M.A.; Brites, D. Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. J. Neuroimmunol. 2004, 153, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Vianello, E.; Zampieri, S.; Marcuzzo, T.; Tordini, F.; Bottin, C.; Dardis, A.; Zanconati, F.; Tiribelli, C.; Gazzin, S. Histone acetylation as a new mechanism for bilirubin-induced encephalopathy in the Gunn rat. Sci. Rep. 2018, 8, 13690. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.S.; Fernandes, A.; Brito, M.A.; Silva, R.F.; Brites, D. Bilirubin-induced inflammatory response, glutamate release, and cell death in rat cortical astrocytes are enhanced in younger cells. Neurobiol. Dis. 2005, 20, 199–206. [Google Scholar] [CrossRef]
- Falcão, A.S.; Fernandes, A.; Brito, M.A.; Silva, R.F.; Brites, D. Bilirubin-induced immunostimulant effects and toxicity vary with neural cell type and maturation state. Acta Neuropathol. 2006, 112, 95–105. [Google Scholar] [CrossRef]
- Bortolussi, G.; Zentillin, L.; Vaníkova, J.; Bockor, L.; Bellarosa, C.; Mancarella, A.; Vianello, E.; Tiribelli, C.; Giacca, M.; Vitek, L.; et al. Life-long correction of hyperbilirubinemia with a neonatal liver-specific AAV-mediated gene transfer in a lethal mouse model of Crigler-Najjar Syndrome. Hum. Gene Ther. 2014, 25, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.S.; Bellarosa, C.; Fernandes, A.; Brito, M.A.; Silva, R.F.M.; Tiribelli, C.; Brites, D. Role of multidrug resistance-associated protein 1 expression in the in vitro susceptibility of rat nerve cell to unconjugated bilirubin. Neuroscience 2007, 144, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Brorson, J.R.; Zhang, H. Ca2+ handling and vulnerability of immature neurons to excitotoxicity. J. Neurosci. Res. 2004, 76, 268–279. [Google Scholar]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef]
- Liang, M.; Yin, X.L.; Shi, H.B.; Li, C.; Li, X.; Song, N.; Shi, H.; Zhao, Y.; Wang, L.; Yin, S. Bilirubin augments Ca2+ load of developing bushy neurons by targeting specific subtype of voltage-gated calcium channels. Sci. Rep. 2017, 7, 431. [Google Scholar] [CrossRef]
- Satrom, K.M.; Rao, R.B.; Tkáč, I. Neonatal hyperbilirubinemia differentially alters the neurochemical profiles of the developing cerebellum and hippocampus in a preterm Gunn rat model. NMR Biomed. 2023, 36, e4946. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.R.; Silva, S.L.; Barateiro, A.; Fernandes, A.; Falcão, A.S.; Brito, M.A.; Brites, D. Pro-inflammatory cytokines intensify the activation of NO/NOS, JNK1/2 and caspase cascades in immature neurons exposed to elevated levels of unconjugated bilirubin. Exp. Neurol. 2011, 229, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.N.; Yoon, S.Y.; Suh, J.; Lee, J.H.; Kim, D. Differential activation of caspase-3 at two maturational stages during okadaic acid-induced rat neuronal death. Neurosci. Lett. 2002, 334, 63–67. [Google Scholar] [CrossRef]
- Hua, J.Y.; Smith, S.J. Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 2004, 7, 327–332. [Google Scholar] [CrossRef]
- Yin, X.L.; Liang, M.; Shi, H.B.; Wang, L.Y.; Li, C.Y.; Yin, S.K. The role of gamma-aminobutyric acid/glycinergic synaptic transmission in mediating bilirubin-induced hyperexcitation in developing auditory neurons. Toxicol. Lett. 2016, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Shi, H.B.; Song, N.Y.; Yin, S.K. Bilirubin enhances neuronal excitability by increasing glutamatergic transmission in the rat lateral superior olive. Toxicology 2011, 284, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Shi, H.B.; Chen, Z.N.; Ye, H.B.; Song, N.Y.; Yin, S.K. Protein kinase A and C signaling induces bilirubin potentiation of GABA/glycinergic synaptic transmission in rat ventral cochlear nucleus neurons. Brain Res. 2010, 1348, 30–41. [Google Scholar] [CrossRef]
- Sipilä, S.T.; Huttu, K.; Soltesz, I.; Voipio, J.; Kaila, K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J. Neurosci. 2005, 25, 5280–5289. [Google Scholar] [CrossRef]
- Hayashida, M.; Miyaoka, T.; Tsuchie, K.; Araki, T.; Izuhara, M.; Miura, S.; Kanayama, M.; Ohtsuki, K.; Nagahama, M.; Azis, I.A.; et al. Parvalbumin-positive GABAergic interneurons deficit in the hippocampus in Gunn rats: A possible hyperbilirubinemia-induced animal model of schizophrenia. Heliyon 2019, 5, e02037. [Google Scholar] [CrossRef]
- Gong, L.; Liu, H.; Lai, K.; Zhang, Z.; Mao, L.; Liu, Z.; Li, M.; Yin, X.; Liang, M.; Shi, H.; et al. Selective Vulnerability of GABAergic Inhibitory Interneurons to Bilirubin Neurotoxicity in the Neonatal Brain. J. Neurosci. 2024, 44, e0442242024. [Google Scholar] [CrossRef]
- Nabekura, J.; Ueno, T.; Okabe, A.; Furuta, A.; Iwaki, T.; Shimizu-Okabe, C.; Fukuda, A.; Akaike, N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 2002, 22, 4412–4417. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Amici, M.; Sobrero, F.; Spinelli, G.; Di, A.S.; Ragozzino, D.; Mascia, A.; Scoppetta, C.; Esposito, V.; Miledi, R.; et al. Anomalous levels of Cl− transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. USA 2006, 103, 8465–8468. [Google Scholar] [CrossRef]
- Jaenisch, N.; Witte, O.W.; Frahm, C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 2010, 41, e151–e159. [Google Scholar] [CrossRef]
- Ma, Y.H.; Zhou, X.G.; Duan, S.H.; Hu, J.H.; Lu, B.F.; Yu, Y.; Mei, Z.T.; Fei, J.; Guo, L.H. Overexpression of gamma-aminobutyric acid transporter subtype I leads to cognitive deterioration in transgenic mice. Acta Pharmacol. Sin. 2001, 22, 340–348. [Google Scholar] [PubMed]
- Liaury, K.; Miyaoka, T.; Tsumori, T.; Furuya, M.; Hashioka, S.; Wake, R.; Tsuchie, K.; Fukushima, M.; Limoa, E.; Tanra, A.J.; et al. Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: A possible hyperbilirubinemia-induced animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 184–190. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cellot, G.; Di Mauro, G.; Ricci, C.; Tiribelli, C.; Bellarosa, C.; Ballerini, L. Bilirubin Triggers Calcium Elevations and Dysregulates Giant Depolarizing Potentials During Rat Hippocampus Maturation. Cells 2025, 14, 172. https://doi.org/10.3390/cells14030172
Cellot G, Di Mauro G, Ricci C, Tiribelli C, Bellarosa C, Ballerini L. Bilirubin Triggers Calcium Elevations and Dysregulates Giant Depolarizing Potentials During Rat Hippocampus Maturation. Cells. 2025; 14(3):172. https://doi.org/10.3390/cells14030172
Chicago/Turabian StyleCellot, Giada, Giuseppe Di Mauro, Chiara Ricci, Claudio Tiribelli, Cristina Bellarosa, and Laura Ballerini. 2025. "Bilirubin Triggers Calcium Elevations and Dysregulates Giant Depolarizing Potentials During Rat Hippocampus Maturation" Cells 14, no. 3: 172. https://doi.org/10.3390/cells14030172
APA StyleCellot, G., Di Mauro, G., Ricci, C., Tiribelli, C., Bellarosa, C., & Ballerini, L. (2025). Bilirubin Triggers Calcium Elevations and Dysregulates Giant Depolarizing Potentials During Rat Hippocampus Maturation. Cells, 14(3), 172. https://doi.org/10.3390/cells14030172






