Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies
2.3. Methods
2.3.1. Induction of EAE in C57BL/6J Mice
2.3.2. Immunofluorescence Microscopy
Embedding of Retinal Samples in Epon for Immunolabelling of 0.5 µm Thin Resin Sections
Immunolabelling of 0.5 µm Thin Resin Sections from the Mouse Retina
Confocal Microscopy
Quantification of Immunosignals in the Outer Plexiform Layer (OPL)
Analyses of MIC60 Immunosignals from Individual Synaptic Mitochondria in the OPL
2.3.3. Transmission Electron Microscopy (TEM) of Mitochondria of Rod Photoreceptor Synapses
Measurement of the Diameter of the Synaptic Mitochondrion in the OPL of Rod Photoreceptor Synapses Using TEM Images
2.3.4. Post-Embedding Immunogold Labelling
Embedding of Retinas in LR Gold Resin for Immunogold Electron Microscopy
Post-Embedding Immunogold Labelling of Ultrathin Sections
Quantification of MIC60/Mitofilin Immunogold Puncta on Presynaptic Mitochondria of Rod Photoreceptor Synapses
2.3.5. Western Blot Analyses of CFA and MOG/CFA Retina Samples and Quantification of Western Blot Bands with the LI-COR System
Quantification of Western Blot Bands
2.3.6. Statistical Analyses
3. Results

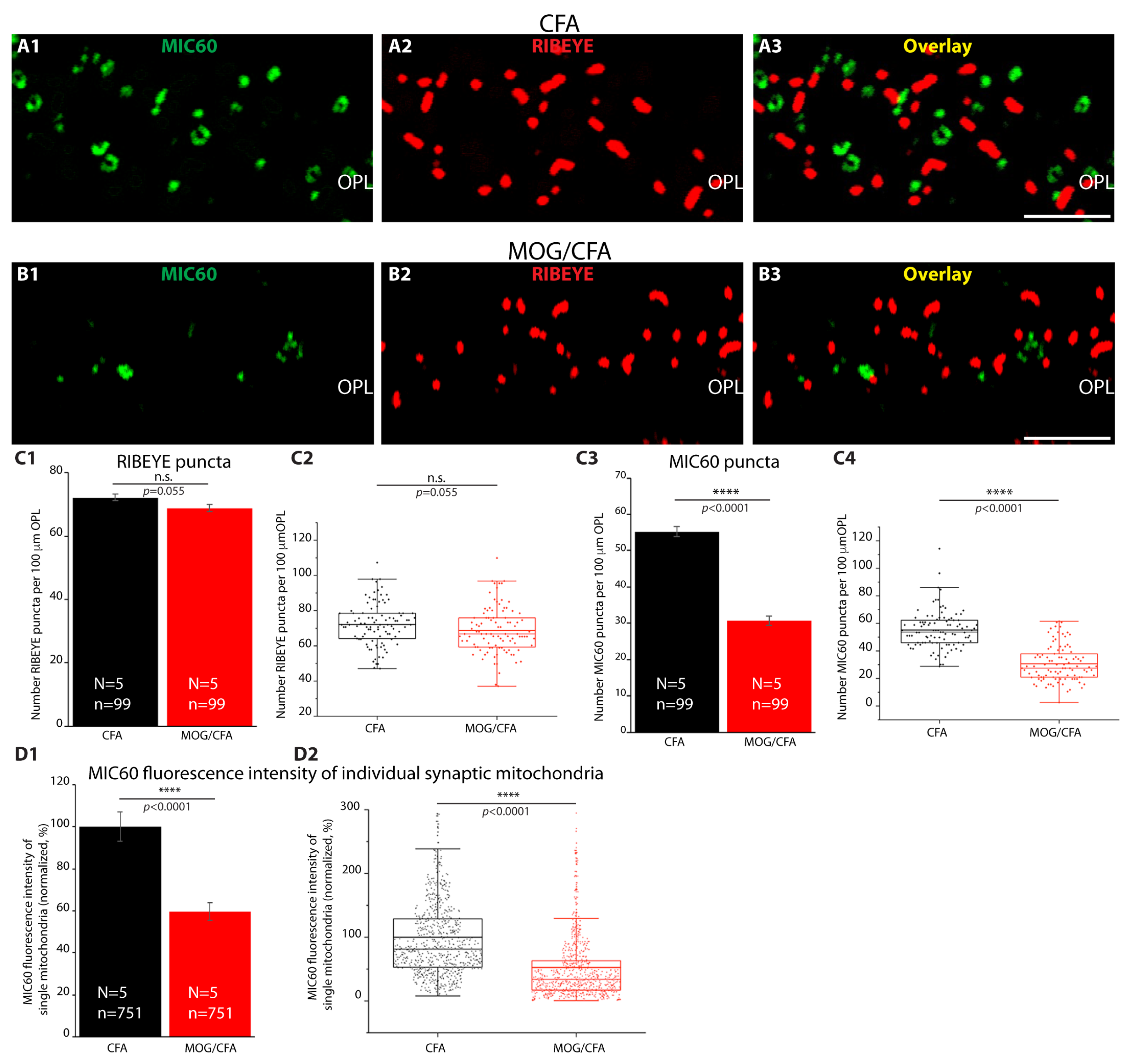
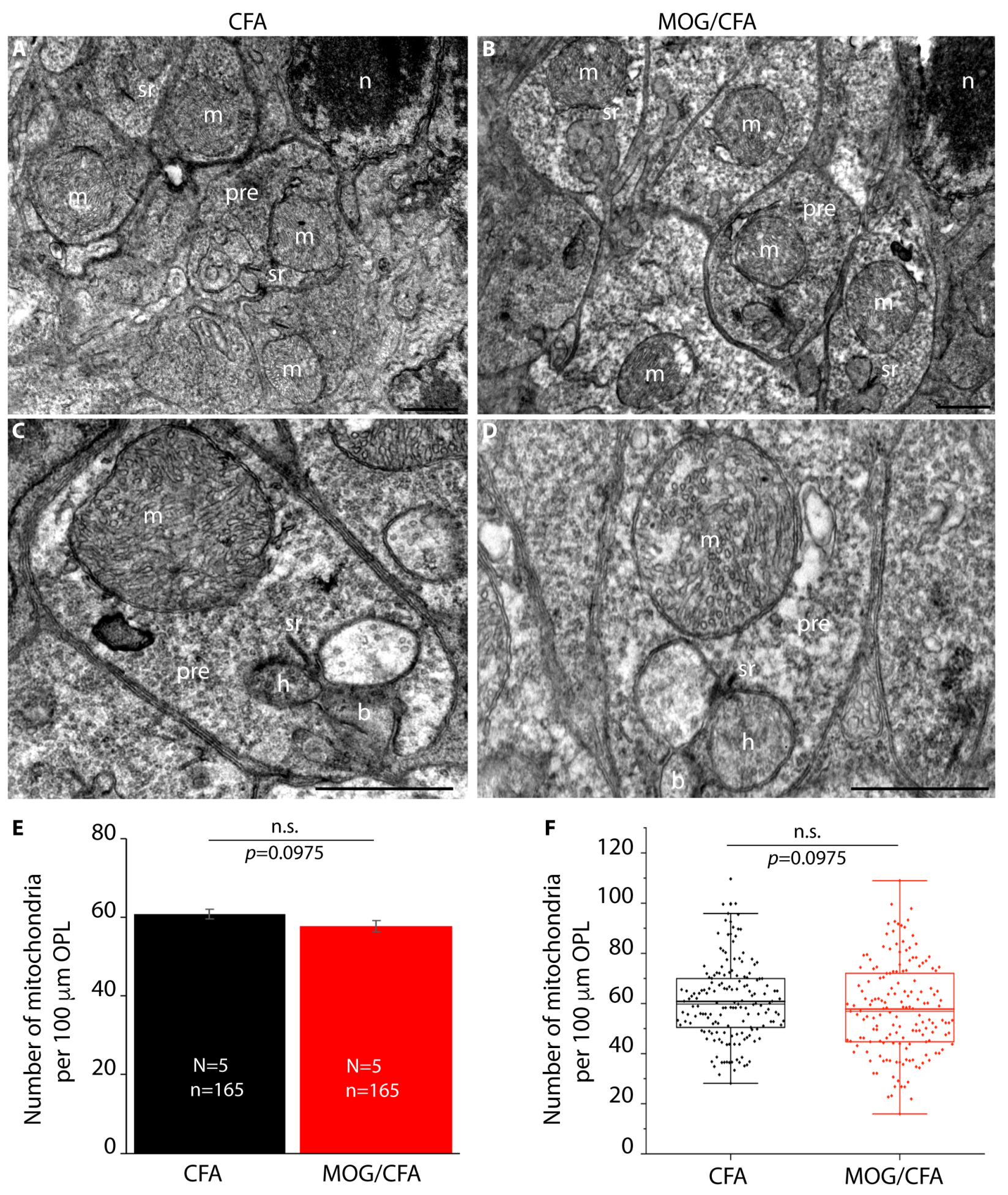


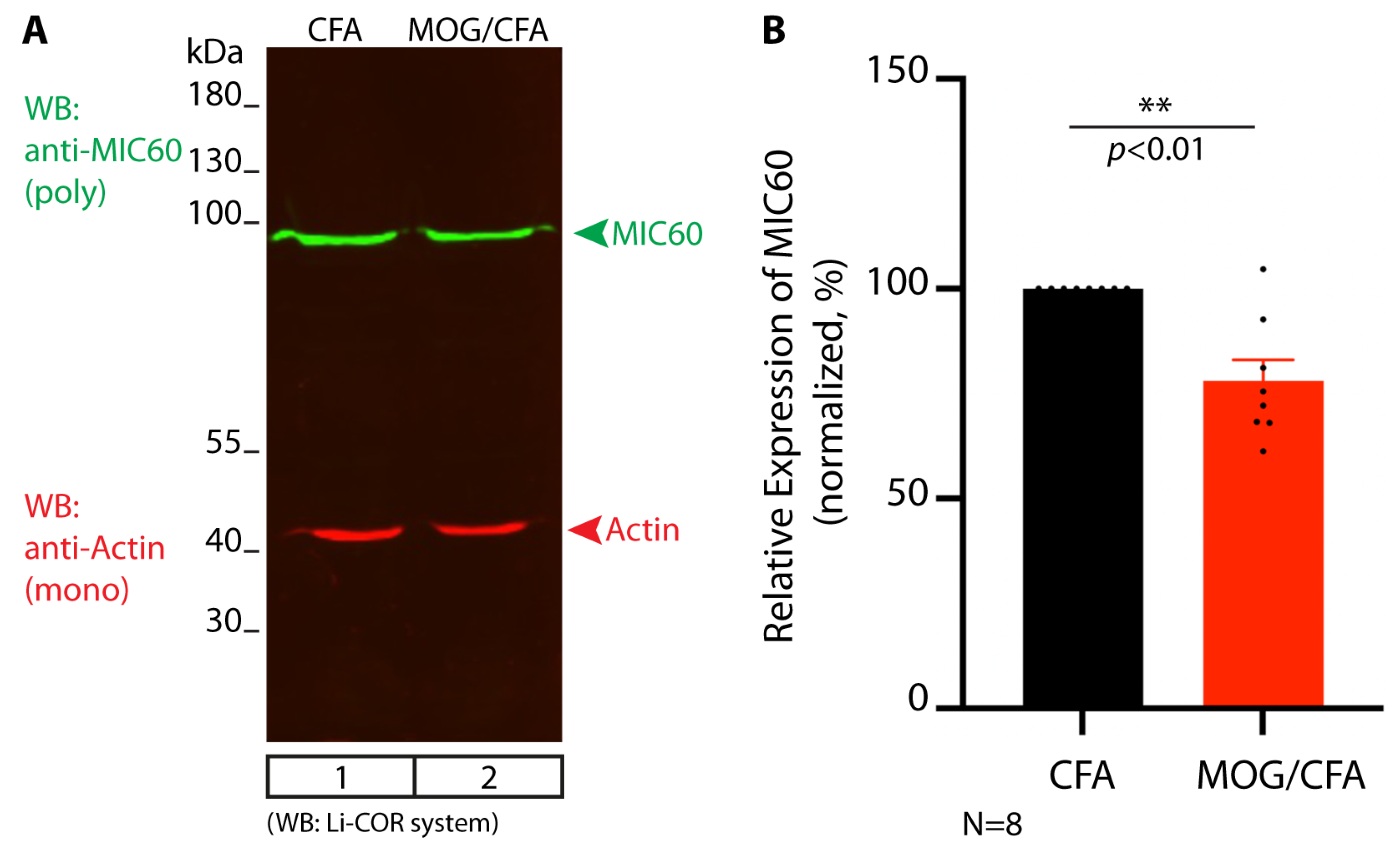


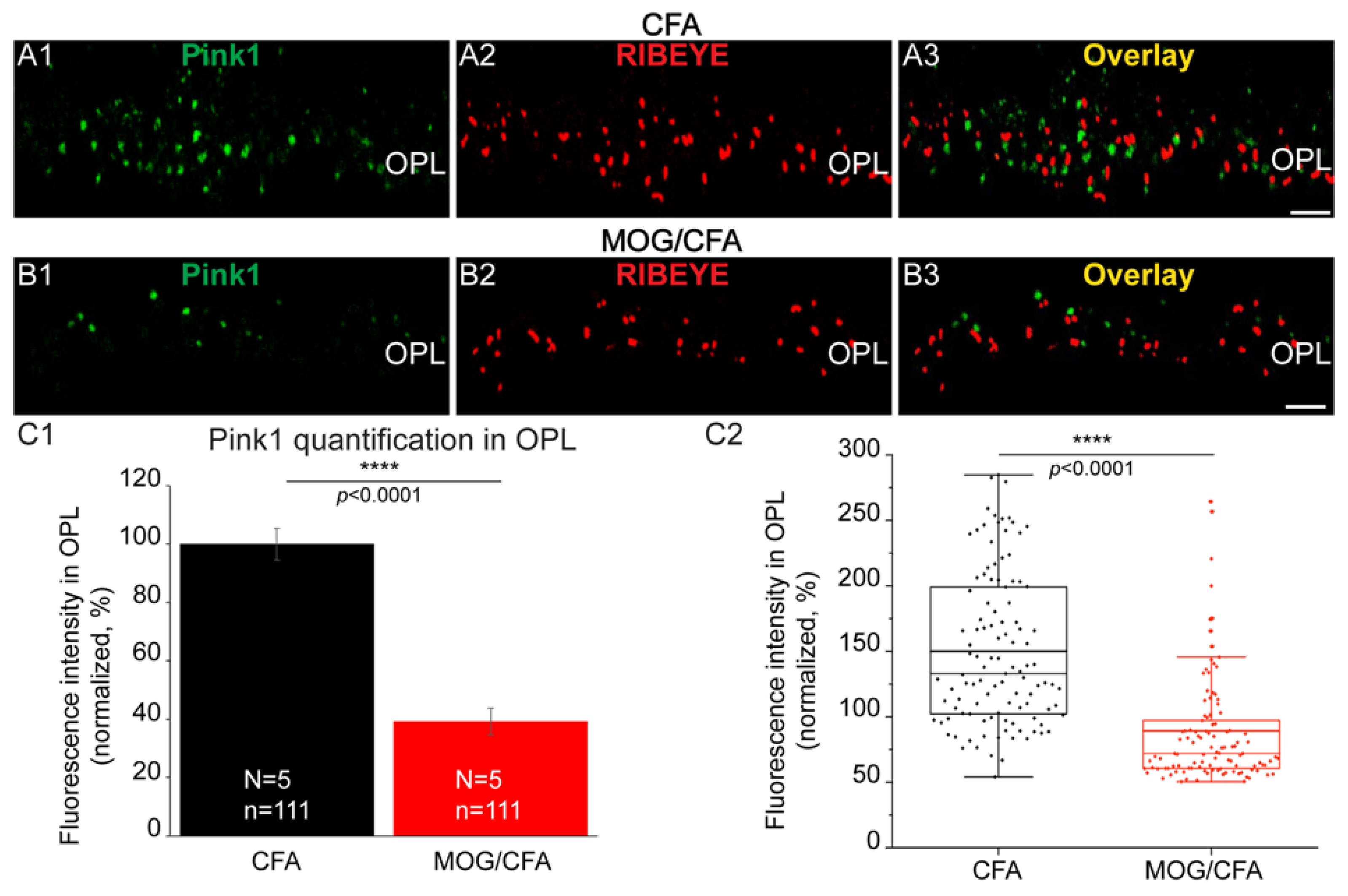
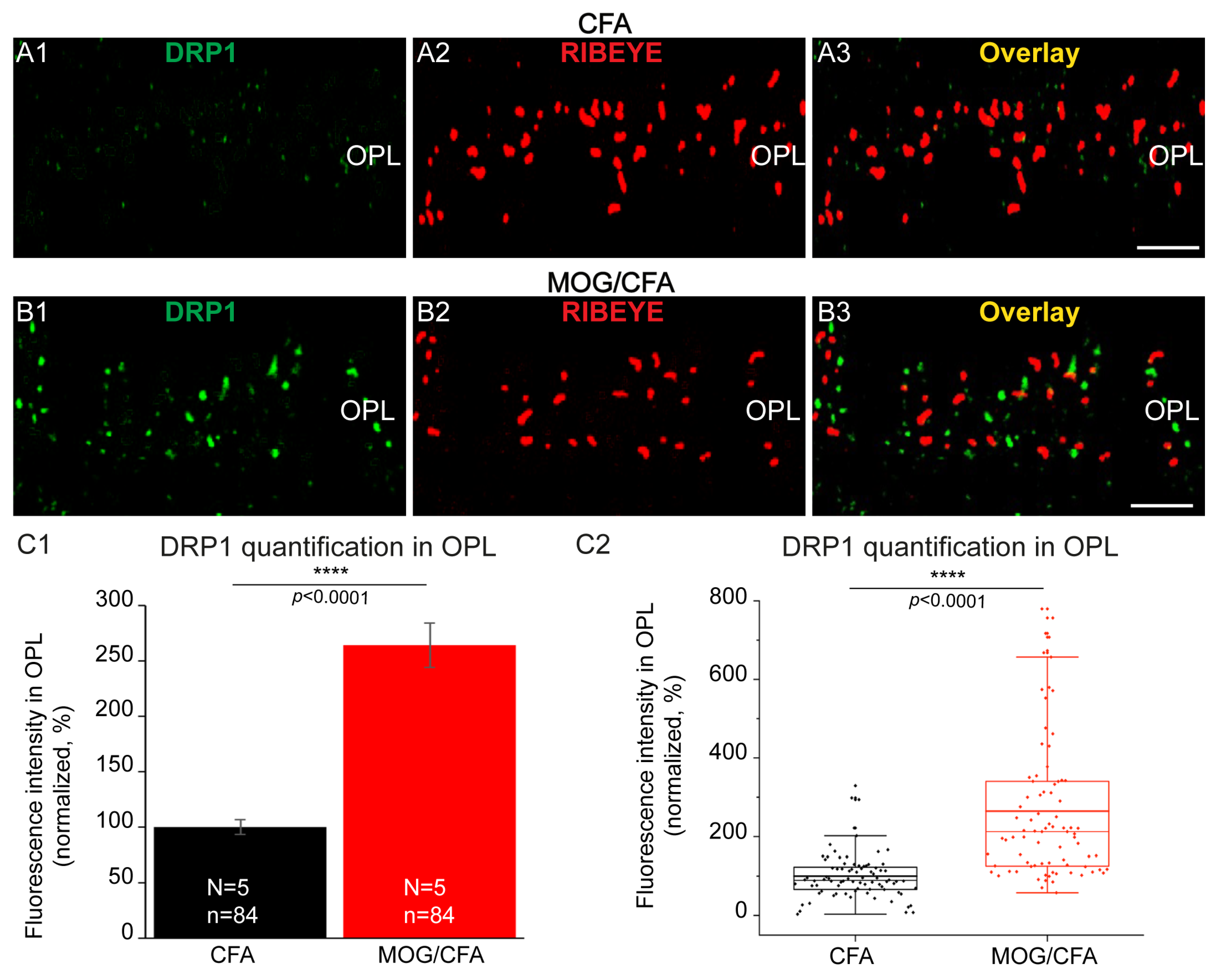


4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korn, T. Pathophysiology of multiple sclerosis. J. Neurol. 2008, 255 (Suppl. 6), 2–6. [Google Scholar] [CrossRef]
- Yamout, B.I.; Alroughani, R. Multiple sclerosis. Sem. Neurol. 2018, 38, 212–225. [Google Scholar]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M. Multiple Sclerosis. Nat. Rev. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Woo, M.S.; Engler, J.B.; Friese, M.A. The neuropathology of multiple sclerosis. Nat. Rev. Neurosci. 2024, 25, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128 Pt 11, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Derfuss, T.; Parikh, K.; Velhin, S.; Braun, M.; Mathey, E.; Krumbholz, M.; Kumpfel, T.; Moldenhauer, A.; Rader, C.; Sonderegger, P.; et al. Contactin-2/TAG-1-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl. Acad. Sci. USA 2009, 106, 8302–8307. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Chang, A.; Doud, M.K.; Kidd, G.J.; Ribaudo, M.V.; Young, E.A.; Fox, R.J.; Staugaitis, S.M.; Trapp, B.D. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011, 69, 445–454. [Google Scholar] [CrossRef]
- Rossi, S.; Motta, C.; Studer, V.; Barbieri, F.; Buttari, F.; Bergami, A.; Sancesario, G.; Gernardini, S.; De Angelis, G.; Martino, G.; et al. Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Mult. Scler. J. 2014, 20, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef]
- Mandolesi, G.; Gentile, A.; Musella, A.; Fresegna, D.; De Vito, F.; Bullitta, S.; Sepman, H.; Marfia, G.A.; Centonze, D. Synaptopathy connects inflammation and neurodegenration in multiple sclerosis. Nat. Rev. Neurol. 2015, 11, 711–724. [Google Scholar] [CrossRef]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Jürgens, T.; Jaffari, M.; Kreutzfeldt, M.; Bahn, E.; Brück, W.; Kerschensteiner, M.; Merkler, D. Reconstructions of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 2016, 139, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Möck, E.E.A.; Honkonen, E.; Airas, L. Synaptic loss in multiple sclerosis: A systematic review of human post-mortem studies. Front. Neurol. 2021, 12, 782599. [Google Scholar] [CrossRef] [PubMed]
- Gillani, R.L.; Kirone, E.N.; Whiteman, S.; Zwang, T.J.; Bacskai, B.J. Instability of excitatory synapses in experimental autoimmune encephalomyelitis and the outcome for excitatory circuits inputs to individual cortical neurons. Brain Behav. Immun. 2024, 119, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gordon, L.K. Ocular manifestations of multiple sclerosis. Curr. Opin. Ophthalmol. 2005, 16, 315–320. [Google Scholar] [CrossRef]
- Martinez-Lapiscina, E.H.; Sanchez-Dalmau, B.; Fraga-Pumar, E.; Ortiz-Perez, S.; Tercero-Uribe, A.I.; Torres-Torres, R.; Villoslada, P. The visual pathway as a model to understand brain damage in multiple sclerosis. Mult. Scler. 2014, 20, 1678–1685. [Google Scholar] [CrossRef]
- Graham, S.L.; Klistorner, A. Afferent visual pathways in multiple sclerosis: A review. Clin. Exp. Ophthalmol. 2017, 45, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polmann, C. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 921–932. [Google Scholar] [CrossRef]
- Toosy, A.T.; Mason, D.F.; Miller, D.H. Optic neuritis. Lancet Neurol. 2014, 13, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kale, N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain 2016, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.M.; Toosy, A.T. Optic neuritis: The eye as a window to the brain. Curr. Opin. Neurol. 2017, 30, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Dembla, M.; Kesharwani, A.; Natarajan, S.; Fecher-Trost, C.; Fairless, R.; Williams, S.K.; Flockerzi, V.; Diem, R.; Schwarz, K.; Schmitz, F. Early auto-immune targeting of photoreceptor ribbon synapses in mouse models of multiple sclerosis. EMBO Mol. Med. 2018, 10, e8926. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Katiyar, R.; Dembla, E.; Dembla, M.; Kumar, P.; Belkacemi, A.; Jung, M.; Beck, A.; Flockerzi, V.; Schwarz, K.; et al. Disturbed presynaptic Ca2+ signaling in photoreceptors in the EAE mouse model of multiple sclerosis. iScience 2020, 23, 101830. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Schwarz, K.; Dembla, E.; Dembla, M.; Schmitz, F. Early changes in exo-and endocytosis in the EAE mouse model of multiple sclerosis correlate with decreased synaptic ribbon size and reduced ribbon-associated vesicle pools in rod photoreceptor synapses. Int. J. Mol. Sci. 2021, 22, 10789. [Google Scholar] [CrossRef]
- Cordano, C.; Werneburg, S.; Abdelhak, A.; Bennett, D.J.; Beaudry-Richard, A.; Duncan, G.J.; Oertel, F.C.; Boscardin, W.J.; Yiu, H.H.; Jabassini, N.; et al. Synaptic injury in the inner plexiform layer of the retina is associated with progression in multiple sclerosis. Cell Rep. Med. 2024, 5, 101490. [Google Scholar] [CrossRef]
- Schwarz, K.; Schmitz, F. Synapse dysfunctions in multiple sclerosis. Int. J. Mol. Sci. 2023, 24, 1639. [Google Scholar] [CrossRef]
- Moser, T.; Grabner, C.P.; Schmitz, F. Sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 2020, 100, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B. Transmission at rod and cone ribbon synapses in the retina. Pflugers Arch. Eur. J. Physiol. 2021, 473, 1469–1491. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Faria-Pereira, A.; Morais, V.A. Synapses: The brain’s energy-demanding sites. Int. J. Mol. Sci. 2022, 23, 3627. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.V.; Ciampi, D.; Duarte, C.B. Mitochondria as central hubs in synaptic modulation. Cell. Mol. Life Sci. 2023, 80, 173. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Jaiswal, M. Mitochondrial calcium at the synapse. Mitochondria 2021, 59, 135–153. [Google Scholar] [CrossRef]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Heidker, R.M.; Emerson, M.R.; LeVine, S.M. Metabolic pathways as possible therapeutic targets for progressive multiple sclerosis. Neural Reg. Res. 2017, 12, 1262–1267. [Google Scholar]
- Mahad, D.J.; Ziabreva, I.; Campbell, G.; Lax, N.; White, K.; Hanson, P.S.; Lassmann, H.; Turnbull, D.M. Mitochondrial changes within axons in multiple sclerosis. Brain 2009, 132, 1161–1174. [Google Scholar] [CrossRef]
- van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef]
- Atkinson, K.C.; Osunde, M.; Tiwari-Woodruff, S.K. The complexities of investigating mitochondria dynamics in multiple sclerosis and mouse models of MS. Front. Neurosci. 2023, 17, 1144896. [Google Scholar] [CrossRef]
- Greeck, V.B.; Williams, S.K.; Haas, J.; Wildemann, B.; Fairless, R. Alterations in lymphocytic metabolism-an emerging hallmark of MS pathophysiology? Int. J. Mol. Sci. 2023, 24, 2094. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; McDonough, J.; Yin, X.G.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zambonin, J.L.; Zhao, C.; Ohno, N.; Campbell, G.R.; Engeham, S.; Ziabreva, I.; Schwarz, N.; Lee, S.E.L.; Frischner, J.M.; Turnbull, D.M.; et al. Increased mitochondrial content in remyelinated axons: Implications for multiple sclerosis. Brain 2011, 134, 1901–1913. [Google Scholar] [CrossRef]
- Witte, M.E.; Nijland, P.G.; Drexhage, J.A.; Gerritsen, W.; Geerts, D.; van Het Hof, B.; Reijerkerk, A.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013, 125, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Licht-Mayer, S.; Campbell, G.R.; Canizares, M.; Mehta, A.R.; Gane, A.B.; McGill, K.; Gosh, A.; Fullerton, A.; Menezes, N.; Dean, J.; et al. Enhanced axonal response of mitochondria in demyelination offers neuroprotection: Implications for multiple sclerosis. Acta Neuropathol. 2020, 140, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Schattling, B.; Engler, J.B.; Volkmann, C.; Rothammer, N.; Woo, M.S.; Petersen, M.; Winkler, I.; Kaufmann, M.; Rosenkranz, S.C.; Fejtova, A.; et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat. Neurosci. 2019, 22, 887–896. [Google Scholar] [CrossRef]
- Rosenkranz, S.C.; Shaposhnykov, A.A.; Träger, S.; Engler, J.B.; Witte, M.E.; Roth, V.; Vieira, V.; Paauw, N.; Bauer, S.; Schwencke-Westphal, C.; et al. Enhancing mitochondrial activity in neurons protects against neurodegeneration in a mouse model of multiple sclerosis. eLife 2021, 10, 61798. [Google Scholar] [CrossRef]
- Johnson, J.E., Jr.; Perkins, G.A.; Chaney, S.; Xiao, W.; White, A.D.; Brown, J.M.; Waggoner, J.; Ellisman, M.H.; Fox, D.A. Spatiotemporal regulation of ATP and Ca2+ in vertebrate rod and cone ribbon synapses. Mol. Vis. 2007, 13, 887–919. [Google Scholar]
- Stone, J.; van Driel, D.; Valter, K.; Rees, S.; Provis, J. The locations of mitochondria in mammalian photoreceptor: Relation to retinal vasculature. Brain Res. 2008, 1189, 58–69. [Google Scholar] [CrossRef]
- Li, S.; Mitchell, J.; Briggs, D.J.; Young, J.K.; Long, S.S.; Fuerst, P.G. Morphological diversity of the rod spherule: A study of serially reconstructed electron micrographs. PLoS ONE 2016, 11, e0150024. [Google Scholar] [CrossRef] [PubMed]
- Gierke, K.; Lux, U.T.; Regus-Leidig, H.; Brandstätter, J.H. The first synapse in vision in the aging mouse retina. Front. Cell. Neurosci. 2023, 17, 1291054. [Google Scholar] [CrossRef] [PubMed]
- El Samad, A.; Jaffal, J.; Ibrahim, D.R.; Schwarz, K.; Schmitz, F. Decreased expression of the EAAT5 glutamate transporter at photoreceptor synapses in early, preclinical experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. Biomedicines 2024, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis as a model of multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef]
- Redler, Y.; Levy, M. Rodent models of optic neuritis. Front. Neurol. 2020, 11, 580951. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Gold, S.M. Sex-related factors in multiple sclerosis: Genetic, hormonal and environmantal contributions. Nat. Rev. Neurol. 2015, 8, 255–263. [Google Scholar] [CrossRef]
- Rahn, E.J.; Iannitti, T.; Donahue, R.R.; Taylor, B.K. Sex differences in a mouse model of multiple sclerosis: Neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol. Sex Differ. 2014, 5, 4. [Google Scholar] [CrossRef]
- Wiedrick, J.; Meza-Romero, R.; Gerstner, G.; Seifert, H.; Chaudhary, P.; Headrick, A.; Kent, G.; Maestas, A.; Offner, H.; Vandenbark, A.A. Sex differences in EAE reveal common and distinct cellular and molecular components. Cellul. Immunol. 2021, 359, 104242. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Greer, J. Effects of biological sex and pregnancy in experimental autoimmune encephalomyelitis: It’s complicated. Front. Immunol. 2022, 13, 1059833. [Google Scholar] [CrossRef] [PubMed]
- Hasselmann, J.P.C.; Karim, H.; Khalaj, A.J.; Gosh, S.; Tiwari-Woodruff, S.K. Consistent induction of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice for the longitudinal study of pathology and repair. J. Neurosci. Methods 2017, 284, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Shankhwar, S.; Schwarz, K.; Katiyar, R.; Jung, M.; Maxeiner, S.; Südhof, T.C.; Schmitz, F. RIBEYE B-domain is essential for RIBEYE A-domain stability and assembly of synaptic ribbons. Front. Mol. Neurosci. 2022, 15, 838311. [Google Scholar] [CrossRef] [PubMed]
- Petrungaro, C.; Zimmermann, K.M.; Küttner, V.; Fischer, M.; Dengjel, J.; Bogeski, I.; Riemer, J. The Ca(2+)-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab. 2015, 22, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Guo, Y.; Xue, B.; Shi, P.; Chen, Y.; Su, Q.P.; Hao, H.; Zhao, S.; Wu, C.; Yu, L.; et al. ER-mitochondria contacts promote mtDNA nucleoids active transportation via mitochondrial dynamic tubulation. Nat. Commun. 2020, 11, 4471. [Google Scholar] [CrossRef]
- Sen, A.; Kallabis, S.; Gaedke, F.; Jüngst, C.; Boix, J.; Nüchel, J.; Maliphol, K.; Hofmann, J.; Schauss, A.C.; Krüger, M.; et al. Mitochondrial membrane proteins and VPS35 orchestrate selective removal of mtDNA. Nat. Commun. 2022, 13, 6704. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factor FOXO1 and FOXO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.C.; Chen, L.C.; Tsang, N.M.; Chuang, W.Y.; Liao, T.C.; Yuan, S.N.; OuYang, C.N.; Ojcius, D.M.; Wu, C.C.; Chang, Y.S. Mitochondrial oxidative complex regulates NLRP3 inflammasome activation and predicts patient survival in nasopharyngeal carcinoma. Mol. Cell. Proteom. 2019, 19, 142–154. [Google Scholar] [CrossRef]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionary conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Gao, Z.Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.P. Icaritin provides neuroprotection in Parkinson’s disease by attenuating neuroinflammation, oxidative stress, and energy deficiency. Antioxidants 2021, 10, 529. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, J.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/Pink1. Redox. Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Wang, X.J.; Qi, L.; Cheng, Y.F.; Ji, X.F.; Chi, T.Y.; Liu, P.; Zou, L.B. Pink1 overexpression prevents forskolin-induced tau hyperphosphorylation and oxidative stress in a rat model of Alzheimer’s disease. Acta Pharmacol. Sin. 2022, 43, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jin, R.; Liu, J.; Ren, L.; Zhang, Y.; Shan, Z.; Peng, X. Diosgenin targets CAMKII to alleviate type II diabetic nephropathy through improving autophagy, mitophagy and mitochondrial dynamics. Nutrients 2023, 15, 3554. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Crisol, B.M.; Brícola, R.S.; Santana, M.R.; Nakandakari, S.C.B.R.; Costa, S.O.; Prada, P.O.; da Silva, A.S.R.; Moura, L.P.; Pauli, J.R.; et al. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci. Rep. 2021, 11, 3183. [Google Scholar] [CrossRef]
- Guo, L.; Cui, C.; Wang, J.; Yuan, J.; Yang, Q.; Zhang, P.; Su, W.; Bao, R.; Ran, J.; Wu, C. PINCH-I regulates mitochondrial dynamics to promote proline synthesis and tumor growth. Nat. Commun. 2020, 11, 4913. [Google Scholar] [CrossRef]
- Xu, Z.; Fu, T.; Guo, Q.; Zhou, D.; Sun, W.; Zhou, Z.; Chen, X.; Zhang, J.; Liu, L.; Xiao, L.; et al. Disuse-associated loss of protease LONP1 in muscle impairs mitochondrial function and causes reduced skeletal muscle mass and strength. Nat. Commun. 2022, 13, 894. [Google Scholar] [CrossRef]
- Qiu, S.; Zhong, X.; Meng, X.; Li, S.; Qian, X.; Lu, H.; Cai, J.; Zhang, Y.; Wang, M.; Ye, Z.; et al. Mitochondria localized cGAS suppresses ferroptosis to promote cancer progression. Cell Res. 2023, 33, 299–311. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Huo, X.K.; Ning, J.; Yu, Z.L.; Morisseu, C.; Sun, C.P.; Hammock, B.D.; Ma, X.C. Macrophage inactivation by small molecule wedelolactone via targeting sEH for the treatment of LPS-induced acute lung injury. ACS Cent. Sci. 2023, 9, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.L. Two monoclonal antibodies to actin: One muscle selective and one generally reactive. Cell Motil. Cytoskelet. 1988, 10, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Williams, S.K.; Hoffman, D.B.; Stojic, A.; Hochmeister, S.; Schmitz, F.; Storch, M.K.; Diem, R. Preclinical retinal neurodegeneration in a model of multiple sclerosis. J. Neurosci. 2012, 32, 5585–5597. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Katiyar, R.; Schmitz, F. A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to the synaptic ribbon. J. Neurosci. 2013, 33, 10278–10300. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, K.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.H.; et al. The disease protein Tulp1 is essential for periactive zone endocytosis in photoreceptor ribbon synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef]
- Dembla, M.; Wahl, S.; Katiyar, R.; Schmitz, F. ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms a NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis. J. Neurosci. 2014, 34, 5245–5260. [Google Scholar] [CrossRef]
- Dembla, E.; Dembla, M.; Maxeiner, S.; Schmitz, F. Synaptic ribbons foster active zone stability and illumination-dependent active zone enrichment of RIM2 and Cav1.4 in photoreceptor synapses. Sci. Rep. 2020, 10, 5957. [Google Scholar] [CrossRef] [PubMed]
- Eich, M.L.; Dembla, E.; Wahl, S.; Dembla, M.; Schwarz, K.; Schmitz, F. The calcineurin-binding, activity-dependent splice variant dynamin1xb is highly enriched in synapses in various regions of the central nervous system. Front. Mol. Neurosci. 2017, 10, 230. [Google Scholar] [CrossRef]
- Suiwal, S.; Dembla, M.; Schwarz, K.; Katiyar, R.; Jung, M.; Carius, Y.; Maxeiner, S.; Lauterbach, M.A.; Lancaster, C.R.D.; Schmitz, F. Ciliary proteins repurposed by the synaptic ribbon: Trafficking myristoylated proteins at rod photoreceptor synapses. Int. J. Mol. Sci. 2022, 23, 7135. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Punge, A.; Hollopeter, G.; Willig, K.I.; Hobson, R.J.; Davis, M.W.; Hell, S.W.; Jorgensen, E.M. Protein localization in electron micrographs using fluorescence nanoscopy. Nat. Methods 2011, 8, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Südhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann-Schuppert, A.; Schnittler, H.-J. A simple assay for quantification of protein in tissue sections, cell cultures, and cell homogenates, and of protein immobilized on solid surfaces. Cell Tissue Res. 1997, 288, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A.G. G*Power 3: A flexibel statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 41, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.G.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009, 6, 1149–1160. [Google Scholar] [CrossRef]
- Hessenberger, M.; Zerbes, R.M.; Ramplet, H.; Kunz, S.; Xavier, A.H.; Purfürst, B.; Lilie, H.; Pfanner, N.; van der Laan, M.; Daumke, O. Regulated membrane modeling by MIC60 controls formation of mitochondrial cristae junctions. Nat. Commun. 2017, 8, 15258. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; van der Laan, M.; Matai, P.; Capaldi, R.A.; Cacidy, A.A.; Chacisnka, A.; Darshi, M.; Deckers, M.; Hoppins, S.; Icho, T.; et al. Unifom nomenclature for the mitochondrial contact site and cristae organizing system. J. Cell Biol. 2014, 204, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, M.; Horvath, S.E.; Pfanner, N. Mitochondrial contact site and cristae organizing system. Curr. Opin. Cell Biol. 2016, 41, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; van der Laan, M. The Yin & Yang of microbial architecture—Interplay of MICOS and F1F0-ATPase synthase in cristae formation. Microb. Cell 2017, 4, 236–239. [Google Scholar] [PubMed]
- Wollweber, F.; von der Malsburg, K.; van der Laan, M. Mitochondrial contact site and cristae organizing system.: A central player in membrane shaping and crosstalk. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Schorr, S.; van der Laan, M. Integrative functions of the mitochondrial contact site and cristae organizing system. Semin. Cell Develop. Biol. 2018, 76, 191–200. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Bopassa, J.C. Mitochondrial inner membrane protein, Mic60/mitofilin in mammalian organ protection. J. Cell Physiol. 2019, 234, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Ellisman, M.H.; Fox, D.A. Three-dimensional analysis of mouse rod and cone mitochondrial cristae architecture: Bioenergetic and functional implications. Mol. Vis. 2003, 9, 60–73. [Google Scholar] [PubMed]
- Meschede, I.P.; Ovenden, N.C.; Seabra, M.C.; Burgoyne, T. Symmetrical arrangements of mitochondria: Plasma membrane contacts between adjacent photoreceptor cells regulated by OPA1. Proc. Natl. Acad. Sci. USA 2020, 117, 15684–15693. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Tracey-White, D.; Kam, J.H.; Powner, M.B.; Jeffery, G. The 3D organization of mitochondria in primate photoreceptors. Sci. Rep. 2021, 11, 18863. [Google Scholar] [CrossRef] [PubMed]
- von der Malsburg, K.; Müller, J.M.; Bohnert, M.; Oeljeklaus, S.; Kwiatkowska, P.; Becker, T.; Loniewska-Lwowska, A.; Wiese, S.; Rao, S.; Milenkovic, D.; et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell 2011, 21, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Hsu, T.; Lin, H.L.; Fu, C.Y. Drosophila MICOS knockdown impairs mitochondrial structure and function and promotes mitophagy. Biol Open 2020, 9, bio054262. [Google Scholar] [CrossRef] [PubMed]
- Dennerlein, S.; Rehling, P. Human mitochondrial COX1 assembly into cytochrome c oxidase at a glance. J. Cell Sci. 2015, 128, 833–837. [Google Scholar] [CrossRef]
- Dennerlein, S.; Rehling, P.; Richter-Dennerlein, R. Cytochrome c oxidase biogenesis: From translation to early assembly of the core subunit COX1. FEBS Lett. 2023, 597, 1569–1578. [Google Scholar] [CrossRef]
- Corti, O.; Lesage, S.; Brice, A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol. Rev. 2011, 91, 1161–1218. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Gelmetti, V.; Torosantucci, L.; Vignone, D.; Lamorte, G.; de Rosa, P.; Cilia, E.; Jonas, E.A.; Valente, E.M. PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro- apoptotic cleavage. Cell Death Differ. 2013, 20, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, F.; Torosantucci, L.; Gelmetti, V.; Franzone, D.; Grünewald, A.; Krüger, R.; Arena, G.; Valente, E.M. PINK1 protects against staurosporine-induced apoptosis by interacting with beclin1 and impairing its pro-apoptotic cleavage. Cells 2022, 11, 678. [Google Scholar] [CrossRef]
- Xian, H.; Liu, Y.H. Functions of outer mitochondrial membrane proteins: Mediating the crosstalk between mitochondrial dynamics and mitophagy. Cell Death Diff. 2021, 28, 827–842. [Google Scholar] [CrossRef]
- Uoselis, L.; Nguyen, T.N.; Lazarou, M. Mitochondrial degradation and beyond. Mol. Cell 2023, 83, 3404–3420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin- mediated mitophagy in neurodegenerative disease. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef]
- Imoto, M.; Tachibana, I.; Urrutia, R. Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p. J. Cell Sci. 1998, 111, 1341–1349. [Google Scholar] [CrossRef]
- Cho, H.M.; Sun, W. Molecular cross talk among the components of the regulatory machinery of mitochondrial structure and quality control. Exp. Mol. Med. 2020, 52, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Zerihun, M.; Sukumaran, S.; Qvit, R. The DRP1-mediated mitochondrial fission protein interactome as an emerging core player in mitochondrial dynamics and cardiovascular disease therapy. Int. J. Mol. Sci. 2023, 24, 5782. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Nandan, P.K.; Job, A.T.; Ramasamy, T. DRP1 association in inflammation and metastasis: A review. Curr. Drug Targets 2024, 25, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Tabara, L.C.; Segawa, M.; Prudent, J. Molecular mechanisms of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ban-Ishihara, B.; Tomohiro-Takamiya, S.; Tani, M.; Baudier, J.; Ishihara, N.; Kuge, O. COX assembly factor ccdc56 regulates mitochondrial morphology by affecting mitochondrial recuitment of Drp1. FEBS Lett. 2015, 589, 3126–3132. [Google Scholar] [CrossRef]
- Yu, R.; Jin, S.B.; Ankarcrona, M.; Lendahl, U.; Nister, M.; Zhao, J. The molecular assembly state of Drp1controls its association with the mitochondrial recruitment receptors Mff and MIEF1/2. Front. Cell Develop Biol. 2021, 9, 706687. [Google Scholar] [CrossRef]
- Duan, C.; Liu, R.; Kuang, L.; Zhang, Z.; Hou, D.; Zheng, D.; Xiang, X.; Huang, H.; Liu, L.; Li, T. Activated DRP1 initiates the formation of endoplasmic reticulum-mitochondrial contacts via Shrm4 mediated actin bundling. Adv. Sci. 2023, 10, 2304885. [Google Scholar] [CrossRef] [PubMed]
- Rochon, K.; Bauer, B.L.; Roethler, N.A.; Buckley, Y.; Su, C.C.; Huang, W.; Ramachandran, R.; Stoll, M.S.K.; Yu, E.W.; Taylor, D.J.; et al. Structural basis for the regulated assembly of the mitochondrial fission Drp1. Nat. Commun. 2024, 15, 1328. [Google Scholar] [CrossRef] [PubMed]
- Zenisek, D.; Matthews, G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron 2000, 25, 229–237. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhose. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Wan, Q.F.; Nixon, E.; Heidelberger, R. Regulation of presynaptic calcium in a mammalian synaptic terminal. J. Neurophysiol. 2012, 108, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Gangwar, P.; Yadav, A.; Yadav, B.; Rao, R.; Kaur, S.; Kumar, P.; Dhiman, M.; Taglialatela, G.; Kumar Manta, A. Understanding the neuronal synapse and challenges associated with the mitochondrial dysfunction in mild cognitive impairment and Alzheimer’s disease. Mitochondrion 2003, 73, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cicali, K.A.; Tapia-Rojas, C. Synaptic mitochondria: A crucial factor in the aged hippocampus. Ageing Res. Rev. 2024, 101, 102524. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.; Erecinska, M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv. Exp. Med. Biol. 1998, 454, 7–16. [Google Scholar]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular tuning of the axonal mitochondrial Ca2+ uniporter ensures metabolic flexibility of neurotransmission. Neuron 2020, 105, 678–687. [Google Scholar] [CrossRef]
- Kann, O.; Kocács, R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 2007, 292, C641–C657. [Google Scholar] [CrossRef] [PubMed]
- Hagenston, A.M.; Bading, H.; Bas-Orth, C. Functional consequences of calcium-dependent synapse-to-nucleus communication: Focus on transcription-dependent metabolic plasticity. Cold Spring Harb. Perspect. Biol. 2020, 12, a035287. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; Monticelli, H.; Rizzuto, R.; Mammucari, C. The mitochondrial Ca2+ uptake and the fine-tuning of aerobic metabolism. Front. Physiol. 2020, 11, 554904. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, R. Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J. Gen. Physiol. 1998, 111, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J. Neurosci. 2001, 21, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, R.; Sterling, P.; Matthews, G. Roles of ATP in depletion and replenishment of the releasable pool of synaptic vesicles. J. Neurophysiol. 2002, 88, 98–106. [Google Scholar] [CrossRef]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008, 18, 1917–1921. [Google Scholar] [CrossRef]
- Wong-Riley, M. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Muangkram, Y.; Himeno, Y.; Amano, A. Clarifying the composition of the ATP consumption factors required for maintaining ion homeostasis in mouse photoreceptors. Sci. Rep. 2023, 13, 14161. [Google Scholar] [CrossRef]
- Rajda, C.; Pukoli, D.; Bende, Z.; Majlath, Z.; Vecsei, L. Excitotoxins, mitochondrial and redox disturbances in multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial dysfunction and multiple sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef]
- Sadeghian, M.; Mastrolia, V.; Haddad, A.R.; Mosley, A.; Mullali, G.; Schiza, D.; Saijic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci. Rep. 2016, 6, 33249. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Arai, K.; Iijima, M.; Sesaki, H. Mitochondrial division, fusion and degradation. J. Biochem. 2020, 167, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell 2023, 83, 857–876. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef]
- Ikeda, A.; Iijima, M.; Sesaki, H. Systemic phospho-defective and phospho-mimetic Drp1 mice exhibit normal growth and development with altered anxiety-like behavior. iScience 2024, 27, 109874. [Google Scholar] [CrossRef]
- Liu, A.; Hatch, A.L.; Higgs, H.N. Effects of phosphorylation on Drp1 activation by its receptors, actin, and cardiolipin. Mol. Biol. Cell 2024, 35, ar16. [Google Scholar] [CrossRef]
- Fecher, C.; Trovo, L.; Müller, S.A.; Snaidero, N.; Wettmarshausen, J.; Heink, S.; Ortiz, O.; Wagner, I.; Kühn, R.; Hartmann, J.; et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular heterogeneity. Nat. Neurosci. 2019, 22, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Kappler, L.; Hoene, M.; Hu, C.; von Toerne, C.; Li, J.; Bleher, D.; Hoffmann, C.; Böhm, A.; Kollipara, L.; Zischka , H.; et al. Linking bioenergetic function of mitochondria to tissue-specific molecular fingerprints. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E374–E387. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.L.; Hagen, J.T.; Coalson, H.S.; Nelson, M.A.; Kew, K.A.; Wooten, A.R.; Fisher-Wellman, K.H. Novel approach to quantify mitochondrial content and intrinsic bioenergetic efficiency across organs. Sci. Rep. 2020, 10, 17599. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Aoki, T.; Endo, Y.; Kazmi, J.; Hagiwara, J.; Kuschner, C.E.; Yin, T.; Kim, J.; Becker, L.B.; Hayashida, K. Organ-specific mitochondrial alterations following ischemia-perfusion injury in post-cardiac arrest syndrome: A comprehensive review. Life 2024, 14, 477. [Google Scholar] [CrossRef]
- Collins, T.J.; Berridge, M.J.; Lipp, P.; Bootman, M.D. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002, 21, 1616–1627. [Google Scholar] [CrossRef]
- Collins, T.J.; Bootman, M.D. Mitochondria are heterogeneous within cells. J. Exp. Biol. 2003, 206, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Usson, Y.; Leverve, X.; Margreiter, R. Subcellular heterogeneity of mitochondrial function and dysfunction: Evidence obtained by confocal imaging. Mol. Cell. Biochem. 2004, 256, 359–365. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Troppmair, J.; Sucher, R.; Herrmann, M.; Saks, V.; Margreiter, R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: Possible physiological role. Biochim. Biophys. Acta 2006, 1757, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R. Heterogeneity of mitochondria and mitochondrial function within cellsas another level of mitochondrial complexity. Int. J. Mol. Sci. 2009, 10, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Raabe, J.; Wittig, I.; Laurette, P.; Stathopoulos, P.; Brand, T.; Schulze, T.; Klampe, B.; Orthey, E.; Cabrera-Orefice, A.; Meisterknecht, J.; et al. Physioxia rewires mitochondrial complex composition to protect stem cell viability. Redox Biol. 2024, 77, 103352. [Google Scholar] [CrossRef]
- Hamilton, J.; Brustovetsky, T.; Rysted, J.E.; Lin, Z.; Usachev, Y.M.; Brustovetsky, N. Deletion of mitochondrial calcium uniporter incompletely inhibits calcium uptake and induction of the permeability transition pore in brain mitochondria. J. Biol. Chem. 2018, 293, 15652–15663. [Google Scholar] [CrossRef]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J. Mol. Cell. Cardiol. 2024, 174, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bayev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of mitochondrial calcium and ROS in neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Bayev, A.Y.; Vinokurov, A.Y.; Potapova, E.V.; Dunaev, A.V.; Angelova, P.R.; Abramov, A.Y. Mitochondrial permeability transition, cell death and neurodegeneration. Cells 2024, 13, 648. [Google Scholar] [CrossRef]
- Bround, M.J.; Abay, E.; Huo, J.; Havens, J.R.; York, A.J.; Bers, D.M.; Molkentin, J.D. MCU-independent Ca2+ uptake mediates mitochondrial overload in a mouse model of Duchenne muscular dystrophy. Sci. Rep. 2024, 14, 6751. [Google Scholar] [CrossRef] [PubMed]
- Hagenston, A.M.; Yan, J.; Bas-Orth, C.; Tan, Y.; Sekler, I.; Bading, H. Disrupted expression of mitochondrial NCLX sensitizes neuroglial networks to excitotoxic stimuli and renders synaptic activity toxic. J. Biol. Chem. 2022, 298, 101508. [Google Scholar] [CrossRef]
- Malvaso, A.; Gatti, A.; Negro, G.; Calatozzolo, C.; Medici, V.; Poloni, T.E. Microglial senescence and activation in healthy aging and Alzheimer’s disease: Systematic review and neuropathological scoring. Cells 2023, 12, 2824. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kishore, A.; Rishi, V.; Aggarwal, A. Mitochondria and its epigenetic dynamics: Insight into synaptic regulation and synaptopathies. Funct. Integr. Genom. 2025, 25, 26. [Google Scholar] [CrossRef]
- Guan, D.; Liang, C.; Zheng, D.; Liu, S.; Luo, J.; Cai, Z.; Zhang, H.; Chen, J. The role of mitochondrial remodeling in neurodegenerative diseases. Neurochem. Int. 2025, 183, 105927. [Google Scholar] [CrossRef] [PubMed]
- McGill Percy, K.C.; Liu, Z.; Qi, X. Mitochondrial dysfunction in Alzheimer’s disease: Guiding the path to targeted therapies. Neurotherapeutics 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Antico, O.; Thompson, P.W.; Hertz, N.T.; Muqit, M.M.K.; Parton, L.E. Targeting mitophagy in neurodegenerative diseases. Nat. Rev. Drug Discov. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Souza, E.M.; Joselevitch, C.; Britto, L.R.G.; Chiavegatto, S. Retinal alterations in a pre-clinical model of an autism spectrum disorder. Mol. Autism 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Ramirez, A.I.; Fernandez-Albarral, J.A.; Lopez-Cuenca, I.; Salobrar-Garcia, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramirez, J.M. Amyotrophic lateral sclerosis: A neurodegenerative disease with ocular involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, F.; Ma, Y.; Xue, T.; Shen, Y. Identification of early-onset photoreceptor degeneration in transgenic mouse models of Alzheimer’s disease. iScience 2021, 24, 103327. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, V.O.; Kukharsky, M.S.; Belykh, A.E.; Sobolev, A.M.; Deykin, A.V. Retinal damage in amyotrophic lateral sclerosis: Underlying mechanisms. Eye Brain 2021, 13, 131–146. [Google Scholar] [CrossRef]
- Gao, J.; Leinonen, H.; Wang, E.J.; Ding, M.; Perry, G.; Palcewski, K.; Wang, X. Sex-specific early retinal dysfunction in mutant TDP-43 transgenic mice. J. Alzheimer Dis. 2024, 97, 927–937. [Google Scholar] [CrossRef]
| Antibody | Source | References | Dilution |
|---|---|---|---|
| RIBEYE(B), rabbit polyclonal (U2656) | Lab-made | [32] | 1:1000 (IF) |
| RIBEYE(B), mouse monoclonal (clone 2D9) | Lab-made | [25,62] | 1:1000 (IF) |
| MIC60/Mitofilin (affinity-purified rabbit polyclonal) | Proteintech (Rosemont, IL, USA), 10179-1-AP | [63,64,65] | 1:1000 (IF) 1:1000 (LI-COR) 1:100 (IG) |
| ATP5B (mouse monoclonal antibody, clone E1), directed against the ATP5B subunit of the F1 complex of the mitochondrial ATPase | Santa Cruz (Dallas, TX, USA) sc-55597 | [66,67,68,69] | 1:20 (IF) |
| PTEN-induced kinase-1 (PINK1), affinity-purified rabbit polyclonal antibody, 23274-1-AP | ProteinTech, 23274-1-AP | [70,71,72] | 1:100 (IF) |
| Cytochrome c oxidase subunit 1 (COX1), aa401-aa500 affinity-purified rabbit polyclonal | Bioss Antibodies (Woburn, MA, USA) (via Biozol) (bs-3953R) | [73] | 1:100 (IF) |
| Dynamin-related protein 1 (DRP1), raised against a C-terminal fusion protein of DRP1, affinity-purified rabbit polyclonal antibody | Proteintech 12957-1-AP | [74,75,76,77] | 1:100 (IF) 1:250 (LI-COR) |
| Actin (mouse monoclonal antibody, clone C4), #1501R | Millipore (Burlington, MA, USA) #1501R | [78] | 1:1000 (LI-COR) |
| Antibody | Source | Dilution |
|---|---|---|
| Chicken anti-mouse-Alexa488 | Invitrogen, Molecular Probes (Waltham, MA, USA), A-21200 | 1:1000 (IF) |
| Donkey anti-rabbit-Alexa568 | Invitrogen, Molecular Probes, A-10042 | 1:1000 (IF) |
| Chicken anti-rabbit-Alexa488 | Invitrogen, Molecular Probes, A-21441 | 1:1000 (IF) |
| Donkey anti-mouse-Alexa568 | Invitrogen, Molecular Probes, A-10037 | 1:1000 (IF) |
| IRDye® 800cw Donkey anti-rabbit | LI-COR, 926-32213 | 1:5000 (LI-COR) |
| IRDye® 680 LT Donkey anti-mouse | LI-COR, 926-68022 | 1:5000 (LI-COR) |
| Goat anti-rabbit secondary antibody conjugated to 5 nm gold particles | Sigma (Taufkirchen, Germany), G7277 | 1:100 (IG) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.R.; Schwarz, K.; Suiwal, S.; Maragkou, S.; Schmitz, F. Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis. Cells 2025, 14, 206. https://doi.org/10.3390/cells14030206
Ibrahim DR, Schwarz K, Suiwal S, Maragkou S, Schmitz F. Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis. Cells. 2025; 14(3):206. https://doi.org/10.3390/cells14030206
Chicago/Turabian StyleIbrahim, Dalia R., Karin Schwarz, Shweta Suiwal, Sofia Maragkou, and Frank Schmitz. 2025. "Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis" Cells 14, no. 3: 206. https://doi.org/10.3390/cells14030206
APA StyleIbrahim, D. R., Schwarz, K., Suiwal, S., Maragkou, S., & Schmitz, F. (2025). Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis. Cells, 14(3), 206. https://doi.org/10.3390/cells14030206








