Three-Dimensional Models: Biomimetic Tools That Recapitulate Breast Tissue Architecture and Microenvironment to Study Ductal Carcinoma In Situ Transition to Invasive Ductal Breast Cancer
Abstract
:1. Introduction
2. The DCIS Microenvironment
2.1. The Biological Significance of the ECM and Its Role in DCIS Transition
2.2. CAFs and Their Role in DCIS Progression
2.3. Other Stromal Cells in DCIS Progression
2.3.1. Myoepithelial Cells
2.3.2. Adipocytes
2.3.3. Tumor-Infiltrating Lymphocytes
2.3.4. Tumor-Associated Macrophages
3. Significance of 3D Models
4. Tumor-on-Chip Models: An Emerging 3D Technology to Study DCIS Transition
4.1. Microfluidics
4.2. Breast Cancer-on-a-Chip
4.3. L-TumorChip
4.4. Microfluidic IDC-on-Chip to Study Epithelial-Endothelial Migration
4.5. Mammary Duct Model Capturing Matrix Mechanics
4.6. Microfluidic Platform for Tumor Spheroid Invasion
4.7. Microfluidic DCIS Model to Study Tumor Metabolism
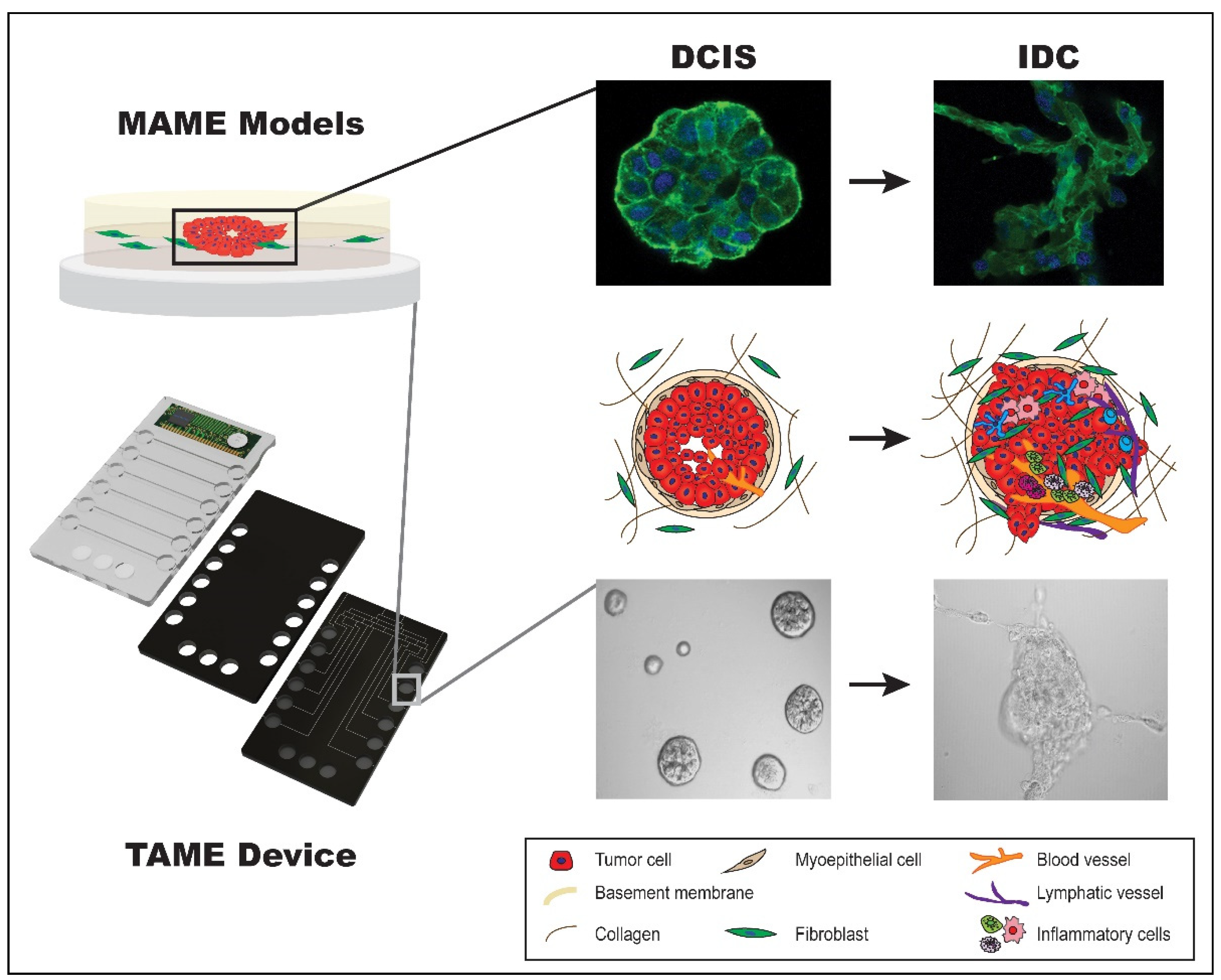
4.8. MAME Model and TAME 3D Cell Culture Device
4.9. 3D Culture Platform Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; Elshof, L.E.; Visser, L.L.; Rutgers, E.J.T.; Winter-Warnars, H.A.O.; Lips, E.H.; Wesseling, J. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017, 31, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; Leon-Sosa, A.; Lugo, P.; Suquillo, D.; Torres, F.; Surre, F.; Trojman, L.; Caicedo, A. Breast cancer, screening and diagnostic tools: All you need to know. Crit. Rev. Oncol. Hematol. 2021, 157, 103174. [Google Scholar] [CrossRef]
- Kaur, H.; Mao, S.; Shah, S.; Gorski, D.H.; Krawetz, S.A.; Sloane, B.F.; Mattingly, R.R. Next-generation sequencing: A powerful tool for the discovery of molecular markers in breast ductal carcinoma in situ. Expert. Rev. Mol. Diagn. 2013, 13, 151–165. [Google Scholar] [CrossRef]
- Rebbeck, C.A.; Xian, J.; Bornelov, S.; Geradts, J.; Hobeika, A.; Geiger, H.; Alvarez, J.F.; Rozhkova, E.; Nicholls, A.; Robine, N.; et al. Gene expression signatures of individual ductal carcinoma in situ lesions identify processes and biomarkers associated with progression towards invasive ductal carcinoma. Nat. Commun. 2022, 13, 3399. [Google Scholar] [CrossRef]
- Doke, K.; Butler, S.; Mitchell, M.P. Current Therapeutic Approaches to DCIS. J. Mammary Gland. Biol. Neoplasia 2018, 23, 279–291. [Google Scholar] [CrossRef]
- Marmot, M.G.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Br. J. Cancer 2013, 108, 2205–2240. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Helvie, M.A.; Hendrick, R.E. Current Issues in the Overdiagnosis and Overtreatment of Breast Cancer. AJR Am. J. Roentgenol. 2018, 210, 285–291. [Google Scholar] [CrossRef]
- Ong, M.S.; Mandl, K.D. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff. 2015, 34, 576–583. [Google Scholar] [CrossRef]
- Ehsan, A.N.; Wu, C.A.; Minasian, A.; Singh, T.; Bass, M.; Pace, L.; Ibbotson, G.C.; Bempong-Ahun, N.; Pusic, A.; Scott, J.W.; et al. Financial Toxicity Among Patients With Breast Cancer Worldwide: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2255388. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Dinh, P.; Pathmanathan, N.; Graham, J.D. Ductal Carcinoma in Situ: Molecular Changes Accompanying Disease Progression. J. Mammary Gland. Biol. Neoplasia 2022, 27, 101–131. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Mariotto, A.; Tangka, F.; Zhao, J.; Islami, F.; Sung, H.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Ward, E.M. Annual Report to the Nation on the Status of Cancer, Part 2: Patient Economic Burden Associated With Cancer Care. J. Natl. Cancer Inst. 2021, 113, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Hanna, W.M.; Parra-Herran, C.; Lu, F.I.; Slodkowska, E.; Rakovitch, E.; Nofech-Mozes, S. Ductal carcinoma in situ of the breast: An update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod. Pathol. 2019, 32, 896–915. [Google Scholar] [CrossRef]
- Solin, L.J.; Gray, R.; Baehner, F.L.; Butler, S.M.; Hughes, L.L.; Yoshizawa, C.; Cherbavaz, D.B.; Shak, S.; Page, D.L.; Sledge, G.W., Jr.; et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. 2013, 105, 701–710. [Google Scholar] [CrossRef]
- Nofech-Mozes, S.; Hanna, W.; Rakovitch, E. Molecular Evaluation of Breast Ductal Carcinoma in Situ with Oncotype DX DCIS. Am. J. Pathol. 2019, 189, 975–980. [Google Scholar] [CrossRef]
- Rakovitch, E.; Parpia, S.; Koch, A.; Grimard, L.; Soliman, H.; Stevens, C.; Perera, F.; Kong, I.; Senthelal, S.; Anthes, M.; et al. DUCHESS: An evaluation of the ductal carcinoma in situ score for decisions on radiotherapy in patients with low/intermediate-risk DCIS. Breast Cancer Res. Treat. 2021, 188, 133–139. [Google Scholar] [CrossRef]
- Manders, J.B.; Kuerer, H.M.; Smith, B.D.; McCluskey, C.; Farrar, W.B.; Frazier, T.G.; Li, L.; Leonard, C.E.; Carter, D.L.; Chawla, S.; et al. Clinical Utility of the 12-Gene DCIS Score Assay: Impact on Radiotherapy Recommendations for Patients with Ductal Carcinoma In Situ. Ann. Surg. Oncol. 2017, 24, 660–668. [Google Scholar] [CrossRef]
- Bremer, T.; Whitworth, P.W.; Patel, R.; Savala, J.; Barry, T.; Lyle, S.; Leesman, G.; Linke, S.P.; Jirstrom, K.; Zhou, W.; et al. A Biological Signature for Breast Ductal Carcinoma In Situ to Predict Radiotherapy Benefit and Assess Recurrence Risk. Clin. Cancer Res. 2018, 24, 5895–5901. [Google Scholar] [CrossRef]
- Warnberg, F.; Karlsson, P.; Holmberg, E.; Sandelin, K.; Whitworth, P.W.; Savala, J.; Barry, T.; Leesman, G.; Linke, S.P.; Shivers, S.C.; et al. Prognostic Risk Assessment and Prediction of Radiotherapy Benefit for Women with Ductal Carcinoma In Situ (DCIS) of the Breast, in a Randomized Clinical Trial (SweDCIS). Cancers 2021, 13, 6103. [Google Scholar] [CrossRef]
- Weinmann, S.; Leo, M.C.; Francisco, M.; Jenkins, C.L.; Barry, T.; Leesman, G.; Linke, S.P.; Whitworth, P.W.; Patel, R.; Pellicane, J.; et al. Validation of a Ductal Carcinoma In Situ Biomarker Profile for Risk of Recurrence after Breast-Conserving Surgery with and without Radiotherapy. Clin. Cancer Res. 2020, 26, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Mann, G.B.; Shah, C.; Weinmann, S.; Leo, M.C.; Whitworth, P.; Rabinovitch, R.; Torres, M.A.; Margenthaler, J.A.; Dabbs, D.; et al. A Novel Biosignature Identifies Patients With DCIS With High Risk of Local Recurrence After Breast Conserving Surgery and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Dabbs, D.; Mittal, K.; Heineman, S.; Whitworth, P.; Shah, C.; Savala, J.; Shivers, S.C.; Bremer, T. Analytical validation of the 7-gene biosignature for prediction of recurrence risk and radiation therapy benefit for breast ductal carcinoma in situ. Front. Oncol. 2023, 13, 1069059. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Whitworth, P.; Vicini, F.A.; Narod, S.; Gerber, N.; Jhawar, S.R.; King, T.A.; Mittendorf, E.A.; Willey, S.C.; Rabinovich, R.; et al. The Clinical Utility of a 7-Gene Biosignature on Radiation Therapy Decision Making in Patients with Ductal Carcinoma In Situ Following Breast-Conserving Surgery: An Updated Analysis of the DCISionRT((R)) PREDICT Study. Ann. Surg. Oncol. 2024, 31, 5919–5928. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Prospective Registry Study to Evaluate the Effect of the DCISionRT Test on Treatment Decisions in Patients with DCIS Following Breast Conserving Therapy. Available online: https://clinicaltrials.gov/study/NCT03448926 (accessed on 23 January 2025).
- Raldow, A.C.; Sher, D.; Chen, A.B.; Recht, A.; Punglia, R.S. Cost Effectiveness of the Oncotype DX DCIS Score for Guiding Treatment of Patients With Ductal Carcinoma In Situ. J. Clin. Oncol. 2016, 34, 3963–3968. [Google Scholar] [CrossRef]
- Alvarado, M.; Lucci, A.; Manders, J. Best practices for multidisciplinary integration of a DCIS genomic assay into clinical practice. J. Surg. Oncol. 2017, 116, 1016–1020. [Google Scholar] [CrossRef]
- Raldow, A.C.; Sher, D.; Chen, A.B.; Punglia, R.S. Cost Effectiveness of DCISionRT for Guiding Treatment of Ductal Carcinoma in Situ. JNCI Cancer Spectr. 2020, 4, pkaa004. [Google Scholar] [CrossRef]
- Ma, X.J.; Dahiya, S.; Richardson, E.; Erlander, M.; Sgroi, D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009, 11, R7. [Google Scholar] [CrossRef]
- Conklin, M.W.; Keely, P.J. Why the stroma matters in breast cancer: Insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef]
- Partridge, A.H.; Hyslop, T.; Rosenberg, S.M.; Bennett, A.V.; Drier, S.; Jonsson, M.; Shimada, A.; Li, Y.; Li, Y.; Lynch, T.; et al. Patient-Reported Outcomes for Low-Risk Ductal Carcinoma In Situ: A Secondary Analysis of the COMET Randomized Clinical Trial. JAMA Oncol. 2024. [Google Scholar] [CrossRef]
- Schmitz, R.; Engelhardt, E.G.; Gerritsma, M.A.; Sondermeijer, C.M.T.; Verschuur, E.; Houtzager, J.; Griffioen, R.; Retel, V.; Bijker, N.; Mann, R.M.; et al. Active surveillance versus treatment in low-risk DCIS: Women’s preferences in the LORD-trial. Eur. J. Cancer 2023, 192, 113276. [Google Scholar] [CrossRef] [PubMed]
- Wheelwright, S.; Matthews, L.; Jenkins, V.; May, S.; Rea, D.; Fairbrother, P.; Gaunt, C.; Young, J.; Pirrie, S.; Wallis, M.G.; et al. Recruiting women with ductal carcinoma in situ to a randomised controlled trial: Lessons from the LORIS study. Trials 2023, 24, 670. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchio, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Osuala, K.O.; Sameni, M.; Shah, S.; Aggarwal, N.; Simonait, M.L.; Franco, O.E.; Hong, Y.; Hayward, S.W.; Behbod, F.; Mattingly, R.R.; et al. Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer 2015, 15, 584. [Google Scholar] [CrossRef]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef]
- Gibson, S.V.; Roozitalab, R.M.; Allen, M.D.; Jones, J.L.; Carter, E.P.; Grose, R.P. Everybody needs good neighbours: The progressive DCIS microenvironment. Trends Cancer 2023, 9, 326–338. [Google Scholar] [CrossRef]
- Nelson, A.C.; Machado, H.L.; Schwertfeger, K.L. Breaking through to the Other Side: Microenvironment Contributions to DCIS Initiation and Progression. J. Mammary Gland. Biol. Neoplasia 2018, 23, 207–221. [Google Scholar] [CrossRef]
- Risom, T.; Glass, D.R.; Averbukh, I.; Liu, C.C.; Baranski, A.; Kagel, A.; McCaffrey, E.F.; Greenwald, N.F.; Rivero-Gutierrez, B.; Strand, S.H.; et al. Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma. Cell 2022, 185, 299–310. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Kaushik, S.; Pickup, M.W.; Weaver, V.M. From transformation to metastasis: Deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev. 2016, 35, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Taufalele, P.V.; VanderBurgh, J.A.; Munoz, A.; Zanotelli, M.R.; Reinhart-King, C.A. Fiber alignment drives changes in architectural and mechanical features in collagen matrices. PLoS ONE 2019, 14, e0216537. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.F.; Aszodi, A.; Alves, F.; Pawson, T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell Biol. 2001, 21, 2906–2917. [Google Scholar] [CrossRef]
- Neuhaus, B.; Buhren, S.; Bock, B.; Alves, F.; Vogel, W.F.; Kiefer, F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol. Life Sci. 2011, 68, 3757–3770. [Google Scholar] [CrossRef]
- Shintani, Y.; Fukumoto, Y.; Chaika, N.; Svoboda, R.; Wheelock, M.J.; Johnson, K.R. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 2008, 180, 1277–1289. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Brett, E.A.; Sauter, M.A.; Machens, H.G.; Duscher, D. Tumor-associated collagen signatures: Pushing tumor boundaries. Cancer Metab. 2020, 8, 14. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Gangnon, R.E.; Sprague, B.L.; Van Gemert, L.; Hampton, J.M.; Eliceiri, K.W.; Bredfeldt, J.S.; Liu, Y.; Surachaicharn, N.; Newcomb, P.A.; et al. Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma In Situ. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.K.; Allen, M.D.; Gomm, J.J.; Goulding, I.; Thompson, C.L.; Knight, M.M.; Marshall, J.F.; Jones, J.L. Mechanostimulation of breast myoepithelial cells induces functional changes associated with DCIS progression to invasion. NPJ Breast Cancer 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the "omics" era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Shao, X.; Gomez, C.D.; Kapoor, N.; Considine, J.M.; Grams, C.; Gao, Y.T.; Naba, A. MatrisomeDB 2.0: 2023 updates to the ECM-protein knowledge database. Nucleic Acids Res. 2023, 51, D1519–D1530. [Google Scholar] [CrossRef]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, e1700167. [Google Scholar] [CrossRef]
- Wiseman, B.S.; Sternlicht, M.D.; Lund, L.R.; Alexander, C.M.; Mott, J.; Bissell, M.J.; Soloway, P.; Itohara, S.; Werb, Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003, 162, 1123–1133. [Google Scholar] [CrossRef]
- Guo, Q.; Betts, C.; Pennock, N.; Mitchell, E.; Schedin, P. Mammary Gland Involution Provides a Unique Model to Study the TGF-beta Cancer Paradox. J. Clin. Med. 2017, 6, 10. [Google Scholar] [CrossRef]
- Buchsbaum, R.J.; Oh, S.Y. Breast Cancer-Associated Fibroblasts: Where We Are and Where We Need to Go. Cancers 2016, 8, 19. [Google Scholar] [CrossRef]
- Fernandez-Nogueira, P.; Fuster, G.; Gutierrez-Uzquiza, A.; Gascon, P.; Carbo, N.; Bragado, P. Cancer-Associated Fibroblasts in Breast Cancer Treatment Response and Metastasis. Cancers 2021, 13, 3146. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, E.; Weigert, A. Breast Cancer CAFs: Spectrum of Phenotypes and Promising Targeting Avenues. Int. J. Mol. Sci. 2021, 22, 11636. [Google Scholar] [CrossRef] [PubMed]
- Arcucci, A.; Ruocco, M.R.; Granato, G.; Sacco, A.M.; Montagnani, S. Cancer: An Oxidative Crosstalk between Solid Tumor Cells and Cancer Associated Fibroblasts. Biomed. Res. Int. 2016, 2016, 4502846. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef]
- Osuala, K.O.; Heyza, J.; Zhao, Z.; Xu, Y.; Moin, K.; Ji, K.; Mattingly, R.R. Carcinoma-Associated Fibroblasts Accelerate Growth and Invasiveness of Breast Cancer Cells in 3D Long-Term Breast Cancer Models. Cancers 2024, 16, 3840. [Google Scholar] [CrossRef]
- Louault, K.; Bonneaud, T.L.; Seveno, C.; Gomez-Bougie, P.; Nguyen, F.; Gautier, F.; Bourgeois, N.; Loussouarn, D.; Kerdraon, O.; Barille-Nion, S.; et al. Interactions between cancer-associated fibroblasts and tumor cells promote MCL-1 dependency in estrogen receptor-positive breast cancers. Oncogene 2019, 38, 3261–3273. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lovrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Pankova, D.; Chen, Y.; Terajima, M.; Schliekelman, M.J.; Baird, B.N.; Fahrenholtz, M.; Sun, L.; Gill, B.J.; Vadakkan, T.J.; Kim, M.P.; et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol. Cancer Res. 2016, 14, 287–295. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Brummer, G.; Acevedo, D.S.; Hu, Q.; Portsche, M.; Fang, W.B.; Yao, M.; Zinda, B.; Myers, M.; Alvarez, N.; Fields, P.; et al. Chemokine Signaling Facilitates Early-Stage Breast Cancer Survival and Invasion through Fibroblast-Dependent Mechanisms. Mol. Cancer Res. 2018, 16, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Peluffo, G.; Chen, H.; Gelman, R.; Schnitt, S.; Polyak, K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc. Natl. Acad. Sci. USA 2009, 106, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.E.; Yang, N.; Pehlke, C.; Keely, P.J.; Eliceiri, K.W.; Friedl, A.; Beebe, D.J. Transition to invasion in breast cancer: A microfluidic in vitro model enables examination of spatial and temporal effects. Integr. Biol. 2011, 3, 439–450. [Google Scholar] [CrossRef]
- Dang, T.T.; Prechtl, A.M.; Pearson, G.W. Breast cancer subtype-specific interactions with the microenvironment dictate mechanisms of invasion. Cancer Res. 2011, 71, 6857–6866. [Google Scholar] [CrossRef]
- Romer, A.M.; Luhr, I.; Klein, A.; Friedl, A.; Sebens, S.; Rosel, F.; Arnold, N.; Strauss, A.; Jonat, W.; Bauer, M. Normal mammary fibroblasts induce reversion of the malignant phenotype in human primary breast cancer. Anticancer. Res. 2013, 33, 1525–1536. [Google Scholar]
- Pandey, P.R.; Saidou, J.; Watabe, K. Role of myoepithelial cells in breast tumor progression. Front. Biosci. 2010, 15, 226–236. [Google Scholar] [CrossRef]
- Sirka, O.K.; Shamir, E.R.; Ewald, A.J. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J. Cell Biol. 2018, 217, 3368–3381. [Google Scholar] [CrossRef]
- Duivenvoorden, H.M.; Rautela, J.; Edgington-Mitchell, L.E.; Spurling, A.; Greening, D.W.; Nowell, C.J.; Molloy, T.J.; Robbins, E.; Brockwell, N.K.; Lee, C.S.; et al. Myoepithelial cell-specific expression of stefin A as a suppressor of early breast cancer invasion. J. Pathol. 2017, 243, 496–509. [Google Scholar] [CrossRef]
- Gudjonsson, T.; Adriance, M.C.; Sternlicht, M.D.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Their origin and function in breast morphogenesis and neoplasia. J. Mammary Gland. Biol. Neoplasia 2005, 10, 261–272. [Google Scholar] [CrossRef]
- Gudjonsson, T.; Ronnov-Jessen, L.; Villadsen, R.; Rank, F.; Bissell, M.J.; Petersen, O.W. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 2002, 115, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
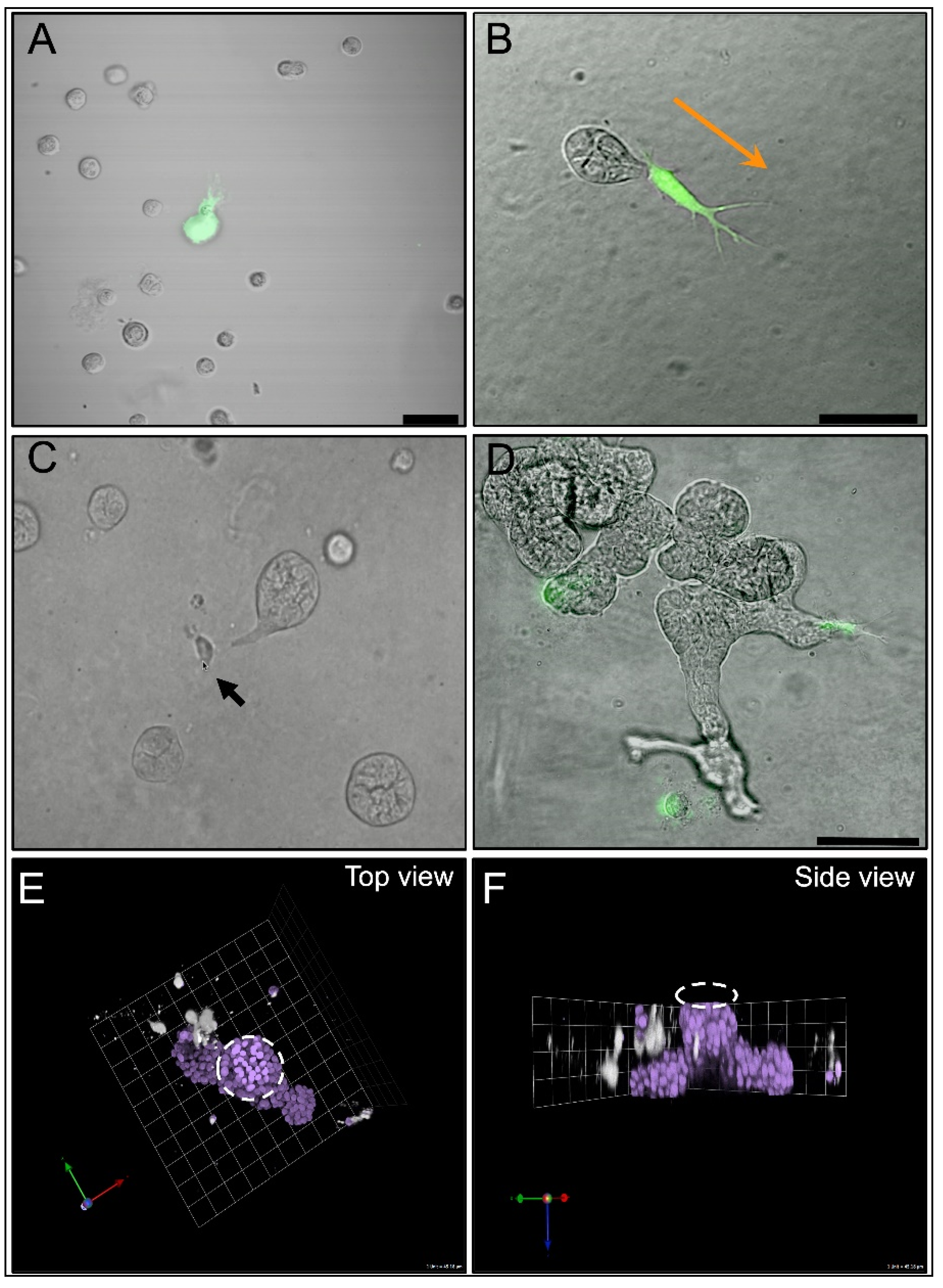
- Sameni, M.; Cavallo-Medved, D.; Franco, O.E.; Chalasani, A.; Ji, K.; Aggarwal, N.; Anbalagan, A.; Chen, X.; Mattingly, R.R.; Hayward, S.W.; et al. Pathomimetic avatars reveal divergent roles of microenvironment in invasive transition of ductal carcinoma in situ. Breast Cancer Res. 2017, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jung, M.; Choi, S.K.; Woo, J.; Piao, Y.J.; Hwang, E.H.; Kim, H.; Kim, S.J.; Moon, W.K. IL-6-mediated cross-talk between human preadipocytes and ductal carcinoma in situ in breast cancer progression. J. Exp. Clin. Cancer Res. 2018, 37, 200. [Google Scholar] [CrossRef]
- Delort, L.; Cholet, J.; Decombat, C.; Vermerie, M.; Dumontet, C.; Castelli, F.A.; Fenaille, F.; Auxenfans, C.; Rossary, A.; Caldefie-Chezet, F. The Adipose Microenvironment Dysregulates the Mammary Myoepithelial Cells and Could Participate to the Progression of Breast Cancer. Front. Cell Dev. Biol. 2020, 8, 571948. [Google Scholar] [CrossRef]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef]
- Shen, J.X.; Couchet, M.; Dufau, J.; de Castro Barbosa, T.; Ulbrich, M.H.; Helmstadter, M.; Kemas, A.M.; Zandi Shafagh, R.; Marques, M.A.; Hansen, J.B.; et al. 3D Adipose Tissue Culture Links the Organotypic Microenvironment to Improved Adipogenesis. Adv. Sci. 2021, 8, e2100106. [Google Scholar] [CrossRef]
- Gil Del Alcazar, C.R.; Aleckovic, M.; Polyak, K. Immune Escape during Breast Tumor Progression. Cancer Immunol. Res. 2020, 8, 422–427. [Google Scholar] [CrossRef]
- Agahozo, M.C.; Westenend, P.J.; van Bockstal, M.R.; Hansum, T.; Giang, J.; Matlung, S.E.; van Deurzen, C.H.M. Immune response and stromal changes in ductal carcinoma in situ of the breast are subtype dependent. Mod. Pathol. 2020, 33, 1773–1782. [Google Scholar] [CrossRef]
- Kim, M.; Chung, Y.R.; Kim, H.J.; Woo, J.W.; Ahn, S.; Park, S.Y. Immune microenvironment in ductal carcinoma in situ: A comparison with invasive carcinoma of the breast. Breast Cancer Res. 2020, 22, 32. [Google Scholar] [CrossRef]
- Agahozo, M.C.; van Bockstal, M.R.; Groenendijk, F.H.; van den Bosch, T.P.P.; Westenend, P.J.; van Deurzen, C.H.M. Ductal carcinoma in situ of the breast: Immune cell composition according to subtype. Mod. Pathol. 2020, 33, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Pang, J.B.; Byrne, D.J.; Lakhani, S.R.; Cummings, M.C.; Campbell, I.G.; Mann, G.B.; Gorringe, K.L.; Fox, S.B. Relationship of the Breast Ductal Carcinoma In Situ Immune Microenvironment with Clinicopathological and Genetic Features. Clin. Cancer Res. 2017, 23, 5210–5217. [Google Scholar] [CrossRef] [PubMed]
- Darvishian, F.; Ozerdem, U.; Adams, S.; Chun, J.; Pirraglia, E.; Kaplowitz, E.; Guth, A.; Axelrod, D.; Shapiro, R.; Price, A.; et al. Tumor-Infiltrating Lymphocytes in a Contemporary Cohort of Women with Ductal Carcinoma In Situ (DCIS). Ann. Surg. Oncol. 2019, 26, 3337–3343. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Aggarwal, A.; Toro, P.; Fu, P.; Badve, S.S.; Cuzick, J.; Madabhushi, A.; Thorat, M.A. A prognostic and predictive computational pathology immune signature for ductal carcinoma in situ: Retrospective results from a cohort within the UK/ANZ DCIS trial. Lancet Digit. Health 2024, 6, e562–e569. [Google Scholar] [CrossRef]
- Phillips, C. First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2024/fda-amtagvi-til-therapy-melanoma (accessed on 17 January 2025).
- Tran, K.Q.; Zhou, J.; Durflinger, K.H.; Langhan, M.M.; Shelton, T.E.; Wunderlich, J.R.; Robbins, P.F.; Rosenberg, S.A.; Dudley, M.E. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 2008, 31, 742–751. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Hwu, P.; Mule, J.J.; Pilon-Thomas, S. Tumor-infiltrating lymphocytes: A new hope. Cancer Cell 2024, 42, 1315–1318. [Google Scholar] [CrossRef]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Leek, R.D.; Harris, A.L. Tumor-associated macrophages in breast cancer. J. Mammary Gland. Biol. Neoplasia 2002, 7, 177–189. [Google Scholar] [CrossRef]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Darvishian, F.; Wu, Y.; Ozerdem, U.; Chun, J.; Adams, S.; Guth, A.; Axelrod, D.; Shapiro, R.; Troxel, A.B.; Schnabel, F.; et al. Macrophage density is an adverse prognosticator for ipsilateral recurrence in ductal carcinoma in situ. Breast 2022, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Weaver, V.M.; Petersen, O.W.; Larabell, C.A.; Dedhar, S.; Briand, P.; Lupu, R.; Bissell, M.J. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 1998, 95, 14821–14826. [Google Scholar] [CrossRef]
- Li, Q.; Chow, A.B.; Mattingly, R.R. Three-dimensional overlay culture models of human breast cancer reveal a critical sensitivity to mitogen-activated protein kinase kinase inhibitors. J. Pharmacol. Exp. Ther. 2010, 332, 821–828. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Invest. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386. [Google Scholar] [CrossRef]
- Behbod, F.; Kittrell, F.S.; LaMarca, H.; Edwards, D.; Kerbawy, S.; Heestand, J.C.; Young, E.; Mukhopadhyay, P.; Yeh, H.W.; Allred, D.C.; et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009, 11, R66. [Google Scholar] [CrossRef]
- Valdez, K.E.; Fan, F.; Smith, W.; Allred, D.C.; Medina, D.; Behbod, F. Human primary ductal carcinoma in situ (DCIS) subtype-specific pathology is preserved in a mouse intraductal (MIND) xenograft model. J. Pathol. 2011, 225, 565–573. [Google Scholar] [CrossRef]
- Hong, Y.; Limback, D.; Elsarraj, H.S.; Harper, H.; Haines, H.; Hansford, H.; Ricci, M.; Kaufman, C.; Wedlock, E.; Xu, M.; et al. Mouse-INtraDuctal (MIND): An in vivo model for studying the underlying mechanisms of DCIS malignancy. J. Pathol. 2022, 256, 186–201. [Google Scholar] [CrossRef]
- Hutten, S.J.; de Bruijn, R.; Lutz, C.; Badoux, M.; Eijkman, T.; Chao, X.; Ciwinska, M.; Sheinman, M.; Messal, H.; Herencia-Ropero, A.; et al. A living biobank of patient-derived ductal carcinoma in situ mouse-intraductal xenografts identifies risk factors for invasive progression. Cancer Cell 2023, 41, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes. Dev. 2009, 23, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Beebe, D.J.; Sung, K.E. Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure. BMC Cancer 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Ghajar, C.M.; Bissell, M.J. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv. Drug Deliv. Rev. 2014, 69–70, 42–51. [Google Scholar] [CrossRef]
- Oskarsson, T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013, 22 Suppl. 2, S66–S72. [Google Scholar] [CrossRef]
- Campbell, J.J.; Watson, C.J. Three-dimensional culture models of mammary gland. Organogenesis 2009, 5, 43–49. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D.C.; Rizki, A.; Weaver, V.M.; Petersen, O.W. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differentiation 2002, 70, 537–546. [Google Scholar] [CrossRef]
- Magdaleno, C.; House, T.; Pawar, J.S.; Carvalho, S.; Rajasekaran, N.; Varadaraj, A. Fibronectin assembly regulates lumen formation in breast acini. J. Cell Biochem. 2021, 122, 524–537. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chang, H.; Giricz, O.; Lee, G.Y.; Baehner, F.L.; Gray, J.W.; Bissell, M.J.; Kenny, P.A.; Parvin, B. Molecular predictors of 3D morphogenesis by breast cancer cell lines in 3D culture. PLoS Comput. Biol. 2010, 6, e1000684. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hall, H.G.; Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982, 99, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, J.; Huang, S.; Wu, X.; Xie, X.; Wang, J.; Liu, F.; Zhang, M.; Peng, Z.; Hu, N. Tumor-on-a-chip: From bioinspired design to biomedical application. Microsyst. Nanoeng. 2021, 7, 50. [Google Scholar] [CrossRef]
- Li, Q.; Mullins, S.R.; Sloane, B.F.; Mattingly, R.R. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 2008, 10, 314–329. [Google Scholar] [CrossRef]
- Sameni, M.; Anbalagan, A.; Olive, M.B.; Moin, K.; Mattingly, R.R.; Sloane, B.F. MAME models for 4D live-cell imaging of tumor: Microenvironment interactions that impact malignant progression. J. Vis. Exp. 2012, e3661. [Google Scholar] [CrossRef]
- Ando, Y.; Ta, H.P.; Yen, D.P.; Lee, S.S.; Raola, S.; Shen, K. A Microdevice Platform Recapitulating Hypoxic Tumor Microenvironments. Sci. Rep. 2017, 7, 15233. [Google Scholar] [CrossRef]
- Polacheck, W.J.; German, A.E.; Mammoto, A.; Ingber, D.E.; Kamm, R.D. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. U S A 2014, 111, 2447–2452. [Google Scholar] [CrossRef]
- Shirure, V.S.; Lezia, A.; Tao, A.; Alonzo, L.F.; George, S.C. Low levels of physiological interstitial flow eliminate morphogen gradients and guide angiogenesis. Angiogenesis 2017, 20, 493–504. [Google Scholar] [CrossRef]
- Fabre, K.M.; Livingston, C.; Tagle, D.A. Organs-on-chips (microphysiological systems): Tools to expedite efficacy and toxicity testing in human tissue. Exp. Biol. Med. 2014, 239, 1073–1077. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Briand, P.; Petersen, O.W.; Van Deurs, B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitr. Cell. Dev. Biol. J. Tissue Cult. Assoc. 1987, 23, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Miller, F.R.; Soule, H.D.; Tait, L.; Pauley, R.J.; Wolman, S.R.; Dawson, P.J.; Heppner, G.H. Xenograft model of progressive human proliferative breast disease. J. Natl. Cancer Inst. 1993, 85, 1725–1732. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer Basic Clin. Res. 2010, 4, 35–41. [Google Scholar] [CrossRef]
- Miller, F.R.; Santner, S.J.; Tait, L.; Dawson, P.J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J. Natl. Cancer Inst. 2000, 92, 1185–1186. [Google Scholar] [CrossRef]
- Fu, J.; Weise, A.M.; Falany, J.L.; Falany, C.N.; Thibodeau, B.J.; Miller, F.R.; Kocarek, T.A.; Runge-Morris, M. Expression of estrogenicity genes in a lineage cell culture model of human breast cancer progression. Breast Cancer Res. Treat. 2010, 120, 35–45. [Google Scholar] [CrossRef]
- Ethier, S.P.; Mahacek, M.L.; Gullick, W.J.; Frank, T.S.; Weber, B.L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993, 53, 627–635. [Google Scholar]
- Barnabas, N.; Cohen, D. Phenotypic and Molecular Characterization of MCF10DCIS and SUM Breast Cancer Cell Lines. Int. J. Breast Cancer 2013, 2013, 872743. [Google Scholar] [CrossRef]
- Band, V.; Zajchowski, D.; Swisshelm, K.; Trask, D.; Kulesa, V.; Cohen, C.; Connolly, J.; Sager, R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990, 50, 7351–7357. [Google Scholar] [PubMed]
- Lee, S.; Stewart, S.; Nagtegaal, I.; Luo, J.; Wu, Y.; Colditz, G.; Medina, D.; Allred, D.C. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 2012, 72, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Brock, E.J.; Ji, K.; Shah, S.; Mattingly, R.R.; Sloane, B.F. In Vitro Models for Studying Invasive Transitions of Ductal Carcinoma In Situ. J. Mammary Gland. Biol. Neoplasia 2019, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.W.; Choong, M.L.; Wang, S.; Wang, Y.; Lim, S.Q.; Lee, M.A. Characterization of ductal carcinoma in situ cell lines established from breast tumor of a Singapore Chinese patient. Cancer Cell Int. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Samson, J.; Derlipanska, M.; Zaheed, O.; Dean, K. Molecular and cellular characterization of two patient-derived ductal carcinoma in situ (DCIS) cell lines, ETCC-006 and ETCC-010. BMC Cancer 2021, 21, 790. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazguez, J.; Long, A.; Albert, S.; Brennan, M. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Santner, S.J.; Dawson, P.J.; Tait, L.; Soule, H.D.; Eliason, J.; Mohamed, A.N.; Wolman, S.R.; Heppner, G.H.; Miller, F.R. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res. Treat. 2001, 65, 101–110. [Google Scholar] [CrossRef]
- Strickland, L.B.; Dawson, P.J.; Santner, S.J.; Miller, F.R. Progression of premalignant MCF10AT generates heterogeneous malignant variants with characteristic histologic types and immunohistochemical markers. Breast Cancer Res. Treat. 2000, 64, 235–240. [Google Scholar] [CrossRef]
- Cailleau, R.; Olive, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitr. Cell Dev. Biol. 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009, 26, 273–287. [Google Scholar] [CrossRef]
- Singhai, R.; Patil, V.W.; Jaiswal, S.R.; Patil, S.D.; Tayade, M.B.; Patil, A.V. E-Cadherin as a diagnostic biomarker in breast cancer. N. Am. J. Med. Sci. 2011, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Sung, K.E.; Jimenez-Torres, J.A.; Mader, B.; Keely, P.J.; Beebe, D.J. The importance of being a lumen. FASEB J. 2014, 28, 4583–4590. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hyun, E.; Seo, J.; Blundell, C.; Kim, H.C.; Lee, E.; Lee, S.H.; Moon, A.; Moon, W.K.; Huh, D. A microengineered pathophysiological model of early-stage breast cancer. Lab. Chip 2015, 15, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.W.; Lao, Y.H.; Ahmed, A.H.R.; Benoy, E.C.; Li, C.; Dereli-Korkut, Z.; Fu, B.M.; Leong, K.W.; Wang, S. High-Throughput Tumor-on-a-Chip Platform to Study Tumor-Stroma Interactions and Drug Pharmacokinetics. Adv. Healthc. Mater. 2020, 9, e2000880. [Google Scholar] [CrossRef] [PubMed]
- Devadas, D.; Moore, T.A.; Walji, N.; Young, E.W.K. A microfluidic mammary gland coculture model using parallel 3D lumens for studying epithelial-endothelial migration in breast cancer. Biomicrofluidics 2019, 13, 064122. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Szot, C.S.; Wilson, T.D.; Akman, S.; Metheny-Barlow, L.J.; Robertson, J.L.; Freeman, J.W.; Rylander, M.N. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J. Cell Biochem. 2012, 113, 1142–1151. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Lin, Y.; Wu, L.; Wu, X.; Wang, K.; He, Q.; Xu, C.; Wan, X.; Wang, X. Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br. J. Cancer 2017, 117, 1371–1382. [Google Scholar] [CrossRef]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef]
- Carpenter, P.M.; Chen, W.P.; Mendez, A.; McLaren, C.E.; Su, M.Y. Angiogenesis in the progression of breast ductal proliferations. Int. J. Surg. Pathol. 2011, 19, 335–341. [Google Scholar] [CrossRef]
- Raica, M.; Cimpean, A.M.; Ribatti, D. Angiogenesis in pre-malignant conditions. Eur. J. Cancer 2009, 45, 1924–1934. [Google Scholar] [CrossRef]
- Teo, N.B.; Shoker, B.S.; Jarvis, C.; Martin, L.; Sloane, J.P.; Holcombe, C. Angiogenesis and invasive recurrence in ductal carcinoma in situ of the breast. Eur. J. Cancer 2003, 39, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Moccia, C.; Haase, K. Engineering Breast Cancer On-chip-Moving Toward Subtype Specific Models. Front. Bioeng. Biotechnol. 2021, 9, 694218. [Google Scholar] [CrossRef] [PubMed]
- Kulwatno, J.; Gong, X.; DeVaux, R.; Herschkowitz, J.I.; Mills, K.L. An Organotypic Mammary Duct Model Capturing Matrix Mechanics-Dependent Ductal Carcinoma In Situ Progression. Tissue Eng. Part. A 2021, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Ma, Y.; Wu, C.; Shiau, C.; Segall, J.E.; Wu, M. Tumor spheroids under perfusion within a 3D microfluidic platform reveal critical roles of cell-cell adhesion in tumor invasion. Sci. Rep. 2020, 10, 9648. [Google Scholar] [CrossRef]
- Shieh, A.C.; Swartz, M.A. Regulation of tumor invasion by interstitial fluid flow. Phys. Biol. 2011, 8, 015012. [Google Scholar] [CrossRef]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef]
- Moon, H.R.; Ospina-Munoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS One 2020, 15, e0234012. [Google Scholar] [CrossRef]
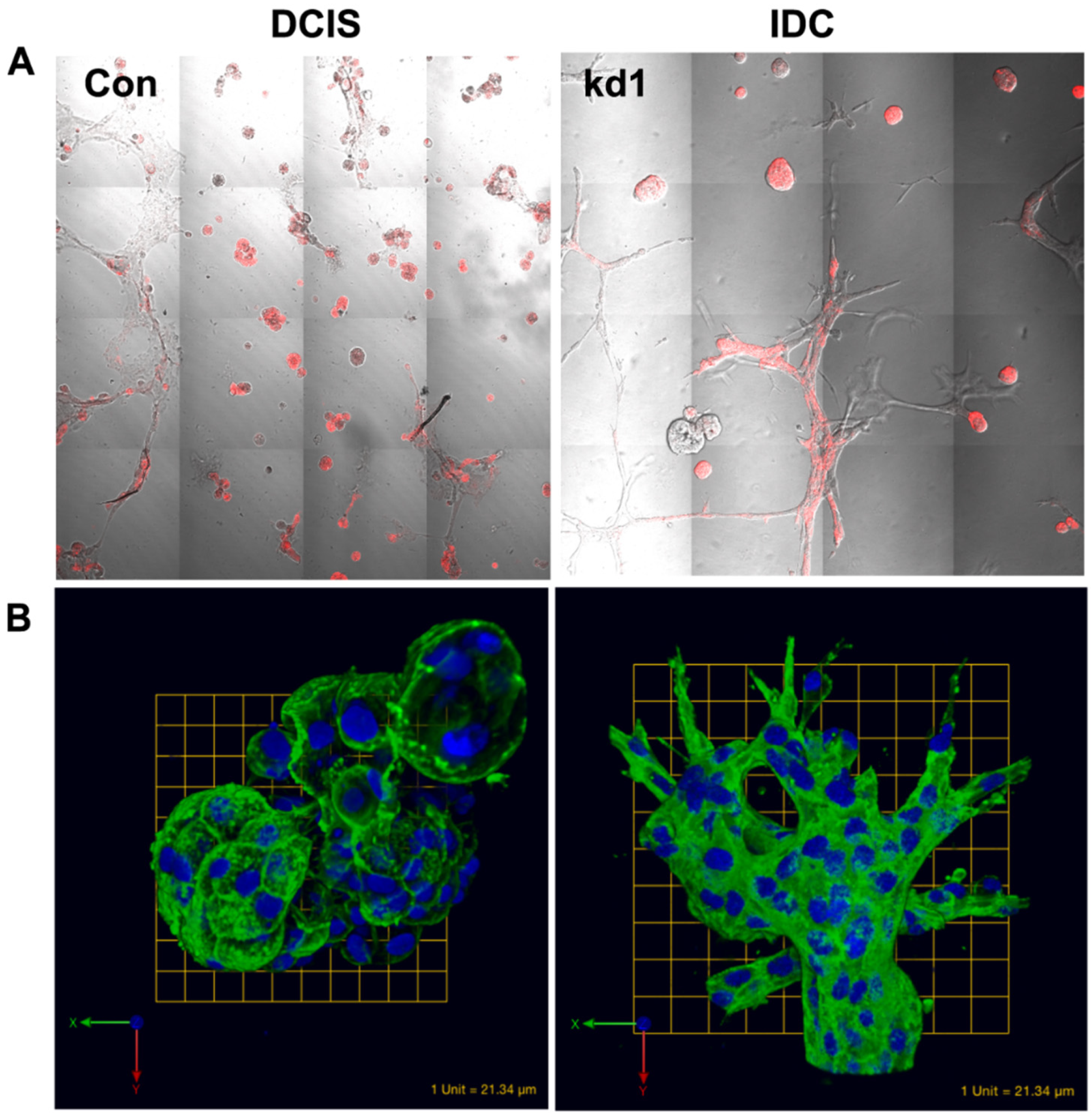
- Shah, S.; Brock, E.J.; Jackson, R.M.; Ji, K.; Boerner, J.L.; Sloane, B.F.; Mattingly, R.R. Downregulation of Rap1Gap: A Switch from DCIS to Invasive Breast Carcinoma via ERK/MAPK Activation. Neoplasia 2018, 20, 951–963. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Lee, S.; Youn, H.; Youn, B. Role of Metabolic Reprogramming in Epithelial(-)Mesenchymal Transition (EMT). Int. J. Mol. Sci. 2019, 20, 2042. [Google Scholar] [CrossRef]
- Espina, V.; Liotta, L.A. What is the malignant nature of human ductal carcinoma in situ? Nat. Rev. Cancer 2011, 11, 68–75. [Google Scholar] [CrossRef]
- Damaghi, M.; Mori, H.; Byrne, S.; Xu, L.; Chen, T.; Johnson, J.; Gallant, N.D.; Marusyk, A.; Borowsky, A.D.; Gillies, R.J. Collagen production and niche engineering: A novel strategy for cancer cells to survive acidosis in DCIS and evolve. Evol. Appl. 2020, 13, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Mayernik, L.; Moin, K.; Sloane, B.F. Acidosis and proteolysis in the tumor microenvironment. Cancer Metastasis Rev. 2019, 38, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Gillette, A.; Lugo-Cintron, K.; Acevedo-Acevedo, S.; Gomez, I.; Morgan, M.; Heaster, T.; Wisinski, K.B.; Palecek, S.P.; Skala, M.C.; et al. Organotypic microfluidic breast cancer model reveals starvation-induced spatial-temporal metabolic adaptations. EBioMedicine 2018, 37, 144–157. [Google Scholar] [CrossRef]
- Kaur, H.; Mao, S.; Li, Q.; Sameni, M.; Krawetz, S.A.; Sloane, B.F.; Mattingly, R.R. RNA-Seq of human breast ductal carcinoma in situ models reveals aldehyde dehydrogenase isoform 5A1 as a novel potential target. PLoS ONE 2012, 7, e50249. [Google Scholar] [CrossRef] [PubMed]
- Brock, E.J.; Jackson, R.M.; Boerner, J.L.; Li, Q.; Tennis, M.A.; Sloane, B.F.; Mattingly, R.R. Sprouty4 negatively regulates ERK/MAPK signaling and the transition from in situ to invasive breast ductal carcinoma. PLoS ONE 2021, 16, e0252314. [Google Scholar] [CrossRef]
- Zuo, H.; Gandhi, M.; Edreira, M.M.; Hochbaum, D.; Nimgaonkar, V.L.; Zhang, P.; Dipaola, J.; Evdokimova, V.; Altschuler, D.L.; Nikiforov, Y.E. Downregulation of Rap1GAP through epigenetic silencing and loss of heterozygosity promotes invasion and progression of thyroid tumors. Cancer Res. 2010, 70, 1389–1397. [Google Scholar] [CrossRef]
- Zhang, Z.; Mitra, R.S.; Henson, B.S.; Datta, N.S.; McCauley, L.K.; Kumar, P.; Lee, J.S.; Carey, T.E.; D’Silva, N.J. Rap1GAP inhibits tumor growth in oropharyngeal squamous cell carcinoma. Am. J. Pathol. 2006, 168, 585–596. [Google Scholar] [CrossRef]
- Zhang, L.; Chenwei, L.; Mahmood, R.; van Golen, K.; Greenson, J.; Li, G.; D’Silva, N.J.; Li, X.; Burant, C.F.; Logsdon, C.D.; et al. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006, 66, 898–906. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Yan, Y.; Cai, H.; Li, M.; Sun, K.; Wang, J.; Liu, X.; Wang, J.; Duan, X. Low expression of Rap1GAP is associated with epithelial-mesenchymal transition (EMT) and poor prognosis in gastric cancer. Oncotarget 2017, 8, 8057–8068. [Google Scholar] [CrossRef]
- Tamate, M.; Tanaka, R.; Osogami, H.; Matsuura, M.; Satohisa, S.; Iwasaki, M.; Saito, T. Rap1GAP inhibits tumor progression in endometrial cancer. Biochem. Biophys. Res. Commun. 2017, 485, 476–483. [Google Scholar] [CrossRef]
- Masoumi-Moghaddam, S.; Amini, A.; Morris, D.L. The developing story of Sprouty and cancer. Cancer Metastasis Rev. 2014, 33, 695–720. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, J.M.; Sameni, M.; Moin, K.; Sloane, B.F. Live-cell imaging of tumor proteolysis: Impact of cellular and non-cellular microenvironment. Biochim. Biophys. Acta 2012, 1824, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Osuala, K.O.; Chalasani, A.; Aggarwal, N.; Ji, K.; Moin, K. Paracrine Activation of STAT3 Drives GM-CSF Expression in Breast Carcinoma Cells, Generating a Symbiotic Signaling Network with Breast Carcinoma-Associated Fibroblasts. Cancers 2024, 16, 2910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tu, H.; Kim, E.G.; Sloane, B.F.; Xu, Y. A flexible Ag/AgCl micro reference electrode based on a parylene tube structure. Sens. Actuators B Chem. 2017, 247, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindstrom, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Ma, Y.; Xing, X.; Zhou, Y.; Xu, X.; Song, J.; Wang, S.; Jiang, W.; Wang, X.; et al. Vimentin plays an important role in the promotion of breast cancer cell migration and invasion by leucine aminopeptidase 3. Cytotechnology 2020, 72, 639–647. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Khillare, C.D.; Sinai Khandeparkar, S.G.; Joshi, A.R.; Kulkarni, M.M.; Gogate, B.P.; Battin, S. Immunohistochemical Expression of Vimentin in Invasive Breast Carcinoma and Its Correlation with Clinicopathological Parameters. Niger. Med. J. 2019, 60, 17–21. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Chia, S.; Wong, S.Q.R.; Zhang, X.; Chua, H.; Loo, J.M.; Chua, W.Y.; Chua, C.; Tan, E.; Hentze, H.; et al. A comparative study of tumour-on-chip models with patient-derived xenografts for predicting chemotherapy efficacy in colorectal cancer patients. Front. Bioeng. Biotechnol. 2022, 10, 952726. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A predictive microfluidic model of human glioblastoma to assess trafficking of blood-brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119. [Google Scholar] [CrossRef] [PubMed]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D cancer models: One step closer to in vitro human studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Peirsman, A.; Tirpakova, Z.; Mandal, K.; Vanlauwe, F.; Maity, S.; Kawakita, S.; Khorsandi, D.; Herculano, R.; Umemura, C.; et al. Engineered Vasculature for Cancer Research and Regenerative Medicine. Micromachines 2023, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The Variety of 3D Breast Cancer Models for the Study of Tumor Physiology and Drug Screening. Int. J. Mol. Sci. 2023, 24, 711. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Clark, S.E.; Warwick, J.; Carpenter, R.; Bowen, R.L.; Duffy, S.W.; Jones, J.L. Molecular subtyping of DCIS: Heterogeneity of breast cancer reflected in pre-invasive disease. Br. J. Cancer 2011, 104, 120–127. [Google Scholar] [CrossRef]
- Livasy, C.A.; Perou, C.M.; Karaca, G.; Cowan, D.W.; Maia, D.; Jackson, S.; Tse, C.K.; Nyante, S.; Millikan, R.C. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum. Pathol. 2007, 38, 197–204. [Google Scholar] [CrossRef]
- Muggerud, A.A.; Hallett, M.; Johnsen, H.; Kleivi, K.; Zhou, W.; Tahmasebpoor, S.; Amini, R.M.; Botling, J.; Borresen-Dale, A.L.; Sorlie, T.; et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol. Oncol. 2010, 4, 357–368. [Google Scholar] [CrossRef]
- Allred, D.C.; Wu, Y.; Mao, S.; Nagtegaal, I.D.; Lee, S.; Perou, C.M.; Mohsin, S.K.; O’Connell, P.; Tsimelzon, A.; Medina, D. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin. Cancer Res. 2008, 14, 370–378. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sharma, M.; Beck, A.H.; Webster, J.A.; Espinosa, I.; Montgomery, K.; Varma, S.; van de Rijn, M.; Jensen, K.C.; West, R.B. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res. Treat. 2010, 123, 397–404. [Google Scholar] [CrossRef]
| Non-Transformed Lines | Origin | ER/PR/HER2 Status | Model |
|---|---|---|---|
| HMT-3522-S1 | Fibrocystic breast tissue [135] | –/–/– [136] | Epithelium |
| MCF10A | Fibrocystic breast tissue [137] | –/–/– [138] | Epithelium |
| DCIS Lines | Origin | ER/PR/HER2 Status | Model |
| MCF10.DCIS.com | Progression from MCF10A [139] | –/–/– [110,140] | DCIS |
| SUM 102 | Surgically resected DCIS with microinvasion [141] | –/–/– [136] | DCIS with microinvasion |
| SUM 225 | DCIS chest wall recurrence [142] | –/–/+ [110,136] | DCIS |
| 21NT | Infiltrating intraductal carcinoma [143] | –/–/+ [136] | DCIS |
| hDCIS.01 | Hyperplastic columnar cell hyperplasia [144] | –/–/– [145] | DCIS |
| FSK-H7 | HER2+ DCIS [110] | –/–/+ [110] | DCIS |
| ETCC006 | Pre-invasive DCIS [146] | U [146,147] | DCIS |
| Transformed Lines | Origin | ER/PR/HER2 Status | Model |
| MCF-7 | Pleural effusion from metastatic carcinoma [148] | +/+/– [136,138] | Luminal A invasive carcinoma |
| MCF10.CA1d | Progression from MCF10A [149] | –/–/– [140,150] | Basal-like invasive carcinoma |
| SUM 159 | Anaplastic carcinoma of the breast [141] | –/–/– [136] | Basal-like invasive carcinoma |
| MDA-MB-231 | Pleural effusion from patient with metastatic adenocarcinoma [151] | –/–/– [136,138] | Basal-like invasive carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.; Osuala, K.O.; Brock, E.J.; Ji, K.; Sloane, B.F.; Mattingly, R.R. Three-Dimensional Models: Biomimetic Tools That Recapitulate Breast Tissue Architecture and Microenvironment to Study Ductal Carcinoma In Situ Transition to Invasive Ductal Breast Cancer. Cells 2025, 14, 220. https://doi.org/10.3390/cells14030220
Shah S, Osuala KO, Brock EJ, Ji K, Sloane BF, Mattingly RR. Three-Dimensional Models: Biomimetic Tools That Recapitulate Breast Tissue Architecture and Microenvironment to Study Ductal Carcinoma In Situ Transition to Invasive Ductal Breast Cancer. Cells. 2025; 14(3):220. https://doi.org/10.3390/cells14030220
Chicago/Turabian StyleShah, Seema, Kingsley O. Osuala, Ethan J. Brock, Kyungmin Ji, Bonnie F. Sloane, and Raymond R. Mattingly. 2025. "Three-Dimensional Models: Biomimetic Tools That Recapitulate Breast Tissue Architecture and Microenvironment to Study Ductal Carcinoma In Situ Transition to Invasive Ductal Breast Cancer" Cells 14, no. 3: 220. https://doi.org/10.3390/cells14030220
APA StyleShah, S., Osuala, K. O., Brock, E. J., Ji, K., Sloane, B. F., & Mattingly, R. R. (2025). Three-Dimensional Models: Biomimetic Tools That Recapitulate Breast Tissue Architecture and Microenvironment to Study Ductal Carcinoma In Situ Transition to Invasive Ductal Breast Cancer. Cells, 14(3), 220. https://doi.org/10.3390/cells14030220











