Generation of a Transgenic Mouse Model for Investigating Mitochondria in Sperm
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
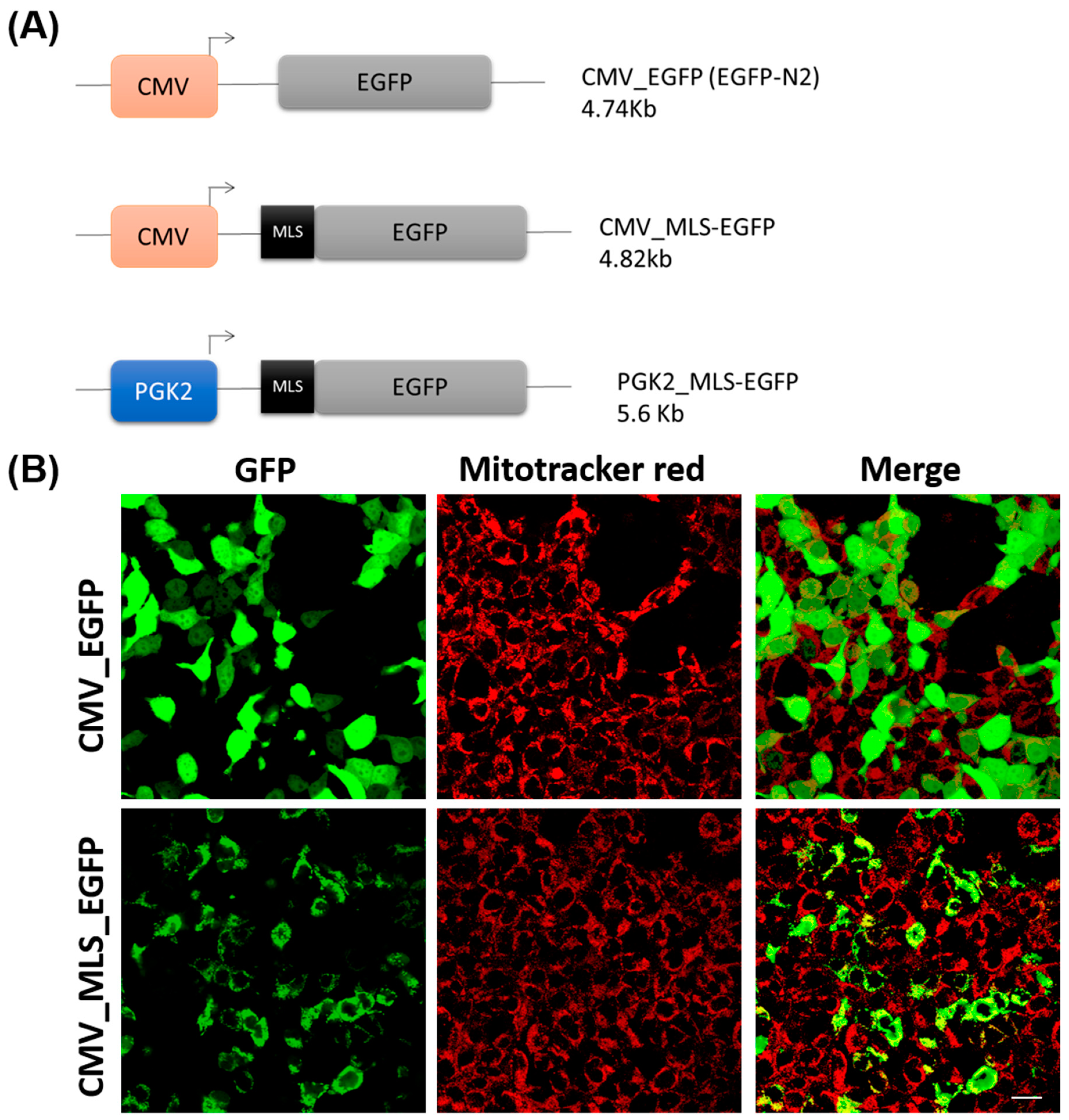
2.2. Transgene Constructs
2.3. Cell Culture and Transfection
2.4. Pronuclear Microinjection of Transgene Cassettes and Oviductal Embryo Transfer
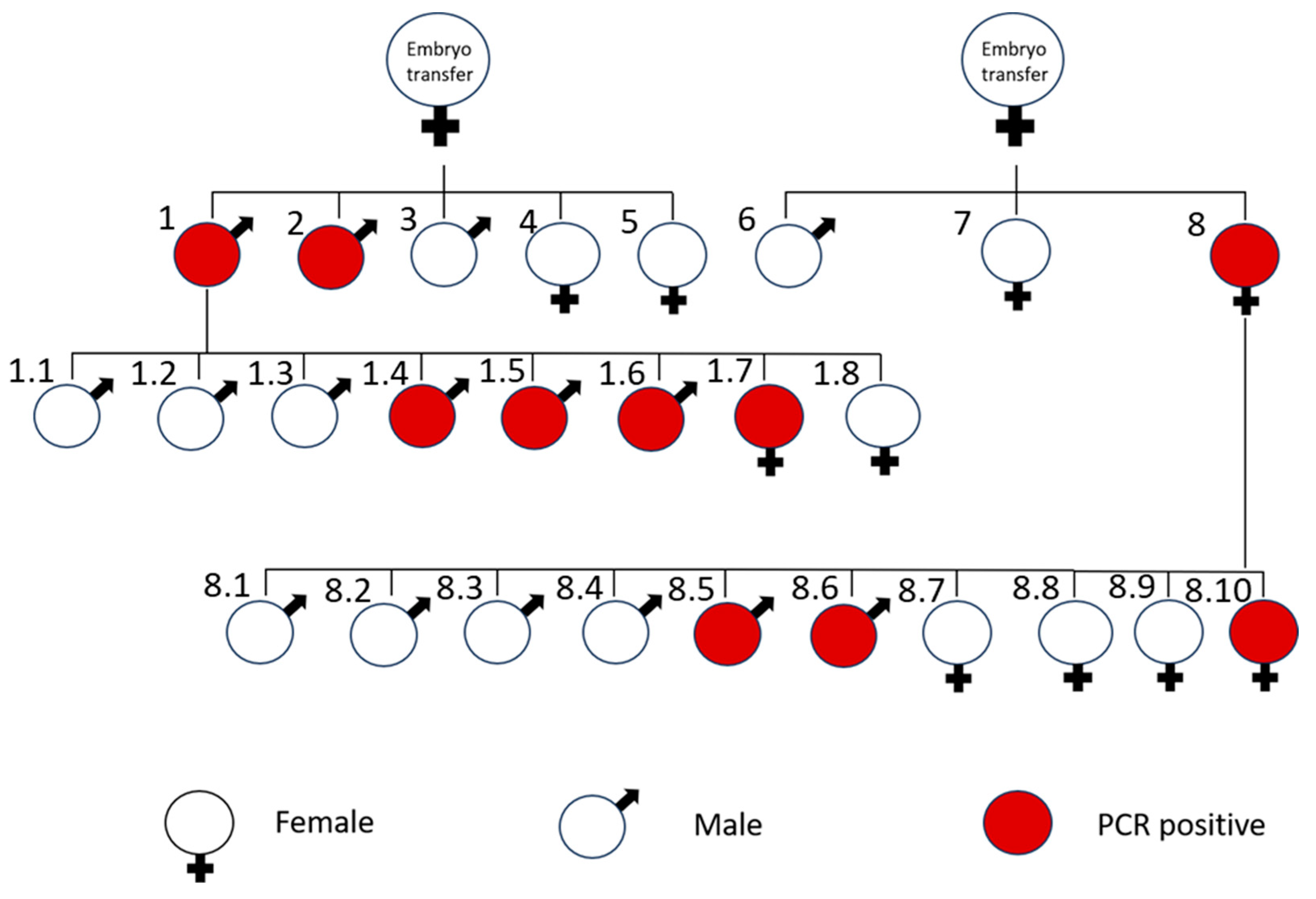
2.5. Establishment of Transgenic Lines
2.6. Sperm Collection
2.7. Fluorescence Microscopy
2.8. Flow Cytometry Analysis
2.9. Statistical Analysis
3. Results
3.1. Validation of MLS Sequence In Vitro
3.2. Generation of Transgenic Mice
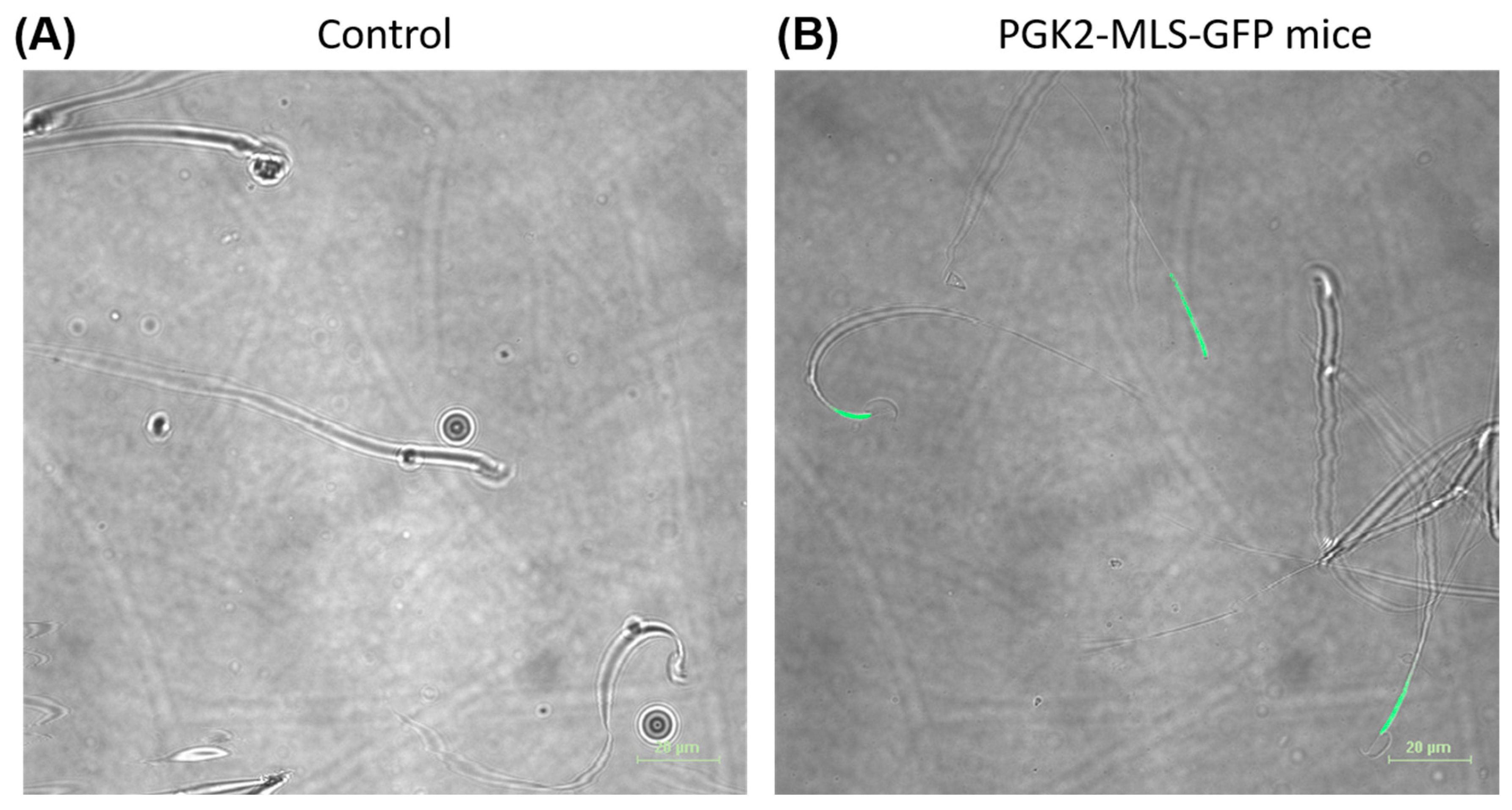
3.3. Localization of the GFP in the Mitochondria of Spermatozoa In Vivo
3.4. Analysis of GFP Expression in the Spermatozoa by Flow Cytometry (FACS)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharpe, R.M. Low sperm counts may be preventable. Science 2011, 333, 1380–1381. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, A.; Wolter, M.; Szardening-Kirchner, C.; Lüers, G.H.; Bergmann, M.; Kliesch, S.; Konrad, L. Reduced numbers of Sertoli, germ, and spermatogonial stem cells in impaired spermatogenesis. Mod. Pathol. 2011, 24, 1380–1389, Erratum in Mod. Pathol. 2017, 30, 311. [Google Scholar] [CrossRef] [PubMed]
- Thirumavalavan, N.; Gabrielsen, J.S.; Lamb, D.J. Where are we going with gene screening for male infertility? Fertil. Steril. 2019, 111, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza-Tesarik, R. Mitochondria in Human Fertility and Infertility. Int. J. Mol. Sci. 2023, 24, 8950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Updat. 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, L.; Luo, Y.X.; Liu, Y.; Liu, H.; Li, H.Z.; Yu, Y. Potential of Mitochondrial Genome Editing for Human Fertility Health. Front. Genet. 2021, 12, 673951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.J.; Liu, X.; Han, J.L.; Wang, Y.W.; Jin, S.H.; Liu, X.X.; Liu, J.; Wang, W.T.; Wang, W.J. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Pang, M.G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Pesini, E.; Diez, C.; Lapeña, A.C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Arenas, J.; López-Pérez, M.J. Correlation of sperm motility with mitochondrial enzymatic activities. Clin. Chem. 1998, 44, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, M.; Kawiak, J. Sperm mitochondria of patients with normal sperm motility and with asthenozoospermia: Morphological and functional study. Folia Histochem. Cytobiol. 2003, 41, 125–139. [Google Scholar] [PubMed]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vadnais, M.L.; Lin, A.M.; Gerton, G.L. Mitochondrial fusion protein MFN2 interacts with the mitostatin-related protein MNS1 required for mouse sperm flagellar structure and function. Cilia 2014, 3, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otera, H.; Ishihara, N.; Mihara, K. New insights into the function and regulation of mitochondrial fission. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Harada, Y.; Miyado, K.; Kono, T. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol. Hum. Reprod. 2014, 20, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, H.; Yao, C.K.; Chen, K.; Jaiswal, M.; Donti, T.; Lin, Y.Q.; Bayat, V.; Xiong, B.; Zhang, K.; David, G.; et al. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. eLife 2014, 3, e03558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bader, G.; Enkler, L.; Araiso, Y.; Hemmerle, M.; Binko, K.; Baranowska, E.; De Craene, J.-O.; Ruer-Laventie, J.; Pieters, J.; Tribouillard-Tanvier, D.; et al. Assigning mitochondrial localization of dual localized proteins using a yeast Bi-Genomic Mitochondrial-Split-GFP. eLife 2020, 9, e56649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, A.; Hossain, T.; Eckmann, D.M. Mitochondrial dynamics involves molecular and mechanical events in motility, fusion and fission. Front. Cell Dev. Biol. 2022, 10, 1010232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.P.; Stroud, J.; Eddy, C.A.; Walter, C.A.; McCarrey, J.R. Multiple elements influence transcriptional regulation from the human testis-specific PGK2 promoter in transgenic mice. Biol. Reprod. 1999, 60, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.M. Immunofluorescent localization of PGK-1 and PGK-2 isozymes within specific cells of the mouse testis. Dev. Biol. 1981, 87, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Danshina, P.V.; Geyer, C.B.; Dai, Q.; Goulding, E.H.; Willis, W.D.; Kitto, G.B.; McCarrey, J.R.; Eddy, E.; O’Brien, D.A. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol. Reprod. 2010, 82, 136–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCarrey, J.R.; Berg, W.M.; Paragioudakis, S.J.; Zhang, P.L.; Dilworth, D.D.; Arnold, B.L.; Rossi, J.J. Differential transcription of Pgk genes during spermatogenesis in the mouse. Dev. Biol. 1992, 154, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Geyer, C.B.; Hornecker, J.L.; Patel, K.T.; McCarrey, J.R. In vivo analysis of developmentally and evolutionarily dynamic protein-DNA interactions regulating transcription of the Pgk2 gene during mammalian spermatogenesis. Mol. Cell. Biol. 2007, 27, 7871–7885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pradhan, B.S.; Majumdar, S.S. An Efficient Method for Generation of Transgenic Rats Avoiding Embryo Manipulation. Mol. Ther. Nucleic Acids 2016, 5, e293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarkar, R.K.; Sharma, S.S.; Mandal, K.; Wadhwa, N.; Kunj, N.; Gupta, A.; Pal, R.; Rai, U.; Majumdar, S.S. Homeobox transcription factor Meis1 is crucial to Sertoli cell mediated regulation of male fertility. Andrology 2021, 9, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, A.B. Production of functional transgenic mice by DNA pronuclear microinjection. Acta Biochim. Pol. 2004, 51, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L.; Chen, H.Y.; Trumbauer, M.; Senear, A.W.; Warren, R.; Palmiter, R.D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell 1981, 27, 223–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costantini, F.; Lacy, E. Introduction of a rabbit beta-globin gene into the mouse germ line. Nature 1981, 294, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Scangos, G.A.; Plotkin, D.J.; Barbosa, J.A.; Ruddle, F.H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 7380–7384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usmani, A.; Ganguli, N.; Sarkar, H.; Dhup, S.; Batta, S.R.; Vimal, M.; Ganguli, N.; Basu, S.; Nagarajan, P.; Majumdar, S.S. A non-surgical approach for male germ cell mediated gene transmission through transgenesis. Sci. Rep. 2013, 3, 3430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzuto, R.; Brini, M.; Pizzo, P.; Murgia, M.; Pozzan, T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr. Biol. 1995, 5, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Brini, M.; De Giorgi, F.; Rossi, R.; Heim, R.; Tsien, R.Y.; Pozzan, T. Double labelling of subcellular structures with organelle-targeted GFP mutants in vivo. Curr. Biol. 1996, 6, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Shitara, H.; Kaneda, H.; Sato, A.; Iwasaki, K.; Hayashi, J.; Taya, C.; Yonekawa, H. Non-invasive visualization of sperm mitochondria behavior in transgenic mice with introduced green fluorescent protein (GFP). FEBS Lett. 2001, 500, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Barrasso, A.P.; Tong, X.; Poché, R.A. The mito::mKate2 mouse: A far-red fluorescent reporter mouse line for tracking mitochondrial dynamics in vivo. Genesis 2017, 56, e23087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, J.; Nishiyama, S.; Shimanuki, M.; Ono, T.; Sato, A.; Nakada, K.; Hayashi, J.; Yonekawa, H.; Shitara, H. Comprehensive application of an mtDsRed2-Tg mouse strain for mitochondrial imaging. Transgenic Res. 2012, 21, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, O.; Quirós, P.M.; Auwerx, J. Mitochondria and Epigenetics—Crosstalk in Homeostasis and Stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Duan, Y.; Chen, B.; Qiu, S.; Huang, T.; Si, W. Generation of Transgenic Sperm Expressing GFP by Lentivirus Transduction of Spermatogonial Stem Cells In vivo in Cynomolgus Monkeys. Veter Sci. 2023, 10, 104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pradhan, B.S.; Bhattacharya, I.; Sarkar, R.; Majumdar, S.S. Pubertal down-regulation of Tetraspanin 8 in testicular Sertoli cells is crucial for male fertility. Mol. Hum. Reprod. 2020, 26, 760–772. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, H.; Batta, S.R.; Wadhwa, N.; Majumdar, S.S.; Pradhan, B.S. Generation of a Transgenic Mouse Model for Investigating Mitochondria in Sperm. Cells 2025, 14, 296. https://doi.org/10.3390/cells14040296
Sarkar H, Batta SR, Wadhwa N, Majumdar SS, Pradhan BS. Generation of a Transgenic Mouse Model for Investigating Mitochondria in Sperm. Cells. 2025; 14(4):296. https://doi.org/10.3390/cells14040296
Chicago/Turabian StyleSarkar, Hironmoy, Suryaprakash R. Batta, Neerja Wadhwa, Subeer S. Majumdar, and Bhola Shankar Pradhan. 2025. "Generation of a Transgenic Mouse Model for Investigating Mitochondria in Sperm" Cells 14, no. 4: 296. https://doi.org/10.3390/cells14040296
APA StyleSarkar, H., Batta, S. R., Wadhwa, N., Majumdar, S. S., & Pradhan, B. S. (2025). Generation of a Transgenic Mouse Model for Investigating Mitochondria in Sperm. Cells, 14(4), 296. https://doi.org/10.3390/cells14040296






