Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression
Abstract
1. Introduction
2. Cells in Cancer Microenvironment
2.1. Non-Immune Components of the TME
2.1.1. Cancer-Associated Fibroblasts
2.1.2. Stellate Cells
2.1.3. Endothelial Cells
2.1.4. Adipocytes
2.1.5. Cancer Stem Cells
2.1.6. Mesenchymal Stem Cells
2.2. Immune Components of the TME
2.2.1. T Lymphocytes
2.2.2. NK Cells
2.2.3. B Cells
2.2.4. Monocytes
2.2.5. Dendritic Cells
2.2.6. Myeloid-Derived Suppressor Cells
2.2.7. Neutrophils
2.2.8. Eosinophils
2.2.9. Basophils
2.2.10. Macrophages
3. The Role of TME Cells in Tumor Development Across Cancer Types
3.1. Head and Neck Cancer
3.1.1. CAFs in Head and Neck Cancer
3.1.2. Adipocytes in Head and Neck Cancer
3.1.3. CSCs in Head and Neck Cancer
3.1.4. T Cells in Head and Neck Cancer
3.1.5. NK Cells in Head and Neck Cancer
3.1.6. B Cells in Head and Neck Cancer
3.1.7. Monocytes and Related Cells in Head and Neck Cancer
3.1.8. Neutrophils in Head and Neck Cancer
3.1.9. Macrophages in Head and Neck Cancer
3.2. Glioma
3.2.1. Non-Immune Cells in Glioma
3.2.2. Immune Cells in Glioma
3.3. Thyroid Cancer
3.3.1. CAFs in Thyroid Cancer
3.3.2. Other Non-Immune Cells in Thyroid Cancer
3.3.3. T Cells in Thyroid Cancer
3.3.4. NK Cells in Thyroid Cancer
3.3.5. Monocyte-Related Cells in Thyroid Cancer
3.3.6. Neutrophils in Thyroid Cancer
3.3.7. Macrophages in Thyroid Cancer
3.4. Esophageal Cancer
3.4.1. Non-Immune Cells in Esophageal Cancer
3.4.2. Immune Cells in Esophageal Cancer
3.5. Gastric Cancer
3.5.1. CAFs in Gastric Cancer
3.5.2. Adipocytes in Gastric Cancer
3.5.3. CSCs in Gastric Cancer
3.5.4. T Cells in Gastric Cancer
3.5.5. NK Cells in Gastric Cancer
3.5.6. B Cells in Gastric Cancer
3.5.7. Monocytes and Related Cells in Gastric Cancer
3.5.8. Other Immune Cells in Gastric Cancer
3.6. Pancreatic Cancer
3.6.1. CAFs in Pancreatic Cancer
3.6.2. Stellate Cells in Pancreatic Cancer
3.6.3. Adipocytes in Pancreatic Cancers
3.6.4. Cancer Stem Cells in Pancreatic Cancer
3.6.5. T Cells in Pancreatic Cancer
3.6.6. NK Cells in Pancreatic Cancer
3.6.7. B Cells in Pancreatic Cancer
3.6.8. Monocytes in Pancreatic Cancer
3.6.9. Dendritic Cells in Pancreatic Cancer
3.6.10. MDSCs in Pancreatic Cancer
3.6.11. Neutrophils in Pancreatic Cancers
3.6.12. Macrophages in Pancreatic Cancers
3.7. Liver Cancer
3.7.1. CAFs in Liver Cancer
3.7.2. Stellate Cells in Liver Cancer
3.7.3. Other Non-Immune Cells in Liver Cancer
3.7.4. T Cells in Liver Cancer
3.7.5. NK Cells in Liver Cancer
3.7.6. B Cells in Liver Cancer
3.7.7. Monocytes and Related Cells in Liver Cancer
3.7.8. Other Immune Cells in Liver Cancer
3.8. Colorectal Cancer
3.8.1. CAFs in Colorectal Cancer
3.8.2. Other Non-Immune Cells in Colorectal Cancer
3.8.3. T Cells in Colorectal Cancer
3.8.4. NK Cells in Colorectal Cancer
3.8.5. B Cells in Colorectal Cancer
3.8.6. Monocytes and Related Cells in Colorectal Cancer
3.8.7. Neutrophils in Colorectal Cancer
3.9. Lung Cancer
3.9.1. CAFs in Lung Cancer
3.9.2. Endothelial Cells in Lung Cancer
3.9.3. Adipocytes in Lung Cancer
3.9.4. CSCs in Lung Cancer
3.9.5. T Cells in Lung Cancer
3.9.6. NK Cells in Lung Cancer
3.9.7. B Cells in Lung Cancer
3.9.8. Monocytes and Related Cells in Lung Cancer
3.9.9. Other Immune Cells in Lung Cancer
3.10. Breast Cancer
3.10.1. CAFs in Breast Cancer
3.10.2. Adipocytes in Breast Cancer
3.10.3. Other Non-Immune Cells in Breast Cancer
3.10.4. T Cells in Breast Cancer
3.10.5. NK Cells in Breast Cancer
3.10.6. B Cells in Breast Cancer
3.10.7. Monocytes and Related Cells in Breast Cancer
3.10.8. Other Immune Cells in Breast Cancer
3.11. Ovarian Cancer
3.11.1. CAFs in Ovarian Cancer
3.11.2. Adipocytes in Ovarian Cancer
3.11.3. CSCs in Ovarian Cancer
3.11.4. T Cells in Ovarian Cancer
3.11.5. NK Cells in Ovarian Cancer
3.11.6. B Cells in Ovarian Cancer
3.11.7. Dendritic Cells in Ovarian Cancer
3.11.8. MDSCs in Ovarian Cancer
3.11.9. Neutrophils in Ovarian Cancer
3.11.10. Macrophages in Ovarian Cancer
3.12. Cervical and Endometrial Cancers
3.12.1. CAFs in Endometrial Cancer
3.12.2. T Cells in Cervical and Endometrial Cancers
3.12.3. NK Cells in Cervical Cancer
3.12.4. B Cells in Cervical Cancer
3.12.5. Dendritic Cells in Cervical Cancer
3.12.6. MDSCs in Cervical and Endometrial Cancer
3.13. Prostate Cancer
3.13.1. CAFs in Prostate Cancer
3.13.2. Adipocytes in Prostate Cancer
3.13.3. CSCs in Prostate Cancer
3.13.4. T Cells in Prostate Cancer
3.13.5. NK Cells in Prostate Cancer
3.13.6. B Cells in Prostate Cancer
3.13.7. Monocytes and Related Cells in Prostate Cancer
3.13.8. Neutrophils in Prostate Cancer
3.13.9. Macrophages in Prostate Cancer
3.14. Kidney Cancer
3.14.1. Non-Immune Cells in Renal Cell Carcinoma
3.14.2. Immune Cells in Renal Cell Carcinoma
3.15. Bladder Cancer
3.15.1. Non-Immune Cells in Bladder Cancer
3.15.2. Immune Cells in Bladder Cancer
3.16. Skin Cancer
3.16.1. CAFs in Skin Cancer
3.16.2. Other Non-Immune Cells in Melanoma
3.16.3. T Cells in Melanoma
3.16.4. NK Cells in Melanoma
3.16.5. B Cells in Melanoma
3.16.6. Monocyte-Related Cells in Melanoma
3.16.7. Macrophages in Melanoma
3.17. Osteosarcoma
3.17.1. CSCs in Osteosarcoma
3.17.2. Neutrophils in Osteosarcoma
3.17.3. Macrophages in Osteosarcoma
4. TME-Targeting Therapies
Tumor Microenvironment Composition Across Cancer Types—A Transcriptomic Perspective
- Immune enriched, fibrotic (IE/F)—characterized by high immune infiltration but also enriched with fibroblasts and pro-fibrotic signaling;
- Immune enriched, non-fibrotic (IE)—characterized by strong immune cell presence with minimal fibroblast activity, associated with high immunogenicity;
- Fibrotic (F)—with low immune infiltration, predominantly fibroblast-rich with angiogenic signatures;
- Immune depleted (D)—composed by scarce immune and stromal cells, with a high malignant cell fraction [865].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the Tumor Microenvironment: Potential Strategy for Cancer Therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, K.; Chen, M.; Liu, L. Metabolic Codependencies in the Tumor Microenvironment and Gastric Cancer: Difficulties and Opportunities. Biomed. Pharmacother. 2023, 162, 114601. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor Microenvironment and Immunotherapy of Oral Cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef] [PubMed]
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.-L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumor-Associated Fibroblasts Predominantly Come from Local and Not Circulating Precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M. Targeted Therapy in Cancer. Cancer Chemother. Pharmacol. 2015, 76, 1113–1132. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Pellinen, T.; Paavolainen, L.; Martín-Bernabé, A.; Papatella Araujo, R.; Strell, C.; Mezheyeuski, A.; Backman, M.; La Fleur, L.; Brück, O.; Sjölund, J.; et al. Fibroblast Subsets in Non-Small Cell Lung Cancer: Associations with Survival, Mutations, and Immune Features. J. Natl. Cancer Inst. 2023, 115, 71–82. [Google Scholar] [CrossRef]
- Rodemann, H.P.; Müller, G.A. Characterization of Human Renal Fibroblasts in Health and Disease: II. In Vitro Growth, Differentiation, and Collagen Synthesis of Fibroblasts from Kidneys with Interstitial Fibrosis. Am. J. Kidney Dis. 1991, 17, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Danø, K.; Behrendt, N.; Høyer-Hansen, G.; Johnsen, M.; Lund, L.R.; Ploug, M.; Rømer, J. Plasminogen Activation and Cancer. Thromb. Haemost. 2005, 93, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Yazdani, S.; Bansal, R.; Prakash, J. Drug Targeting to Myofibroblasts: Implications for Fibrosis and Cancer. Adv. Drug Deliv. Rev. 2017, 121, 101–116. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Cukierman, E.; Bassi, D.E. Physico-Mechanical Aspects of Extracellular Matrix Influences on Tumorigenic Behaviors. Semin. Cancer Biol. 2010, 20, 139–145. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-Led Collective Invasion of Carcinoma Cells with Differing Roles for RhoGTPases in Leading and Following Cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- Anderberg, C.; Li, H.; Fredriksson, L.; Andrae, J.; Betsholtz, C.; Li, X.; Eriksson, U.; Pietras, K. Paracrine Signaling by Platelet-Derived Growth Factor-CC Promotes Tumor Growth by Recruitment of Cancer-Associated Fibroblasts. Cancer Res. 2009, 69, 369–378. [Google Scholar] [CrossRef]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.F.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.-F.; et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Scholer-Dahirel, A.; Mechta-Grigoriou, F. The Role of Reactive Oxygen Species and Metabolism on Cancer Cells and Their Microenvironment. Semin. Cancer Biol. 2014, 25, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Acar, A.; Eaton, E.N.; Mellody, K.T.; Scheel, C.; Ben-Porath, I.; Onder, T.T.; Wang, Z.C.; Richardson, A.L.; Weinberg, R.A.; et al. Autocrine TGF-Beta and Stromal Cell-Derived Factor-1 (SDF-1) Signaling Drives the Evolution of Tumor-Promoting Mammary Stromal Myofibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 20009–20014. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhou, Q.; Li, P.; Yu, B.; Li, J.; Qu, Y.; Yan, J.; Yu, Y.; Yan, M.; et al. Hepatocyte Growth Factor Activates Tumor Stromal Fibroblasts to Promote Tumorigenesis in Gastric Cancer. Cancer Lett. 2013, 335, 128–135. [Google Scholar] [CrossRef]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-Associated Fibroblast Classification in Single-Cell and Spatial Proteomics Data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Barrera, L.N.; Ridley, P.M.; Bermejo-Rodriguez, C.; Costello, E.; Perez-Mancera, P.A. The Role of microRNAs in the Modulation of Cancer-Associated Fibroblasts Activity during Pancreatic Cancer Pathogenesis. J. Physiol. Biochem. 2023, 79, 193–204. [Google Scholar] [CrossRef]
- Geng, X.; Chen, H.; Zhao, L.; Hu, J.; Yang, W.; Li, G.; Cheng, C.; Zhao, Z.; Zhang, T.; Li, L.; et al. Cancer-Associated Fibroblast (CAF) Heterogeneity and Targeting Therapy of CAFs in Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 655152. [Google Scholar] [CrossRef]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct Populations of Inflammatory Fibroblasts and Myofibroblasts in Pancreatic Cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Ge, W.; Yue, M.; Lin, R.; Zhou, T.; Xu, H.; Wang, Y.; Mao, T.; Li, S.; Wu, X.; Zhang, X.; et al. PLA2G2A+ Cancer-Associated Fibroblasts Mediate Pancreatic Cancer Immune Escape via Impeding Antitumor Immune Response of CD8+ Cytotoxic T Cells. Cancer Lett. 2023, 558, 216095. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-Associated Fibroblasts in the Single-Cell Era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The Pancreatic Stellate Cell: A Star on the Rise in Pancreatic Diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-Delivered CD44v6/C1QBP Complex Drives Pancreatic Cancer Liver Metastasis by Promoting Fibrotic Liver Microenvironment. Gut 2022, 71, 568–579. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Selvan, S.R.; Santin, A.D.; Bellone, S.; Saravanan, T.S.; Morton, D.L. Immunogenic Gangliosides in Human Ovarian Carcinoma. Biochem. Biophys. Res. Commun. 2007, 353, 251–258. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Pinzani, M.; Palù, G.; Martines, D. Exposure to Bacterial Cell Wall Products Triggers an Inflammatory Phenotype in Hepatic Stellate Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G571–G578. [Google Scholar] [CrossRef]
- Winau, F.; Hegasy, G.; Weiskirchen, R.; Weber, S.; Cassan, C.; Sieling, P.A.; Modlin, R.L.; Liblau, R.S.; Gressner, A.M.; Kaufmann, S.H.E. Ito Cells Are Liver-Resident Antigen-Presenting Cells for Activating T Cell Responses. Immunity 2007, 26, 117–129. [Google Scholar] [CrossRef]
- Yu, M.-C.; Chen, C.-H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-Cell Responses by Hepatic Stellate Cells via B7-H1-Mediated T-Cell Apoptosis in Mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef]
- Farran, B.; Nagaraju, G.P. The Dynamic Interactions between the Stroma, Pancreatic Stellate Cells and Pancreatic Tumor Development: Novel Therapeutic Targets. Cytokine Growth Factor. Rev. 2019, 48, 11–23. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Ou-Yang, F.; Hou, M.-F.; Huang, H.-W.; Wang, H.-R.; Li, K.-T.; Fayyaz, S.; Shu, C.-W.; Chang, H.-W. Oxidative Stress-Modulating Drugs Have Preferential Anticancer Effects—Involving the Regulation of Apoptosis, DNA Damage, Endoplasmic Reticulum Stress, Autophagy, Metabolism, and Migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J.; Sun, B.; Xu, W.; Zhong, M.; Li, Y.; He, C.; Chen, Y.; Wang, X.; Jones, P.M.; et al. Vitamin A Deficiency Causes Islet Dysfunction by Inducing Islet Stellate Cell Activation via Cellular Retinol Binding Protein 1. Int. J. Biol. Sci. 2020, 16, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Drummer, C.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by Endothelial Cells—Partnering up with the Immune System? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A.; McKay, C.; Pendeville-Samain, H.; Nilsson, J.A.; Maclean, K.H.; White, E.L.; Davis, A.C.; Ihle, J.N.; Cleveland, J.L. C-Myc Is Essential for Vasculogenesis and Angiogenesis during Development and Tumor Progression. Genes. Dev. 2002, 16, 2530–2543. [Google Scholar] [CrossRef]
- Ciesielski, O.; Biesiekierska, M.; Panthu, B.; Vialichka, V.; Pirola, L.; Balcerczyk, A. The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2606. [Google Scholar] [CrossRef]
- Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. A Dialogue between the Hypoxia-Inducible Factor and the Tumor Microenvironment. Cancer Microenviron. 2008, 1, 53–68. [Google Scholar] [CrossRef]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.-C.; Pircher, A.; Geldhof, V.; de Rooij, L.P.M.H.; Kalucka, J.; Sokol, L.; et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 21–36.e13. [Google Scholar] [CrossRef]
- Hida, K.; Hida, Y.; Amin, D.N.; Flint, A.F.; Panigrahy, D.; Morton, C.C.; Klagsbrun, M. Tumor-Associated Endothelial Cells with Cytogenetic Abnormalities. Cancer Res. 2004, 64, 8249–8255. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype Molding of Stromal Cells in the Lung Tumor Microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef] [PubMed]
- Mer, A.S.; Ba-Alawi, W.; Smirnov, P.; Wang, Y.X.; Brew, B.; Ortmann, J.; Tsao, M.-S.; Cescon, D.W.; Goldenberg, A.; Haibe-Kains, B. Integrative Pharmacogenomics Analysis of Patient-Derived Xenografts. Cancer Res. 2019, 79, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment—New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Naschberger, E.; Schellerer, V.S.; Rau, T.T.; Croner, R.S.; Stürzl, M. Isolation of Endothelial Cells from Human Tumors. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 209–218. ISBN 978-1-61779-080-5. [Google Scholar]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in Pericytes on Blood Vessels and Endothelial Sprouts in Tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Sievert, W.; Tapio, S.; Breuninger, S.; Gaipl, U.; Andratschke, N.; Trott, K.-R.; Multhoff, G. Adhesion Molecule Expression and Function of Primary Endothelial Cells in Benign and Malignant Tissues Correlates with Proliferation. PLoS ONE 2014, 9, e91808. [Google Scholar] [CrossRef]
- Carman, C.V.; Martinelli, R. T Lymphocyte-Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity. Front. Immunol. 2015, 6, 603. [Google Scholar] [CrossRef]
- Doak, G.R.; Schwertfeger, K.L.; Wood, D.K. Distant Relations: Macrophage Functions in the Metastatic Niche. Trends Cancer 2018, 4, 445–459. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour Hypoxia Promotes Tolerance and Angiogenesis via CCL28 and T(Reg) Cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Irjala, H.; Elima, K.; Johansson, E.-L.; Merinen, M.; Kontula, K.; Alanen, K.; Grenman, R.; Salmi, M.; Jalkanen, S. The Same Endothelial Receptor Controls Lymphocyte Traffic Both in Vascular and Lymphatic Vessels. Eur. J. Immunol. 2003, 33, 815–824. [Google Scholar] [CrossRef]
- De Sanctis, F.; Ugel, S.; Facciponte, J.; Facciabene, A. The Dark Side of Tumor-Associated Endothelial Cells. Semin. Immunol. 2018, 35, 35–47. [Google Scholar] [CrossRef]
- Dalton, H.J.; Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Lopez-Berestein, G.; Bar-Eli, M.; Sood, A.K. Monocyte Subpopulations in Angiogenesis. Cancer Res. 2014, 74, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Silina, K.; van den Broek, M.; Hirahara, K.; Yanagita, M. The Roles of Tertiary Lymphoid Structures in Chronic Diseases. Nat. Rev. Nephrol. 2023, 19, 525–537. [Google Scholar] [CrossRef]
- Yeo, E.-J.; Cassetta, L.; Qian, B.-Z.; Lewkowich, I.; Li, J.; Stefater, J.A.; Smith, A.N.; Wiechmann, L.S.; Wang, Y.; Pollard, J.W.; et al. Myeloid WNT7b Mediates the Angiogenic Switch and Metastasis in Breast Cancer. Cancer Res. 2014, 74, 2962–2973. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Mousa, M.; Nadukkandy, A.S.; Franssens, L.; Alnaqbi, H.; Alshamsi, F.Y.; Safar, H.A.; Carmeliet, P. Understanding Tumour Endothelial Cell Heterogeneity and Function from Single-Cell Omics. Nat. Rev. Cancer 2023, 23, 544–564. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, P.-P.; Wang, L.; Zhao, H.-J.; Weng, X.-Q.; Zhong, H.-J.; Qu, B.; Xiong, J.; Zhao, Y.; Wang, X.-F.; et al. MiR21 Sensitized B-Lymphoma Cells to ABT-199 via ICOS/ICOSL-Mediated Interaction of Treg Cells with Endothelial Cells. J. Exp. Clin. Cancer Res. 2017, 36, 82. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered Adipose Tissue and Adipocyte Function in the Pathogenesis of Metabolic Syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Lu, Y.; Liu, C.; Yang, W. Tumor Microenvironment-Mediated Immune Tolerance in Development and Treatment of Gastric Cancer. Front. Immunol. 2022, 13, 1016817. [Google Scholar] [CrossRef]
- Cao, Y. Adipocyte and Lipid Metabolism in Cancer Drug Resistance. J. Clin. Investig. 2019, 129, 3006–3017. [Google Scholar] [CrossRef]
- Blade, S.P.; Falkowski, D.J.; Bachand, S.N.; Pagano, S.J.; Chin, L. Mechanobiology of Adipocytes. Biology 2024, 13, 434. [Google Scholar] [CrossRef]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Wang, X.; Wu, Q.; Zhong, M.; Chen, Y.; Wang, Y.; Li, X.; Zhao, W.; Ge, C.; Wang, X.; Yu, Y.; et al. Adipocyte-Derived Ferroptotic Signaling Mitigates Obesity. Cell Metab. 2024. [Google Scholar] [CrossRef] [PubMed]
- Otley, M.O.C.; Sinal, C.J. Adipocyte-Cancer Cell Interactions in the Bone Microenvironment. Front. Endocrinol. 2022, 13, 903925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, M.; Tu, J.; Li, F.; Deng, Q.; Xu, J.; He, X.; Ding, J.; Xia, J.; Sheng, D.; et al. PMN-MDSCs Modulated by CCL20 from Cancer Cells Promoted Breast Cancer Cell Stemness through CXCL2-CXCR2 Pathway. Signal Transduct. Target. Ther. 2023, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, Y.; Luo, M.; Mäkilä, E.; Day, B.W.; Voelcker, N.H.; Tong, W.Y. Effectiveness of Porous Silicon Nanoparticle Treatment at Inhibiting the Migration of a Heterogeneous Glioma Cell Population. J. Nanobiotechnol. 2021, 19, 60. [Google Scholar] [CrossRef]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer Stem Cells: A Review from Origin to Therapeutic Implications. J. Cell Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, T.; Liu, A.Y.; Ouyang, G. Differentiation and Transdifferentiation Potentials of Cancer Stem Cells. Oncotarget 2015, 6, 39550–39563. [Google Scholar] [CrossRef]
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 31–42. [Google Scholar] [CrossRef]
- Bartsch, J.E.; Staren, E.D.; Appert, H.E. Matrix Metalloproteinase Expression in Breast Cancer. J. Surg. Res. 2003, 110, 383–392. [Google Scholar] [CrossRef]
- Hass, R. Role of MSC in the Tumor Microenvironment. Cancers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with Tumor Cells. Cell Commun. Signal 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Naderi, E.H.; Skah, S.; Ugland, H.; Myklebost, O.; Sandnes, D.L.; Torgersen, M.L.; Josefsen, D.; Ruud, E.; Naderi, S.; Blomhoff, H.K. Bone Marrow Stroma-Derived PGE2 Protects BCP-ALL Cells from DNA Damage-Induced P53 Accumulation and Cell Death. Mol. Cancer 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Rafii, A.; Mirshahi, P.; Poupot, M.; Faussat, A.-M.; Simon, A.; Ducros, E.; Mery, E.; Couderc, B.; Lis, R.; Capdet, J.; et al. Oncologic Trogocytosis of an Original Stromal Cells Induces Chemoresistance of Ovarian Tumours. PLoS ONE 2008, 3, e3894. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Oliveira, R.H.M.; Zhao, C.; Popel, A.S. Systems Biology of Angiogenesis Signaling: Computational Models and Omics. WIREs Mech. Dis. 2022, 14, e1550. [Google Scholar] [CrossRef]
- van de Ven, R.; Bifulco, C.B.; Luke, J.J.; Church, S.E. Editorial: Mechanisms of Lymphocyte Exclusion in the Tumor Microenvironment. Front. Immunol. 2022, 13, 908612. [Google Scholar] [CrossRef]
- Nakiboneka, R.; Mugaba, S.; Auma, B.O.; Kintu, C.; Lindan, C.; Nanteza, M.B.; Kaleebu, P.; Serwanga, J. Interferon Gamma (IFN-γ) Negative CD4+ and CD8+ T-Cells Can Produce Immune Mediators in Response to Viral Antigens. Vaccine 2019, 37, 113–122. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T Cells in Tumor Microenvironment: New Mechanisms, Potential Therapeutic Strategies and Future Prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-Infiltrating Regulatory T Cells as Targets of Cancer Immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef]
- Feng, B.; Bai, Z.; Zhou, X.; Zhao, Y.; Xie, Y.-Q.; Huang, X.; Liu, Y.; Enbar, T.; Li, R.; Wang, Y.; et al. The Type 2 Cytokine Fc-IL-4 Revitalizes Exhausted CD8+ T Cells against Cancer. Nature 2024, 634, 712–720. [Google Scholar] [CrossRef]
- Blunt, M.D.; Khakoo, S.I. Activating Killer Cell Immunoglobulin-like Receptors: Detection, Function and Therapeutic Use. Int. J. Immunogenet. 2020, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Shah, M.H.; Turner, M.J.; VanDeusen, J.B.; Whitman, S.P.; Cooper, M.A.; Suzuki, K.; Wechser, M.; Goodsaid, F.; Caligiuri, M.A. Differential Cytokine and Chemokine Gene Expression by Human NK Cells Following Activation with IL-18 or IL-15 in Combination with IL-12: Implications for the Innate Immune Response. J. Immunol. 1999, 162, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Nagler, A.; Lanier, L.L.; Cwirla, S.; Phillips, J.H. Comparative Studies of Human FcRIII-Positive and Negative Natural Killer Cells. J. Immunol. 1989, 143, 3183–3191. [Google Scholar] [CrossRef]
- Weil, S.; Memmer, S.; Lechner, A.; Huppert, V.; Giannattasio, A.; Becker, T.; Müller-Runte, A.; Lampe, K.; Beutner, D.; Quaas, A.; et al. Natural Killer Group 2D Ligand Depletion Reconstitutes Natural Killer Cell Immunosurveillance of Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2017, 8, 387. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Loder, B.F.; Mutschler, B.; Ray, R.J.; Paige, C.J.; Sideras, P.; Torres, R.; Lamers, M.C.; Carsetti, R. B Cell Development in the Spleen Takes Place in Discrete Steps and Is Determined by the Quality of B Cell Receptor–Derived Signals. J. Exp. Med. 1999, 190, 75–90. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Veneri, D.; Ortolani, R.; Franchini, M.; Tridente, G.; Pizzolo, G.; Vella, A. Expression of CD27 and CD23 on Peripheral Blood B Lymphocytes in Humans of Different Ages. Blood Transfus. 2009, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.-D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, Y.-X.; Lao, X.-M.; Liang, Y.-J.; Liao, G.-Q. CD19(+)IL-10(+) Regulatory B Cells Affect Survival of Tongue Squamous Cell Carcinoma Patients and Induce Resting CD4(+) T Cells to CD4(+)Foxp3(+) Regulatory T Cells. Oral. Oncol. 2016, 53, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte Heterogeneity and Functions in Cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Dress, R.J.; Zhu, Y.; Zhang, S.; De Feo, D.; Kong, W.T.; Cai, P.; Shin, A.; et al. Dendritic Cell Type 3 Arises from Ly6C+ Monocyte-Dendritic Cell Progenitors. Immunity 2023, 56, 1761–1777.e6. [Google Scholar] [CrossRef]
- Tak, T.; Drylewicz, J.; Conemans, L.; de Boer, R.J.; Koenderman, L.; Borghans, J.A.M.; Tesselaar, K. Circulatory and Maturation Kinetics of Human Monocyte Subsets in Vivo. Blood 2017, 130, 1474–1477. [Google Scholar] [CrossRef]
- Edwards, C.V.; Hassan, H.; Yildirim, C.; Ferri, G.; Verma, K.P.; Murray Horwitz, M.E.; Fillmore, N.R.; Munshi, N.C. Peripheral Blood Monocyte Count Is a Dynamic Prognostic Biomarker in Multiple Myeloma. Blood Adv. 2023, 7, 482–490. [Google Scholar] [CrossRef]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of Human Monocyte Subpopulations in Health and Disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Soltani, S.; Mahmoudi, M.; Farhadi, E. Dendritic Cells Currently under the Spotlight; Classification and Subset Based upon New Markers. Immunol. Investig. 2021, 50, 646–661. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. Systems Immunology Allows a New View on Human Dendritic Cells. Semin. Cell Dev. Biol. 2019, 86, 15–23. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Andersen, M.H. The Role of Dendritic Cells in Cancer. Semin. Immunopathol. 2017, 39, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Fricke, I.; Gabrilovich, D.I. Dendritic Cells and Tumor Microenvironment: A Dangerous Liaison. Immunol. Investig. 2006, 35, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen Presentation in Cancer: Insights into Tumour Immunogenicity and Immune Evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, M.; Sun, H.; Feng, Y.; Xu, L.; Chan, A.W.H.; Tong, J.H.; Wong, J.; Chong, C.C.N.; Lai, P.B.S.; et al. Hepatoma-Intrinsic CCRK Inhibition Diminishes Myeloid-Derived Suppressor Cell Immunosuppression and Enhances Immune-Checkpoint Blockade Efficacy. Gut 2018, 67, 931–944. [Google Scholar] [CrossRef]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-Type Oxidized LDL Receptor-1 Distinguishes Population of Human Polymorphonuclear Myeloid-Derived Suppressor Cells in Cancer Patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-Derived Suppressor Cells as Immunosuppressive Regulators and Therapeutic Targets in Cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Baniyash, M. Myeloid-Derived Suppressor Cells as Intruders and Targets: Clinical Implications in Cancer Therapy. Cancer Immunol. Immunother. 2016, 65, 857–867. [Google Scholar] [CrossRef]
- Lopez-Bujanda, Z.; Drake, C.G. Myeloid-Derived Cells in Prostate Cancer Progression: Phenotype and Prospective Therapies. J. Leukoc. Biol. 2017, 102, 393–406. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Carus, A.; Ladekarl, M.; Hager, H.; Pilegaard, H.; Nielsen, P.S.; Donskov, F. Tumor-Associated Neutrophils and Macrophages in Non-Small Cell Lung Cancer: No Immediate Impact on Patient Outcome. Lung Cancer 2013, 81, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, P.C.; Vieira, É.L.M.; Sousa, A.A.; Teixeira, A.L.; Aguiar, M.C.F. Immunophenotype of Neutrophils in Oral Squamous Cell Carcinoma Patients. J. Oral. Pathol. Med. 2017, 46, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, Y.-J.; Wang, X.-Y.; Qiu, S.-J.; Shi, Y.-H.; Sun, J.; Yi, Y.; Shi, J.-Y.; Shi, G.-M.; Ding, Z.-B.; et al. CXCR6 Upregulation Contributes to a Proinflammatory Tumor Microenvironment That Drives Metastasis and Poor Patient Outcomes in Hepatocellular Carcinoma. Cancer Res. 2012, 72, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.-M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.-Y.; Zheng, L. Peritumoral Neutrophils Link Inflammatory Response to Disease Progression by Fostering Angiogenesis in Hepatocellular Carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J.-S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Rubenich, D.S.; Omizzollo, N.; Szczepański, M.J.; Reichert, T.E.; Whiteside, T.L.; Ludwig, N.; Braganhol, E. Small Extracellular Vesicle-Mediated Bidirectional Crosstalk between Neutrophils and Tumor Cells. Cytokine Growth Factor. Rev. 2021, 61, 16–26. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils Orchestrate Cancer Rejection by Normalizing Tumor Vessels and Enhancing Infiltration of CD8(+) T Cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. [Google Scholar] [CrossRef]
- Johnston, L.K.; Bryce, P.J. Understanding Interleukin 33 and Its Roles in Eosinophil Development. Front. Med. 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 Restricts Tumor Growth and Inhibits Pulmonary Metastasis in Melanoma-Bearing Mice through Eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Ishida, T.; Inagaki, A.; Ishii, T.; Yano, H.; Komatsu, H.; Iida, S.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; et al. Clinical Significance of a Blood Eosinophilia in Adult T-Cell Leukemia/Lymphoma: A Blood Eosinophilia Is a Significant Unfavorable Prognostic Factor. Leuk. Res. 2007, 31, 915–920. [Google Scholar] [CrossRef]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Khiabany, A.; Nakamura, M.; Pellizzari, G.; Ilieva, K.M.; Lombardi, S.; Gould, H.J.; Corrigan, C.J.; et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells 2020, 9, 1631. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tüting, T.; Rudensky, A.Y.; Hämmerling, G.J. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Schwartz, C.; Panzer, M.; Schiedewitz, I.; Naumann, R.; Voehringer, D. Basophils Orchestrate Chronic Allergic Dermatitis and Protective Immunity against Helminths. Immunity 2010, 33, 364–374. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Westra, W.H.; Taube, J.M.; Poeta, M.L.; Begum, S.; Sidransky, D.; Koch, W.M. Inverse Relationship between Human Papillomavirus-16 Infection and Disruptive P53 Gene Mutations in Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2008, 14, 366–369. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhai, Y.; Li, F.; Shi, X.; Ying, M. Single-Cell Profiling Reveals Heterogeneity of Primary and Lymph Node Metastatic Tumors and Immune Cell Populations and Discovers Important Prognostic Significance of CCDC43 in Oral Squamous Cell Carcinoma. Front. Immunol. 2022, 13, 843322. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Xia, C.; Ding, L.; Pu, Y.; Hu, X.; Cai, H.; Hu, Q. Integrated Analysis of Single-Cell RNA-Seq and Bulk RNA-Seq Reveals Distinct Cancer-Associated Fibroblasts in Head and Neck Squamous Cell Carcinoma. Ann. Transl. Med. 2021, 9, 1017. [Google Scholar] [CrossRef]
- Alcolea, S.; Antón, R.; Camacho, M.; Soler, M.; Alfranca, A.; Avilés-Jurado, F.-X.; Redondo, J.-M.; Quer, M.; León, X.; Vila, L. Interaction between Head and Neck Squamous Cell Carcinoma Cells and Fibroblasts in the Biosynthesis of PGE2. J. Lipid Res. 2012, 53, 630–642. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Vitek, R.; Swick, A.D.; Skala, M.C.; Wisinski, K.B.; Kimple, R.J.; Lambert, P.F.; Beebe, D.J. Effects of Culture Method on Response to EGFR Therapy in Head and Neck Squamous Cell Carcinoma Cells. Sci. Rep. 2019, 9, 12480. [Google Scholar] [CrossRef]
- Custódio, M.; Biddle, A.; Tavassoli, M. Portrait of a CAF: The Story of Cancer-Associated Fibroblasts in Head and Neck Cancer. Oral. Oncol. 2020, 110, 104972. [Google Scholar] [CrossRef]
- Galbo, P.M.; Zang, X.; Zheng, D. Molecular Features of Cancer-Associated Fibroblast Subtypes and Their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Heesom, K.; Parkinson, E.K.; Prime, S.S. Senescent Cancer-Associated Fibroblasts Secrete Active MMP-2 That Promotes Keratinocyte Dis-Cohesion and Invasion. Br. J. Cancer 2014, 111, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.-C.; Ansell, A.; Jerhammar, F.; Lindh, M.B.; Grénman, R.; Munck-Wikland, E.; Östman, A.; Roberg , K. Cancer-Associated Fibroblasts Induce Matrix Metalloproteinase-Mediated Cetuximab Resistance in Head and Neck Squamous Cell Carcinoma Cells. Mol. Cancer Res. 2012, 10, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; Humayun, M.; Gong, M.M.; Kerr, S.C.; Ponik, S.M.; Harari, P.M.; Virumbrales-Muñoz, M.; Beebe, D.J. Primary Head and Neck Tumour-Derived Fibroblasts Promote Lymphangiogenesis in a Lymphatic Organotypic Co-Culture Model. eBioMedicine 2021, 73, 103634. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Rokudai, S.; Kawabata-Iwakawa, R.; Sakakura, K.; Oyama, T.; Nishiyama, M.; Chikamatsu, K. AKT3 Is a Novel Regulator of Cancer-Associated Fibroblasts in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1233. [Google Scholar] [CrossRef]
- Qin, X.; Yan, M.; Wang, X.; Xu, Q.; Wang, X.; Zhu, X.; Shi, J.; Li, Z.; Zhang, J.; Chen, W. Cancer-Associated Fibroblast-Derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-Kappa B Signaling Pathway. Theranostics 2018, 8, 921–940. [Google Scholar] [CrossRef]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.; Lu, E.; Chen, W.; et al. Exosomal miR-196a Derived from Cancer-Associated Fibroblasts Confers Cisplatin Resistance in Head and Neck Cancer through Targeting CDKN1B and ING5. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef]
- Yegodayev, K.M.; Novoplansky, O.; Golden, A.; Prasad, M.; Levin, L.; Jagadeeshan, S.; Zorea, J.; Dimitstein, O.; Joshua, B.-Z.; Cohen, L.; et al. TGF-Beta-Activated Cancer-Associated Fibroblasts Limit Cetuximab Efficacy in Preclinical Models of Head and Neck Cancer. Cancers 2020, 12, 339. [Google Scholar] [CrossRef]
- Principe, S.; Mejia-Guerrero, S.; Ignatchenko, V.; Sinha, A.; Ignatchenko, A.; Shi, W.; Pereira, K.; Su, S.; Huang, S.H.; O’Sullivan, B.; et al. Proteomic Analysis of Cancer-Associated Fibroblasts Reveals a Paracrine Role for MFAP5 in Human Oral Tongue Squamous Cell Carcinoma. J. Proteome Res. 2018, 17, 2045–2059. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Zhao, M.; Ding, L.; Yang, X.; Jing, Y.; Song, Y.; Chen, S.; Hu, Q.; Ni, Y. ITGB2-Mediated Metabolic Switch in CAFs Promotes OSCC Proliferation by Oxidation of NADH in Mitochondrial Oxidative Phosphorylation System. Theranostics 2020, 10, 12044–12059. [Google Scholar] [CrossRef]
- Herrera-Vargas, A.K.; García-Rodríguez, E.; Olea-Flores, M.; Mendoza-Catalán, M.A.; Flores-Alfaro, E.; Navarro-Tito, N. Pro-Angiogenic Activity and Vasculogenic Mimicry in the Tumor Microenvironment by Leptin in Cancer. Cytokine Growth Factor. Rev. 2021, 62, 23–41. [Google Scholar] [CrossRef]
- Miao, X.; Wang, B.; Chen, K.; Ding, R.; Wu, J.; Pan, Y.; Ji, P.; Ye, B.; Xiang, M. Perspectives of Lipid Metabolism Reprogramming in Head and Neck Squamous Cell Carcinoma: An Overview. Front. Oncol. 2022, 12, 1008361. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wang, H.; Fan, C.; Song, Q.; Ding, R.; Wu, J.; Hu, H.; Chen, K.; Ji, P.; Wen, Q.; et al. Enhancing Prognostic Accuracy in Head and Neck Squamous Cell Carcinoma Chemotherapy via a Lipid Metabolism-Related Clustered Polygenic Model. Cancer Cell Int. 2023, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Mfuna Endam, L.; Cormier, C.; Bossé, Y.; Filali-Mouhim, A.; Desrosiers, M. Association of IL1A, IL1B, and TNF Gene Polymorphisms with Chronic Rhinosinusitis with and without Nasal Polyposis: A Replication Study. Arch. Otolaryngol. Head. Neck Surg. 2010, 136, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar] [CrossRef]
- Damasio, M.P.S.; Nascimento, C.S.; Andrade, L.M.; de Oliveira, V.L.; Calzavara-Silva, C.E. The Role of T-Cells in Head and Neck Squamous Cell Carcinoma: From Immunity to Immunotherapy. Front. Oncol. 2022, 12, 1021609. [Google Scholar] [CrossRef]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor Infiltrating Lymphocytes and Survival in Patients with Head and Neck Squamous Cell Carcinoma. Head. Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- Schneider, S.; Kadletz, L.; Wiebringhaus, R.; Kenner, L.; Selzer, E.; Füreder, T.; Rajky, O.; Berghoff, A.S.; Preusser, M.; Heiduschka, G. PD-1 and PD-L1 Expression in HNSCC Primary Cancer and Related Lymph Node Metastasis—Impact on Clinical Outcome. Histopathology 2018, 73, 573–584. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The Urgent Need to Recover MHC Class I in Cancers for Effective Immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef]
- Charap, A.J.; Enokida, T.; Brody, R.; Sfakianos, J.; Miles, B.; Bhardwaj, N.; Horowitz, A. Landscape of Natural Killer Cell Activity in Head and Neck Squamous Cell Carcinoma. J. Immunother. Cancer 2020, 8, e001523. [Google Scholar] [CrossRef]
- Gavrielatou, N.; Vathiotis, I.; Economopoulou, P.; Psyrri, A. The Role of B Cells in Head and Neck Cancer. Cancers 2021, 13, 5383. [Google Scholar] [CrossRef]
- Wood, O.; Woo, J.; Seumois, G.; Savelyeva, N.; McCann, K.J.; Singh, D.; Jones, T.; Peel, L.; Breen, M.S.; Ward, M.; et al. Gene Expression Analysis of TIL Rich HPV-Driven Head and Neck Tumors Reveals a Distinct B-Cell Signature When Compared to HPV Independent Tumors. Oncotarget 2016, 7, 56781–56797. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Schlößer, H.A.; Thelen, M.; Wennhold, K.; Rothschild, S.I.; Gilles, R.; Quaas, A.; Siefer, O.G.; Huebbers, C.U.; Cukuroglu, E.; et al. Tumor-Associated B Cells and Humoral Immune Response in Head and Neck Squamous Cell Carcinoma. OncoImmunology 2019, 8, 1535293. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Takahashi, H.; Motegi, S.-I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological Features of Circulating Monocyte Subsets in Patients with Squamous Cell Carcinoma of the Head and Neck. Clin. Immunol. 2021, 225, 108677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef]
- Lohse, S.; Loew, S.; Kretschmer, A.; Jansen, J.H.M.; Meyer, S.; Ten Broeke, T.; Rösner, T.; Dechant, M.; Derer, S.; Klausz, K.; et al. Effector Mechanisms of IgA Antibodies against CD20 Include Recruitment of Myeloid Cells for Antibody-Dependent Cell-Mediated Cytotoxicity and Complement-Dependent Cytotoxicity. Br. J. Haematol. 2018, 181, 413–417. [Google Scholar] [CrossRef]
- Mattavelli, D.; Lombardi, D.; Missale, F.; Calza, S.; Battocchio, S.; Paderno, A.; Bozzola, A.; Bossi, P.; Vermi, W.; Piazza, C.; et al. Prognostic Nomograms in Oral Squamous Cell Carcinoma: The Negative Impact of Low Neutrophil to Lymphocyte Ratio. Front. Oncol. 2019, 9, 339. [Google Scholar] [CrossRef]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hütte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear Granulocytes in Human Head and Neck Cancer: Enhanced Inflammatory Activity, Modulation by Cancer Cells and Expansion in Advanced Disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ Neutrophils as Functionally Activated Neutrophils Negatively Regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, K.; Song, Y.; Yang, W.; Shu, W.; Yu, T.; Yu, L.; Lin, M.; Wei, Q.; Chen, C.; et al. CD177+ Neutrophils Suppress Epithelial Cell Tumourigenesis in Colitis-Associated Cancer and Predict Good Prognosis in Colorectal Cancer. Carcinogenesis 2018, 39, 272–282. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Zhong, W.-Q.; Liu, Z.-J.; Li, H.-M.; Yu, Z.-L.; Zhao, Y.-F. Tumor Associated Macrophages Induce Epithelial to Mesenchymal Transition via the EGFR/ERK1/2 Pathway in Head and Neck Squamous Cell Carcinoma. Oncol. Rep. 2018, 40, 2558–2572. [Google Scholar] [CrossRef]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journé, F.; Saussez, S. High Infiltration of CD68+ Macrophages Is Associated with Poor Prognoses of Head and Neck Squamous Cell Carcinoma Patients and Is Influenced by Human Papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N. Molecular Pathology of Malignant Gliomas. Annu. Rev. Pathol. 2006, 1, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Nutt, C.L.; Betensky, R.A.; Iafrate, A.J.; Han, M.; Ligon, K.L.; Rowitch, D.H.; Louis, D.N. Expression of Oligodendroglial and Astrocytic Lineage Markers in Diffuse Gliomas: Use of YKL-40, ApoE, ASCL1, and NKX2-2. J. Neuropathol. Exp. Neurol. 2006, 65, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Armstrong, T.S.; Gilbert, M.R. Biology and Management of Ependymomas. Neuro Oncol. 2016, 18, 902–913. [Google Scholar] [CrossRef]
- Schneider, T.; Mawrin, C.; Scherlach, C.; Skalej, M.; Firsching, R. Gliomas in Adults. Dtsch. Arztebl. Int. 2010, 107, 799–807, quiz 808. [Google Scholar] [CrossRef]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular Signature and Crossroads with Tumor Microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef]
- Fuchs, Q.; Pierrevelcin, M.; Messe, M.; Lhermitte, B.; Blandin, A.-F.; Papin, C.; Coca, A.; Dontenwill, M.; Entz-Werlé, N. Hypoxia Inducible Factors’ Signaling in Pediatric High-Grade Gliomas: Role, Modelization and Innovative Targeted Approaches. Cancers 2020, 12, 979. [Google Scholar] [CrossRef]
- Balaziova, E.; Vymola, P.; Hrabal, P.; Mateu, R.; Zubal, M.; Tomas, R.; Netuka, D.; Kramar, F.; Zemanova, Z.; Svobodova, K.; et al. Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers 2021, 13, 3304. [Google Scholar] [CrossRef]
- Garnier, D.; Ratcliffe, E.; Briand, J.; Cartron, P.-F.; Oliver, L.; Vallette, F.M. The Activation of Mesenchymal Stem Cells by Glioblastoma Microvesicles Alters Their Exosomal Secretion of miR-100-5p, miR-9-5p and Let-7d-5p. Biomedicines 2022, 10, 112. [Google Scholar] [CrossRef]
- Jain, S.; Rick, J.W.; Joshi, R.S.; Beniwal, A.; Spatz, J.; Gill, S.; Chang, A.C.-C.; Choudhary, N.; Nguyen, A.T.; Sudhir, S.; et al. Single-Cell RNA Sequencing and Spatial Transcriptomics Reveal Cancer-Associated Fibroblasts in Glioblastoma with Protumoral Effects. J. Clin. Investig. 2023, 133, e147087. [Google Scholar] [CrossRef]
- Yao, Y.; Tan, X.; Yin, W.; Kou, Y.; Wang, X.; Jiang, X.; Chen, S.; Liu, Y.; Dang, J.; Yin, J.; et al. Performance of 18 F-FAPI PET/CT in Assessing Glioblastoma before Radiotherapy: A Pilot Study. BMC Med. Imaging 2022, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of Glioblastoma Stem Cells in Cancer Therapeutic Resistance: A Perspective on Antineoplastic Agents from Natural Sources and Chemical Derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, X.; Zhao, C.; Zhao, Z.; Hu, L.; Wang, D.; Wang, R.; Fang, Z. Molecular Subtyping of Glioblastoma Based on Immune-Related Genes for Prognosis. Sci. Rep. 2020, 10, 15495. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Lesniak, M.S. CD4+ CD25+ FoxP3+ T-Cell Infiltration and Heme Oxygenase-1 Expression Correlate with Tumor Grade in Human Gliomas. J. Neurooncol. 2007, 83, 145–152. [Google Scholar] [CrossRef]
- Sugihara, A.Q.; Rolle, C.E.; Lesniak, M.S. Regulatory T Cells Actively Infiltrate Metastatic Brain Tumors. Int. J. Oncol. 2009, 34, 1533–1540. [Google Scholar] [CrossRef]
- Gras Navarro, A.; Kmiecik, J.; Leiss, L.; Zelkowski, M.; Engelsen, A.; Bruserud, Ø.; Zimmer, J.; Enger, P.Ø.; Chekenya, M. NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage to Efficiently Kill Glioblastoma and Prolong Animal Survival. J. Immunol. 2014, 193, 6192–6206. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The Cancer-Natural Killer Cell Immunity Cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ Tumor-Infiltrating Immune Cells Correlate with Prolonged Survival in Glioblastoma Patients despite Integrated Immunosuppressive Mechanisms in the Tumor Microenvironment and at the Systemic Level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef]
- Levy, E.M.; Roberti, M.P.; Mordoh, J. Natural Killer Cells in Human Cancer: From Biological Functions to Clinical Applications. J. Biomed. Biotechnol. 2011, 2011, 676198. [Google Scholar] [CrossRef]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C.; et al. Tumor Infiltrating Immune Cells in Gliomas and Meningiomas. Brain Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, C.; Li, Q.; Dong, J.; Liu, Y.; Huang, Y.; Jiang, T.; Wu, A. Tumour-Infiltrating CD4+ and CD8+ Lymphocytes as Predictors of Clinical Outcome in Glioma. Br. J. Cancer 2014, 110, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; Gonzalez, G.C.; Zhang, L.; Ibrahim, G.; Kelly, J.J.; Gustafson, M.P.; Lin, Y.; Dietz, A.B.; Forsyth, P.A.; Yong, V.W.; et al. Normal Human Monocytes Exposed to Glioma Cells Acquire Myeloid-Derived Suppressor Cell-like Properties. Neuro Oncol. 2010, 12, 351–365. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Ugolini, A.; Veglia, F. Myeloid Cells in Glioblastoma Microenvironment. Cells 2020, 10, 18. [Google Scholar] [CrossRef]
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S.; et al. Immune Profiling of Human Tumors Identifies CD73 as a Combinatorial Target in Glioblastoma. Nat. Med. 2020, 26, 39–46. [Google Scholar] [CrossRef]
- Massara, M.; Persico, P.; Bonavita, O.; Mollica Poeta, V.; Locati, M.; Simonelli, M.; Bonecchi, R. Neutrophils in Gliomas. Front. Immunol. 2017, 8, 01349. [Google Scholar] [CrossRef]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; de Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef]
- Pang, L.; Khan, F.; Heimberger, A.B.; Chen, P. Mechanism and Therapeutic Potential of Tumor-Immune Symbiosis in Glioblastoma. Trends Cancer 2022, 8, 839–854. [Google Scholar] [CrossRef]
- Simonds, E.F.; Lu, E.D.; Badillo, O.; Karimi, S.; Liu, E.V.; Tamaki, W.; Rancan, C.; Downey, K.M.; Stultz, J.; Sinha, M.; et al. Deep Immune Profiling Reveals Targetable Mechanisms of Immune Evasion in Immune Checkpoint Inhibitor-Refractory Glioblastoma. J. Immunother. Cancer 2021, 9, e002181. [Google Scholar] [CrossRef]
- Xuan, W.; Lesniak, M.S.; James, C.D.; Heimberger, A.B.; Chen, P. Context-Dependent Glioblastoma-Macrophage/Microglia Symbiosis and Associated Mechanisms. Trends Immunol. 2021, 42, 280–292. [Google Scholar] [CrossRef]
- Bogović Crnčić, T. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L. An Overview of the Management of Papillary and Follicular Thyroid Carcinoma. Thyroid 1999, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Koo, J.S. Cell Component and Function of Tumor Microenvironment in Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 12578. [Google Scholar] [CrossRef]
- Pu, W.; Shi, X.; Yu, P.; Zhang, M.; Liu, Z.; Tan, L.; Han, P.; Wang, Y.; Ji, D.; Gan, H.; et al. Single-Cell Transcriptomic Analysis of the Tumor Ecosystems Underlying Initiation and Progression of Papillary Thyroid Carcinoma. Nat. Commun. 2021, 12, 6058. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.K.; Pienta, K.J. The Biology and Treatment of Oligometastatic Cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef]
- Saitoh, O.; Mitsutake, N.; Nakayama, T.; Nagayama, Y. Fibroblast-Mediated In Vivo and In Vitro Growth Promotion of Tumorigenic Rat Thyroid Carcinoma Cells but Not Normal Fisher Rat Thyroid Follicular Cells. Thyroid 2009, 19, 735–742. [Google Scholar] [CrossRef]
- Sun, R.; Luo, H.; Su, J.; Di, S.; Zhou, M.; Shi, B.; Sun, Y.; Du, G.; Zhang, H.; Jiang, H.; et al. Olaparib Suppresses MDSC Recruitment via SDF1α/CXCR4 Axis to Improve the Anti-Tumor Efficacy of CAR-T Cells on Breast Cancer in Mice. Mol. Ther. 2021, 29, 60–74. [Google Scholar] [CrossRef]
- Jolly, L.A.; Novitskiy, S.; Owens, P.; Massoll, N.; Cheng, N.; Fang, W.; Moses, H.L.; Franco, A.T. Fibroblast-Mediated Collagen Remodeling Within the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by BrafV600E and Pten Loss. Cancer Res. 2016, 76, 1804–1813. [Google Scholar] [CrossRef]
- Rotondi, M.; Chiovato, L.; Pacini, F.; Bartalena, L.; Vitti, P. Management of Subclinical Hypothyroidism in Pregnancy: A Comment from the Italian Society of Endocrinology and the Italian Thyroid Association to the 2017 American Thyroid Association Guidelines-“The Italian Way”. Thyroid 2018, 28, 551–555. [Google Scholar] [CrossRef]
- Wen, S.; Qu, N.; Ma, B.; Wang, X.; Luo, Y.; Xu, W.; Jiang, H.; Zhang, Y.; Wang, Y.; Ji, Q. Cancer-Associated Fibroblasts Positively Correlate with Dedifferentiation and Aggressiveness of Thyroid Cancer. Onco. Targets Ther. 2021, 14, 1205–1217. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.; Pan, Y.; Xu, J.; Si, Y.; Min, Z.; Yu, B. A New Risk Factor Indicator for Papillary Thyroid Cancer Based on Immune Infiltration. Cell Death Dis. 2021, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Zhou, T.; Li, B.; Wang, Z. Adiponectin and Thyroid Cancer: Insight into the Association between Adiponectin and Obesity. Aging Dis. 2021, 12, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Frasca, F.; Garozzo, A.; Gianì, F.; Pandini, G.; Vella, V.; Vigneri, R.; Belfiore, A. Insulin Receptor Isoforms and Insulin-like Growth Factor Receptor in Human Follicular Cell Precursors from Papillary Thyroid Cancer and Normal Thyroid. J. Clin. Endocrinol. Metab. 2011, 96, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Iovino, F.; Eterno, V.; Cammareri, P.; Gambara, G.; Espina, V.; Gulotta, G.; Dieli, F.; Giordano, S.; De Maria, R.; et al. Tumorigenic and Metastatic Activity of Human Thyroid Cancer Stem Cells. Cancer Res. 2010, 70, 8874–8885. [Google Scholar] [CrossRef]
- Cunha, L.L.; Morari, E.C.; Guihen, A.C.T.; Razolli, D.; Gerhard, R.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Infiltration of a Mixture of Immune Cells May Be Related to Good Prognosis in Patients with Differentiated Thyroid Carcinoma. Clin. Endocrinol. 2012, 77, 918–925. [Google Scholar] [CrossRef]
- Weber, F. Lymphocytes and Thyroid Cancer: More to It than Meets the Eye? Endocr. Relat. Cancer 2014, 21, C1–C5. [Google Scholar] [CrossRef]
- Provinciali, M.; Muzzioli, M.; Di Stefano, G.; Fabris, N. Recovery of Spleen Cell Natural Killer Activity by Thyroid Hormone Treatment in Old Mice. Nat. Immun. Cell Growth Regul. 1991, 10, 226–236. [Google Scholar]
- Sharma, S.D.; Tsai, V.; Proffitt, M.R. Enhancement of Mouse Natural Killer Cell Activity by Thyroxine. Cell Immunol. 1982, 73, 83–97. [Google Scholar] [CrossRef]
- Gogali, F.; Paterakis, G.; Rassidakis, G.Z.; Liakou, C.I.; Liapi, C. CD3(-)CD16(-)CD56(Bright) Immunoregulatory NK Cells Are Increased in the Tumor Microenvironment and Inversely Correlate with Advanced Stages in Patients with Papillary Thyroid Cancer. Thyroid 2013, 23, 1561–1568. [Google Scholar] [CrossRef]
- Lee, E.K.; Sunwoo, J.B. Natural Killer Cells and Thyroid Diseases. Endocrinol. Metab. 2019, 34, 132–137. [Google Scholar] [CrossRef]
- Park, A.; Lee, Y.; Kim, M.S.; Kang, Y.J.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. Prostaglandin E2 Secreted by Thyroid Cancer Cells Contributes to Immune Escape Through the Suppression of Natural Killer (NK) Cell Cytotoxicity and NK Cell Differentiation. Front. Immunol. 2018, 9, 01859. [Google Scholar] [CrossRef] [PubMed]
- Menicali, E.; Guzzetti, M.; Morelli, S.; Moretti, S.; Puxeddu, E. Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. 2020, 11, 637826. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Takeda, H.; Kawada, S.; Maeda, K.; Yamakawa, M. Characterization of Dendritic Cells in Differentiated Thyroid Cancer. J. Pathol. 2005, 205, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Liu, X.; Jin, H.; Zhang, G.; Zhang, Q.; Yu, J. Regulatory T Cells and Plasmacytoid Dendritic Cells Contribute to the Immune Escape of Papillary Thyroid Cancer Coexisting with Multinodular Non-Toxic Goiter. Endocrine 2013, 44, 172–181. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Bellevicine, C.; Lansione, T.; Ferrara, A.L.; Iannone, R.; di Somma, S.; Borriello, F.; Clery, E.; et al. Potential Involvement of Neutrophils in Human Thyroid Cancer. PLoS ONE 2018, 13, e0199740. [Google Scholar] [CrossRef]
- Hattar, K.; Franz, K.; Ludwig, M.; Sibelius, U.; Wilhelm, J.; Lohmeyer, J.; Savai, R.; Subtil, F.S.B.; Dahlem, G.; Eul, B.; et al. Interactions between Neutrophils and Non-Small Cell Lung Cancer Cells: Enhancement of Tumor Proliferation and Inflammatory Mediator Synthesis. Cancer Immunol. Immunother. 2014, 63, 1297–1306. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Chen, J.; Zhang, P.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Interaction with Neutrophils Promotes Gastric Cancer Cell Migration and Invasion by Inducing Epithelial-Mesenchymal Transition. Oncol. Rep. 2017, 38, 2959–2966. [Google Scholar] [CrossRef]
- Fiumara, A.; Belfiore, A.; Russo, G.; Salomone, E.; Santonocito, G.M.; Ippolito, O.; Vigneri, R.; Gangemi, P. In Situ Evidence of Neoplastic Cell Phagocytosis by Macrophages in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 1997, 82, 1615–1620. [Google Scholar]
- Mantovani, A.; Allavena, P. The Interaction of Anticancer Therapies with Tumor-Associated Macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.M.; Knauf, J.A.; Fagin, J.A. Increased Density of Tumor-Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef]
- Qing, W.; Fang, W.-Y.; Ye, L.; Shen, L.-Y.; Zhang, X.-F.; Fei, X.-C.; Chen, X.; Wang, W.-Q.; Li, X.-Y.; Xiao, J.-C.; et al. Density of Tumor-Associated Macrophages Correlates with Lymph Node Metastasis in Papillary Thyroid Carcinoma. Thyroid 2012, 22, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Caillou, B.; Talbot, M.; Weyemi, U.; Pioche-Durieu, C.; Al Ghuzlan, A.; Bidart, J.M.; Chouaib, S.; Schlumberger, M.; Dupuy, C. Tumor-Associated Macrophages (TAMs) Form an Interconnected Cellular Supportive Network in Anaplastic Thyroid Carcinoma. PLoS ONE 2011, 6, e22567. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.; Mauro, G.; Erreni, M.; Romeo, P.; Minna, E.; Vizioli, M.G.; Belgiovine, C.; Rizzetti, M.G.; Pagliardini, S.; Avigni, R.; et al. Senescent Thyrocytes and Thyroid Tumor Cells Induce M2-like Macrophage Polarization of Human Monocytes via a PGE2-Dependent Mechanism. J. Exp. Clin. Cancer Res. 2019, 38, 208. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.M.; Chadwick, J.A.; Owen, R.P.; White, M.J.; Kaplinsky, J.; Peneva, I.; Frangou, A.; Xie, P.F.; Chang, J.; Roth, A.; et al. Tumor Monocyte Content Predicts Immunochemotherapy Outcomes in Esophageal Adenocarcinoma. Cancer Cell 2023, 41, 1222–1241.e7. [Google Scholar] [CrossRef]
- Jain, S.; Dhingra, S. Pathology of Esophageal Cancer and Barrett’s Esophagus. Ann. Cardiothorac. Surg. 2017, 6, 99–109. [Google Scholar] [CrossRef]
- Koizumi, W.; Kitago, M.; Shinoda, M.; Yagi, H.; Abe, Y.; Oshima, G.; Hori, S.; Inomata, K.; Kawakubo, H.; Kawaida, M.; et al. Successful Resection of Pancreatic Metastasis from Oesophageal Squamous Cell Carcinoma: A Case Report and Review of the Literature. BMC Cancer 2019, 19, 320. [Google Scholar] [CrossRef]
- Huang, T.-X.; Fu, L. The Immune Landscape of Esophageal Cancer. Cancer Commun. 2019, 39, 79. [Google Scholar] [CrossRef]
- Kam, N.W.; Wu, K.C.; Dai, W.; Wang, Y.; Yan, L.Y.C.; Shakya, R.; Khanna, R.; Qin, Y.; Law, S.; Lo, A.W.I.; et al. Peritumoral B Cells Drive Proangiogenic Responses in HMGB1-Enriched Esophageal Squamous Cell Carcinoma. Angiogenesis 2022, 25, 181–203. [Google Scholar] [CrossRef]
- Dinh, H.Q.; Pan, F.; Wang, G.; Huang, Q.-F.; Olingy, C.E.; Wu, Z.-Y.; Wang, S.-H.; Xu, X.; Xu, X.-E.; He, J.-Z.; et al. Integrated Single-Cell Transcriptome Analysis Reveals Heterogeneity of Esophageal Squamous Cell Carcinoma Microenvironment. Nat. Commun. 2021, 12, 7335. [Google Scholar] [CrossRef]
- Dunbar, K.J.; Karakasheva, T.A.; Tang, Q.; Efe, G.; Lin, E.W.; Harris, M.; Sahu, V.; Sachdeva, U.M.; Hu, J.; Klein-Szanto, A.J.; et al. Tumor-Derived CCL5 Recruits Cancer-Associated Fibroblasts and Promotes Tumor Cell Proliferation in Esophageal Squamous Cell Carcinoma. Mol. Cancer Res. 2023, 21, 741–752. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018, 78, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Morishima, K.; Ui, T.; Hoshino, H.; Matsubara, D.; Ishikawa, S.; Aburatani, H.; Fukayama, M.; Hosoya, Y.; Sata, N.; et al. The Role of HGF/MET and FGF/FGFR in Fibroblast-Derived Growth Stimulation and Lapatinib-Resistance of Esophageal Squamous Cell Carcinoma. BMC Cancer 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yang, L.; Yu, C.; Zhu, W.; Zhou, X.; Xiong, Y.; Wang, W.; Ji, F.; He, D.; Cao, X. Tumor-Secreted Exosomal lncRNA POU3F3 Promotes Cisplatin Resistance in ESCC by Inducing Fibroblast Differentiation into CAFs. Mol. Ther. Oncolytics 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Qiu, L.; Yue, J.; Ding, L.; Yin, Z.; Zhang, K.; Zhang, H. Cancer-Associated Fibroblasts: An Emerging Target against Esophageal Squamous Cell Carcinoma. Cancer Lett. 2022, 546, 215860. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Luo, Y.; Zhang, S.; Pu, Y.; Chen, Y.; Guo, W.; Yao, J.; Shao, M.; Fan, W.; et al. Dissecting Esophageal Squamous-Cell Carcinoma Ecosystem by Single-Cell Transcriptomic Analysis. Nat. Commun. 2021, 12, 5291. [Google Scholar] [CrossRef]
- Enzinger, P.C.; Mayer, R.J. Esophageal Cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef]
- Jacobson, B.C.; Somers, S.C.; Fuchs, C.S.; Kelly, C.P.; Camargo, C.A. Body-Mass Index and Symptoms of Gastroesophageal Reflux in Women. N. Engl. J. Med. 2006, 354, 2340–2348. [Google Scholar] [CrossRef]
- Kayani, B.; Okabayashi, K.; Ashrafian, H.; Harling, L.; Rao, C.; Darzi, A.; Kitagawa, Y.; Athanasiou, T.; Zacharakis, E. Does Obesity Affect Outcomes in Patients Undergoing Esophagectomy for Cancer? A Meta-Analysis. World J. Surg. 2012, 36, 1785–1795. [Google Scholar] [CrossRef]
- Perumal, K.; Mun, K.S.; Yap, N.Y.; Razack, A.H.A.; Gobe, G.C.; Ong, T.A.; Kuppusamy, S.; Rajandram, R. A Study on the Immunohistochemical Expressions of Leptin and Leptin Receptor in Clear Cell Renal Cell Carcinoma. Biomed. Res. Int. 2020, 2020, 3682086. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Ma, N.; Yang, Y.; Ma, Y.; Wang, F.; Wang, Y.; Wei, J.; Chen, H.; Tartarone, A.; et al. Progenitor-like Exhausted SPRY1+CD8+ T Cells Potentiate Responsiveness to Neoadjuvant PD-1 Blockade in Esophageal Squamous Cell Carcinoma. Cancer Cell 2023, 41, 1852–1870.e9. [Google Scholar] [CrossRef] [PubMed]
- Croft, W.; Evans, R.P.T.; Pearce, H.; Elshafie, M.; Griffiths, E.A.; Moss, P. The Single Cell Transcriptional Landscape of Esophageal Adenocarcinoma and Its Modulation by Neoadjuvant Chemotherapy. Mol. Cancer 2022, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Z.; Han, Y.; Han, L.; Zou, X.; Zhou, B.; Hu, R.; Hao, J.; Bai, S.; Xiao, H.; et al. Immune Suppressive Landscape in the Human Esophageal Squamous Cell Carcinoma Microenvironment. Nat. Commun. 2020, 11, 6268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Zhai, T.-T.; Su, M.; Pan, H.-C.; Tang, Q.; Huang, B.-H.; Chi, X.-R.; Li, N.; Xie, L.-H.; Qiu, S.-Q.; et al. EZH2 Elicits CD8+ T-Cell Desert in Esophageal Squamous Cell Carcinoma via Suppressing CXCL9 and Dendritic Cells. Commun. Biol. 2024, 7, 1–16. [Google Scholar] [CrossRef]
- Qian, L.; Bian, G.-R.; Zhou, Y.; Wang, Y.; Hu, J.; Liu, X.; Xu, Y. Clinical Significance of Regulatory B Cells in the Peripheral Blood of Patients with Oesophageal Cancer. Cent. Eur. J. Immunol. 2015, 40, 263–265. [Google Scholar] [CrossRef]
- Girolomoni, G.; Caux, C.; Lebecque, S.; Dezutter-Dambuyant, C.; Ricciardi-Castagnoli, P. Langerhans Cells: Still a Fundamental Paradigm for Studying the Immunobiology of Dendritic Cells. Trends Immunol. 2002, 23, 6–8. [Google Scholar] [CrossRef]
- Zavala, W.D.; De Simone, D.S.; Sacerdote, F.L.; Cavicchia, J.C. Variation in Langerhans Cell Number and Morphology between the Upper and Lower Regions of the Human Esophageal Epithelium. Anat. Rec. 2002, 268, 360–364. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J. Immunologic Function of Dendritic Cells in Esophageal Cancer. Dig. Dis. Sci. 2008, 53, 1739–1746. [Google Scholar] [CrossRef]
- Chen, S.-R.; Luo, Y.-P.; Zhang, J.-K.; Yang, W.; Zhen, Z.-C.; Chen, L.-X.; Zhang, W. Study on Immune Function of Dendritic Cells in Patients with Esophageal Carcinoma. World J. Gastroenterol. 2004, 10, 934–939. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, P.; Song, M.; Qin, G.; Gao, Q.; Zhang, Z.; Yue, D.; Wang, D.; Nan, S.; et al. Dual TGF-β and PD-1 Blockade Synergistically Enhances MAGE-A3-Specific CD8+ T Cell Response in Esophageal Squamous Cell Carcinoma. Int. J. Cancer 2018, 143, 2561–2574. [Google Scholar] [CrossRef]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current Developments in Gastric Cancer: From Molecular Profiling to Treatment Strategy. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter Pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Chia, N.-Y.; Tan, P. Molecular Classification of Gastric Cancer. Ann. Oncol. 2016, 27, 763–769. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining Cell Type Abundance and Expression from Bulk Tissues with Digital Cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Gao, L.-M.; Wang, F.; Zheng, Y.; Fu, Z.-Z.; Zheng, L.; Chen, L.-L. Roles of Fibroblast Activation Protein and Hepatocyte Growth Factor Expressions in Angiogenesis and Metastasis of Gastric Cancer. Pathol. Oncol. Res. 2019, 25, 369–376. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Liu, X.; Kammertoens, T.; Blankenstein, T.; Qin, Z. Fibroblast-Specific Protein 1/S100A4-Positive Cells Prevent Carcinoma through Collagen Production and Encapsulation of Carcinogens. Cancer Res. 2013, 73, 2770–2781. [Google Scholar] [CrossRef]
- Zhan, S.; Liu, Z.; Zhang, M.; Guo, T.; Quan, Q.; Huang, L.; Guo, L.; Cao, L.; Zhang, X. Overexpression of B7-H3 in α-SMA-Positive Fibroblasts Is Associated with Cancer Progression and Survival in Gastric Adenocarcinomas. Front. Oncol. 2019, 9, 1466. [Google Scholar] [CrossRef]
- Kushiyama, S.; Yashiro, M.; Yamamoto, Y.; Sera, T.; Sugimoto, A.; Nishimura, S.; Togano, S.; Kuroda, K.; Okuno, T.; Miki, Y.; et al. Dipeptidyl Peptidase-4 from Cancer-Associated Fibroblasts Stimulates the Proliferation of Scirrhous-Type Gastric Cancer Cells. Anticancer Res. 2022, 42, 501–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, X.; Li, Z.; Xue, Y. Estrogen Facilitates Gastric Cancer Cell Proliferation and Invasion through Promoting the Secretion of Interleukin-6 by Cancer-Associated Fibroblasts. Int. Immunopharmacol. 2020, 78, 105937. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Yashiro, M.; Nishii, T.; Matsuoka, J.; Fuyuhiro, Y.; Morisaki, T.; Fukuoka, T.; Shimizu, K.; Shimizu, T.; Miwa, A.; et al. Cancer-Associated Fibroblasts Might Sustain the Stemness of Scirrhous Gastric Cancer Cells via Transforming Growth Factor-β Signaling. Int. J. Cancer 2014, 134, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Izumi, D.; Ishimoto, T.; Miyake, K.; Sugihara, H.; Eto, K.; Sawayama, H.; Yasuda, T.; Kiyozumi, Y.; Kaida, T.; Kurashige, J.; et al. CXCL12/CXCR4 Activation by Cancer-Associated Fibroblasts Promotes Integrin Β1 Clustering and Invasiveness in Gastric Cancer. Int. J. Cancer 2016, 138, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shan, T.; Qiao, L. Collagen Type IV Alpha 1 (COL4A1) Silence Hampers the Invasion, Migration and Epithelial–Mesenchymal Transition (EMT) of Gastric Cancer Cells through Blocking Hedgehog Signaling Pathway. Bioengineered 2022, 13, 8972–8981. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, B.; Ye, J.; Cao, S.; Shi, J.; Zhao, Y.; Wang, Y.; Sang, J.; Yao, Y.; Guan, W.; et al. Exosomal miRNA-139 in Cancer-Associated Fibroblasts Inhibits Gastric Cancer Progression by Repressing MMP11 Expression. Int. J. Biol. Sci. 2019, 15, 2320–2329. [Google Scholar] [CrossRef]
- Tang, D.; Gao, J.; Wang, S.; Ye, N.; Chong, Y.; Huang, Y.; Wang, J.; Li, B.; Yin, W.; Wang, D. Cancer-Associated Fibroblasts Promote Angiogenesis in Gastric Cancer through Galectin-1 Expression. Tumour Biol. 2016, 37, 1889–1899. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, Y.; Wu, X.; Pan, T.; Yu, Z.; Hou, J.; Wu, A.; Li, J.; Yang, Z.; Li, C.; et al. The Cross-Talk between Tumor Cells and Activated Fibroblasts Mediated by Lactate/BDNF/TrkB Signaling Promotes Acquired Resistance to Anlotinib in Human Gastric Cancer. Redox Biol. 2021, 46, 102076. [Google Scholar] [CrossRef]
- Uchihara, T.; Miyake, K.; Yonemura, A.; Komohara, Y.; Itoyama, R.; Koiwa, M.; Yasuda, T.; Arima, K.; Harada, K.; Eto, K.; et al. Extracellular Vesicles from Cancer-Associated Fibroblasts Containing Annexin A6 Induces FAK-YAP Activation by Stabilizing Β1 Integrin, Enhancing Drug Resistance. Cancer Res. 2020, 80, 3222–3235. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF Secreted miR-522 Suppresses Ferroptosis and Promotes Acquired Chemo-Resistance in Gastric Cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Hamabe-Horiike, T.; Harada, S.-I.; Yoshida, K.; Kinoshita, J.; Yamaguchi, T.; Fushida, S. Adipocytes Contribute to Tumor Progression and Invasion of Peritoneal Metastasis by Interacting with Gastric Cancer Cells as Cancer Associated Fibroblasts. Cancer Rep. 2023, 6, e1647. [Google Scholar] [CrossRef]
- Park, K.B.; Kim, E.Y.; Chin, H.; Yoon, D.J.; Jun, K.-H. Leptin Stimulates Migration and Invasion and Maintains Cancer Stem-like Properties in Gastric Cancer Cells. Oncol. Rep. 2022, 48, 162. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Wu, K.; Liu, Y.; Shi, L.; Wang, D.; Li, G.; Tao, K.; Wang, G. Omental Adipocytes Enhance the Invasiveness of Gastric Cancer Cells by Oleic Acid-Induced Activation of the PI3K-Akt Signaling Pathway. Int. J. Biochem. Cell Biol. 2017, 84, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.-R.; Zhu, J.; Bai, L.; Kulabiek, D.; Zhai, X.-X.; Zheng, X.; Qian, J. Adipocytes Impact on Gastric Cancer Progression: Prognostic Insights and Molecular Features. World J. Gastrointest. Oncol. 2024, 16, 3011–3031. [Google Scholar] [CrossRef] [PubMed]
- Brungs, D.; Aghmesheh, M.; Vine, K.L.; Becker, T.M.; Carolan, M.G.; Ranson, M. Gastric Cancer Stem Cells: Evidence, Potential Markers, and Clinical Implications. J. Gastroenterol. 2016, 51, 313–326. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schreiber, S. Diagnostics of Inflammatory Bowel Disease. Gastroenterology 2007, 133, 1670–1689. [Google Scholar] [CrossRef]
- Ramage, J.I.J.; Rumalla, A.; Baron, T.H.; Pochron, N.L.; Zinsmeister, A.R.; Murray, J.A.; Norton, I.D.; Diehl, N.; Romero, Y. A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial of Endoscopic Steroid Injection Therapy for Recalcitrant Esophageal Peptic Strictures. Off. J. Am. Coll. Gastroenterol. ACG 2005, 100, 2419. [Google Scholar] [CrossRef]
- Yu, K.; Gu, Y.; Zhang, P.; Fang, H.; Cao, Y.; Wang, J.; Lin, C.; Liu, H.; Zhang, H.; He, H.; et al. Intratumoral PD-1+CD8+ T Cells Associate Poor Clinical Outcomes and Adjuvant Chemotherapeutic Benefit in Gastric Cancer. Br. J. Cancer 2022, 127, 1709–1717. [Google Scholar] [CrossRef]
- Peng, L.-S.; Zhang, J.-Y.; Teng, Y.-S.; Zhao, Y.-L.; Wang, T.-T.; Mao, F.-Y.; Lv, Y.-P.; Cheng, P.; Li, W.-H.; Chen, N.; et al. Tumor-Associated Monocytes/Macrophages Impair NK-Cell Function via TGFβ1 in Human Gastric Cancer. Cancer Immunol. Res. 2017, 5, 248–256. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Q.; Jiang, Y.; Yu, J.; Hu, Y.; Mou, T.; Chen, G.; Li, G. Gastric Cancer Cells Inhibit Natural Killer Cell Proliferation and Induce Apoptosis via Prostaglandin E2. Oncoimmunology 2016, 5, e1069936. [Google Scholar] [CrossRef]
- Xing, R.; Li, L.; Chen, L.; Gao, Z.; Wang, H.; Li, W.; Cui, J.; Tian, G.; Liang, Q.; Yu, J.; et al. Copy Number Variations of HLA-I and Activation of NKp30 Pathway Determine the Sensitivity of Gastric Cancer Cells to the Cytotoxicity of Natural Killer Cells. Oncogene 2016, 35, 2584–2591. [Google Scholar] [CrossRef]
- Katoh, H.; Komura, D.; Konishi, H.; Suzuki, R.; Yamamoto, A.; Kakiuchi, M.; Sato, R.; Ushiku, T.; Yamamoto, S.; Tatsuno, K.; et al. Immunogenetic Profiling for Gastric Cancers Identifies Sulfated Glycosaminoglycans as Major and Functional B Cell Antigens in Human Malignancies. Cell Rep. 2017, 20, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Zeng, B.; Hu, G.; Gan, R. Epstein–Barr Virus-Associated Gastric Cancer: A Distinct Subtype. Cancer Lett. 2020, 495, 191–199. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, D.; Huang, X.; Zhang, M.; Zhu, W.; Xu, C. Significance of Monocyte Infiltration in Patients with Gastric Cancer: A Combined Study Based on Single Cell Sequencing and TCGA. Front. Oncol. 2022, 12, 1001307. [Google Scholar] [CrossRef] [PubMed]
- Eo, W.K.; Jeong, D.W.; Chang, H.J.; Won, K.Y.; Choi, S.I.; Kim, S.H.; Chun, S.W.; Oh, Y.L.; Lee, T.H.; Kim, Y.O.; et al. Absolute Monocyte and Lymphocyte Count Prognostic Score for Patients with Gastric Cancer. World J. Gastroenterol. 2015, 21, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Mei, P.; Feng, W.; Zhan, Y.; Guo, X. Prognostic Value of Lymphocyte-to-Monocyte Ratio in Gastric Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1321584. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Sohma, Y.; Nagafune, J.; Cella, M.; Colonna, M.; Facchetti, F.; Günther, G.; Johnston, I.; Lanzavecchia, A.; Nagasaka, T.; et al. BDCA-2, a Novel Plasmacytoid Dendritic Cell-Specific Type II C-Type Lectin, Mediates Antigen Capture and Is a Potent Inhibitor of Interferon Alpha/Beta Induction. J. Exp. Med. 2001, 194, 1823–1834. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J.; Li, Q.; Wang, Q.; Zhou, Y.; Tong, Z. Gastric Cancer Patients Have Elevated Plasmacytoid and CD1c+ Dendritic Cells in the Peripheral Blood. Oncol. Lett. 2018, 15, 5087–5092. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Ohuchida, K.; Katayama, N.; Yamada, Y.; Nakamura, S.; Okuda, S.; Otsubo, Y.; Iwamoto, C.; Torata, N.; Horioka, K.; et al. Tumor-Infiltrating Monocytic Myeloid-Derived Suppressor Cells Contribute to the Development of an Immunosuppressive Tumor Microenvironment in Gastric Cancer. Gastric Cancer 2024, 27, 248–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlossman, S.F.; Edwards, R.A.; Ou, C.-N.; Gu, J.; Wu, M.X. Impaired Apoptosis, Extended Duration of Immune Responses, and a Lupus-like Autoimmune Disease in IEX-1-Transgenic Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 878–883. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-Associated Neutrophils Induce EMT by IL-17a to Promote Migration and Invasion in Gastric Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 6. [Google Scholar] [CrossRef]
- Wang, J.-B.; Gao, Y.-X.; Ye, Y.-H.; Lin, T.-X.; Li, P.; Lin, J.-X.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; Tu, R.-H.; et al. CDK5RAP3 Acts as a Tumour Suppressor in Gastric Cancer through the Infiltration and Polarization of Tumour-Associated Macrophages. Cancer Gene Ther. 2023, 30, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Sung, J.-Y.; Lee, J.; Park, Y.-K.; Kim, Y.W.; Kim, G.Y.; Won, K.Y.; Lim, S.-J. Polarized CD163+ Tumor-Associated Macrophages Are Associated with Increased Angiogenesis and CXCL12 Expression in Gastric Cancer. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Pancreas—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 21 January 2025).
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Löhr, J.-M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.-L.; Heinemann, V. Addressing the Challenges of Pancreatic Cancer: Future Directions for Improving Outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic Cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Manoukian, P.; Bijlsma, M.; van Laarhoven, H. The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth. Front. Cell Dev. Biol. 2021, 9, 743907. [Google Scholar] [CrossRef]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front. Oncol. 2019, 9, 1482. [Google Scholar] [CrossRef]
- Foster, D.S.; Januszyk, M.; Delitto, D.; Yost, K.E.; Griffin, M.; Guo, J.; Guardino, N.; Delitto, A.E.; Chinta, M.; Burcham, A.R.; et al. Multiomic Analysis Reveals Conservation of Cancer-Associated Fibroblast Phenotypes across Species and Tissue of Origin. Cancer Cell 2022, 40, 1392–1406.e7. [Google Scholar] [CrossRef]
- Furini, S.; Falciani, C. Expression and Role of Heparan Sulfated Proteoglycans in Pancreatic Cancer. Front. Oncol. 2021, 11, 695858. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Chen, J.; Song, E. Targeting CAFs to Overcome Anticancer Therapeutic Resistance. Trends Cancer 2022, 8, 527–555. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; DeCant, B.; Mascariñas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGFβ Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Inman, K.S.; Francis, A.A.; Murray, N.R. Complex Role for the Immune System in Initiation and Progression of Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 11160–11181. [Google Scholar] [CrossRef]
- McClanahan, F.; Riches, J.C.; Miller, S.; Day, W.P.; Kotsiou, E.; Neuberg, D.; Croce, C.M.; Capasso, M.; Gribben, J.G. Mechanisms of PD-L1/PD-1-Mediated CD8 T-Cell Dysfunction in the Context of Aging-Related Immune Defects in the Eµ-TCL1 CLL Mouse Model. Blood 2015, 126, 212–221. [Google Scholar] [CrossRef]
- Vennin, C.; Mélénec, P.; Rouet, R.; Nobis, M.; Cazet, A.S.; Murphy, K.J.; Herrmann, D.; Reed, D.A.; Lucas, M.C.; Warren, S.C.; et al. CAF Hierarchy Driven by Pancreatic Cancer Cell P53-Status Creates a pro-Metastatic and Chemoresistant Environment via Perlecan. Nat. Commun. 2019, 10, 3637. [Google Scholar] [CrossRef]
- Wehr, A.Y.; Furth, E.E.; Sangar, V.; Blair, I.A.; Yu, K.H. Analysis of the Human Pancreatic Stellate Cell Secreted Proteome. Pancreas 2011, 40, 557–566. [Google Scholar] [CrossRef]
- Bailey, J.M.; Swanson, B.J.; Hamada, T.; Eggers, J.P.; Singh, P.K.; Caffery, T.; Ouellette, M.M.; Hollingsworth, M.A. Sonic Hedgehog Promotes Desmoplasia in Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 5995–6004. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Wu, Z.; Zhang, L.; Liang, C.; Chen, X. Exosomal LINC00355 Derived from Cancer-Associated Fibroblasts Promotes Bladder Cancer Cell Resistance to Cisplatin by Regulating miR-34b-5p/ABCB1 Axis. Acta Biochim. Biophys. Sin. 2021, 53, 558–566. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-Associated Fibroblasts Promote Progression and Gemcitabine Resistance via the SDF-1/SATB-1 Pathway in Pancreatic Cancer. Cell Death Dis. 2018, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G. CAFs Interacting with TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef] [PubMed]
- Louault, K.; Li, R.-R.; DeClerck, Y.A. Cancer-Associated Fibroblasts: Understanding Their Heterogeneity. Cancers 2020, 12, 3108. [Google Scholar] [CrossRef] [PubMed]
- Ohshio, Y.; Teramoto, K.; Hanaoka, J.; Tezuka, N.; Itoh, Y.; Asai, T.; Daigo, Y.; Ogasawara, K. Cancer-Associated Fibroblast-Targeted Strategy Enhances Antitumor Immune Responses in Dendritic Cell-Based Vaccine. Cancer Sci. 2015, 106, 134–142. [Google Scholar] [CrossRef]
- Kim, P.K.; Halbrook, C.J.; Kerk, S.A.; Radyk, M.; Wisner, S.; Kremer, D.M.; Sajjakulnukit, P.; Andren, A.; Hou, S.W.; Trivedi, A.; et al. Hyaluronic Acid Fuels Pancreatic Cancer Cell Growth. eLife 2021, 10, e62645. [Google Scholar] [CrossRef]
- Li, J.; Eu, J.Q.; Kong, L.R.; Wang, L.; Lim, Y.C.; Goh, B.C.; Wong, A.L.A. Targeting Metabolism in Cancer Cells and the Tumour Microenvironment for Cancer Therapy. Molecules 2020, 25, 4831. [Google Scholar] [CrossRef]
- Mochizuki, S.; Miki, H.; Zhou, R.; Kido, Y.; Nishimura, W.; Kikuchi, M.; Noda, Y. Oxysterol-Binding Protein-Related Protein (ORP) 6 Localizes to the ER and ER-Plasma Membrane Contact Sites and Is Involved in the Turnover of PI4P in Cerebellar Granule Neurons. Exp. Cell Res. 2018, 370, 601–612. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The Reverse Warburg Effect: Aerobic Glycolysis in Cancer Associated Fibroblasts and the Tumor Stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- Pavlides, S.; Tsirigos, A.; Migneco, G.; Whitaker-Menezes, D.; Chiavarina, B.; Flomenberg, N.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Pestell, R.G.; et al. The Autophagic Tumor Stroma Model of Cancer: Role of Oxidative Stress and Ketone Production in Fueling Tumor Cell Metabolism. Cell Cycle 2010, 9, 3485–3505. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Tseng, T.W.; Sousa, C.M.; Liu, S.; Truitt, M.L.; He, N.; Ding, N.; Liddle, C.; Atkins, A.R.; et al. Stromal Cues Regulate the Pancreatic Cancer Epigenome and Metabolome. Proc. Natl. Acad. Sci. USA 2017, 114, 1129–1134. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define Cancer-Associated Fibroblasts (CAFs) in the Tumor Microenvironment: New Opportunities in Cancer Immunotherapy and Advances in Clinical Trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Kanat, O.; Ertas, H. Shattering the Castle Walls: Anti-Stromal Therapy for Pancreatic Cancer. World J. Gastrointest. Oncol. 2018, 10, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Verloy, R.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Treatment for Pancreatic Cancer—The Importance of Pancreatic Stellate Cells. Cancers 2020, 12, 2782. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef]
- Garg, B.; Giri, B.; Modi, S.; Sethi, V.; Castro, I.; Umland, O.; Ban, Y.; Lavania, S.; Dawra, R.; Banerjee, S.; et al. NFκB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-Regulation of CXCL12. Gastroenterology 2018, 155, 880–891.e8. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, M.; Meng, F.; Sun, R. Activated Pancreatic Stellate Cells Inhibit NK Cell Function in the Human Pancreatic Cancer Microenvironment. Cell. Mol. Immunol. 2019, 16, 87–89. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Liu, X.; Guo, W.; Liu, Q.; Chen, L.; Pang, J.; Liu, X.; Li, R.; Tong, W.-M.; et al. Pancreatic Stellate Cells Exploit Wnt/β-Catenin/TCF7-Mediated Glutamine Metabolism to Promote Pancreatic Cancer Cells Growth. Cancer Lett. 2023, 555, 216040. [Google Scholar] [CrossRef]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic Stellate Cells Support Tumour Metabolism through Autophagic Alanine Secretion. Nature 2016, 536, 479–483. [Google Scholar] [CrossRef]
- Takikawa, T.; Hamada, S.; Matsumoto, R.; Tanaka, Y.; Kataoka, F.; Sasaki, A.; Masamune, A. Senescent Human Pancreatic Stellate Cells Secrete CXCR2 Agonist CXCLs to Promote Proliferation and Migration of Human Pancreatic Cancer AsPC-1 and MIAPaCa-2 Cell Lines. Int. J. Mol. Sci. 2022, 23, 9275. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-Mediated Paracrine Interaction for Pancreatic Cancer Therapy and Monitoring. Nature 2019, 569, 131–135. [Google Scholar] [CrossRef]
- Okumura, N.; Hashimoto, K.; Kitahara, M.; Okuda, H.; Ueda, E.; Watanabe, K.; Nakahara, M.; Sato, T.; Kinoshita, S.; Tourtas, T.; et al. Activation of TGF-β Signaling Induces Cell Death via the Unfolded Protein Response in Fuchs Endothelial Corneal Dystrophy. Sci. Rep. 2017, 7, 6801. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Taylor, R. Intra-Pancreatic Fat Deposition: Bringing Hidden Fat to the Fore. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Fan, Z.; Zhou, B.; Zhan, H. Crosstalk between Pancreatic Cancer and Adipose Tissue: Molecular Mechanisms and Therapeutic Implications. Biochem. Biophys. Res. Commun. 2024, 740, 151012. [Google Scholar] [CrossRef] [PubMed]
- Quiclet, C.; Dittberner, N.; Gässler, A.; Stadion, M.; Gerst, F.; Helms, A.; Baumeier, C.; Schulz, T.J.; Schürmann, A. Pancreatic Adipocytes Mediate Hypersecretion of Insulin in Diabetes-Susceptible Mice. Metab.-Clin. Exp. 2019, 97, 9–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, C.; Zhao, B.; Wang, Y.; Li, Z.; Li, T.; Yang, X.; Wang, W. Pancreatic Cancer Stemness: Dynamic Status in Malignant Progression. J. Exp. Clin. Cancer Res. 2023, 42, 122. [Google Scholar] [CrossRef]
- Liudahl, S.M.; Betts, C.B.; Sivagnanam, S.; Morales-Oyarvide, V.; da Silva, A.; Yuan, C.; Hwang, S.; Grossblatt-Wait, A.; Leis, K.R.; Larson, W.; et al. Leukocyte Heterogeneity in Pancreatic Ductal Adenocarcinoma: Phenotypic and Spatial Features Associated with Clinical Outcome. Cancer Discov. 2021, 11, 2014–2031. [Google Scholar] [CrossRef]
- Barman, S.; Fatima, I.; Singh, A.B.; Dhawan, P. Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 4765. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Ajina, R.; Malchiodi, Z.X.; Fitzgerald, A.A.; Zuo, A.; Wang, S.; Moussa, M.; Cooper, C.J.; Shen, Y.; Johnson, Q.R.; Parks, J.M.; et al. Antitumor T-Cell Immunity Contributes to Pancreatic Cancer Immune Resistance. Cancer Immunol. Res. 2021, 9, 386–400. [Google Scholar] [CrossRef]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ Regulatory T Cells Induce Cytokine Deprivation-Mediated Apoptosis of Effector CD4+ T Cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Fong, Y.; Ko, M.-W.; Safaei, T.; Perišić Nanut, M.; Kaur, K. NK Cells Shape Pancreatic and Oral Tumor Microenvironments; Role in Inhibition of Tumor Growth and Metastasis. Semin. Cancer Biol. 2018, 53, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Herter-Sprie, G.; Zhang, H.; Lin, E.Y.; Biancur, D.; Wang, X.; Deng, J.; Hai, J.; Yang, S.; Wong, K.-K.; et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov. 2018, 8, 276–287. [Google Scholar] [CrossRef]
- Mirlekar, B.; Michaud, D.; Lee, S.J.; Kren, N.P.; Harris, C.; Greene, K.; Goldman, E.C.; Gupta, G.P.; Fields, R.C.; Hawkins, W.G.; et al. B Cell-Derived IL35 Drives STAT3-Dependent CD8+ T-Cell Exclusion in Pancreatic Cancer. Cancer Immunol. Res. 2020, 8, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Castino, G.F.; Cortese, N.; Capretti, G.; Serio, S.; Di Caro, G.; Mineri, R.; Magrini, E.; Grizzi, F.; Cappello, P.; Novelli, F.; et al. Spatial Distribution of B Cells Predicts Prognosis in Human Pancreatic Adenocarcinoma. Oncoimmunology 2016, 5, e1085147. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Das, S.; Handler, J.S.; Hajdu, C.H.; Coffre, M.; Koralov, S.B.; Bar-Sagi, D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016, 6, 247–255. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, S.Y.; Jeong, H.; Han, J.; Chae, H.Y.; Yang, H.; Sung, C.O.; Choi, Y.-L.; Shin, Y.K.; Kwon, M.J. Matrix Metalloproteinase 11 (MMP11) in Macrophages Promotes the Migration of HER2-Positive Breast Cancer Cells and Monocyte Recruitment through CCL2–CCR2 Signaling. Lab. Investig. 2022, 102, 376–390. [Google Scholar] [CrossRef]
- Markowitz, J.; Wesolowski, R.; Papenfuss, T.; Brooks, T.R.; Carson, W.E. Myeloid-Derived Suppressor Cells in Breast Cancer. Breast Cancer Res. Treat. 2013, 140, 13–21. [Google Scholar] [CrossRef]
- Isherwood, J.; Arshad, A.; Chung, W.Y.; Runau, F.; Cooke, J.; Pollard, C.; Howells, L.; Fishwick, J.; Thompson, J.; Metcalfe, M.; et al. Myeloid Derived Suppressor Cells Are Reduced and T Regulatory Cells Stabilised in Patients with Advanced Pancreatic Cancer Treated with Gemcitabine and Intravenous Omega 3. Ann. Transl. Med. 2020, 8, 172. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human Neutrophils Uniquely Release TIMP-Free MMP-9 to Provide a Potent Catalytic Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix Metalloproteinase-9 Triggers the Angiogenic Switch during Carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Nocelo, M.; Ruiz-Cañas, L.; Sancho, P.; Görgülü, K.; Alcalá, S.; Pedrero, C.; Vallespinos, M.; López-Gil, J.C.; Ochando, M.; García-García, E.; et al. Macrophages Direct Cancer Cells through a LOXL2-Mediated Metastatic Cascade in Pancreatic Ductal Adenocarcinoma. Gut 2023, 72, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Reshkin, S.J.; Carvalho, T.M.A.; Di Molfetta, D.; Greco, M.R.; Alfarouk, K.O.; Cardone, R.A. Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma: A Physiopathologic and Pharmacologic Review. Cancers 2022, 14, 2486. [Google Scholar] [CrossRef] [PubMed]
- Olson, O.C.; Kim, H.; Quail, D.F.; Foley, E.A.; Joyce, J.A. Tumor-Associated Macrophages Suppress the Cytotoxic Activity of Antimitotic Agents. Cell Rep. 2017, 19, 101–113. [Google Scholar] [CrossRef]
- Yu, M.; Guan, R.; Hong, W.; Zhou, Y.; Lin, Y.; Jin, H.; Hou, B.; Jian, Z. Prognostic Value of Tumor-Associated Macrophages in Pancreatic Cancer: A Meta-Analysis. Cancer Manag. Res. 2019, 11, 4041–4058. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Chen, W.; Wang, X.; Cao, M.; Han, X.; Zhang, K.; Teng, B.; Cao, J.; Wu, W.; et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Mol. Ther. 2021, 29, 1226–1238. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Donne, R.; Lujambio, A. The Liver Cancer Immune Microenvironment: Therapeutic Implications for Hepatocellular Carcinoma. Hepatology 2023, 77, 1773–1796. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence Rates. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Milette, S.; Sicklick, J.K.; Lowy, A.M.; Brodt, P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin. Cancer Res. 2017, 23, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor Metastasis: Mechanistic Insights and Clinical Challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Chiavarina, B.; Ronca, R.; Otaka, Y.; Sutton, R.B.; Rezzola, S.; Yokobori, T.; Chiodelli, P.; Souche, R.; Pourquier, D.; Maraver, A.; et al. Fibroblast-Derived Prolargin Is a Tumor Suppressor in Hepatocellular Carcinoma. Oncogene 2022, 41, 1410–1420. [Google Scholar] [CrossRef]
- Khan, G.J.; Sun, L.; Khan, S.; Yuan, S.; Nongyue, H. Versatility of Cancer Associated Fibroblasts: Commendable Targets for Anti-Tumor Therapy. Curr. Drug Targets 2018, 19, 1573–1588. [Google Scholar] [CrossRef]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-Tissue Organization of the Fibroblast Lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-Associated Fibroblasts Induce Metalloprotease-Independent Cancer Cell Invasion of the Basement Membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef]
- Santamato, A.; Fransvea, E.; Dituri, F.; Caligiuri, A.; Quaranta, M.; Niimi, T.; Pinzani, M.; Antonaci, S.; Giannelli, G. Hepatic Stellate Cells Stimulate HCC Cell Migration via Laminin-5 Production. Clin. Sci. 2011, 121, 159–168. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Yin, Z.; Su, W.; Ren, G.; Zhou, C.; You, J.; Fan, J.; Wang, X. Activated Hepatic Stellate Cells Promote Hepatocellular Carcinoma Development in Immunocompetent Mice. Int. J. Cancer 2011, 129, 2651–2661. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Wang, S.-S.; Liebe, R.; Ding, H.-G. Crosstalk between Hepatic Stellate Cells and Tumor Cells in the Development of Hepatocellular Carcinoma. Chin. Med. J. 2021, 134, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, X.; Lu, J.; Salfenmoser, M.; Wirsik, N.M.; Schleussner, N.; Imle, A.; Freire Valls, A.; Radhakrishnan, P.; Liang, J.; et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell 2020, 37, 800–817.e7. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.; Guimaraes, J.C.; Auf der Maur, P.; De Silva, D.; Trefny, M.P.; Okamoto, R.; Bruno, S.; Schmidt, A.; Mertz, K.; Volkmann, K.; et al. Hepatic Stellate Cells Suppress NK Cell-Sustained Breast Cancer Dormancy. Nature 2021, 594, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; et al. Non-Cell-Autonomous Tumor Suppression by P53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef]
- Sakano, Y.; Noda, T.; Kobayashi, S.; Sasaki, K.; Iwagami, Y.; Yamada, D.; Tomimaru, Y.; Akita, H.; Gotoh, K.; Takahashi, H.; et al. Tumor Endothelial Cell-Induced CD8+ T-Cell Exhaustion via GPNMB in Hepatocellular Carcinoma. Cancer Sci. 2022, 113, 1625–1638. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Zhang, T.; Yue, L.; Xiao, Y.; Guo, C. LAG-3 Is a Promising Inhibitory Immune Checkpoint for Antitumor Immunotherapy. Expert. Rev. Anticancer Ther. 2022, 22, 289–296. [Google Scholar] [CrossRef]
- Dalerba, P.; Clarke, M.F. Cancer Stem Cells and Tumor Metastasis: First Steps into Uncharted Territory. Cell Stem Cell 2007, 1, 241–242. [Google Scholar] [CrossRef]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and Functional Alterations of Tumor-Associated Antigen-Specific CD8+ T-Cell Responses in Hepatocellular Carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef]
- Kim, H.-D.; Song, G.-W.; Park, S.; Jung, M.K.; Kim, M.H.; Kang, H.J.; Yoo, C.; Yi, K.; Kim, K.H.; Eo, S.; et al. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8+ T Cells and Features of Hepatocellular Carcinoma. Gastroenterology 2018, 155, 1936–1950.e17. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Qin, S.; Ma, S.; Huang, X.; Lu, D.; Zhou, Y.; Jiang, H. Th22 Cells Are Associated with Hepatocellular Carcinoma Development and Progression. Chin. J. Cancer Res. 2014, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.-A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-Fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A Expression Contributes to NK Cell Exhaustion and Predicts a Poor Prognosis of Patients with Liver Cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Bi, Y.; Yang, W.; Guo, X.; Jiang, Y.; Wan, C.; Li, L.; Bai, Y.; Guo, J.; Wang, Y.; et al. Ca2+ Regulates T-Cell Receptor Activation by Modulating the Charge Property of Lipids. Nature 2013, 493, 111–115. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef]
- Faggioli, F.; Palagano, E.; Di Tommaso, L.; Donadon, M.; Marrella, V.; Recordati, C.; Mantero, S.; Villa, A.; Vezzoni, P.; Cassani, B. B Lymphocytes Limit Senescence-Driven Fibrosis Resolution and Favor Hepatocarcinogenesis in Mouse Liver Injury. Hepatology 2018, 67, 1970–1985. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Tacke, F. Role of Lymphocytes in Liver Cancer. Oncoimmunology 2013, 2, e26468. [Google Scholar] [CrossRef]
- Shao, D.D.; Xue, W.; Krall, E.B.; Bhutkar, A.; Piccioni, F.; Wang, X.; Schinzel, A.C.; Sood, S.; Rosenbluh, J.; Kim, J.W.; et al. KRAS and YAP1 Converge to Regulate EMT and Tumor Survival. Cell 2014, 158, 171–184. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Beckebaum, S.; Zhang, X.; Chen, X.; Yu, Z.; Frilling, A.; Dworacki, G.; Grosse-Wilde, H.; Broelsch, C.E.; Gerken, G.; Cicinnati, V.R. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clin. Cancer Res. 2004, 10, 7260–7269. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M.; Banerjee, D.; Connolly, J.; Kukreja, A.; Matayeva, E.; Veri, M.C.; Ravetch, J.V.; Steinman, R.M.; Dhodapkar, M.V. Selective Blockade of the Inhibitory Fcgamma Receptor (FcgammaRIIB) in Human Dendritic Cells and Monocytes Induces a Type I Interferon Response Program. J. Exp. Med. 2007, 204, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Kunitani, H.; Shimizu, Y.; Murata, H.; Higuchi, K.; Watanabe, A. Phenotypic Analysis of Circulating and Intrahepatic Dendritic Cell Subsets in Patients with Chronic Liver Diseases. J. Hepatol. 2002, 36, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chikina, M.; Szymczak-Workman, A.L.; Horne, W.; Kolls, J.K.; Vignali, K.M.; Normolle, D.; Bettini, M.; Workman, C.J.; Vignali, D.A.A. LAG3 Limits Regulatory T Cell Proliferation and Function in Autoimmune Diabetes. Sci. Immunol. 2017, 2, eaah4569. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.-F.; Lei, A.-H.; Xiao, Q.; Lin, Z.-H.; Hong, Y.-F.; Wu, X.-Y.; Zhou, J. Neutrophil Count Is Associated with Myeloid Derived Suppressor Cell Level and Presents Prognostic Value for Hepatocellular Carcinoma Patients. Oncotarget 2017, 8, 24380–24388. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Shi, M.; Chen, Y.; Yu, D.; Zhao, C.; Gu, Y.; Yang, B.; Guo, S.; Ding, G.; et al. CD13hi Neutrophil-like Myeloid-Derived Suppressor Cells Exert Immune Suppression through Arginase 1 Expression in Pancreatic Ductal Adenocarcinoma. OncoImmunology 2017, 6, e1258504. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef]
- Quail, D.F.; Bowman, R.L.; Akkari, L.; Quick, M.L.; Schuhmacher, A.J.; Huse, J.T.; Holland, E.C.; Sutton, J.C.; Joyce, J.A. The Tumor Microenvironment Underlies Acquired Resistance to CSF-1R Inhibition in Gliomas. Science 2016, 352, aad3018. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2019, 51, 411–412. [Google Scholar] [CrossRef]
- Yao, R.-R.; Li, J.-H.; Zhang, R.; Chen, R.-X.; Wang, Y.-H. M2-Polarized Tumor-Associated Macrophages Facilitated Migration and Epithelial-Mesenchymal Transition of HCC Cells via the TLR4/STAT3 Signaling Pathway. World J. Surg. Oncol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M. Genetic Testing for High-Risk Colon Cancer Patients. Gastroenterology 2003, 124, 1574–1594. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Brouquet, A.; Cervantes, A.; ESMO Guidelines Working Group. Primary Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Adjuvant Treatment and Follow-Up. Ann. Oncol. 2010, 21 (Suppl. S5), v70–v77. [Google Scholar] [CrossRef]
- Wu, C. Systemic Therapy for Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 235–242. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906. [Google Scholar] [CrossRef]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference Component Analysis of Single-Cell Transcriptomes Elucidates Cellular Heterogeneity in Human Colorectal Tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef]
- Allam, A.; Yakou, M.; Pang, L.; Ernst, M.; Huynh, J. Exploiting the STAT3 Nexus in Cancer-Associated Fibroblasts to Improve Cancer Therapy. Front. Immunol. 2021, 12, 767939. [Google Scholar] [CrossRef] [PubMed]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.-M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 Activation through IL-6/IL-11 in Cancer-Associated Fibroblasts Promotes Colorectal Tumour Development and Correlates with Poor Prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-T.; Zhang, Y.; Liu, M.; Qiuwaxi, J.; Ashwood, P.; Cho, S.C.; Huan, Y.; Ge, R.-C.; Chen, X.-W.; Wang, Z.-J.; et al. Transplantation of Human Cord Blood Mononuclear Cells and Umbilical Cord-Derived Mesenchymal Stem Cells in Autism. J. Transl. Med. 2013, 11, 196. [Google Scholar] [CrossRef]
- Mochizuki, S.; Ao, T.; Sugiura, T.; Yonemura, K.; Shiraishi, T.; Kajiwara, Y.; Okamoto, K.; Shinto, E.; Okada, Y.; Ueno, H. Expression and Function of a Disintegrin and Metalloproteinases in Cancer-Associated Fibroblasts of Colorectal Cancer. Digestion 2020, 101, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-Associated Fibroblast-Derived WNT2 Increases Tumor Angiogenesis in Colon Cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Torres, S.; Bartolomé, R.A.; Mendes, M.; Barderas, R.; Fernandez-Aceñero, M.J.; Peláez-García, A.; Peña, C.; Lopez-Lucendo, M.; Villar-Vázquez, R.; de Herreros, A.G.; et al. Proteome Profiling of Cancer-Associated Fibroblasts Identifies Novel Proinflammatory Signatures and Prognostic Markers for Colorectal Cancer. Clin. Cancer Res. 2013, 19, 6006–6019. [Google Scholar] [CrossRef]
- Lu, J.; Ye, X.; Fan, F.; Xia, L.; Bhattacharya, R.; Bellister, S.; Tozzi, F.; Sceusi, E.; Zhou, Y.; Tachibana, I.; et al. Endothelial Cells Promote the Colorectal Cancer Stem Cell Phenotype through a Soluble Form of Jagged-1. Cancer Cell 2013, 23, 171–185. [Google Scholar] [CrossRef]
- Wang, R.; Bhattacharya, R.; Ye, X.; Fan, F.; Boulbes, D.R.; Ellis, L.M. Endothelial Cells Promote Colorectal Cancer Cell Survival by Activating the HER3-AKT Pathway in a Paracrine Fashion. Mol. Cancer Res. 2019, 17, 20–29. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Wherry, E.J.; Teichgräber, V.; Becker, T.C.; Masopust, D.; Kaech, S.M.; Antia, R.; von Andrian, U.H.; Ahmed, R. Lineage Relationship and Protective Immunity of Memory CD8 T Cell Subsets. Nat. Immunol. 2003, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Smyrk, T.C.; Watson, P.; Kaul, K.; Lynch, H.T. Tumor-Infiltrating Lymphocytes Are a Marker for Microsatellite Instability in Colorectal Carcinoma. Cancer 2001, 91, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Corsale, A.M.; Di Simone, M.; Lo Presti, E.; Dieli, F.; Meraviglia, S. Γδ T Cells and Their Clinical Application in Colon Cancer. Front. Immunol. 2023, 14, 1098847. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.; Schimanski, C.-C.; Neurath, M.-F. Colon Cancer and the Immune System: The Role of Tumor Invading T Cells. World J. Gastroenterol. 2006, 12, 7233–7238. [Google Scholar] [CrossRef]
- Ghazvinian, Z.; Abdolahi, S.; Tokhanbigli, S.; Tarzemani, S.; Piccin, A.; Reza Zali, M.; Verdi, J.; Baghaei, K. Contribution of Natural Killer Cells in Innate Immunity against Colorectal Cancer. Front. Oncol. 2022, 12, 1077053. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xing, B.; Luo, N.; Gao, R.; Yu, K.; Hu, X.; Bu, Z.; Peng, J.; Ren, X.; et al. Immune Phenotypic Linkage between Colorectal Cancer and Liver Metastasis. Cancer Cell. 2022, 40, 424–437.e5. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human Natural Killer Cells: A Unique Innate Immunoregulatory Role for the CD56bright Subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef]
- Edin, S.; Kaprio, T.; Hagström, J.; Larsson, P.; Mustonen, H.; Böckelman, C.; Strigård, K.; Gunnarsson, U.; Haglund, C.; Palmqvist, R. The Prognostic Importance of CD20+ B Lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell Subsets. Sci. Rep. 2019, 9, 19997. [Google Scholar] [CrossRef]
- Xia, J.; Xie, Z.; Niu, G.; Lu, Z.; Wang, Z.; Xing, Y.; Ren, J.; Hu, Z.; Hong, R.; Cao, Z.; et al. Single-Cell Landscape and Clinical Outcomes of Infiltrating B Cells in Colorectal Cancer. Immunology 2023, 168, 135–151. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Yu, Y.; Zhao, H.-P.; Guo, L.; Dai, K.; Lv, J. Mechanisms of Tumor Immunosuppressive Microenvironment Formation in Esophageal Cancer. World J. Gastroenterol. 2024, 30, 2195–2208. [Google Scholar] [CrossRef]
- Alzamami, A. Implications of Single-Cell Immune Landscape of Tumor Microenvironment for the Colorectal Cancer Diagnostics and Therapy. Med. Oncol. 2023, 40, 352. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.V.; Ananiev, J.R.; Vlaykova, T.I.; Yovchev, Y.; Tsoneva, V.; Manolova, I.M. Role of Dendritic Cells in Progression and Clinical Outcome of Colon Cancer. Int. J. Color. Dis. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Sandel, M.H.; Dadabayev, A.R.; Menon, A.G.; Morreau, H.; Melief, C.J.M.; Offringa, R.; van der Burg, S.H.; Janssen-van Rhijn, C.M.; Ensink, N.G.; Tollenaar, R.A.E.M.; et al. Prognostic Value of Tumor-Infiltrating Dendritic Cells in Colorectal Cancer: Role of Maturation Status and Intratumoral Localization. Clin. Cancer Res. 2005, 11, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-P.; Liao, R.-Q.; Tu, H.-Y.; Wang, W.-J.; Dong, Z.-Y.; Huang, S.-M.; Guo, W.-B.; Gou, L.-Y.; Sun, H.-W.; Zhang, Q.; et al. Stromal PD-L1-Positive Regulatory T Cells and PD-1-Positive CD8-Positive T Cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy. J. Thorac. Oncol. 2018, 13, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Wu, L.; Zhang, M.; Li, W.; Ding, J.; Zhu, J.; Wei, H.; Zhao, K. Circulating and Tumor-Infiltrating Myeloid-Derived Suppressor Cells in Patients with Colorectal Carcinoma. PLoS ONE 2013, 8, e57114. [Google Scholar] [CrossRef]
- Berry, R.S.; Xiong, M.-J.; Greenbaum, A.; Mortaji, P.; Nofchissey, R.A.; Schultz, F.; Martinez, C.; Luo, L.; Morris, K.T.; Hanson, J.A. High Levels of Tumor-Associated Neutrophils Are Associated with Improved Overall Survival in Patients with Stage II Colorectal Cancer. PLoS ONE 2017, 12, e0188799. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-Associated Neutrophils Stimulate T Cell Responses in Early-Stage Human Lung Cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Governa, V.; Trella, E.; Mele, V.; Tornillo, L.; Amicarella, F.; Cremonesi, E.; Muraro, M.G.; Xu, H.; Droeser, R.; Däster, S.R.; et al. The Interplay Between Neutrophils and CD8+ T Cells Improves Survival in Human Colorectal Cancer. Clin. Cancer Res. 2017, 23, 3847–3858. [Google Scholar] [CrossRef]
- He, Y.; Li, T.; Liu, J.; Ou, Q.; Zhou, J. Early Onset Neutropenia: A Useful Predictor of Chemosensitivity and Favorable Prognosis in Patients with Serous Ovarian Cancer. BMC Cancer 2020, 20, 116. [Google Scholar] [CrossRef]
- Lepsenyi, M.; Algethami, N.; Al-Haidari, A.A.; Algaber, A.; Syk, I.; Rahman, M.; Thorlacius, H. CXCL2-CXCR2 Axis Mediates αV Integrin-Dependent Peritoneal Metastasis of Colon Cancer Cells. Clin. Exp. Metastasis 2021, 38, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.-Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, V.M.L.; Carvalho, L. Heterogeneity in Lung Cancer. Pathobiology 2018, 85, 96–107. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Hou, J.-M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients with Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung Cancer: Biology and Treatment Options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Mathieson, L.; Koppensteiner, L.; Dorward, D.A.; O’Connor, R.A.; Akram, A.R. Cancer-Associated Fibroblasts Expressing Fibroblast Activation Protein and Podoplanin in Non-Small Cell Lung Cancer Predict Poor Clinical Outcome. Br. J. Cancer 2024, 130, 1758–1769. [Google Scholar] [CrossRef]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, e1700167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Ho, C.-C.; Chang, Y.-L.; Chen, H.-Y.; Lin, C.-A.; Ling, T.-Y.; Yu, S.-L.; Yuan, S.-S.; Chen, Y.-J.L.; Lin, C.-Y.; et al. Cancer-Associated Fibroblasts Regulate the Plasticity of Lung Cancer Stemness via Paracrine Signalling. Nat. Commun. 2014, 5, 3472. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Fujiwara, A.; Kimura, T.; Kawamura, T.; Funaki, S.; Minami, M.; Okumura, M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J. Thorac. Oncol. 2016, 11, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The Potential and Controversy of Targeting STAT Family Members in Cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-Associated Fibroblasts Enhance Metastatic Potential of Lung Cancer Cells through IL-6/STAT3 Signaling Pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef]
- Zhang, J.; Han, L.; Yu, J.; Li, H.; Li, Q. miR-224 Aggravates Cancer-Associated Fibroblast-Induced Progression of Non-Small Cell Lung Cancer by Modulating a Positive Loop of the SIRT3/AMPK/mTOR/HIF-1α Axis. Aging 2021, 13, 10431–10449. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, F.-H.; Wang, J.-Y.; Liu, F.; Yuan, H.-H.; Zhang, W.-Y.; Jiang, B. JAK2/STAT3 Signaling Pathway Activation Mediates Tumor Angiogenesis by Upregulation of VEGF and bFGF in Non-Small-Cell Lung Cancer. Lung Cancer 2011, 73, 366–374. [Google Scholar] [CrossRef]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-Associated Fibroblasts Induce Antigen-Specific Deletion of CD8 + T Cells to Protect Tumour Cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef]
- Antony, J.; Huang, R.Y.-J. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017, 77, 3725–3732. [Google Scholar] [CrossRef]
- German, P.; Bai, S.; Liu, X.-D.; Sun, M.; Zhou, L.; Kalra, S.; Zhang, X.; Minelli, R.; Scott, K.L.; Mills, G.B.; et al. Phosphorylation-Dependent Cleavage Regulates von Hippel Lindau Proteostasis and Function. Oncogene 2016, 35, 4973–4980. [Google Scholar] [CrossRef]
- Kanzaki, R.; Naito, H.; Kise, K.; Takara, K.; Eino, D.; Minami, M.; Shintani, Y.; Funaki, S.; Kawamura, T.; Kimura, T.; et al. Gas6 Derived from Cancer-Associated Fibroblasts Promotes Migration of Axl-Expressing Lung Cancer Cells during Chemotherapy. Sci. Rep. 2017, 7, 10613. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, J.; Long, X.; Wang, Y.; Xiang, X. Mir-1-Mediated Paracrine Effect of Cancer-Associated Fibroblasts on Lung Cancer Cell Proliferation and Chemoresistance. Oncol. Rep. 2016, 35, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-Associated Fibroblasts Treated with Cisplatin Facilitates Chemoresistance of Lung Adenocarcinoma through IL-11/IL-11R/STAT3 Signaling Pathway. Sci. Rep. 2016, 6, 38408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Ren, Y.; Geng, H.; Zhang, Q.; Cao, L.; Meng, Z.; Wu, X.; Xu, M.; Xu, K. Cancer-Associated Fibroblasts Contribute to Cisplatin Resistance by Modulating ANXA3 in Lung Cancer Cells. Cancer Sci. 2019, 110, 1609–1620. [Google Scholar] [CrossRef]
- Ying, L.; Zhu, Z.; Xu, Z.; He, T.; Li, E.; Guo, Z.; Liu, F.; Jiang, C.; Wang, Q. Cancer Associated Fibroblast-Derived Hepatocyte Growth Factor Inhibits the Paclitaxel-Induced Apoptosis of Lung Cancer A549 Cells by Up-Regulating the PI3K/Akt and GRP78 Signaling on a Microfluidic Platform. PLoS ONE 2015, 10, e0129593. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Bai, J.; Ren, J. Reverse of Non-Small Cell Lung Cancer Drug Resistance Induced by Cancer-Associated Fibroblasts via a Paracrine Pathway. Cancer Sci. 2018, 109, 944–955. [Google Scholar] [CrossRef]
- Dimitrova, N.; Gocheva, V.; Bhutkar, A.; Resnick, R.; Jong, R.M.; Miller, K.M.; Bendor, J.; Jacks, T. Stromal Expression of miR-143/145 Promotes Neoangiogenesis in Lung Cancer Development. Cancer Discov. 2016, 6, 188–201. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H.H. IFN-γ, IL-17A, or Zonulin Rapidly Increase the Permeability of the Blood-Brain and Small Intestinal Epithelial Barriers: Relevance for Neuro-Inflammatory Diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef]
- Xie, M.; Liu, M.; He, C.-S. SIRT1 Regulates Endothelial Notch Signaling in Lung Cancer. PLoS ONE 2012, 7, e45331. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Wang, S.; Chen, X.; Su, J. Bone Marrow Adipocytes: A Critical Player in the Bone Marrow Microenvironment. Front. Cell Dev. Biol. 2021, 9, 770705. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.B.; Weide, B.; Gries, M.; Reith, M.; Tarnanidis, K.; Schuermans, V.; Kemper, C.; Kehrel, C.; Funder, A.; Lichtenberger, R.; et al. Tumor Microenvironment-Derived S100A8/A9 Is a Novel Prognostic Biomarker for Advanced Melanoma Patients and during Immunotherapy with Anti-PD-1 Antibodies. J. Immunother. Cancer 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018, 9, 3018. [Google Scholar] [CrossRef]
- Raniszewska, A.; Polubiec-Kownacka, M.; Rutkowska, E.; Domagala-Kulawik, J. PD-L1 Expression on Lung Cancer Stem Cells in Metastatic Lymph Nodes Aspirates. Stem Cell Rev. Rep. 2019, 15, 324–330. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, B.; Xu, W.; Zhao, W.; Wu, J. Clinicopathological Significance of CD133 in Lung Cancer: A Meta-Analysis. Mol. Clin. Oncol. 2014, 2, 111–115. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef]
- Bravaccini, S.; Bronte, G.; Ulivi, P. TMB in NSCLC: A Broken Dream? Int. J. Mol. Sci. 2021, 22, 6536. [Google Scholar] [CrossRef]
- Donnem, T.; Hald, S.M.; Paulsen, E.-E.; Richardsen, E.; Al-Saad, S.; Kilvaer, T.K.; Brustugun, O.T.; Helland, A.; Lund-Iversen, M.; Poehl, M.; et al. Stromal CD8+ T-Cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 2635–2643. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Nie, X.; Desai, S.S.; Villaroel-Espindola, F.; Badri, T.; Zhao, D.; Kim, A.W.; Ji, L.; Zhang, T.; Quinlan, E.; et al. A Burned-Out CD8+ T-Cell Subset Expands in the Tumor Microenvironment and Curbs Cancer Immunotherapy. Cancer Discov. 2021, 11, 1700–1715. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Wang, X.; Zheng, X.; Wang, D.; Fu, B.; Sun, R.; Tian, Z.; Wei, H. Dysfunction of Natural Killer Cells by FBP1-Induced Inhibition of Glycolysis during Lung Cancer Progression. Cell Metab. 2018, 28, 243–255.e5. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Deng, Y.; Hao, J.-W.; Li, Y.; Liu, B.; Yu, Y.; Shi, F.-D.; Zhou, Q.-H. NK Cell Phenotypic Modulation in Lung Cancer Environment. PLoS ONE 2014, 9, e109976. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Caligiuri, M.A.; Yu, J. Harnessing Natural Killer Cells for Lung Cancer Therapy. Cancer Res. 2023, 83, 3327–3339. [Google Scholar] [CrossRef]
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-Small Cell Lung Cancer. Front. Immunol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; André, P.; Dieu-Nosjean, M.-C.; Alifano, M.; Régnard, J.-F.; et al. Profound Coordinated Alterations of Intratumoral NK Cell Phenotype and Function in Lung Carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 Expression in Peripheral NK Cells Predicts a Poorer Prognosis and Tim-3 Blockade Improves NK Cell-Mediated Cytotoxicity in Human Lung Adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef]
- Russell, J.H.; Ley, T.J. Lymphocyte-Mediated Cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, Z.; Bi, J. Intracellular Checkpoints for NK Cell Cancer Immunotherapy. Front. Med. 2024, 18, 763–777. [Google Scholar] [CrossRef]
- Bruno, T.C.; Ebner, P.J.; Moore, B.L.; Squalls, O.G.; Waugh, K.A.; Eruslanov, E.B.; Singhal, S.; Mitchell, J.D.; Franklin, W.A.; Merrick, D.T.; et al. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non–Small Cell Lung Cancer Patients. Cancer Immunol. Res. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B Cells in Tertiary Lymphoid Structures Is Associated with a Protective Immunity in Patients with Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, P.H.; Ivanova, E.V.; Awad, M.M.; Jones, R.E.; Keogh, L.; Liu, H.; Dries, R.; Almonte, C.; Herter-Sprie, G.S.; Santos, A.; et al. Multiparametric Profiling of Non-Small-Cell Lung Cancers Reveals Distinct Immunophenotypes. JCI Insight 2016, 1, e89014. [Google Scholar] [CrossRef]
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Blood Monocyte Subsets with Activation Markers in Relation with Macrophages in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2513. [Google Scholar] [CrossRef] [PubMed]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.-A.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef]
- Wang, J.-B.; Huang, X.; Li, F.-R. Impaired Dendritic Cell Functions in Lung Cancer: A Review of Recent Advances and Future Perspectives. Cancer Commun. 2019, 39, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Han, B.; Zhong, H. A Prospective Study of the Efficacy of a Combination of Autologous Dendritic Cells, Cytokine-Induced Killer Cells, and Chemotherapy in Advanced Non-Small Cell Lung Cancer Patients. Tumour Biol. 2014, 35, 987–994. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.S.; Kota, V.; Rojiani, M.V.; Kolhe, R. Natural Killer Cells and Dendritic Cells: Expanding Clinical Relevance in the Non-Small Cell Lung Cancer (Nsclc) Tumor Microenvironment. Cancers 2021, 13, 4037. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Huang, G.; Cheng, L.; Lv, J.; Zheng, D.; Lin, S.; Wang, S.; Wu, Q.; Long, Y.; et al. Myeloid-Derived Suppressor Cells Promote Lung Cancer Metastasis by CCL11 to Activate ERK and AKT Signaling and Induce Epithelial-Mesenchymal Transition in Tumor Cells. Oncogene 2021, 40, 1476–1489. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Yang, Y.; Zhao, M.; Zhu, G.; Hou, Z. The Prognostic Landscape of Tumor-Infiltrating Immune Cell and Immunomodulators in Lung Cancer. Biomed. Pharmacother. 2017, 95, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.T.; Raj, R.; Prasad, R.; Barney, C.; Brownstein, J.; Grecula, J.; Haglund, K.; Xu-Welliver, M.; Williams, T.M.; Bazan, J.G. Association of Pre- and Posttreatment Neutrophil-Lymphocyte Ratio with Recurrence and Mortality in Locally Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 598873. [Google Scholar] [CrossRef] [PubMed]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of M1 and M2 Macrophages in Tumor Islets and Stroma in Relation to Prognosis of Non-Small Cell Lung Cancer. BMC Immunol. 2018, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.-L. M2 Tumor-Associated Macrophages Promote Tumor Progression in Non-Small-Cell Lung Cancer. Exp. Ther. Med. 2019, 18, 4490–4498. [Google Scholar] [CrossRef]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast Cancer: Presentation, Investigation and Management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-Associated Fibroblasts: Orchestrating the Composition of Malignancy. Genes. Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Raz, Y.; Cohen, N.; Shani, O.; Bell, R.E.; Novitskiy, S.V.; Abramovitz, L.; Levy, C.; Milyavsky, M.; Leider-Trejo, L.; Moses, H.L.; et al. Bone Marrow-Derived Fibroblasts Are a Functionally Distinct Stromal Cell Population in Breast Cancer. J. Exp. Med. 2018, 215, 3075–3093. [Google Scholar] [CrossRef]
- Tchou, J.; Kossenkov, A.V.; Chang, L.; Satija, C.; Herlyn, M.; Showe, L.C.; Puré, E. Human Breast Cancer Associated Fibroblasts Exhibit Subtype Specific Gene Expression Profiles. BMC Med. Genom. 2012, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.Q.; Herum, K.M.; Brakebusch, C. Cancer-Associated Fibroblasts: How Do They Contribute to Metastasis? Clin. Exp. Metastasis 2019, 36, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.-L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting Stromal Remodeling and Cancer Stem Cell Plasticity Overcomes Chemoresistance in Triple Negative Breast Cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nogueira, P.; Mancino, M.; Fuster, G.; López-Plana, A.; Jauregui, P.; Almendro, V.; Enreig, E.; Menéndez, S.; Rojo, F.; Noguera-Castells, A.; et al. Tumor-Associated Fibroblasts Promote HER2-Targeted Therapy Resistance through FGFR2 Activation. Clin. Cancer Res. 2020, 26, 1432–1448. [Google Scholar] [CrossRef]
- Hu, M.; Yao, J.; Carroll, D.K.; Weremowicz, S.; Chen, H.; Carrasco, D.; Richardson, A.; Violette, S.; Nikolskaya, T.; Nikolsky, Y.; et al. Regulation of In Situ to Invasive Breast Carcinoma Transition. Cancer Cell 2008, 13, 394–406. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Zhang, X.; Wang, Y. Cancer-Associated Fibroblast-Secreted Collagen Triple Helix Repeat Containing-1 Promotes Breast Cancer Cell Migration, Invasiveness and Epithelial-Mesenchymal Transition by Activating the Wnt/β-Catenin Pathway. Oncol. Lett. 2021, 22, 814. [Google Scholar]
- O’Connell, J.T.; Sugimoto, H.; Cooke, V.G.; MacDonald, B.A.; Mehta, A.I.; LeBleu, V.S.; Dewar, R.; Rocha, R.M.; Brentani, R.R.; Resnick, M.B.; et al. VEGF-A and Tenascin-C Produced by S100A4+ Stromal Cells Are Important for Metastatic Colonization. Proc. Natl. Acad. Sci. USA 2011, 108, 16002–16007. [Google Scholar] [CrossRef]
- Ren, J.; Smid, M.; Iaria, J.; Salvatori, D.C.F.; van Dam, H.; Zhu, H.J.; Martens, J.W.M.; Ten Dijke, P. Cancer-Associated Fibroblast-Derived Gremlin 1 Promotes Breast Cancer Progression. Breast Cancer Res. 2019, 21, 109. [Google Scholar] [CrossRef]
- Wen, S.; Hou, Y.; Fu, L.; Xi, L.; Yang, D.; Zhao, M.; Qin, Y.; Sun, K.; Teng, Y.; Liu, M. Cancer-Associated Fibroblast (CAF)-Derived IL32 Promotes Breast Cancer Cell Invasion and Metastasis via Integrin Β3-P38 MAPK Signalling. Cancer Lett. 2019, 442, 320–332. [Google Scholar] [CrossRef]
- Xu, K.; Tian, X.; Oh, S.Y.; Movassaghi, M.; Naber, S.P.; Kuperwasser, C.; Buchsbaum, R.J. The Fibroblast Tiam1-Osteopontin Pathway Modulates Breast Cancer Invasion and Metastasis. Breast Cancer Res. 2016, 18, 14. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.-H.; Tan, L.-D.; Wang, Q.-S.; Li, X.-Q.; Feng, Y.-M. Cancer-Associated Fibroblasts Induce Epithelial-Mesenchymal Transition of Breast Cancer Cells through Paracrine TGF-β Signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, L.; Guo, X.; Li, Z.; Liao, X.; Zhang, X.; Huang, L.; He, J. HMGB1-Activated Fibroblasts Promote Breast Cancer Cells Metastasis via RAGE/Aerobic Glycolysis. Neoplasma 2021, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Prisco, M.; Ertel, A.; Tsirigos, A.; Lin, Z.; Pavlides, S.; Wang, C.; Flomenberg, N.; Knudsen, E.S.; Howell, A.; et al. Ketones and Lactate Increase Cancer Cell “Stemness,” Driving Recurrence, Metastasis and Poor Clinical Outcome in Breast Cancer: Achieving Personalized Medicine via Metabolo-Genomics. Cell Cycle 2011, 10, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.-H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-Cell-Secreted Exosomal miR-105 Promotes Tumour Growth through the MYC-Dependent Metabolic Reprogramming of Stromal Cells. Nat. Cell Biol. 2018, 20, 597–609. [Google Scholar] [CrossRef]
- Yu, T.; Yang, G.; Hou, Y.; Tang, X.; Wu, C.; Wu, X.-A.; Guo, L.; Zhu, Q.; Luo, H.; Du, Y.-E.; et al. Cytoplasmic GPER Translocation in Cancer-Associated Fibroblasts Mediates cAMP/PKA/CREB/Glycolytic Axis to Confer Tumor Cells with Multidrug Resistance. Oncogene 2017, 36, 2131–2145. [Google Scholar] [CrossRef]
- Liao, D.; Luo, Y.; Markowitz, D.; Xiang, R.; Reisfeld, R.A. Cancer Associated Fibroblasts Promote Tumor Growth and Metastasis by Modulating the Tumor Immune Microenvironment in a 4T1 Murine Breast Cancer Model. PLoS ONE 2009, 4, e7965. [Google Scholar] [CrossRef]
- Al-Jomah, N.; Al-Mohanna, F.H.; Aboussekhra, A. Tocilizumab Suppresses the Pro-Carcinogenic Effects of Breast Cancer-Associated Fibroblasts through Inhibition of the STAT3/AUF1 Pathway. Carcinogenesis 2021, 42, 1439–1448. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Lappano, R.; Santolla, M.F.; Marsico, S.; Caruso, A.; Maggiolini, M. HIF-1α/GPER Signaling Mediates the Expression of VEGF Induced by Hypoxia in Breast Cancer Associated Fibroblasts (CAFs). Breast Cancer Res. 2013, 15, R64. [Google Scholar] [CrossRef]
- Choi, J.; Cha, Y.J.; Koo, J.S. Adipocyte Biology in Breast Cancer: From Silent Bystander to Active Facilitator. Prog. Lipid Res. 2018, 69, 11–20. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Iyengar, P.; Espina, V.; Williams, T.W.; Lin, Y.; Berry, D.; Jelicks, L.A.; Lee, H.; Temple, K.; Graves, R.; Pollard, J.; et al. Adipocyte-Derived Collagen VI Affects Early Mammary Tumor Progression In Vivo, Demonstrating a Critical Interaction in the Tumor/Stroma Microenvironment. J. Clin. Investig. 2005, 115, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Lapeire, L.; Hendrix, A.; Lambein, K.; Van Bockstal, M.; Braems, G.; Van Den Broecke, R.; Limame, R.; Mestdagh, P.; Vandesompele, J.; Vanhove, C.; et al. Cancer-Associated Adipose Tissue Promotes Breast Cancer Progression by Paracrine Oncostatin M and Jak/STAT3 Signaling. Cancer Res. 2014, 74, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Vona-Davis, L.; Rose, D.P. Adipokines as Endocrine, Paracrine, and Autocrine Factors in Breast Cancer Risk and Progression. Endocr. Relat. Cancer 2007, 14, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936.e10. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Liu, S.; Chen, C. Targeting Breast Cancer Stem Cells. Int. J. Biol. Sci. 2023, 19, 552–570. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Pruneri, G.; Vingiani, A.; Denkert, C. Tumor Infiltrating Lymphocytes in Early Breast Cancer. Breast 2018, 37, 207–214. [Google Scholar] [CrossRef]
- Thompson, E.; Taube, J.M.; Elwood, H.; Sharma, R.; Meeker, A.; Warzecha, H.N.; Argani, P.; Cimino-Mathews, A.; Emens, L.A. The Immune Microenvironment of Breast Ductal Carcinoma In Situ. Mod. Pathol. 2016, 29, 249–258. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-Cell Infiltration and Breast Cancer Survival in 12,439 Patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T Regulatory Cells in Cancer: Prognostic Biomarkers and Therapeutic Targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Liu, F.; Lang, R.; Zhao, J.; Zhang, X.; Pringle, G.A.; Fan, Y.; Yin, D.; Gu, F.; Yao, Z.; Fu, L. CD8+ Cytotoxic T Cell and FOXP3+ Regulatory T Cell Infiltration in Relation to Breast Cancer Survival and Molecular Subtypes. Breast Cancer Res. Treat. 2011, 130, 645–655. [Google Scholar] [CrossRef]
- Bouzidi, L.; Triki, H.; Charfi, S.; Kridis, W.B.; Derbel, M.; Ayadi, L.; Sellami-Boudawara, T.; Cherif, B. Prognostic Value of Natural Killer Cells Besides Tumor-Infiltrating Lymphocytes in Breast Cancer Tissues. Clin. Breast Cancer 2021, 21, e738–e747. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Z.; Hu, X.; Zhang, J.; Gao, Z.; Han, X.; Qin, J.; Li, C.; Wang, Y. The PI3K/Akt/GSK-3β/ROS/eIF2B Pathway Promotes Breast Cancer Growth and Metastasis via Suppression of NK Cell Cytotoxicity and Tumor Cell Susceptibility. Cancer Biol. Med. 2019, 16, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human Breast Cancer Cells Enhance Self Tolerance by Promoting Evasion from NK Cell Antitumor Immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H.; et al. Tumor-Infiltrating Lymphocyte Composition, Organization and PD-1/PD-L1 Expression Are Linked in Breast Cancer. Oncoimmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Guan, H.; Wan, Y.; Lan, J.; Wang, Q.; Wang, Z.; Li, Y.; Zheng, J.; Zhang, X.; Wang, Z.; Shen, Y.; et al. PD-L1 Is a Critical Mediator of Regulatory B Cells and T Cells in Invasive Breast Cancer. Sci. Rep. 2016, 6, 35651. [Google Scholar] [CrossRef]
- Minnich, M.; Tagoh, H.; Bönelt, P.; Axelsson, E.; Fischer, M.; Cebolla, B.; Tarakhovsky, A.; Nutt, S.L.; Jaritz, M.; Busslinger, M. Multifunctional Role of the Transcription Factor Blimp-1 in Coordinating Plasma Cell Differentiation. Nat. Immunol. 2016, 17, 331–343. [Google Scholar] [CrossRef]
- Patysheva, M.; Larionova, I.; Stakheyeva, M.; Grigoryeva, E.; Iamshchikov, P.; Tarabanovskaya, N.; Weiss, C.; Kardashova, J.; Frolova, A.; Rakina, M.; et al. Effect of Early-Stage Human Breast Carcinoma on Monocyte Programming. Front. Oncol. 2022, 11, 800235. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malfroy, M.; Thys, S.; Treilleux, I.; Labidi-Galy, S.I.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast Cancer-Derived Transforming Growth Factor-β and Tumor Necrosis Factor-α Compromise Interferon-α Production by Tumor-Associated Plasmacytoid Dendritic Cells. Int. J. Cancer 2013, 133, 771–778. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kini Bailur, J.; Gueckel, B.; Pawelec, G. Prognostic Impact of High Levels of Circulating Plasmacytoid Dendritic Cells in Breast Cancer. J. Transl. Med. 2016, 14, 151. [Google Scholar] [CrossRef]
- Kumar, S.; Lindsay, D.; Chen, Q.B.; Garrett, A.L.; Tan, X.M.; Anders, C.K.; Carey, L.A.; Gupta, G.P. Tracking Plasma DNA Mutation Dynamics in Estrogen Receptor Positive Metastatic Breast Cancer with dPCR-SEQ. NPJ Breast Cancer 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased Circulating Myeloid-Derived Suppressor Cells Correlate with Clinical Cancer Stage, Metastatic Tumor Burden, and Doxorubicin-Cyclophosphamide Chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Jian, J.-W.; Hung, C.-F.; Peng, H.-P.; Yang, C.-F.; Cheng, H.-C.S.; Yang, A.-S. Germline Breast Cancer Susceptibility Gene Mutations and Breast Cancer Outcomes. BMC Cancer 2018, 18, 315. [Google Scholar] [CrossRef]
- Carnevale, S.; Di Ceglie, I.; Grieco, G.; Rigatelli, A.; Bonavita, E.; Jaillon, S. Neutrophil Diversity in Inflammation and Cancer. Front. Immunol. 2023, 14, 1180810. [Google Scholar] [CrossRef]
- SenGupta, S.; Hein, L.E.; Parent, C.A. The Recruitment of Neutrophils to the Tumor Microenvironment Is Regulated by Multiple Mediators. Front. Immunol. 2021, 12, 734188. [Google Scholar] [CrossRef]
- Ma, R.-Y.; Zhang, H.; Li, X.-F.; Zhang, C.-B.; Selli, C.; Tagliavini, G.; Lam, A.D.; Prost, S.; Sims, A.H.; Hu, H.-Y.; et al. Monocyte-Derived Macrophages Promote Breast Cancer Bone Metastasis Outgrowth. J. Exp. Med. 2020, 217, e20191820. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human Breast Cancer Cells Educate Macrophages toward the M2 Activation Status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Vargas, A.N. Natural History of Ovarian Cancer. Ecancermedicalscience 2014, 8, 465. [Google Scholar] [CrossRef]
- Duarte Mendes, A.; Freitas, A.R.; Vicente, R.; Vitorino, M.; Vaz Batista, M.; Silva, M.; Braga, S. Adipocyte Microenvironment in Ovarian Cancer: A Critical Contributor? Int. J. Mol. Sci. 2023, 24, 16589. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Takahashi, T.; Sawai, K.; Taniguchi, H.; Shimotsuma, M.; Okano, S.; Sakakura, C.; Tsujimoto, H.; Osaki, K.; Sasaki, S. Milky Spots as the Implantation Site for Malignant Cells in Peritoneal Dissemination in Mice. Cancer Res. 1993, 53, 687–692. [Google Scholar]
- Sorensen, E.W.; Gerber, S.A.; Sedlacek, A.L.; Rybalko, V.Y.; Chan, W.M.; Lord, E.M. Omental Immune Aggregates and Tumor Metastasis within the Peritoneal Cavity. Immunol. Res. 2009, 45, 185–194. [Google Scholar] [CrossRef]
- Desbois, M.; Wang, Y. Cancer-Associated Fibroblasts: Key Players in Shaping the Tumor Immune Microenvironment. Immunol. Rev. 2021, 302, 241–258. [Google Scholar] [CrossRef]
- Givel, A.-M.; Kieffer, Y.; Scholer-Dahirel, A.; Sirven, P.; Cardon, M.; Pelon, F.; Magagna, I.; Gentric, G.; Costa, A.; Bonneau, C.; et al. miR200-Regulated CXCL12β Promotes Fibroblast Heterogeneity and Immunosuppression in Ovarian Cancers. Nat. Commun. 2018, 9, 1056. [Google Scholar] [CrossRef]
- Hornburg, M.; Desbois, M.; Lu, S.; Guan, Y.; Lo, A.A.; Kaufman, S.; Elrod, A.; Lotstein, A.; DesRochers, T.M.; Munoz-Rodriguez, J.L.; et al. Single-Cell Dissection of Cellular Components and Interactions Shaping the Tumor Immune Phenotypes in Ovarian Cancer. Cancer Cell 2021, 39, 928–944.e6. [Google Scholar] [CrossRef]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.-J.; Shah, P.; et al. A Single-Cell Landscape of High-Grade Serous Ovarian Cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; Maniati, E.; Malacrida, B.; Nichols, S.; Roozitalab, R.; Jones, R.R.; Lecker, L.S.M.; Pearce, O.M.T.; Knight, M.M.; Balkwill, F.R. Modelling TGFβR and Hh Pathway Regulation of Prognostic Matrisome Molecules in Ovarian Cancer. iScience 2021, 24, 102674. [Google Scholar] [CrossRef]
- Yeung, T.-L.; Leung, C.S.; Wong, K.-K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-β Modulates Ovarian Cancer Invasion by Upregulating CAF-Derived Versican in the Tumor Microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, W.; Chen, R.; Wang, J.; Lu, X.; Li, J. Stromal POSTN Induced by TGF-Β1 Facilitates the Migration and Invasion of Ovarian Cancer. Gynecol. Oncol. 2021, 160, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Huang, Y.-F.; Chang, T.-H.; Chen, C.-C.; Wu, P.-Y.; Huang, S.-C.; Chou, C.-Y. COL11A1 Activates Cancer-Associated Fibroblasts by Modulating TGF-Β3 through the NF-κB/IGFBP2 Axis in Ovarian Cancer Cells. Oncogene 2021, 40, 4503–4519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, X.; Evans, B.J.; VanBlaricom, J.L.; Weroha, S.J.; Cliby, W.A. LY2157299 Monohydrate, a TGF-βR1 Inhibitor, Suppresses Tumor Growth and Ascites Development in Ovarian Cancer. Cancers 2018, 10, 260. [Google Scholar] [CrossRef]
- Deying, W.; Feng, G.; Shumei, L.; Hui, Z.; Ming, L.; Hongqing, W. CAF-Derived HGF Promotes Cell Proliferation and Drug Resistance by up-Regulating the c-Met/PI3K/Akt and GRP78 Signalling in Ovarian Cancer Cells. Biosci. Rep. 2017, 37, BSR20160470. [Google Scholar] [CrossRef]
- Ortiz, M.R. Best Practices in Patient-Centered Care: Nursing Theory Reflections. Nurs. Sci. Q. 2021, 34, 322–327. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastatis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Cybulska, P.; Mager, K.L.; Koplev, S.; Cast, O.; Couturier, D.-L.; Memon, D.; Selenica, P.; Nikolovski, I.; Mazaheri, Y.; et al. Unraveling Tumor-Immune Heterogeneity in Advanced Ovarian Cancer Uncovers Immunogenic Effect of Chemotherapy. Nat. Genet. 2020, 52, 582–593. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-Catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, R.; Zhou, J.; Wang, J.; Xu, Y.; Zhang, H.; Gu, Y.; Fu, F.; Shen, Y.; Zhang, G.; et al. CTL Attenuation Regulated by PS1 in Cancer-Associated Fibroblast. Front. Immunol. 2020, 11, 999. [Google Scholar] [CrossRef]
- Taki, M.; Abiko, K.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Murakami, R.; Yamanoi, K.; Horikawa, N.; Hosoe, Y.; Nakamura, E.; et al. Snail Promotes Ovarian Cancer Progression by Recruiting Myeloid-Derived Suppressor Cells via CXCR2 Ligand Upregulation. Nat. Commun. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29, 141–155.e9. [Google Scholar] [CrossRef] [PubMed]
- Demircioglu, F.; Wang, J.; Candido, J.; Costa, A.S.H.; Casado, P.; de Luxan Delgado, B.; Reynolds, L.E.; Gomez-Escudero, J.; Newport, E.; Rajeeve, V.; et al. Cancer Associated Fibroblast FAK Regulates Malignant Cell Metabolism. Nat. Commun. 2020, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ji, G.; Le, X.; Wang, C.; Xu, L.; Feng, M.; Zhang, Y.; Yang, H.; Xuan, Y.; Yang, Y.; et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017, 77, 1369–1382. [Google Scholar] [CrossRef]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting Metastasis-Initiating Cells through the Fatty Acid Receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Chehade, H.; Tedja, R.; Ramos, H.; Bawa, T.S.; Adzibolosu, N.; Gogoi, R.; Mor, G.; Alvero, A.B. Regulatory Role of the Adipose Microenvironment on Ovarian Cancer Progression. Cancers 2022, 14, 2267. [Google Scholar] [CrossRef]
- Hao, D.; Liu, J.; Chen, M.; Li, J.; Wang, L.; Li, X.; Zhao, Q.; Di, L. Immunogenomic Analyses of Advanced Serous Ovarian Cancer Reveal Immune Score Is a Strong Prognostic Factor and an Indicator of Chemosensitivity. Clin. Cancer Res. 2018, 24, 3560–3571. [Google Scholar] [CrossRef]
- Borah, A.; Raveendran, S.; Rochani, A.; Maekawa, T.; Kumar, D.S. Targeting Self-Renewal Pathways in Cancer Stem Cells: Clinical Implications for Cancer Therapy. Oncogenesis 2015, 4, e177. [Google Scholar] [CrossRef]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt Pathway in Human Cancers: Therapeutic Targeting with a Focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 Produced by Tumor Microenvironment Promotes Self-Renewal of CD133+ Cancer Stem-like Cells in Ovarian Cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, T.; Hong, W.; Ye, H.; Hu, C.; Zheng, Y. Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis. Cell Physiol. Biochem. 2018, 50, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Varier, L.; Sundaram, S.M.; Gamit, N.; Warrier, S. An Overview of Ovarian Cancer: The Role of Cancer Stem Cells in Chemoresistance and a Precision Medicine Approach Targeting the Wnt Pathway with the Antagonist sFRP4. Cancers 2023, 15, 1275. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Nephew, K.P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting. Cancers 2019, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Almeida-Nunes, D.L.; Mendes-Frias, A.; Silvestre, R.; Dinis-Oliveira, R.J.; Ricardo, S. Immune Tumor Microenvironment in Ovarian Cancer Ascites. Int. J. Mol. Sci. 2022, 23, 10692. [Google Scholar] [CrossRef]
- Chen, S.S.; Michael, A.; Butler-Manuel, S.A. Advances in the Treatment of Ovarian Cancer: A Potential Role of Antiinflammatory Phytochemicals. Discov. Med. 2012, 13, 7–17. [Google Scholar]
- Fialová, A.; Partlová, S.; Sojka, L.; Hromádková, H.; Brtnický, T.; Fučíková, J.; Kocián, P.; Rob, L.; Bartůňková, J.; Spíšek, R. Dynamics of T-Cell Infiltration during the Course of Ovarian Cancer: The Gradual Shift from a Th17 Effector Cell Response to a Predominant Infiltration by Regulatory T-Cells. Int. J. Cancer 2013, 132, 1070–1079. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Zhang, L.; Zhou, Y.; Zhang, W.; Wan, T.; Gu, H.; Ouyang, Y.; Zheng, X.; Liu, P.; et al. Targeting the Cdc2-like Kinase 2 for Overcoming Platinum Resistance in Ovarian Cancer. MedComm (2020) 2024, 5, e537. [Google Scholar] [CrossRef]
- Li, K.; Mandai, M.; Hamanishi, J.; Matsumura, N.; Suzuki, A.; Yagi, H.; Yamaguchi, K.; Baba, T.; Fujii, S.; Konishi, I. Clinical Significance of the NKG2D Ligands, MICA/B and ULBP2 in Ovarian Cancer: High Expression of ULBP2 Is an Indicator of Poor Prognosis. Cancer Immunol. Immunother. 2009, 58, 641–652. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Nham, T.; Chew, M.V.; Lee, A.J.; Hammill, J.A.; Fan, I.Y.; Butcher, M.; Bramson, J.L.; Lee, D.A.; Hirte, H.W.; et al. Expanded CD56superbrightCD16+ NK Cells from Ovarian Cancer Patients Are Cytotoxic against Autologous Tumor in a Patient-Derived Xenograft Murine Model. Cancer Immunol. Res. 2018, 6, 1174–1185. [Google Scholar] [CrossRef]
- Vazquez, J.; Chavarria, M.; Lopez, G.E.; Felder, M.A.; Kapur, A.; Romo Chavez, A.; Karst, N.; Barroilhet, L.; Patankar, M.S.; Stanic, A.K. Identification of Unique Clusters of T, Dendritic, and Innate Lymphoid Cells in the Peritoneal Fluid of Ovarian Cancer Patients. Am. J. Reprod. Immunol. 2020, 84, e13284. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; Moyano-Galceran, L.; Lheureux, S.; Mattsson, J.; Lehti, K.; Dahlstrand, H.; Magalhaes, I. Molecular, Cellular and Systemic Aspects of Epithelial Ovarian Cancer and Its Tumor Microenvironment. Semin. Cancer Biol. 2022, 86, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Elstrand, M.B.; Holth, A.; Silins, I.; Berner, A.; Trope, C.G.; Davidson, B.; Risberg, B. NK- and B-Cell Infiltration Correlates with Worse Outcome in Metastatic Ovarian Carcinoma. Am. J. Clin. Pathol. 2006, 125, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chen, C.; Chaluvally-Raghavan, P.; Pradeep, S. B Cells as an Immune-Regulatory Signature in Ovarian Cancer. Cancers 2019, 11, 894. [Google Scholar] [CrossRef]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic Impact of Tumour-Associated B Cells and Plasma Cells in Epithelial Ovarian Cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-Cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef]
- Wei, X.; Jin, Y.; Tian, Y.; Zhang, H.; Wu, J.; Lu, W.; Lu, X. Regulatory B Cells Contribute to the Impaired Antitumor Immunity in Ovarian Cancer Patients. Tumour Biol. 2016, 37, 6581–6588. [Google Scholar] [CrossRef]
- He, Y.; Qian, H.; Liu, Y.; Duan, L.; Li, Y.; Shi, G. The Roles of Regulatory B Cells in Cancer. J. Immunol. Res. 2014, 2014, 215471. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The Lung Microenvironment: An Important Regulator of Tumour Growth and Metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Sisirak, V.; Meeus, P.; Gobert, M.; Treilleux, I.; Bajard, A.; Combes, J.-D.; Faget, J.; Mithieux, F.; Cassignol, A.; et al. Quantitative and Functional Alterations of Plasmacytoid Dendritic Cells Contribute to Immune Tolerance in Ovarian Cancer. Cancer Res. 2011, 71, 5423–5434. [Google Scholar] [CrossRef]
- Wefers, C.; Duiveman-de Boer, T.; Yigit, R.; Zusterzeel, P.L.M.; van Altena, A.M.; Massuger, L.F.A.G.; De Vries, I.J.M. Survival of Ovarian Cancer Patients Is Independent of the Presence of DC and T Cell Subsets in Ascites. Front. Immunol. 2019, 9, 03156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, T.; Li, Y.; Chen, L.; Liu, H.; Wu, Y.; Guo, H. Dendritic Cell Vaccines in Ovarian Cancer. Front. Immunol. 2021, 11, 613773. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Baba, T.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Konishi, I. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin. Cancer Res. 2017, 23, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.E.; Abrams, S.I.; Joseph, J.M.; Goode, E.L.; Tario, J.D., Jr.; Wallace, P.K.; Kaur, D.; Adamson, A.-K.; Buas, M.F.; Lugade, A.A.; et al. Circulating CD14+HLA-DRlo/− Monocytic Cells as a Biomarker for Epithelial Ovarian Cancer Progression. Am. J. Reprod. Immunol. 2021, 85, e13343. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils Facilitate Ovarian Cancer Premetastatic Niche Formation in the Omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Denizli, M.; Kanlikilicer, P.; Bayraktar, R.; Ivan, C.; Rashed, M.; Kabil, N.; Ozpolat, B.; Calin, G.A.; Salama, S.A.; et al. GATA3 as a Master Regulator for Interactions of Tumor-Associated Macrophages with High-Grade Serous Ovarian Carcinoma. Cell Signal 2020, 68, 109539. [Google Scholar] [CrossRef]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef]
- Chen, X.; Ying, X.; Wang, X.; Wu, X.; Zhu, Q.; Wang, X. Exosomes Derived from Hypoxic Epithelial Ovarian Cancer Deliver microRNA-940 to Induce Macrophage M2 Polarization. Oncol. Rep. 2017, 38, 522–528. [Google Scholar] [CrossRef]
- Lan, C.; Huang, X.; Lin, S.; Huang, H.; Cai, Q.; Wan, T.; Lu, J.; Liu, J. Expression of M2-Polarized Macrophages Is Associated with Poor Prognosis for Advanced Epithelial Ovarian Cancer. Technol. Cancer Res. Treat. 2013, 12, 259–267. [Google Scholar] [CrossRef]
- Xiong, J.; He, X.; Xu, Y.; Zhang, W.; Fu, F. MiR-200b Is Upregulated in Plasma-Derived Exosomes and Functions as an Oncogene by Promoting Macrophage M2 Polarization in Ovarian Cancer. J. Ovarian Res. 2021, 14, 74. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic Significance of Tumor-Associated Macrophages in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-Associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial Ovarian Cancer-Secreted Exosomal miR-222-3p Induces Polarization of Tumor-Associated Macrophages. Oncotarget 2016, 7, 43076–43087. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Xie, H.; Yuan, J.; Jiang, X.-Y.; Yong, J.-H.; Zeng, D.; Dou, Y.-Y.; Xiao, S.-S. M2-like Tumor-Associated Macrophages-Secreted EGF Promotes Epithelial Ovarian Cancer Metastasis via Activating EGFR-ERK Signaling and Suppressing lncRNA LIMT Expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, K.; Wu, L.; Duan, T. Interaction of Monocytes/Macrophages with Ovarian Cancer Cells Promotes Angiogenesis in Vitro. Cancer Sci. 2013, 104, 516–523. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef]
- Margul, D.; Yu, C.; AlHilli, M.M. Tumor Immune Microenvironment in Gynecologic Cancers. Cancers 2023, 15, 3849. [Google Scholar] [CrossRef]
- Meyer, L.A.; Broaddus, R.R.; Lu, K.H. Endometrial Cancer and Lynch Syndrome: Clinical and Pathologic Considerations. Cancer Control 2009, 16, 14–22. [Google Scholar] [CrossRef]
- Aprelikova, O.; Yu, X.; Palla, J.; Wei, B.-R.; John, S.; Yi, M.; Stephens, R.; Simpson, R.M.; Risinger, J.I.; Jazaeri, A.; et al. The Role of miR-31 and Its Target Gene SATB2 in Cancer-Associated Fibroblasts. Cell Cycle 2010, 9, 4387–4398. [Google Scholar] [CrossRef]
- Baker, A.-M.; Bird, D.; Lang, G.; Cox, T.R.; Erler, J.T. Lysyl Oxidase Enzymatic Function Increases Stiffness to Drive Colorectal Cancer Progression through FAK. Oncogene 2013, 32, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- De Nola, R.; Menga, A.; Castegna, A.; Loizzi, V.; Ranieri, G.; Cicinelli, E.; Cormio, G. The Crowded Crosstalk between Cancer Cells and Stromal Microenvironment in Gynecological Malignancies: Biological Pathways and Therapeutic Implication. Int. J. Mol. Sci. 2019, 20, 2401. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Konno, Y.; Watari, H.; Hosaka, M.; Noguchi, M.; Sakuragi, N. The Impact of microRNA-Mediated PI3K/AKT Signaling on Epithelial-Mesenchymal Transition and Cancer Stemness in Endometrial Cancer. J. Transl. Med. 2014, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yi, Z.; Jin, L.; Zhao, L.; Raskind, A.; Yeomans, L.; Nwosu, Z.C.; Simeone, D.M.; Lyssiotis, C.A.; Stringer, K.A.; et al. Cyst Fluid Metabolites Distinguish Malignant from Benign Pancreatic Cysts. Neoplasia 2021, 23, 1078–1088. [Google Scholar] [CrossRef]
- Subramaniam, K.S.; Omar, I.S.; Kwong, S.C.; Mohamed, Z.; Woo, Y.L.; Mat Adenan, N.A.; Chung, I. Cancer-Associated Fibroblasts Promote Endometrial Cancer Growth via Activation of Interleukin-6/STAT-3/c-Myc Pathway. Am. J. Cancer Res. 2016, 6, 200–213. [Google Scholar]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.E.; Kwappenberg, K.M.C.; van der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Fleuren, G.J.; Offringa, R.; et al. High Number of Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes Is Associated with the Absence of Lymph Node Metastases in Patients with Large Early-Stage Cervical Cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef]
- Yu, Q.; Lou, X.; He, Y. Preferential Recruitment of Th17 Cells to Cervical Cancer via CCR6-CCL20 Pathway. PLoS ONE 2015, 10, e0120855. [Google Scholar] [CrossRef]
- Cicchini, L.; Westrich, J.A.; Xu, T.; Vermeer, D.W.; Berger, J.N.; Clambey, E.T.; Lee, D.; Song, J.I.; Lambert, P.F.; Greer, R.O.; et al. Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14. mBio 2016, 7, e00270-16. [Google Scholar] [CrossRef]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef]
- Jenkins, D.; Tay, S.K.; Singer, A.; Tay, S.K. Natural Killer Cells in Cervical Intraepithelial Neoplasia and Human Papillomavirus Infection. BJOG Int. J. Obstet. Gynaecol. 1987, 94, 901–906. [Google Scholar] [CrossRef]
- Textor, S.; Dürst, M.; Jansen, L.; Accardi, R.; Tommasino, M.; Trunk, M.J.; Porgador, A.; Watzl, C.; Gissmann, L.; Cerwenka, A. Activating NK Cell Receptor Ligands Are Differentially Expressed during Progression to Cervical Cancer. Int. J. Cancer 2008, 123, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Y.; Du, R.; Pang, N.; Zhang, F.; Dong, D.; Ding, J.; Ding, Y. Role of Regulatory B Cells in the Progression of Cervical Cancer. Mediat. Inflamm. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-N.; Follen, M.; Rodriquez, G.; Shen, D.-Y.; Malpica, A.; Shearer, W.T.; Reuben, J.M. Deficiencies in Myeloid Antigen-Presenting Cells in Women with Cervical Squamous Intraepithelial Lesions. Cancer 2006, 107, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, M.; Bieda, T.; Bobek, M.; Zrubek, H. Dynamics of Cervical Langerhans Cell Counts in the Course of HPV-Positive CIN Treatment with the Use of Human Recombinant Interferon Gamma. Eur. J. Gynaecol. Oncol. 2005, 26, 294–298. [Google Scholar]
- Pahne-Zeppenfeld, J.; Schröer, N.; Walch-Rückheim, B.; Oldak, M.; Gorter, A.; Hegde, S.; Smola, S. Cervical Cancer Cell-Derived Interleukin-6 Impairs CCR7-Dependent Migration of MMP-9-Expressing Dendritic Cells. Int. J. Cancer 2014, 134, 2061–2073. [Google Scholar] [CrossRef]
- Walch-Rückheim, B.; Ströder, R.; Theobald, L.; Pahne-Zeppenfeld, J.; Hegde, S.; Kim, Y.-J.; Bohle, R.M.; Juhasz-Böss, I.; Solomayer, E.-F.; Smola, S. Cervical Cancer-Instructed Stromal Fibroblasts Enhance IL23 Expression in Dendritic Cells to Support Expansion of Th17 Cells. Cancer Res. 2019, 79, 1573–1586. [Google Scholar] [CrossRef]
- Kawano, M.; Mabuchi, S.; Matsumoto, Y.; Sasano, T.; Takahashi, R.; Kuroda, H.; Kozasa, K.; Hashimoto, K.; Isobe, A.; Sawada, K.; et al. The Significance of G-CSF Expression and Myeloid-Derived Suppressor Cells in the Chemoresistance of Uterine Cervical Cancer. Sci. Rep. 2015, 5, 18217. [Google Scholar] [CrossRef]
- Mabuchi, S.; Matsumoto, Y.; Kawano, M.; Minami, K.; Seo, Y.; Sasano, T.; Takahashi, R.; Kuroda, H.; Hisamatsu, T.; Kakigano, A.; et al. Uterine Cervical Cancer Displaying Tumor-Related Leukocytosis: A Distinct Clinical Entity with Radioresistant Feature. J. Natl. Cancer Inst. 2014, 106, dju147. [Google Scholar] [CrossRef]
- Mabuchi, S.; Sasano, T. Myeloid-Derived Suppressor Cells as Therapeutic Targets in Uterine Cervical and Endometrial Cancers. Cells 2021, 10, 1073. [Google Scholar] [CrossRef]
- Bluemn, E.G.; Nelson, P.S. The Androgen/Androgen Receptor Axis in Prostate Cancer. Curr. Opin. Oncol. 2012, 24, 251–257. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational Bioinformatics for Diagnostic and Prognostic Prediction of Prostate Cancer in the Next-Generation Sequencing Era. Biomed. Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.-I. Splicing Factors Have an Essential Role in Prostate Cancer Progression and Androgen Receptor Signaling. Biomolecules 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Eder, T.; Weber, A.; Neuwirt, H.; Grünbacher, G.; Ploner, C.; Klocker, H.; Sampson, N.; Eder, I.E. Cancer-Associated Fibroblasts Modify the Response of Prostate Cancer Cells to Androgen and Anti-Androgens in Three-Dimensional Spheroid Culture. Int. J. Mol. Sci. 2016, 17, 1458. [Google Scholar] [CrossRef] [PubMed]
- Levesque, C.; Nelson, P.S. Cellular Constituents of the Prostate Stroma: Key Contributors to Prostate Cancer Progression and Therapy Resistance. Cold Spring Harb. Perspect. Med. 2018, 8, a030510. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Su, H.; Wang, Y.-H.; Zhao, X.-K. Role of Tumor-Associated Immune Cells in Prostate Cancer: Angel or Devil? Asian J. Androl. 2019, 21, 433–437. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Chiarugi, P.; Paoli, P.; Cirri, P. Tumor Microenvironment and Metabolism in Prostate Cancer. Semin. Oncol. 2014, 41, 267–280. [Google Scholar] [CrossRef]
- Sun, D.-Y.; Wu, J.-Q.; He, Z.-H.; He, M.-F.; Sun, H.-B. Cancer-Associated Fibroblast Regulate Proliferation and Migration of Prostate Cancer Cells through TGF-β Signaling Pathway. Life Sci. 2019, 235, 116791. [Google Scholar] [CrossRef]
- Bonollo, F.; Thalmann, G.N.; Kruithof-de Julio, M.; Karkampouna, S. The Role of Cancer-Associated Fibroblasts in Prostate Cancer Tumorigenesis. Cancers 2020, 12, 1887. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-de-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-Associated Fibroblasts and M2-Polarized Macrophages Synergize during Prostate Carcinoma Progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Batson, J.; Kadir, S.; Charlet, J.; Persad, R.A.; Gillatt, D.; Oxley, J.D.; Nobes, C.D. Competition amongst Eph Receptors Regulates Contact Inhibition of Locomotion and Invasiveness in Prostate Cancer Cells. Nat. Cell Biol. 2010, 12, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-Associated Fibroblasts Promote Directional Cancer Cell Migration by Aligning Fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Jiang, M.; Strand, D.W.; Peacock, J.; Fernandez, S.; Jackson, R.S.; Revelo, M.P.; Bhowmick, N.A.; Hayward, S.W. Altered TGF-β Signaling in a Subpopulation of Human Stromal Cells Promotes Prostatic Carcinogenesis. Cancer Res. 2011, 71, 1272–1281. [Google Scholar] [CrossRef]
- Romero, D.; Al-Shareef, Z.; Gorroño-Etxebarria, I.; Atkins, S.; Turrell, F.; Chhetri, J.; Bengoa-Vergniory, N.; Zenzmaier, C.; Berger, P.; Waxman, J.; et al. Dickkopf-3 Regulates Prostate Epithelial Cell Acinar Morphogenesis and Prostate Cancer Cell Invasion by Limiting TGF-β-Dependent Activation of Matrix Metalloproteases. Carcinogenesis 2016, 37, 18–29. [Google Scholar] [CrossRef]
- Scott, L.E.; Weinberg, S.H.; Lemmon, C.A. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, B.; Zhang, L.; Dang, T.; Rowley, D.; Ittmann, M.; Xin, L. Jagged1 Upregulation in Prostate Epithelial Cells Promotes Formation of Reactive Stroma in the Pten Null Mouse Model for Prostate Cancer. Oncogene 2017, 36, 618–627. [Google Scholar] [CrossRef]
- Castoria, G.; Giovannelli, P.; Di Donato, M.; Ciociola, A.; Hayashi, R.; Bernal, F.; Appella, E.; Auricchio, F.; Migliaccio, A. Role of Non-Genomic Androgen Signalling in Suppressing Proliferation of Fibroblasts and Fibrosarcoma Cells. Cell Death Dis. 2014, 5, e1548. [Google Scholar] [CrossRef]
- Liu, X.; Ge, X. Classical Cell Cycle Kinase Limits Tubulin Polyglutamylation and Cilium Stability. J. Cell Biol. 2025, 224, e202412034. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- Ku, S.-Y.; Gleave, M.E.; Beltran, H. Towards Precision Oncology in Advanced Prostate Cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.M.; Kawasaki, B.T.; Klarmann, G.J.; Thomas, S.B.; Farrar, W.L. CD44+ CD24(-) Prostate Cells Are Early Cancer Progenitor/Stem Cells That Provide a Model for Patients with Poor Prognosis. Br. J. Cancer 2008, 98, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Ramírez, N.; Völler, M.; Wetterwald, A.; Germann, M.; Cross, N.A.; Rentsch, C.A.; Schalken, J.; Thalmann, G.N.; Cecchini, M.G. In Vitro Propagation and Characterization of Neoplastic Stem/Progenitor-like Cells from Human Prostate Cancer Tissue. Prostate 2009, 69, 1683–1693. [Google Scholar] [CrossRef]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly Purified CD44+ Prostate Cancer Cells from Xenograft Human Tumors Are Enriched in Tumorigenic and Metastatic Progenitor Cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef]
- Sharpe, B.; Beresford, M.; Bowen, R.; Mitchard, J.; Chalmers, A.D. Searching for Prostate Cancer Stem Cells: Markers and Methods. Stem Cell Rev. Rep. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Williams, K.; Motiani, K.; Giridhar, P.V.; Kasper, S. CD44 Integrates Signaling in Normal Stem Cell, Cancer Stem Cell and (Pre)Metastatic Niches. Exp. Biol. Med. 2013, 238, 324–338. [Google Scholar] [CrossRef]
- Aldinucci, D.; Colombatti, A. The Inflammatory Chemokine CCL5 and Cancer Progression. Mediat. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef]
- Flammiger, A.; Weisbach, L.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; Trepel, M. High Tissue Density of FOXP3+ T Cells Is Associated with Clinical Outcome in Prostate Cancer. Eur. J. Cancer 2013, 49, 1273–1279. [Google Scholar] [CrossRef]
- Hu, S.; Li, L.; Yeh, S.; Cui, Y.; Li, X.; Chang, H.-C.; Jin, J.; Chang, C. Infiltrating T Cells Promote Prostate Cancer Metastasis via Modulation of FGF11→miRNA-541→androgen Receptor (AR)→MMP9 Signaling. Mol. Oncol. 2015, 9, 44–57. [Google Scholar] [CrossRef]
- Nardone, V.; Botta, C.; Caraglia, M.; Martino, E.C.; Ambrosio, M.R.; Carfagno, T.; Tini, P.; Semeraro, L.; Misso, G.; Grimaldi, A.; et al. Tumor Infiltrating T Lymphocytes Expressing FoxP3, CCR7 or PD-1 Predict the Outcome of Prostate Cancer Patients Subjected to Salvage Radiotherapy after Biochemical Relapse. Cancer Biol. Ther. 2016, 17, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Wang, L.; Dai, J.; Kryczek, I.; Wei, S.; Vatan, L.; Altuwaijri, S.; Sparwasser, T.; Wang, G.; Keller, E.T.; et al. Regulatory T Cells in the Bone Marrow Microenvironment in Patients with Prostate Cancer. Oncoimmunology 2012, 1, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.C.; Shim, D.H.; Yang, C.M.; Lee, S.-B.; Kim, S.M.; Shin, T.Y.; Kim, K.H.; Yoon, H.G.; Rha, K.H.; Lee, J.M.; et al. Reduction of the CD16(-)CD56bright NK Cell Subset Precedes NK Cell Dysfunction in Prostate Cancer. PLoS ONE 2013, 8, e78049. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, W.-J.; Sun, H.-J.; Yang, X.; Wu, Y.-Z. C5b-9 Staining Correlates With Clinical and Tumor Stage in Gastric Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 470–475. [Google Scholar] [CrossRef]
- Flynn, N.J.; Somasundaram, R.; Arnold, K.M.; Sims-Mourtada, J. The Multifaceted Roles of B Cells in Solid Tumors: Emerging Treatment Opportunities. Target. Oncol. 2017, 12, 139–152. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The Effects of Diet-Induced Obesity on B Cell Function. Clin. Exp. Immunol. 2015, 179, 90–99. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B.; Zheng, S.; Wen, Y.; Xu, A.; Xu, K.; Li, B.; Liu, C. IgG Silencing Induces Apoptosis and Suppresses Proliferation, Migration and Invasion in LNCaP Prostate Cancer Cells. Cell Mol. Biol. Lett. 2016, 21, 27. [Google Scholar] [CrossRef]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Chi, N.; Tan, Z.; Ma, K.; Bao, L.; Yun, Z. Increased Circulating Myeloid-Derived Suppressor Cells Correlate with Cancer Stages, Interleukin-8 and -6 in Prostate Cancer. Int. J. Clin. Exp. Med. 2014, 7, 3181–3192. [Google Scholar]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 Secreted by Myeloid Cells Drives Castration-Resistant Prostate Cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Dey, P.; Deng, P.; Wu, C.C.; Jiang, S.; Fang, Z.; Zhao, K.; Konaparthi, R.; Hua, S.; et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016, 6, 80–95. [Google Scholar] [CrossRef]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; Thor Straten, P. Correlation between Frequencies of Blood Monocytic Myeloid-Derived Suppressor Cells, Regulatory T Cells and Negative Prognostic Markers in Patients with Castration-Resistant Metastatic Prostate Cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, T.J.; Xie, J.; LeBaron, M.J.; Zhu, J.; Nurmi, M.; Alanen, K.; Rui, H.; Nevalainen, M.T. Inhibition of Transcription Factor Stat5 Induces Cell Death of Human Prostate Cancer Cells. J. Biol. Chem. 2003, 278, 39259. [Google Scholar] [CrossRef]
- Costanzo-Garvey, D.L.; Keeley, T.; Case, A.J.; Watson, G.F.; Alsamraae, M.; Yu, Y.; Su, K.; Heim, C.E.; Kielian, T.; Morrissey, C.; et al. Neutrophils Are Mediators of Metastatic Prostate Cancer Progression in Bone. Cancer Immunol. Immunother. 2020, 69, 1113–1130. [Google Scholar] [CrossRef]
- Fiévez, L.; Desmet, C.; Henry, E.; Pajak, B.; Hegenbarth, S.; Garzé, V.; Bex, F.; Jaspar, F.; Boutet, P.; Gillet, L.; et al. STAT5 Is an Ambivalent Regulator of Neutrophil Homeostasis. PLoS ONE 2007, 2, e727. [Google Scholar] [CrossRef][Green Version]
- De Marzo, A.M.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative Inflammatory Atrophy of the Prostate: Implications for Prostatic Carcinogenesis. Am. J. Pathol. 1999, 155, 1985–1992. [Google Scholar] [CrossRef]
- Lanciotti, M.; Masieri, L.; Raspollini, M.R.; Minervini, A.; Mari, A.; Comito, G.; Giannoni, E.; Carini, M.; Chiarugi, P.; Serni, S. The Role of M1 and M2 Macrophages in Prostate Cancer in Relation to Extracapsular Tumor Extension and Biochemical Recurrence after Radical Prostatectomy. Biomed. Res. Int. 2014, 2014, 486798. [Google Scholar] [CrossRef]
- Lissbrant, I.F.; Stattin, P.; Wikstrom, P.; Damber, J.E.; Egevad, L.; Bergh, A. Tumor Associated Macrophages in Human Prostate Cancer: Relation to Clinicopathological Variables and Survival. Int. J. Oncol. 2000, 17, 445–451. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Miller, L.S.; O’Connell, R.M.; Gutierrez, M.A.; Pietras, E.M.; Shahangian, A.; Gross, C.E.; Thirumala, A.; Cheung, A.L.; Cheng, G.; Modlin, R.L. MyD88 Mediates Neutrophil Recruitment Initiated by IL-1R but Not TLR2 Activation in Immunity against Staphylococcus Aureus. Immunity 2006, 24, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.Y.; Harrison, D. WHO/ISUP Classification, Grading and Pathological Staging of Renal Cell Carcinoma: Standards and Controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Ambrosetti, D.; Coutts, M.; Paoli, C.; Durand, M.; Borchiellini, D.; Montemagno, C.; Rastoin, O.; Borderie, A.; Grepin, R.; Rioux-Leclercq, N.; et al. Cancer-Associated Fibroblasts in Renal Cell Carcinoma: Implication in Prognosis and Resistance to Anti-Angiogenic Therapy. BJU Int. 2022, 129, 80–92. [Google Scholar] [CrossRef]
- Kuzet, S.-E.; Gaggioli, C. Fibroblast Activation in Cancer: When Seed Fertilizes Soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef]
- Abdu Allah, A.M.; El-Hefnway, S.M.; Alhanafy, A.M.; Zahran, A.M.; Kasem, H.E. Leptin Receptor Gene (A/G) Polymorphism Rs1137101 and Renal Cell Carcinoma. Mol. Cell Biochem. 2018, 448, 137–144. [Google Scholar] [CrossRef]
- Zhang, G.; Gu, C.; Zhu, Y.; Luo, L.; Dong, D.; Wan, F.; Zhang, H.; Shi, G.; Sun, L.; Ye, D. ADIPOQ Polymorphism Rs182052 Is Associated with Clear Cell Renal Cell Carcinoma. Cancer Sci. 2015, 106, 687–691. [Google Scholar] [CrossRef]
- Arik, D.; Can, C.; Dündar, E.; Kabukçuoğlu, S.; Paşaoğlu, Ö. Prognostic Significance of CD24 in Clear Cell Renal Cell Carcinoma. Pathol. Oncol. Res. 2017, 23, 409–416. [Google Scholar] [CrossRef]
- Wang, J.; Sakariassen, P.Ø.; Tsinkalovsky, O.; Immervoll, H.; Bøe, S.O.; Svendsen, A.; Prestegarden, L.; Røsland, G.; Thorsen, F.; Stuhr, L.; et al. CD133 Negative Glioma Cells Form Tumors in Nude Rats and Give Rise to CD133 Positive Cells. Int. J. Cancer 2008, 122, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Zarif, T.E.; Dudani, S.; Wells, J.C.; Gan, C.L.; Donskov, F.; Shapiro, J.; Davis, I.D.; Parnis, F.; Ravi, P.; et al. Upfront Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors or Targeted Therapy: An Observational Study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2023, 83, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.-F.; Liu, H.; Dotti, G.; Balyasnikova, I.V.; Gottschalk, S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, C.; Slots, C.; Vasdev, N.; Rottey, S.; Fonteyne, V.; Andras, I.; Albersen, M.; De Meerleer, G.; Bex, A.; Decaestecker, K. Current Role of Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma. Turk. J. Urol. 2021, 47, S79–S84. [Google Scholar] [CrossRef]
- Yue, D.; Wang, W.; Liu, H.; Chen, Q.; Chen, C.; Zhang, J.; Bai, F.; Wang, C. LBA58 Pathological Response to Neoadjuvant Tislelizumab (TIS) plus Platinum-Doublet (PtDb) Chemotherapy (CT) in Resectable Stage II-IIIA NSCLC Patients (Pts) in the Phase III (Ph3) RATIONALE-315 Trial. Ann. Oncol. 2023, 34, S1299. [Google Scholar] [CrossRef]
- Laumont, C.M.; Nelson, B.H. B Cells in the Tumor Microenvironment: Multi-Faceted Organizers, Regulators, and Effectors of Anti-Tumor Immunity. Cancer Cell 2023, 41, 466–489. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, D.; Ji, C.; Wang, M.; Fu, M.; Qian, Y.; Zhang, X.; Ji, R.; Li, C.; Gu, J.; et al. Exosomal miR-4745-5p/3911 from N2-Polarized Tumor-Associated Neutrophils Promotes Gastric Cancer Metastasis by Regulating SLIT2. Mol. Cancer 2024, 23, 198. [Google Scholar] [CrossRef]
- Daurkin, I.; Eruslanov, E.; Stoffs, T.; Perrin, G.Q.; Algood, C.; Gilbert, S.M.; Rosser, C.J.; Su, L.-M.; Vieweg, J.; Kusmartsev, S. Tumor-Associated Macrophages Mediate Immunosuppression in the Renal Cancer Microenvironment by Activating the 15-Lipoxygenase-2 Pathway. Cancer Res. 2011, 71, 6400–6409. [Google Scholar] [CrossRef]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef]
- Komohara, Y.; Hasita, H.; Ohnishi, K.; Fujiwara, Y.; Suzu, S.; Eto, M.; Takeya, M. Macrophage Infiltration and Its Prognostic Relevance in Clear Cell Renal Cell Carcinoma. Cancer Sci. 2011, 102, 1424–1431. [Google Scholar] [CrossRef]
- Zou, M.; Lu, N.; Hu, C.; Liu, W.; Sun, Y.; Wang, X.; You, Q.; Gu, C.; Xi, T.; Guo, Q. Beclin 1-Mediated Autophagy in Hepatocellular Carcinoma Cells: Implication in Anticancer Efficiency of Oroxylin A via Inhibition of mTOR Signaling. Cell Signal 2012, 24, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between Smoking and Risk of Bladder Cancer among Men and Women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.-C.; Huang, J.; Ko, W.Y.-K.; Lok, V.; Choi, P.; Ng, C.-F.; Sengupta, S.; Mostafid, H.; Kamat, A.M.; Black, P.C.; et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur. Urol. 2020, 78, 893–906. [Google Scholar] [CrossRef]
- van Osch, F.H.; Jochems, S.H.; van Schooten, F.-J.; Bryan, R.T.; Zeegers, M.P. Quantified Relations between Exposure to Tobacco Smoking and Bladder Cancer Risk: A Meta-Analysis of 89 Observational Studies. Int. J. Epidemiol. 2016, 45, 857–870. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in Bladder Cancer Biology and Therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, H.; Wang, L.; Cao, Y.; Liu, P.; Xu, X.; Wang, Y.; Sun, L.; Niu, H. Overexpression of Monocarboxylate Anion Transporter 1 and 4 in T24-Induced Cancer-Associated Fibroblasts Regulates the Progression of Bladder Cancer Cells in a 3D Microfluidic Device. Cell Cycle 2015, 14, 3058–3065. [Google Scholar] [CrossRef]
- Wiseman, O.; Fowler, C.; Landon, D. The Role of the Human Bladder Lamina Propria Myofibroblast. BJU Int. 2003, 91, 89–93. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Lu, T.; Han, D.; Zhang, K.; Gan, L.; Wu, X.; Li, Y.; Zhao, X.; Li, Z.; et al. Single-Cell Analysis Reveals the COL11A1+ Fibroblasts Are Cancer-Specific Fibroblasts That Promote Tumor Progression. Front. Pharmacol. 2023, 14, 1121586. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFβ1 Secreted by Cancer-Associated Fibroblasts Induces Epithelial-Mesenchymal Transition of Bladder Cancer Cells through lncRNA-ZEB2NAT. Sci. Rep. 2015, 5, 11924. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, Z.; He, C.; Qing, L.; Wang, H.; Wu, J.; Lu, X. Cancer-Associated Fibroblasts Promote Cell Proliferation and Invasion via Paracrine Wnt/IL1β Signaling Pathway in Human Bladder Cancer. Neoplasma 2021, 68, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Yao, Y.; Song, J.; Sun, L.; Zhang, G. Cancer-Associated Fibroblasts Regulate Bladder Cancer Invasion and Metabolic Phenotypes through Autophagy. Dis. Markers 2021, 2021, 6645220. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamawaki, H.; Usui, T.; Sasaki, K. Emerging Roles of Cancer Stem Cells in Bladder Cancer Progression, Tumorigenesis, and Resistance to Chemotherapy: A Potential Therapeutic Target for Bladder Cancer. Cells 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Volkmer, J.-P.; Weissman, I. Cancer Stem Cells in Bladder Cancer: A Revisited and Evolving Concept. Curr. Opin. Urol. 2010, 20, 393–397. [Google Scholar] [CrossRef]
- Van Wilpe, S.; Koornstra, R.; Den Brok, M.; De Groot, J.W.; Blank, C.; De Vries, J.; Gerritsen, W.; Mehra, N. Lactate Dehydrogenase: A Marker of Diminished Antitumor Immunity. Oncoimmunology 2020, 9, 1731942. [Google Scholar] [CrossRef]
- Krpina, K.; Babarović, E.; Jonjić, N. Correlation of Tumor-Infiltrating Lymphocytes with Bladder Cancer Recurrence in Patients with Solitary Low-Grade Urothelial Carcinoma. Virchows Arch. 2015, 467, 443–448. [Google Scholar] [CrossRef]
- Ayari, C.; LaRue, H.; Hovington, H.; Caron, A.; Bergeron, A.; Têtu, B.; Fradet, V.; Fradet, Y. High Level of Mature Tumor-Infiltrating Dendritic Cells Predicts Progression to Muscle Invasion in Bladder Cancer. Hum. Pathol. 2013, 44, 1630–1637. [Google Scholar] [CrossRef]
- Ornstein, M.C.; Diaz-Montero, C.M.; Rayman, P.; Elson, P.; Haywood, S.; Finke, J.H.; Kim, J.S.; Pavicic, P.G.; Lamenza, M.; Devonshire, S.; et al. Myeloid-Derived Suppressors Cells (MDSC) Correlate with Clinicopathologic Factors and Pathologic Complete Response (pCR) in Patients with Urothelial Carcinoma (UC) Undergoing Cystectomy. Urol. Oncol. 2018, 36, 405–412. [Google Scholar] [CrossRef]
- Yang, G.; Liu, M.; Liu, Q.; Duan, X.; Chen, H.; Zhang, L.; Bo, J. Granulocytic Myeloid-Derived Suppressor Cells Correlate with Outcomes Undergoing Neoadjuvant Chemotherapy for Bladder Cancer. Urol. Oncol. 2020, 38, 5.e17–15.e23. [Google Scholar] [CrossRef]
- Butala, A.A.; Jain, V.; Reddy, V.K.; Sebro, R.A.; Song, Y.; Karakousis, G.; Mitchell, T.C.; Lukens, J.N.; Shabason, J.E. Impact of Tumor-Infiltrating Lymphocytes on Overall Survival in Merkel Cell Carcinoma. Oncologist 2021, 26, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.A.; ElBanna, A.K.; Noufal, N.; El-Assmy, M.; Lotfy, H.; Ali, R.I. Significance of Tumor-Associated Neutrophils, Lymphocytes, and Neutrophil-to-Lymphocyte Ratio in Non-Invasive and Invasive Bladder Urothelial Carcinoma. J. Pathol. Transl. Med. 2023, 57, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ku, J.H. Prognostic Impact of Tumor Infiltrating Lymphocytes in Bladder Urothelial Carcinoma. Transl. Androl. Urol. 2019, 8, S291–S292. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic Value of Tumor-Associated Macrophage Count in Human Bladder Cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef]
- Leblond, M.M.; Tillé, L.; Nassiri, S.; Gilfillan, C.B.; Imbratta, C.; Schmittnaegel, M.; Ries, C.H.; Speiser, D.E.; Verdeil, G. CD40 Agonist Restores the Antitumor Efficacy of Anti-PD1 Therapy in Muscle-Invasive Bladder Cancer in an IFN I/II-Mediated Manner. Cancer Immunol. Res. 2020, 8, 1180–1192. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Dong, X.; Wu, S.; Zeng, H.; Liu, Z.; Wan, D.; Dong, W.; He, W.; Chen, X.; et al. High CD204+ Tumor-Infiltrating Macrophage Density Predicts a Poor Prognosis in Patients with Urothelial Cell Carcinoma of the Bladder. Oncotarget 2015, 6, 20204–20214. [Google Scholar] [CrossRef]
- Hasan, N.; Nadaf, A.; Imran, M.; Jiba, U.; Sheikh, A.; Almalki, W.H.; Almujri, S.S.; Mohammed, Y.H.; Kesharwani, P.; Ahmad, F.J. Skin Cancer: Understanding the Journey of Transformation from Conventional to Advanced Treatment Approaches. Mol. Cancer 2023, 22, 168. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Incidence, Risk Factors, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Coenye, T.; He, L.; Kabashima, K.; Kobayashi, T.; Niemann, C.; Nomura, T.; Oláh, A.; Picardo, M.; Quist, S.R.; et al. Sebaceous Immunobiology—Skin Homeostasis, Pathophysiology, Coordination of Innate Immunity and Inflammatory Response and Disease Associations. Front. Immunol. 2022, 13, 1029818. [Google Scholar] [CrossRef]
- Azoury, S.C.; Lange, J.R. Epidemiology, Risk Factors, Prevention, and Early Detection of Melanoma. Surg. Clin. N. Am. 2014, 94, 945–962, vii. [Google Scholar] [CrossRef]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Avagliano, A.; Arcucci, A. Insights into Melanoma Fibroblast Populations and Therapeutic Strategy Perspectives: Friends or Foes? Curr. Med. Chem. 2022, 29, 6159–6168. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zeng, W.; Zhang, J.; Cai, L.; Wu, Z.; Su, J.; Xiao, Y.; Liu, N.; Tang, L.; et al. TRAF6 Activates Fibroblasts to Cancer-Associated Fibroblasts through FGF19 in Tumor Microenvironment to Benefit the Malignant Phenotype of Melanoma Cells. J. Investig. Dermatol. 2020, 140, 2268–2279.e11. [Google Scholar] [CrossRef]
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous Blocking of IL-6 and IL-8 Is Sufficient to Fully Inhibit CAF-Induced Human Melanoma Cell Invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef]
- Lacina, L.; Plzak, J.; Kodet, O.; Szabo, P.; Chovanec, M.; Dvorankova, B.; Smetana, K., Jr. Cancer Microenvironment: What Can We Learn from the Stem Cell Niche. Int. J. Mol. Sci. 2015, 16, 24094–24110. [Google Scholar] [CrossRef]
- Romano, V.; Belviso, I.; Venuta, A.; Ruocco, M.R.; Masone, S.; Aliotta, F.; Fiume, G.; Montagnani, S.; Avagliano, A.; Arcucci, A. Influence of Tumor Microenvironment and Fibroblast Population Plasticity on Melanoma Growth, Therapy Resistance and Immunoescape. Int. J. Mol. Sci. 2021, 22, 5283. [Google Scholar] [CrossRef]
- Strnadová, K.; Pfeiferová, L.; Přikryl, P.; Dvořánková, B.; Vlčák, E.; Frýdlová, J.; Vokurka, M.; Novotný, J.; Šáchová, J.; Hradilová, M.; et al. Exosomes Produced by Melanoma Cells Significantly Influence the Biological Properties of Normal and Cancer-Associated Fibroblasts. Histochem. Cell Biol. 2022, 157, 153–172. [Google Scholar] [CrossRef]
- Arumi-Planas, M.; Rodriguez-Baena, F.J.; Cabello-Torres, F.; Gracia, F.; Lopez-Blau, C.; Nieto, M.A.; Sanchez-Laorden, B. Microenvironmental Snail1-Induced Immunosuppression Promotes Melanoma Growth. Oncogene 2023, 42, 2659–2672. [Google Scholar] [CrossRef]
- Çakır, U.; Hajdara, A.; Széky, B.; Mayer, B.; Kárpáti, S.; Mezey, É.; Silló, P.; Szakács, G.; Füredi, A.; Pós, Z.; et al. Mesenchymal-Stromal Cell-like Melanoma-Associated Fibroblasts Increase IL-10 Production by Macrophages in a Cyclooxygenase/Indoleamine 2,3-Dioxygenase-Dependent Manner. Cancers 2021, 13, 6173. [Google Scholar] [CrossRef]
- Hu, G.; Cheng, P.; Pan, J.; Wang, S.; Ding, Q.; Jiang, Z.; Cheng, L.; Shao, X.; Huang, L.; Huang, J. An IL6-Adenosine Positive Feedback Loop between CD73+ γδTregs and CAFs Promotes Tumor Progression in Human Breast Cancer. Cancer Immunol. Res. 2020, 8, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Peng, X.; Yang, L.; Yang, S.; Li, X.; Tang, S.; Li, B.; Jin, H.; Wu, B.; Liu, J.; et al. Non-Coding RNA Derived from Extracellular Vesicles in Cancer Immune Escape: Biological Functions and Potential Clinical Applications. Cancer Lett. 2021, 501, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, J.; Zhang, J.; Li, S.; Wang, H.; Du, J. Cancer-Associated Fibroblasts Promote PD-L1 Expression in Mice Cancer Cells via Secreting CXCL5. Int. J. Cancer 2019, 145, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-Driven and Amyloid β-Dependent Induction of Neutrophil Extracellular Traps Modulates Tumor Growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- Ziani, L.; Buart, S.; Chouaib, S.; Thiery, J. Hypoxia Increases Melanoma-Associated Fibroblasts Immunosuppressive Potential and Inhibitory Effect on T Cell-Mediated Cytotoxicity. OncoImmunology 2021, 10, 1950953. [Google Scholar] [CrossRef]
- Yamashita, T.; Negishi, K.; Hariya, T.; Kunizawa, N.; Ikuta, K.; Yanai, M.; Wakamatsu, S. Intense Pulsed Light Therapy for Superficial Pigmented Lesions Evaluated by Reflectance-Mode Confocal Microscopy and Optical Coherence Tomography. J. Investig. Dermatol. 2006, 126, 2281–2286. [Google Scholar] [CrossRef]
- Rogava, M.; Braun, A.D.; van der Sluis, T.C.; Shridhar, N.; Tüting, T.; Gaffal, E. Tumor Cell Intrinsic Toll-like Receptor 4 Signaling Promotes Melanoma Progression and Metastatic Dissemination. Int. J. Cancer 2022, 150, 142–151. [Google Scholar] [CrossRef]
- Neagu, M.; Dobre, E.-G. New Insights into the Link Between Melanoma and Obesity. Adv. Exp. Med. Biol. 2024, 1460, 851–867. [Google Scholar] [CrossRef]
- Olszańska, J.; Pietraszek-Gremplewicz, K.; Domagalski, M.; Nowak, D. Mutual Impact of Adipocytes and Colorectal Cancer Cells Growing in Co-Culture Conditions. Cell Commun. Signal 2023, 21, 130. [Google Scholar] [CrossRef]
- Wu, Q.; Li, B.; Li, J.; Sun, S.; Yuan, J.; Sun, S. Cancer-Associated Adipocytes as Immunomodulators in Cancer. Biomark. Res. 2021, 9, 2. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.M.; Wu, B.P.; Zhao, S.; Wu, H.; Klein-Szanto, A.J.P.; Tahan, S.R. Increased Expression of Stem Cell Markers in Malignant Melanoma. Mod. Pathol. 2007, 20, 102–107. [Google Scholar] [CrossRef]
- Marzagalli, M.; Raimondi, M.; Fontana, F.; Montagnani Marelli, M.; Moretti, R.M.; Limonta, P. Cellular and Molecular Biology of Cancer Stem Cells in Melanoma: Possible Therapeutic Implications. Semin. Cancer Biol. 2019, 59, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Anichini, A.; Mortarini, R.; Parmiani, G. Interaction of Cytotoxic T Lymphocytes with Autologous Melanoma: Role of Adhesion Molecules and Β1 Integrins. In Cell Adhesion Molecules: Cellular Recognition Mechanisms; Hemler, M.E., Mihich, E., Eds.; Springer US: Boston, MA, USA, 1993; pp. 209–219. ISBN 978-1-4615-2830-2. [Google Scholar]
- Tarazona, R.; Durán, E.; Solana, R. Natural Killer Cell Recognition of Melanoma: New Clues for a More Effective Immunotherapy. Front. Immunol. 2016, 6, 649. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; De Marco, C.; Cristiani, C.M. NK Cells in the Tumor Microenvironment as New Potential Players Mediating Chemotherapy Effects in Metastatic Melanoma. Front. Oncol. 2021, 11, 754541. [Google Scholar] [CrossRef]
- Vujanovic, L.; Chuckran, C.; Lin, Y.; Ding, F.; Sander, C.A.; Santos, P.M.; Lohr, J.; Mashadi-Hossein, A.; Warren, S.; White, A.; et al. CD56dim CD16− Natural Killer Cell Profiling in Melanoma Patients Receiving a Cancer Vaccine and Interferon-α. Front. Immunol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK Cells and ILCs in Tumor Immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Bosisio, F.M.; Wilmott, J.S.; Volders, N.; Mercier, M.; Wouters, J.; Stas, M.; Blokx, W.A.; Massi, D.; Thompson, J.F.; Scolyer, R.A.; et al. Plasma Cells in Primary Melanoma. Prognostic Significance and Possible Role of IgA. Mod. Pathol. 2016, 29, 347–358. [Google Scholar] [CrossRef]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L. Immunotype and Immunohistologic Characteristics of Tumor-Infiltrating Immune Cells Are Associated with Clinical Outcome in Metastatic Melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Harris, R.J.; Crescioli, S.; Hussein, K.; Kakkassery, H.; Thapa, D.; Cheung, A.; Chauhan, J.; Bax, H.J.; Chenoweth, A.; et al. B Cells in Patients With Melanoma: Implications for Treatment with Checkpoint Inhibitor Antibodies. Front. Immunol. 2021, 11, 622442. [Google Scholar] [CrossRef]
- Hemmi, H.; Akira, S. TLR Signalling and the Function of Dendritic Cells. Chem. Immunol. Allergy 2005, 86, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef] [PubMed]
- Tel, J.; Aarntzen, E.H.J.G.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.G.; van Rossum, M.; et al. Natural Human Plasmacytoid Dendritic Cells Induce Antigen-Specific T-Cell Responses in Melanoma Patients. Cancer Res. 2013, 73, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; West, M.A.; Zaru, R. TLR Signalling Regulated Antigen Presentation in Dendritic Cells. Curr. Opin. Immunol. 2010, 22, 124–130. [Google Scholar] [CrossRef]
- Jiang, H.; Gebhardt, C.; Umansky, L.; Beckhove, P.; Schulze, T.J.; Utikal, J.; Umansky, V. Elevated Chronic Inflammatory Factors and Myeloid-Derived Suppressor Cells Indicate Poor Prognosis in Advanced Melanoma Patients. Int. J. Cancer 2015, 136, 2352–2360. [Google Scholar] [CrossRef]
- Marguier, A.; Laheurte, C.; Lecoester, B.; Malfroy, M.; Boullerot, L.; Renaudin, A.; Seffar, E.; Kumar, A.; Nardin, C.; Aubin, F.; et al. TIE-2 Signaling Activation by Angiopoietin 2 On Myeloid-Derived Suppressor Cells Promotes Melanoma-Specific T-Cell Inhibition. Front. Immunol. 2022, 13, 932298. [Google Scholar] [CrossRef]
- Schilling, B.; Sucker, A.; Griewank, K.; Zhao, F.; Weide, B.; Görgens, A.; Giebel, B.; Schadendorf, D.; Paschen, A. Vemurafenib Reverses Immunosuppression by Myeloid Derived Suppressor Cells. Int. J. Cancer 2013, 133, 1653–1663. [Google Scholar] [CrossRef]
- Kou, Y.; Ji, L.; Wang, H.; Wang, W.; Zheng, H.; Zou, J.; Liu, L.; Qi, X.; Liu, Z.; Du, B.; et al. Connexin 43 Upregulation by Dioscin Inhibits Melanoma Progression via Suppressing Malignancy and Inducing M1 Polarization. Int. J. Cancer 2017, 141, 1690–1703. [Google Scholar] [CrossRef]
- Muniz-Bongers, L.R.; McClain, C.B.; Saxena, M.; Bongers, G.; Merad, M.; Bhardwaj, N. MMP2 and TLRs Modulate Immune Responses in the Tumor Microenvironment. JCI Insight 2021, 6, e144913. [Google Scholar] [CrossRef]
- Su, X.; Esser, A.K.; Amend, S.R.; Xiang, J.; Xu, Y.; Ross, M.H.; Fox, G.C.; Kobayashi, T.; Steri, V.; Roomp, K.; et al. Antagonizing Integrin Β3 Increases Immunosuppression in Cancer. Cancer Res. 2016, 76, 3484–3495. [Google Scholar] [CrossRef]
- Egas-Bejar, D.; Anderson, P.M.; Agarwal, R.; Corrales-Medina, F.; Devarajan, E.; Huh, W.W.; Brown, R.E.; Subbiah, V. Theranostic Profiling for Actionable Aberrations in Advanced High Risk Osteosarcoma with Aggressive Biology Reveals High Molecular Diversity: The Human Fingerprint Hypothesis. Oncoscience 2014, 1, 167–179. [Google Scholar] [CrossRef]
- Friebele, J.C.; Peck, J.; Pan, X.; Abdel-Rasoul, M.; Mayerson, J.L. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am. J. Orthop. 2015, 44, 547–553. [Google Scholar]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21 (Suppl. S7), vii320–vii325. [Google Scholar] [CrossRef]
- Colombo, M.; Platonova, N.; Giannandrea, D.; Palano, M.T.; Basile, A.; Chiaramonte, R. Re-Establishing Apoptosis Competence in Bone Associated Cancers via Communicative Reprogramming Induced Through Notch Signaling Inhibition. Front. Pharmacol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Ji, P.; Yu, L.; Guo, W.-C.; Mei, H.-J.; Wang, X.-J.; Chen, H.; Fang, S.; Yang, J. Doxorubicin Inhibits Proliferation of Osteosarcoma Cells Through Upregulation of the Notch Signaling Pathway. Oncol. Res. 2014, 22, 185–191. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The Role of Notch Ligand Jagged1 in Osteosarcoma Proliferation, Metastasis, and Recurrence. J. Orthop. Surg. Res. 2021, 16, 226. [Google Scholar] [CrossRef]
- Demkow, U. Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis. Cancers 2021, 13, 4495. [Google Scholar] [CrossRef]
- Lu, J.; Rui, J.; Xu, X.-Y.; Shen, J.-K. Exploring the Role of Neutrophil-Related Genes in Osteosarcoma via an Integrative Analysis of Single-Cell and Bulk Transcriptome. Biomedicines 2024, 12, 1513. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Deng, C.; Xu, Y.; Fu, J.; Zhu, X.; Chen, H.; Xu, H.; Wang, G.; Song, Y.; Song, G.; Lu, J.; et al. Reprograming the Tumor Immunologic Microenvironment Using Neoadjuvant Chemotherapy in Osteosarcoma. Cancer Sci. 2020, 111, 1899–1909. [Google Scholar] [CrossRef]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of Macrophage Polarization Is Associated with the Metastatic Process in Osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-Positive Tumor-Associated Macrophages and CD8-Positive Cytotoxic Lymphocytes Are Powerful Diagnostic Markers for the Therapeutic Stratification of Osteosarcoma Patients: An Immunohistochemical Analysis of the Biopsies Fromthe French OS2006 Phase 3 Trial. Oncoimmunology 2017, 6, e1331193. [Google Scholar] [CrossRef]
- Han, Q.; Shi, H.; Liu, F. CD163(+) M2-Type Tumor-Associated Macrophage Support the Suppression of Tumor-Infiltrating T Cells in Osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-Associated Macrophages Promote Lung Metastasis and Induce Epithelial-Mesenchymal Transition in Osteosarcoma by Activating the COX-2/STAT3 Axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef]
- Markel, J.E.; Noore, J.; Emery, E.J.; Bobnar, H.J.; Kleinerman, E.S.; Lindsey, B.A. Using the Spleen as an In Vivo Systemic Immune Barometer Alongside Osteosarcoma Disease Progression and Immunotherapy with α-PD-L1. Sarcoma 2018, 2018, 8694397. [Google Scholar] [CrossRef]
- Maloney, C.; Kallis, M.P.; Edelman, M.; Tzanavaris, C.; Lesser, M.; Soffer, S.Z.; Symons, M.; Steinberg, B.M. Gefitinib Inhibits Invasion and Metastasis of Osteosarcoma via Inhibition of Macrophage Receptor Interacting Serine-Threonine Kinase 2. Mol. Cancer Ther. 2020, 19, 1340–1350. [Google Scholar] [CrossRef]
- Shao, X.-J.; Xiang, S.-F.; Chen, Y.-Q.; Zhang, N.; Cao, J.; Zhu, H.; Yang, B.; Zhou, Q.; Ying, M.-D.; He, Q.-J. Inhibition of M2-like Macrophages by All-Trans Retinoic Acid Prevents Cancer Initiation and Stemness in Osteosarcoma Cells. Acta Pharmacol. Sin. 2019, 40, 1343–1350. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Xu, P. NK Cell and Macrophages Confer Prognosis and Reflect Immune Status in Osteosarcoma. J. Cell. Biochem. 2019, 120, 8792–8797. [Google Scholar] [CrossRef]
- Filipazzi, P.; Valenti, R.; Huber, V.; Pilla, L.; Canese, P.; Iero, M.; Castelli, C.; Mariani, L.; Parmiani, G.; Rivoltini, L. Identification of a New Subset of Myeloid Suppressor Cells in Peripheral Blood of Melanoma Patients with Modulation by a Granulocyte-Macrophage Colony-Stimulation Factor-Based Antitumor Vaccine. J. Clin. Oncol. 2007, 25, 2546–2553. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, X.; Lynn, K.D.; Thorpe, P.E. Phosphatidylserine-Targeting Antibody Induces M1 Macrophage Polarization and Promotes Myeloid-Derived Suppressor Cell Differentiation. Cancer Immunol. Res. 2013, 1, 256–268. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 Can Function as a Negative Regulator of T Cell Activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Toor, S.M.; Sasidharan Nair, V.; Decock, J.; Elkord, E. Immune Checkpoints in the Tumor Microenvironment. Semin. Cancer Biol. 2020, 65, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Quail, D.; Joyce, J. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Follicular Lymphoma: The Phase 2 ELARA Trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Xiao, B.-F.; Zhang, J.-T.; Cui, X.-R.; Lu, Z.-M.; Wu, N. Genetically Modified T Cells for Esophageal Cancer Therapy: A Promising Clinical Application. Front. Oncol. 2021, 11, 763806. [Google Scholar] [CrossRef]
- Jin, X.; Xie, D.; Sun, R.; Lu, W.; Xiao, X.; Yu, Y.; Meng, J.; Zhao, M. CAR-T Cells Dual-Target CD123 and NKG2DLs to Eradicate AML Cells and Selectively Target Immunosuppressive Cells. Oncoimmunology 2023, 12, 2248826. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, Q.; Song, Y.; Liu, D. Universal CARs, Universal T Cells, and Universal CAR T Cells. J. Hematol. Oncol. 2018, 11, 132. [Google Scholar] [CrossRef]
- Shan, F.; Somasundaram, A.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Therapeutic Targeting of Regulatory T Cells in Cancer. Trends Cancer 2022, 8, 944–961. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Kim, K.W.; Heo, M.-G.; Park, D.-I.; Lee, J.-H.; Lim, N.J.; Min, D.-H.; Won, C. Nanoparticle Delivery of Recombinant IL-2 (BALLkine-2) Achieves Durable Tumor Control with Less Systemic Adverse Effects in Cancer Immunotherapy. Biomaterials 2022, 280, 121257. [Google Scholar] [CrossRef]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.-M.; Ries, C.H.; Rüttinger, D. Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors in Cancer Therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient Presentation of Soluble Antigen by Cultured Human Dendritic Cells Is Maintained by Granulocyte/Macrophage Colony-Stimulating Factor plus Interleukin 4 and Downregulated by Tumor Necrosis Factor Alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.A.; Brault, M.; Bleakley, M. TCR-Based Immunotherapy for Hematologic Malignancies. Cancer J. 2019, 25, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic Viruses for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Muthukutty, P.; Yoo, S.Y. Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective. Viruses 2023, 15, 1645. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, H.; Lin, L.; Li, S.; Yang, Y.; Han, Y.; Huang, W.; Yang, J. Application of Oncolytic Virus in Tumor Therapy. J. Med. Virol. 2023, 95, e28729. [Google Scholar] [CrossRef]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Bagaev, A.; Kotlov, N.; Nomie, K.; Svekolkin, V.; Gafurov, A.; Isaeva, O.; Osokin, N.; Kozlov, I.; Frenkel, F.; Gancharova, O.; et al. Conserved Pan-Cancer Microenvironment Subtypes Predict Response to Immunotherapy. Cancer Cell 2021, 39, 845–865.e7. [Google Scholar] [CrossRef]
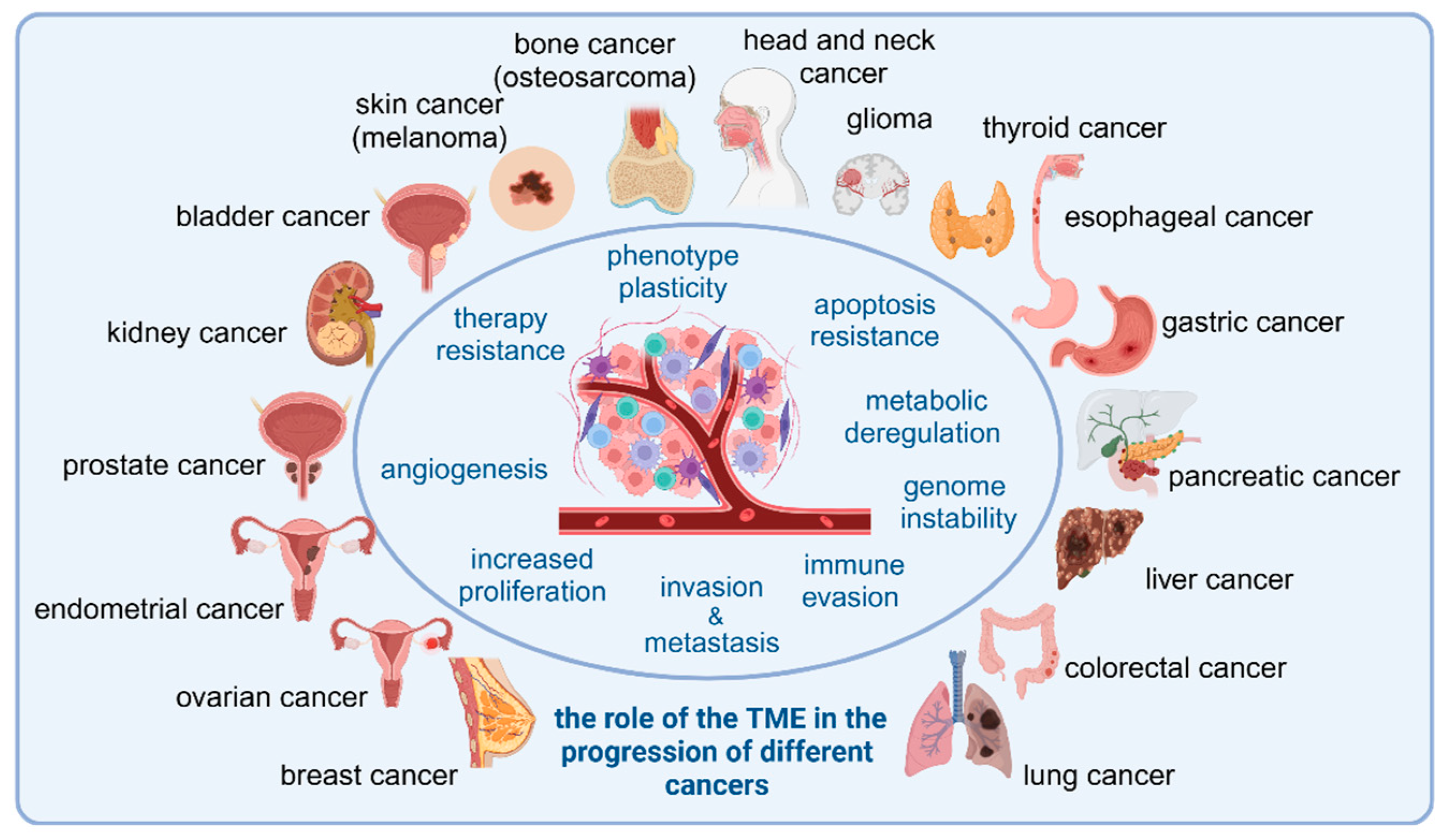
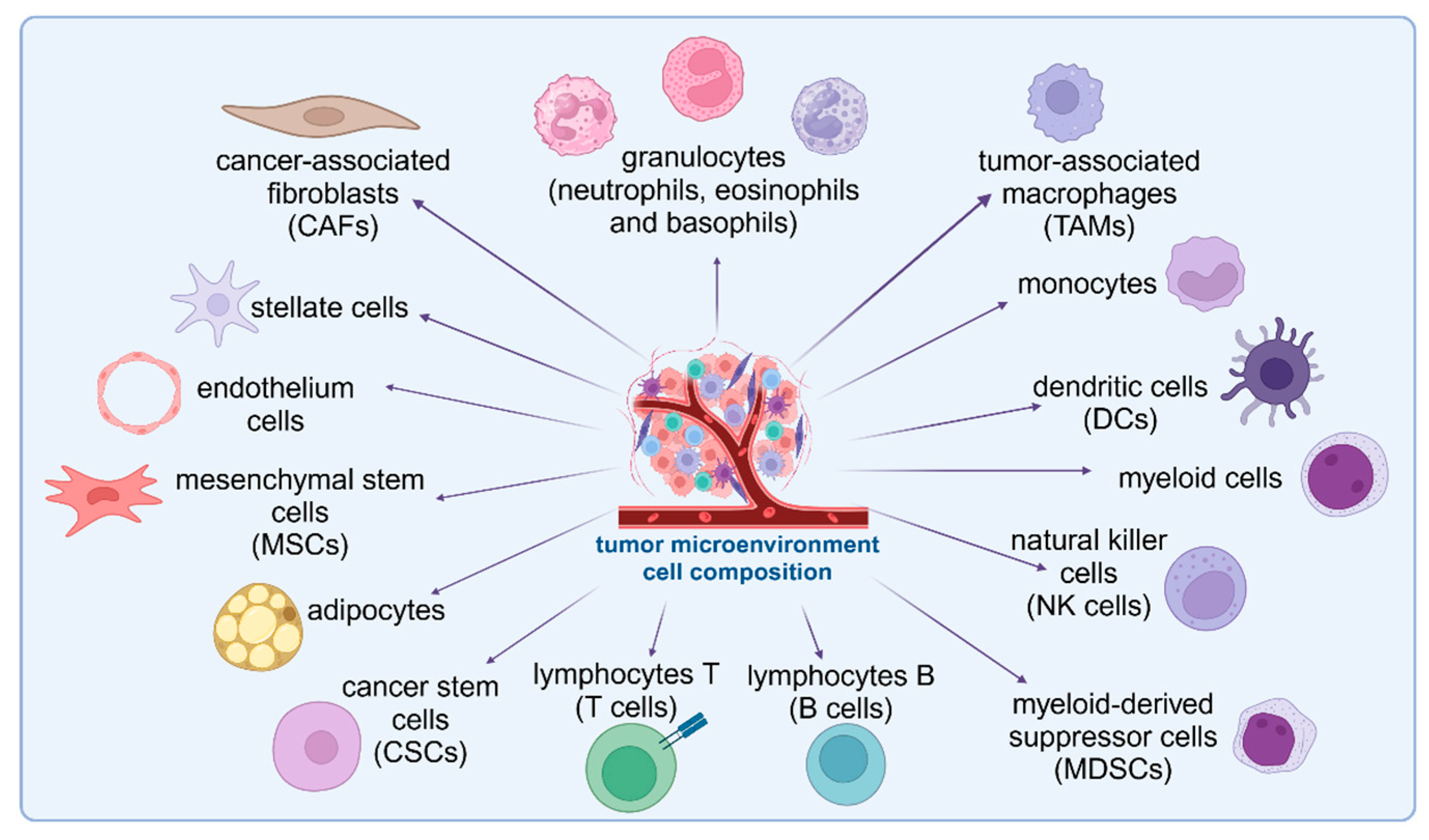
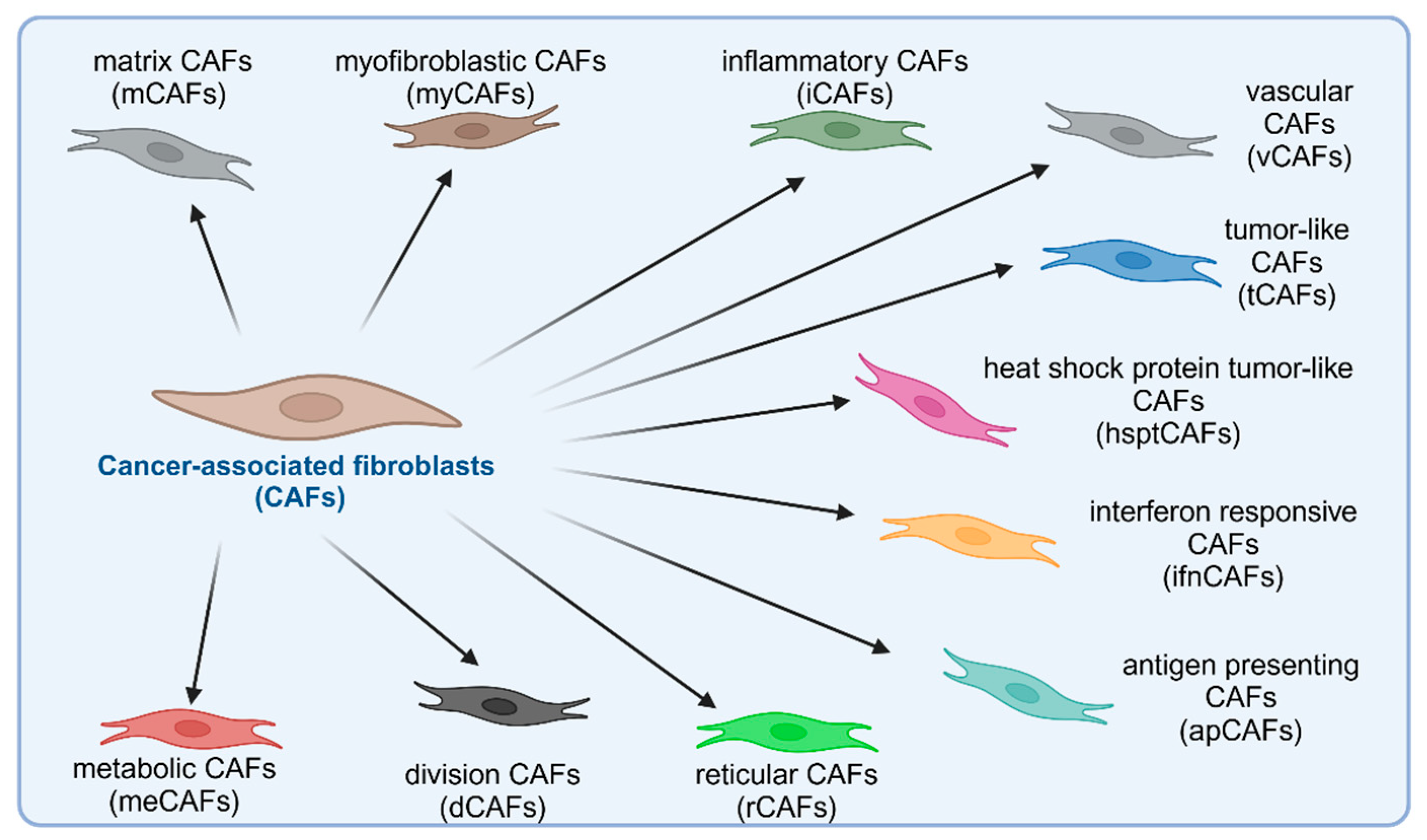
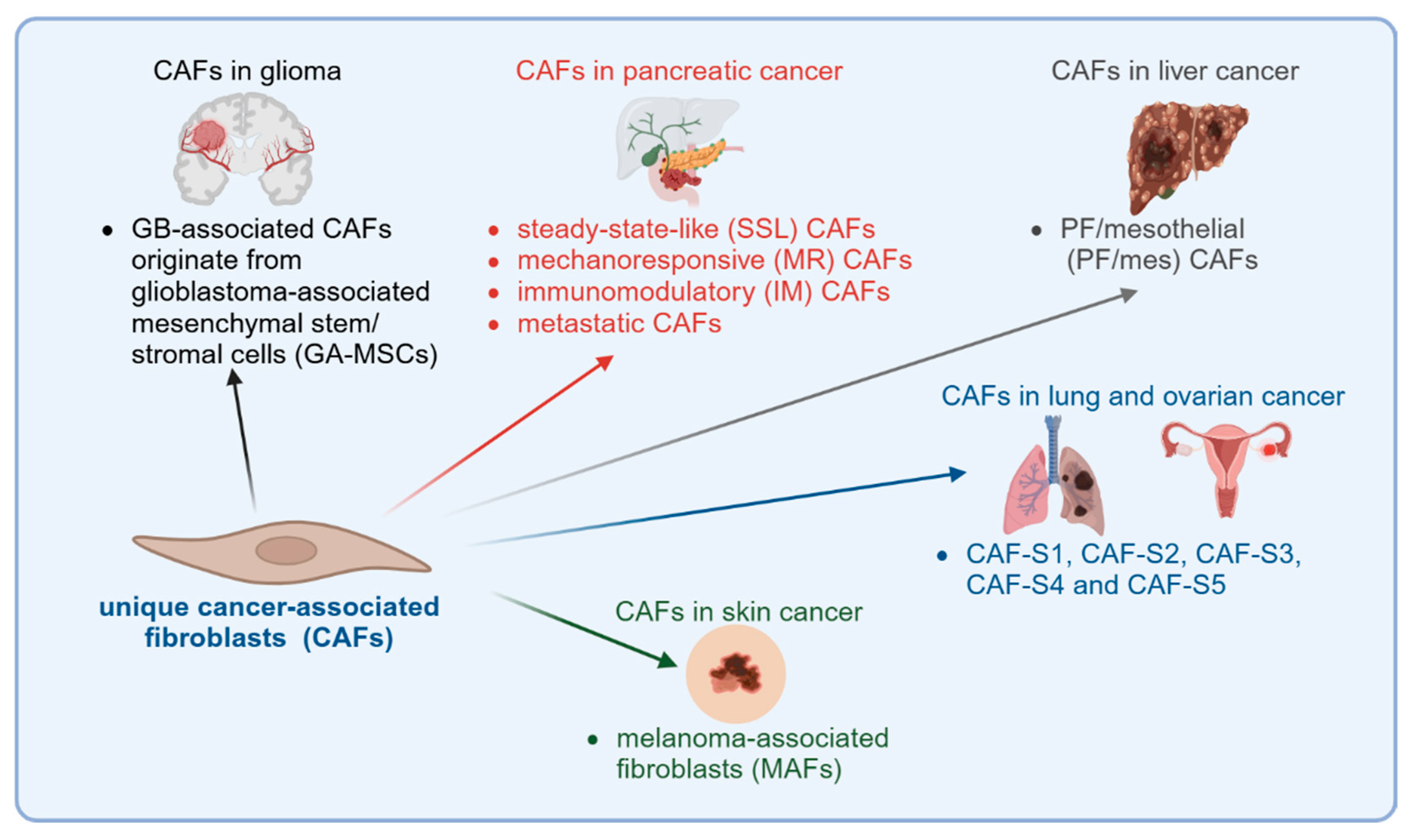
| CAF Subtype | Specific Markers | Functions |
|---|---|---|
| matrix CAFs | MMP11, COL10A1, COL11A1, COL8A1, COL1A2, COL12A1, COL3A1, COMP, POSTN, LRRC15, LRRC17, ASPN, SULF1, INHBA, VCAN, TGF-β, KRAS | ECM remodeling, collagen deposition, tissue stiffness, tumor invasion |
| (mCAFs) | ||
| myofibroblastic CAFs (myCAFs) | TAGLN, MYL9, TPM1, TPM2, MMP11, POSTN, αSMA, COL1A1 | ECM contraction, mechanical force transmission, tumor progression |
| inflammatory CAFs (iCAFs) | IL-6, IL-8, CXCL1, CXCL2, CCL2, CXCL12, CFD, LMNA, DPT, HAS1, HAS2, AGTR1, PLA2G2A | Inflammatory cytokines secretion, immune cell recruitment, tumor-promoting inflammation |
| vascular CAFs (vCAFs) | NOTCH3, COL18A1 | Angiogenesis |
| tumor-like CAFs (tCAFs) | PDPN, MME, TMEM158, NDRG1, ENO1, GAPDH, VEGFA | Tumor growth, metabolic support, immune evasion |
| heat shock protein tumor-like CAFs (hsptCAFs) | HSPH1, HSP90AA1, PDPN, MME, TMEM158, NDRG1, ENO1, GAPDH, VEGFA, TGF-β, KRAS, MTORC1 | Stress response, therapy resistance, tumor survival |
| interferon-responsive CAFs (ifnCAFs) | IL-32, CXCL9, CXCL10, CXCL11, IDO1, TAT5, TNF-α, IL-6, KRAS | Immune signaling, T cell response, immune suppression |
| antigen-presenting CAFs (apCAFs) | HLA-DRA, HLA-DRB1, CD74 | Interactions with immune cells, potential role in antigen presentation, immune modulation |
| reticular CAFs (rCAFs) | CCL21 and CCL19 | Lymphoid-like structure formation, immune response, T cell recruitment |
| division CAFs (dCAFs) | TUBA1B, MKI67 | Cell division, tumorigenesis, tumor growth and survival |
| metabolic CAFs (meCAFs) | PLA2G2A | Energy supply to tumor cells, adaptation to hypoxia |
| Cell Type | IE (%) * | IE/F (%) * | F (%) ** | D (%) ** |
|---|---|---|---|---|
| CD8+ T Cells | 20–30 | 10–20 | <5 | <5 |
| CD4+ T Cells | 10–15 | 5–10 | <5 | <5 |
| Tregs | 5–10 | 5–10 | <5 | <5 |
| Macrophages (M1) | 10–15 | 5–10 | <5 | <5 |
| Macrophages (M2) | <5 | 10–15 | 10–20 | <5 |
| Fibroblasts | <5 | 15–25 | 40–50 | <5 |
| Endothelial Cells | <5 | 5–10 | 20–30 | <5 |
| Cancer Cells | 30–40 | 30–40 | 30–40 | >60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turlej, E.; Domaradzka, A.; Radzka, J.; Drulis-Fajdasz, D.; Kulbacka, J.; Gizak, A. Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression. Cells 2025, 14, 403. https://doi.org/10.3390/cells14060403
Turlej E, Domaradzka A, Radzka J, Drulis-Fajdasz D, Kulbacka J, Gizak A. Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression. Cells. 2025; 14(6):403. https://doi.org/10.3390/cells14060403
Chicago/Turabian StyleTurlej, Eliza, Aleksandra Domaradzka, Justyna Radzka, Dominika Drulis-Fajdasz, Julita Kulbacka, and Agnieszka Gizak. 2025. "Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression" Cells 14, no. 6: 403. https://doi.org/10.3390/cells14060403
APA StyleTurlej, E., Domaradzka, A., Radzka, J., Drulis-Fajdasz, D., Kulbacka, J., & Gizak, A. (2025). Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression. Cells, 14(6), 403. https://doi.org/10.3390/cells14060403











