Rac1 Temporarily Suppresses Fertilization Envelope Formation Immediately After 1-Methyladenine Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Oocyte Preparation
2.2. Observation of the Maturing Process and Inhibitor Treatment
2.3. Observation of Fertilization Envelope Formation
2.4. Sample Preparation and SDS-PAGE
2.5. Western Blotting
2.6. cDNA Cloning of Rac1
2.7. DNA Constructs
2.8. Microinjection
2.9. Rac1 Pull Down
2.10. FRET Measurement Using Confocal Microscopy
3. Results and Discussion
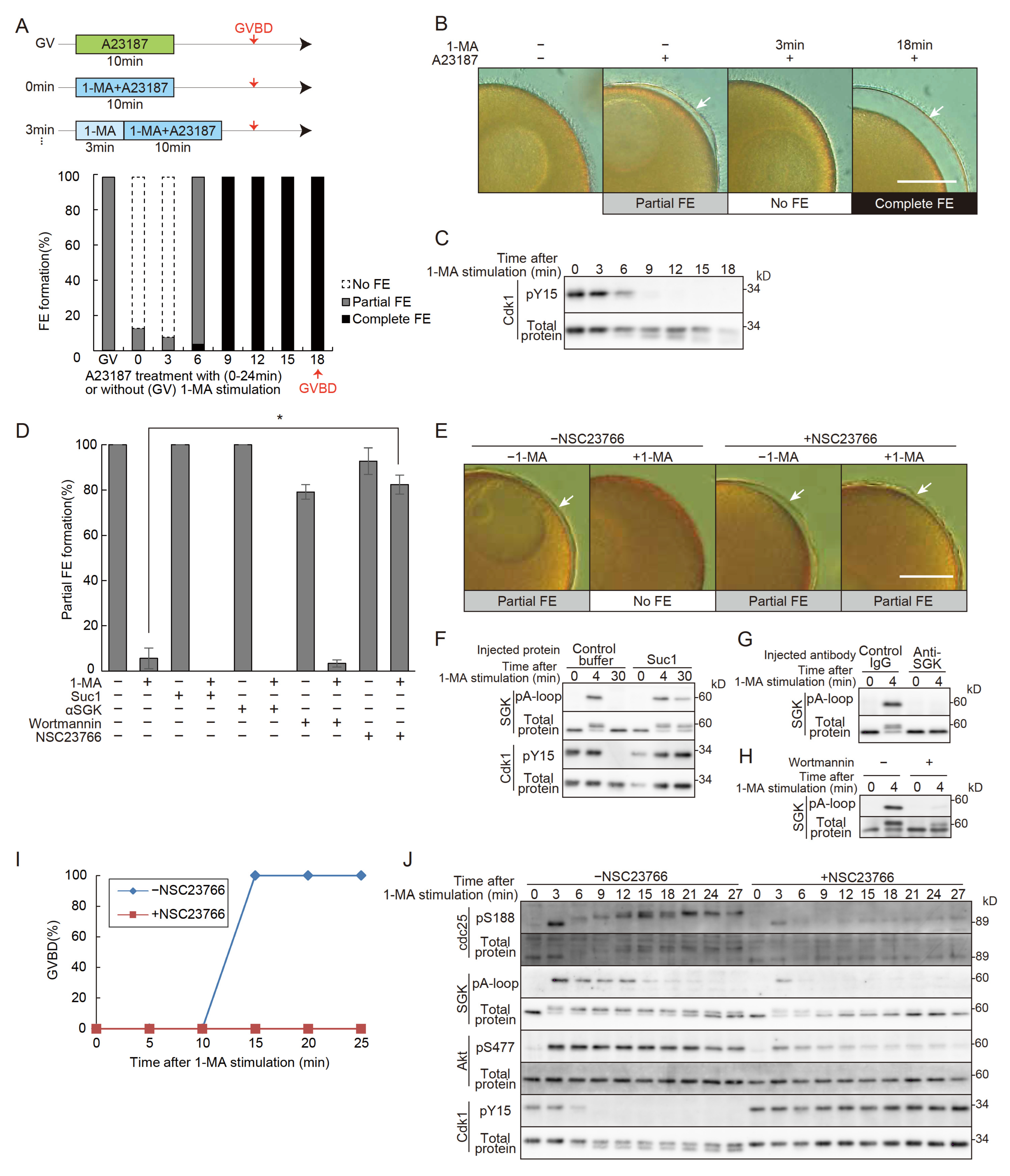
3.1. Changes in FE Formation upon 1-MA Stimulation and the Involvement of Rac1 in the No FE Phase
3.2. Presence and Activity of Rac1 Protein in Starfish Oocytes
3.3. Constitutively Active Rac1 Induces the No FE Phase
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanatani, H.; Shirai, H.; Nakanishi, K.; Kurokawa, T. Isolation and Indentification on Meiosis Inducing Substance in Starfish Asterias Amurensis. Nature 1969, 221, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Hirai, S. Differences in Starfish Oocyte Susceptibility to Polyspermy during the Course of Maturation. Biol. Bull. 1979, 157, 249–257. [Google Scholar] [CrossRef]
- Chiba, K.; Hoshi, M. Three Phases of Cortical Maturation during Meiosis Reinitiation in Starfish Oocytes. Dev. Growth Differ. 1989, 31, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kado, R.T.; Jaffe, L.A. Development of Calcium Release Mechanisms during Starfish Oocyte Maturation. Dev. Biol. 1990, 140, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Chiba, K.; Uchiyama, T.; Yoshikawa, F.; Suzuki, F.; Ikeda, M.; Furuichi, T.; Mikoshiba, K. Molecular Characterization of the Starfish Inositol 1,4,5-Trisphosphate Receptor and Its Role during Oocyte Maturation and Fertilization. J. Biol. Chem. 2002, 277, 2763–2772. [Google Scholar] [CrossRef]
- Chiba, K.; Tadenuma, H.; Matsumoto, M.; Takahashi, K.; Katada, T.; Hoshi, M. The Primary Structure of the Alpha Subunit of a Starfish Guanosine-Nucleotide-Binding Regulatory Protein Involved in 1-Methyladenine-Induced Oocyte Maturation. Eur. J. Biochem. 1992, 207, 833–838. [Google Scholar] [CrossRef]
- Shilling, F.; Chiba, K.; Hoshi, M.; Kishimoto, T.; Jaffe, L.A. Pertussis Toxin Inhibits 1-Methyladenine-Induced Maturation in Starfish Oocytes. Dev. Biol. 1989, 133, 605–608. [Google Scholar] [CrossRef]
- Chiba, K.; Kontani, K.; Tadenuma, H.; Katada, T.; Hoshi, M. Induction of Starfish Oocyte Maturation by the Beta Gamma Subunit of Starfish G Protein and Possible Existence of the Subsequent Effector in Cytoplasm. Mol. Biol. Cell 1993, 4, 1027–1034. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Gallo, C.J.; Lee, R.H.; Ho, Y.K.; Jones, T.L. Oocyte Maturation in Starfish Is Mediated by the Beta Gamma-Subunit Complex of a G-Protein. J. Cell Biol. 1993, 121, 775–783. [Google Scholar] [CrossRef]
- Sadler, K.C.; Ruderman, J.V. Components of the Signaling Pathway Linking the 1-Methyladenine Receptor to MPF Activation and Maturation in Starfish Oocytes. Dev. Biol. 1998, 197, 25–38. [Google Scholar] [CrossRef]
- Hosoda, E.; Hiraoka, D.; Hirohashi, N.; Omi, S.; Kishimoto, T.; Chiba, K. SGK Regulates pH Increase and Cyclin B–Cdk1 Activation to Resume Meiosis in Starfish Ovarian Oocytes. J. Cell Biol. 2019, 218, 3612–3629. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, D.; Hosoda, E.; Chiba, K.; Kishimoto, T. SGK Phosphorylates Cdc25 and Myt1 to Trigger Cyclin B-Cdk1 Activation at the Meiotic G2/M Transition. J. Cell Biol. 2019, 218, 3597–3611. [Google Scholar] [CrossRef]
- Hiraoka, D.; Aono, R.; Hanada, S.-I.; Okumura, E.; Kishimoto, T. Two New Competing Pathways Establish the Threshold for Cyclin-B-Cdk1 Activation at the Meiotic G2/M Transition. J. Cell Sci. 2016, 129, 3153–3166. [Google Scholar] [CrossRef][Green Version]
- Coso, O.A.; Teramoto, H.; Simonds, W.F.; Gutkind, J.S. Signaling from G Protein-Coupled Receptors to c-Jun Kinase Involves Βγ Subunits of Heterotrimeric G Proteins Acting on a Ras and Rac1-Dependent Pathway. J. Biol. Chem. 1996, 271, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Profirovic, J.; Pan, H.; Vaiskunaite, R.; Voyno-Yasenetskaya, T. G Protein Betagamma Subunits Stimulate p114RhoGEF, a Guanine Nucleotide Exchange Factor for RhoA and Rac1: Regulation of Cell Shape and Reactive Oxygen Species Production. Circ. Res. 2003, 93, 848–856. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Adame-García, S.R.; García-Jiménez, I.; Color-Aparicio, V.M.; Beltrán-Navarro, Y.M.; König, G.M.; Kostenis, E.; Reyes-Cruz, G.; Gutkind, J.S.; Vázquez-Prado, J. Gβγ Signaling to the Chemotactic Effector P-REX1 and Mammalian Cell Migration Is Directly Regulated by Gαq and Gα13 Proteins. J. Biol. Chem. 2019, 294, 531–546. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; De Riso, L.; Gragnaniello, G.; Kyozuka, K. Separate Activation of the Cytoplasmic and Nuclear Calcium Pools in Maturing Starfish Oocytes. Biochem. Biophys. Res. Commun. 1998, 252, 1–4. [Google Scholar] [CrossRef]
- Kyozuka, K.; Chun, J.T.; Puppo, A.; Gragnaniello, G.; Garante, E.; Santella, L. Guanine Nucleotides in the Meiotic Maturation of Starfish Oocytes: Regulation of the Actin Cytoskeleton and of Ca2+ Signaling. PLoS ONE 2009, 4, e6296. [Google Scholar] [CrossRef]
- Kyozuka, K.; Chun, J.T.; Puppo, A.; Gragnaniello, G.; Garante, E.; Santella, L. Actin Cytoskeleton Modulates Calcium Signaling during Maturation of Starfish Oocytes. Dev. Biol. 2008, 320, 426–435. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Vasilev, F.; Chun, J.T. Maturation and Fertilization of Echinoderm Eggs: Role of Actin Cytoskeleton Dynamics. Biochem. Biophys. Res. Commun. 2018, 506, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dickerson, J.B.; Guo, F.; Zheng, J.; Zheng, Y. Rational Design and Characterization of a Rac GTPase-Specific Small Molecule Inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 7618–7623. [Google Scholar] [CrossRef]
- Stephens, L.; Smrcka, A.; Cooke, F.T.; Jackson, T.R.; Sternweis, P.C.; Hawkins, P.T. A Novel Phosphoinositide 3 Kinase Activity in Myeloid-Derived Cells Is Activated by G Protein Βγ Subunits. Cell 1994, 77, 83–93. [Google Scholar] [CrossRef]
- Reed, P.W.; Lardy, H.A. A23187: A Divalent Cation Ionophore. J. Biol. Chem. 1972, 247, 6970–6977. [Google Scholar] [CrossRef]
- Steinhardt, R.A.; Epel, D. Activation of Sea-Urchin Eggs by a Calcium Ionophore. Proc. Natl. Acad. Sci. USA 1974, 71, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Okumura, E.; Sekiai, T.; Hisanaga, S.; Tachibana, K.; Kishimoto, T. Initial Triggering of M-Phase in Starfish Oocytes: A Possible Novel Component of Maturation-Promoting Factor besides Cdc2 Kinase. J. Cell Biol. 1996, 132, 125–135. [Google Scholar] [CrossRef]
- Hiraoka, D.; Okumura, E.; Kishimoto, T. Turn Motif Phosphorylation Negatively Regulates Activation Loop Phosphorylation in Akt. Oncogene 2011, 30, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Okumura, E.; Fukuhara, T.; Yoshida, H.; Hanada Si, S.; Kozutsumi, R.; Mori, M.; Tachibana, K.; Kishimoto, T. Akt Inhibits Myt1 in the Signalling Pathway That Leads to Meiotic G2/M-Phase Transition. Nat. Cell Biol. 2002, 4, 111–116. [Google Scholar] [CrossRef]
- Komatsu, N.; Aoki, K.; Yamada, M.; Yukinaga, H.; Fujita, Y.; Kamioka, Y.; Matsuda, M. Development of an Optimized Backbone of FRET Biosensors for Kinases and GTPases. Mol. Biol. Cell 2011, 22, 4647–4656. [Google Scholar] [CrossRef]
- Nakamura, T.; Aoki, K.; Matsuda, M. Monitoring Spatio-Temporal Regulation of Ras and Rho GTPases with GFP-Based FRET Probes. Methods 2005, 37, 146–153. [Google Scholar] [CrossRef]
- Kishimoto, T. MPF-Based Meiotic Cell Cycle Control: Half a Century of Lessons from Starfish Oocytes. Proc. Jpn. Acad. Ser. B 2018, 94, 180–203. [Google Scholar] [CrossRef]
- Kusubata, M.; Tokui, T.; Matsuoka, Y.; Okumura, E.; Tachibana, K.; Hisanaga, S.; Kishimoto, T.; Yasuda, H.; Kamijo, M.; Ohba, Y. P13suc1 Suppresses the Catalytic Function of P34cdc2 Kinase for Intermediate Filament Proteins, in Vitro. J. Biol. Chem. 1992, 267, 20937–20942. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.A.; Steen, H.; Shapiro, P.; Lewis, T.; Ahn, N.; Shaw, P.E.; Cobb, M.H. Cross-cascade Activation of ERKs and Ternary Complex Factors by Rho Family Proteins. EMBO J. 1997, 16, 6426–6438. [Google Scholar] [CrossRef] [PubMed]
- Itoh, R.E.; Kurokawa, K.; Ohba, Y.; Yoshizaki, H.; Mochizuki, N.; Matsuda, M. Activation of Rac and Cdc42 Video Imaged by Fluorescent Resonance Energy Transfer-Based Single-Molecule Probes in the Membrane of Living Cells. Mol. Cell. Biol. 2002, 22, 6582–6591. [Google Scholar] [CrossRef]
- Kuhn, T.B.; Meberg, P.J.; Brown, M.D.; Bernstein, B.W.; Minamide, L.S.; Jensen, J.R.; Okada, K.; Soda, E.A.; Bamburg, J.R. Regulating Actin Dynamics in Neuronal Growth Cones by ADF/Cofilin and Rho Family GTPases. J. Neurobiol. 2000, 44, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chou, H.-T.; Brautigam, C.A.; Xing, W.; Yang, S.; Henry, L.; Doolittle, L.K.; Walz, T.; Rosen, M.K. Rac1 GTPase Activates the WAVE Regulatory Complex through Two Distinct Binding Sites. eLife 2017, 6, e29795. [Google Scholar] [CrossRef]
- Schroeder, T.E. Microfilament-Mediated Surface Change in Starfish Oocytes in Response to 1-Methyladenine: Implications for Identifying the Pathway and Receptor Sites for Maturation-Inducing Hormones. J. Cell Biol. 1981, 90, 362–371. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Kyozuka, K.; Santella, L. Novel Ca2+ Increases in the Maturing Oocytes of Starfish during the Germinal Vesicle Breakdown. Cell Calcium 2015, 58, 500–510. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Xue, M.; Zhao, T.; Song, J.; Zhang, W.; Chang, Y.; Zhan, Y. Dynamic Molecular Responses of the Sea Urchin Strongylocentrotus Intermedius to Pathogen Infection: Insights from a Serial Comparative Transcriptome Analysis. Fish Shellfish Immunol. 2025, 158, 110176. [Google Scholar] [CrossRef]
- Li, K.; Liu, L.; Shang, S.; Wang, Y.; Zhan, Y.; Song, J.; Zhang, X.; Chang, Y. cDNA Cloning, Expression and Immune Function Analysis of a Novel Rac1 Gene (AjRac1) in the Sea Cucumber Apostichopus Japonicus. Fish Shellfish Immunol. 2017, 69, 218–226. [Google Scholar] [CrossRef]
- Ramírez-Ramírez, D.; Salgado-Lucio, M.L.; Roa-Espitia, A.L.; Fierro, R.; González-Márquez, H.; Cordero-Martínez, J.; Hernández-González, E.O. Rac1 Is Necessary for Capacitation and Acrosome Reaction in Guinea Pig Spermatozoa. J. Cell. Biochem. 2020, 121, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Herrmann, B.G. RAC1 Controls Progressive Movement and Competitiveness of Mammalian Spermatozoa. PLoS Genet. 2021, 17, e1009308. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aida, S.; Matsumoto, T.; Yamazaki, Y.; Limatola, N.; Santella, L.; Chiba, K. Rac1 Temporarily Suppresses Fertilization Envelope Formation Immediately After 1-Methyladenine Stimulation. Cells 2025, 14, 405. https://doi.org/10.3390/cells14060405
Aida S, Matsumoto T, Yamazaki Y, Limatola N, Santella L, Chiba K. Rac1 Temporarily Suppresses Fertilization Envelope Formation Immediately After 1-Methyladenine Stimulation. Cells. 2025; 14(6):405. https://doi.org/10.3390/cells14060405
Chicago/Turabian StyleAida, Sakurako, Takako Matsumoto, Yuna Yamazaki, Nunzia Limatola, Luigia Santella, and Kazuyoshi Chiba. 2025. "Rac1 Temporarily Suppresses Fertilization Envelope Formation Immediately After 1-Methyladenine Stimulation" Cells 14, no. 6: 405. https://doi.org/10.3390/cells14060405
APA StyleAida, S., Matsumoto, T., Yamazaki, Y., Limatola, N., Santella, L., & Chiba, K. (2025). Rac1 Temporarily Suppresses Fertilization Envelope Formation Immediately After 1-Methyladenine Stimulation. Cells, 14(6), 405. https://doi.org/10.3390/cells14060405







