Ameliorative Effect of Itaconic Acid/IRG1 Against Endoplasmic Reticulum Stress-Induced Necroptosis in Granulosa Cells via PERK-ATF4-AChE Pathway in Bovine
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Ethics Statement
2.2. Primary Cell Culture
2.3. Cell Viability Assays
2.4. Quantitative Reverse Transcription PCR (qRT-PCR)
2.5. Western Blot Assay
2.6. Flow Cytometry Assay
2.7. Transmission Electron Microscopy
2.8. Transient Cell Transfection
2.9. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.10. Ellman Assay
2.11. Statistical Analysis
3. Results
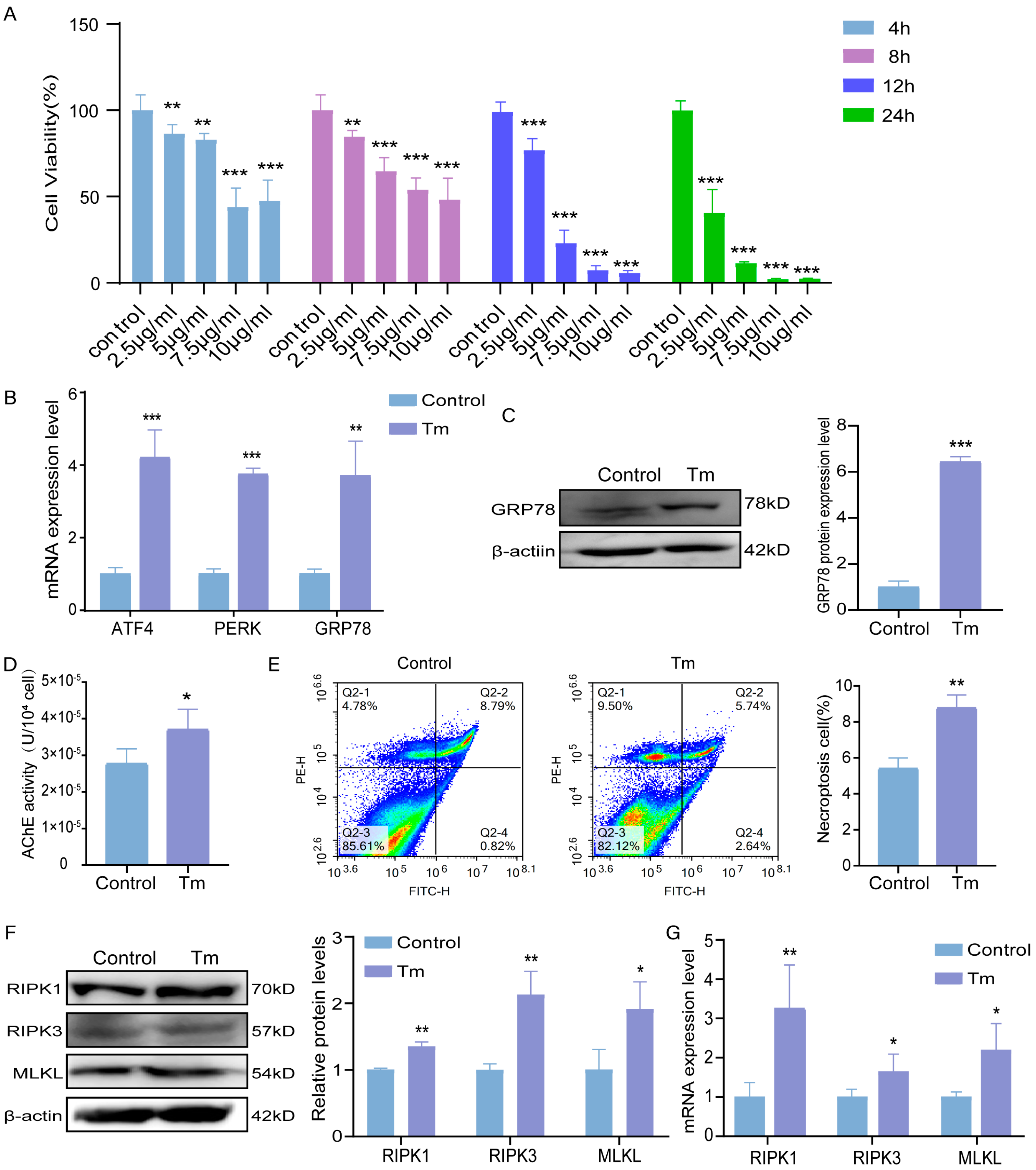
3.1. Effect of Tm on Bovine Granulosa Cells
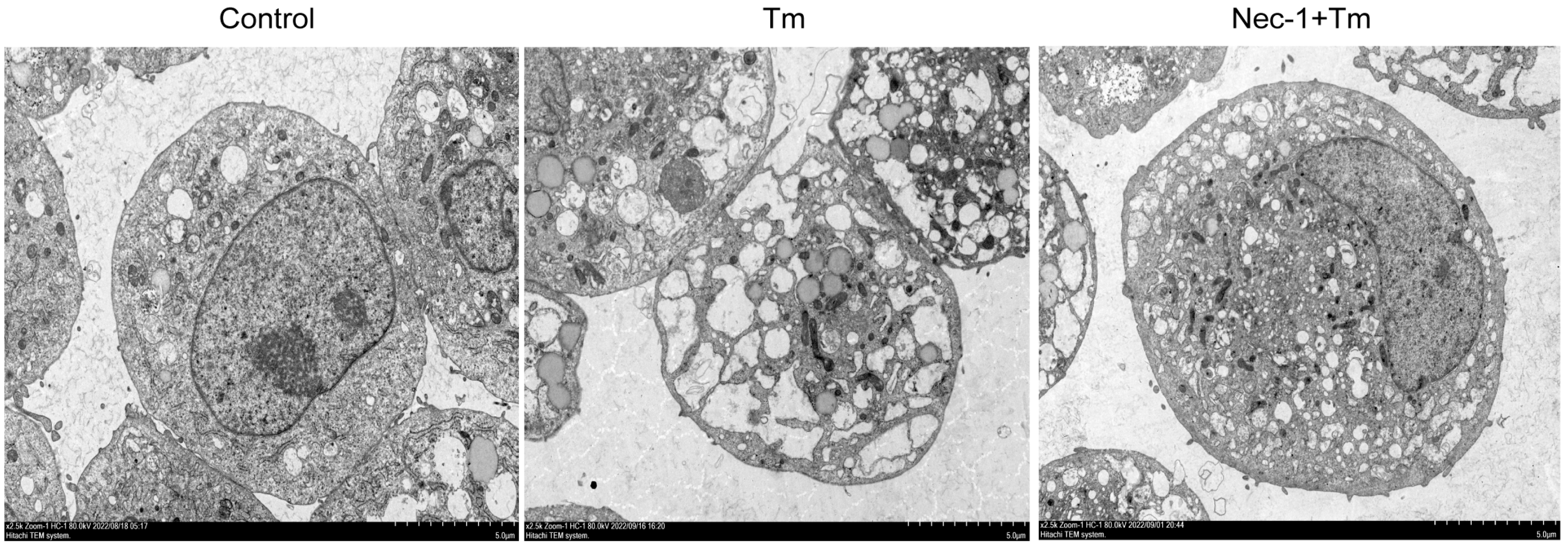
3.2. Protective Effect of Nec-1 Against Tm on Bovine Granulosa Cells
3.3. Protective Effect of HupA Against Tm on Bovine Granulosa Cells
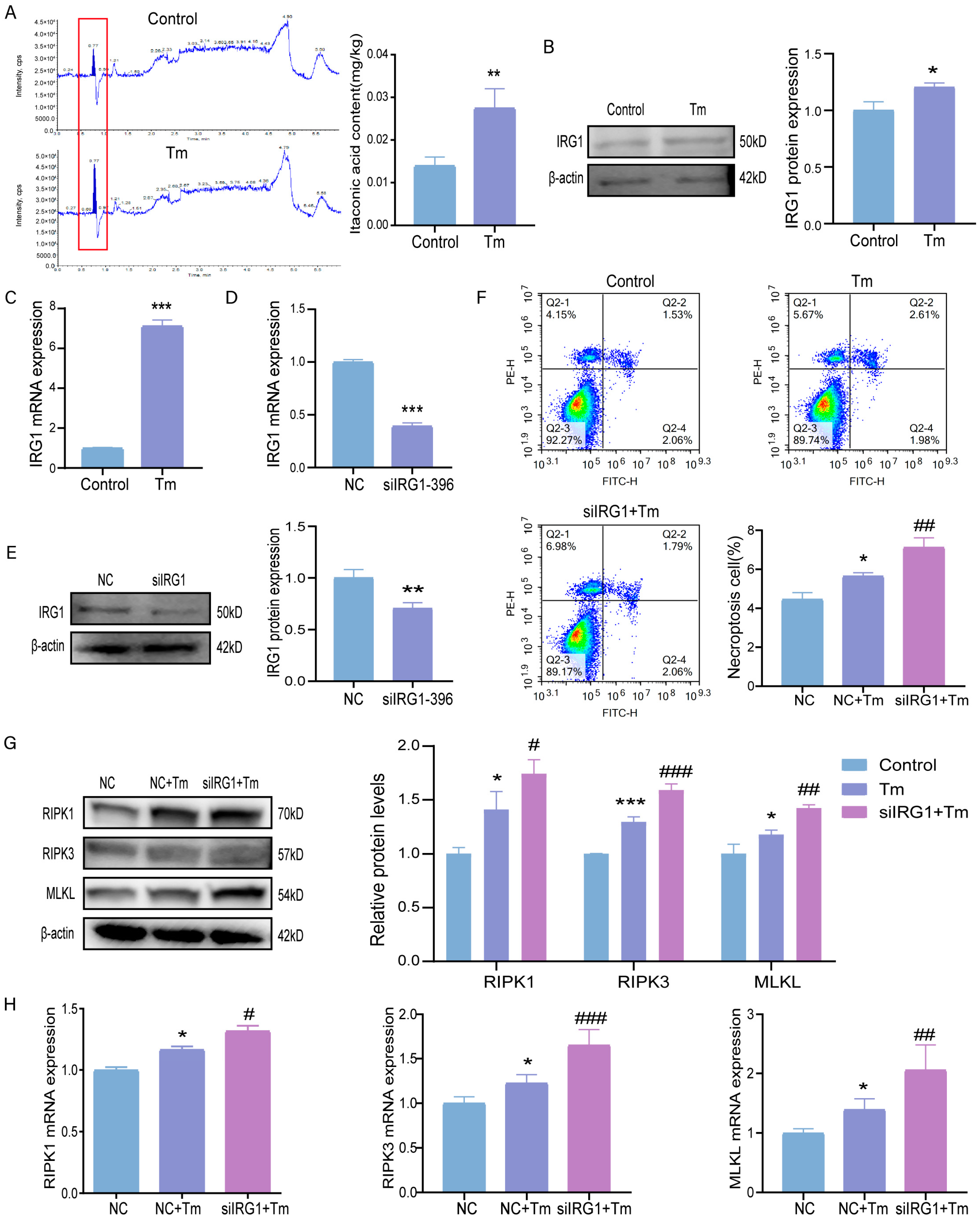
3.4. Knockdown of the IRG1 Exacerbates ERS-Induced Necroptosis
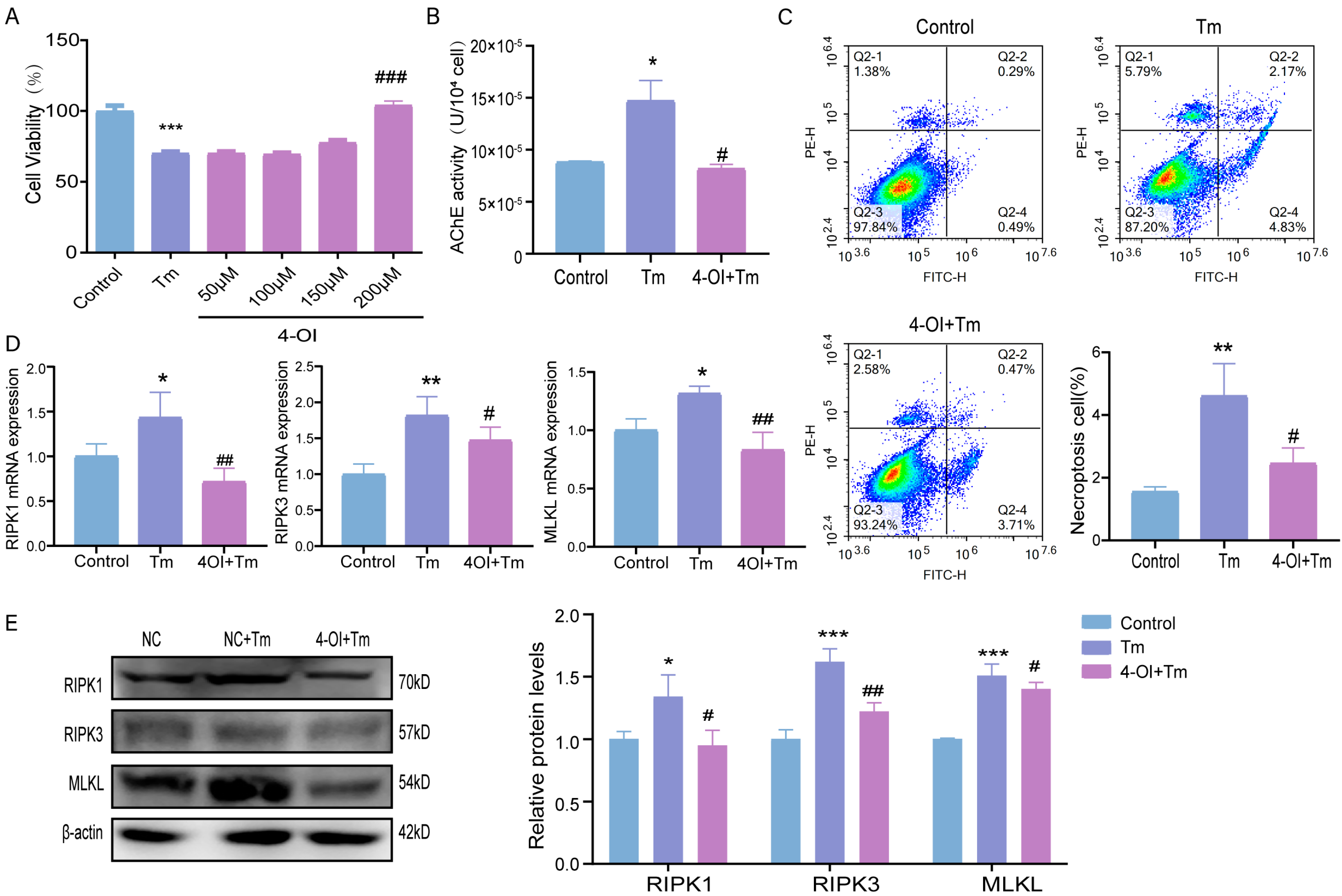
3.5. Effects of Itaconic Acid Supplementation on ERS-Induced Necroptosis in Bovine Granulosa Cells
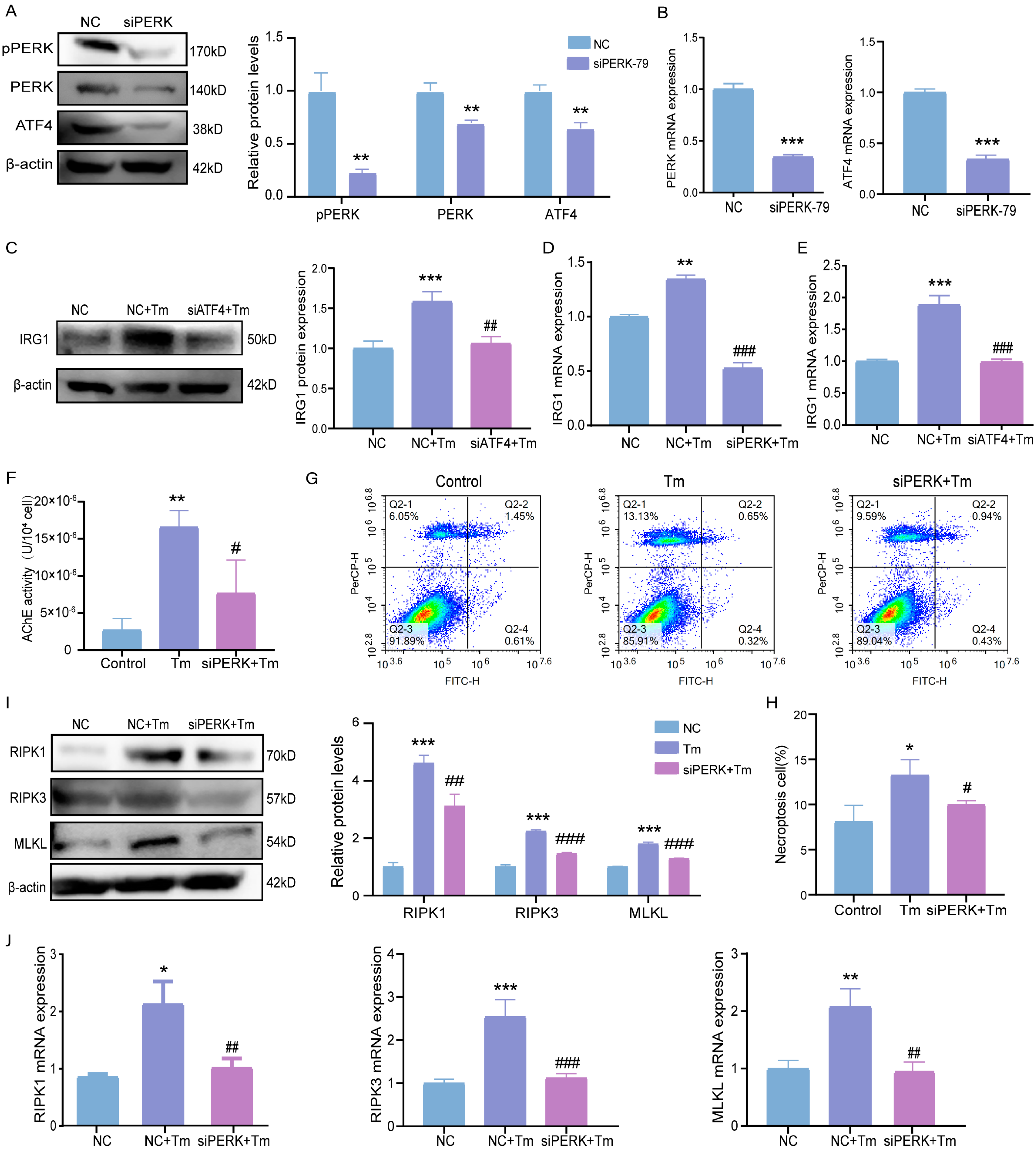
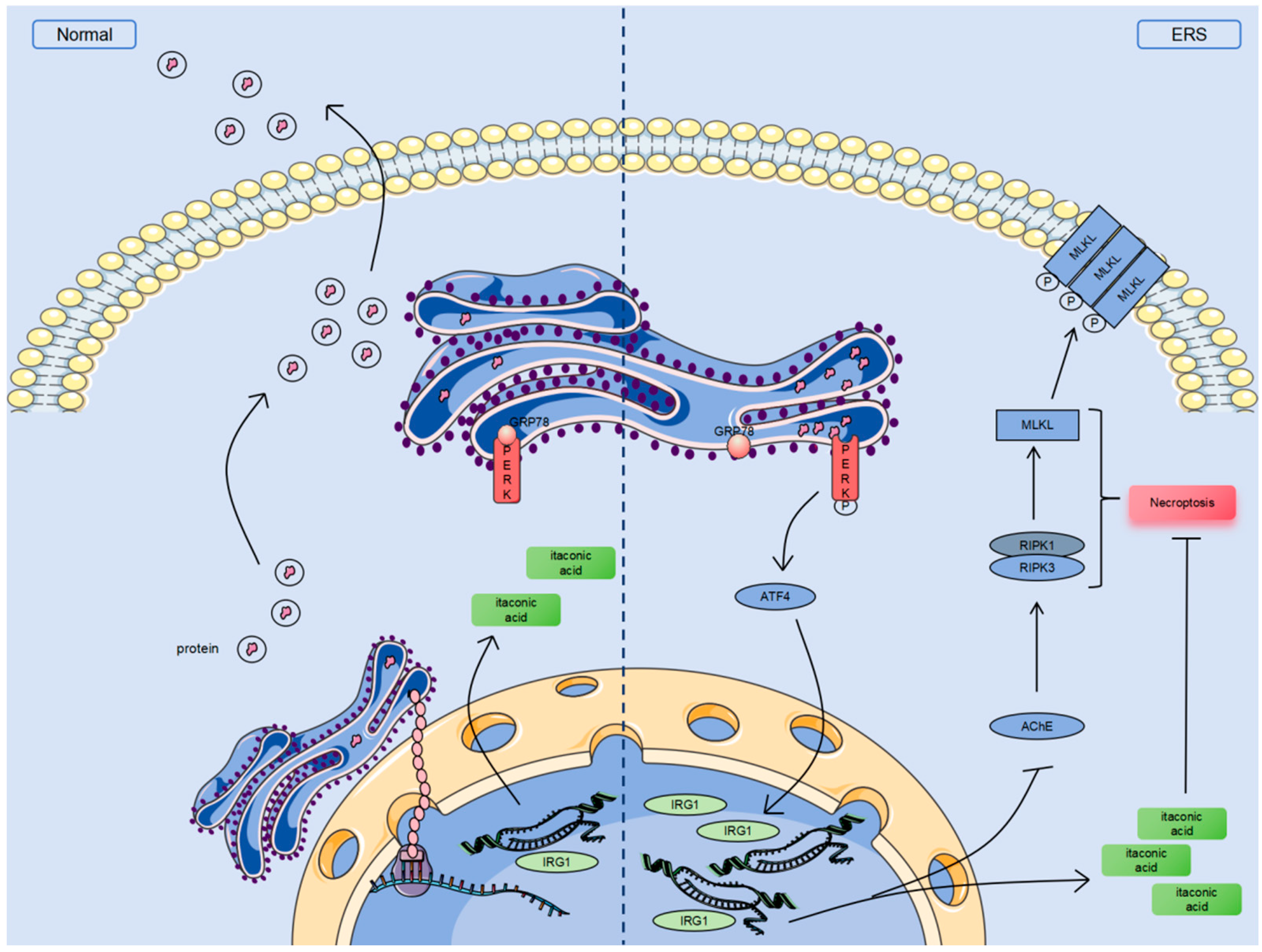
3.6. ERS Activates the PERK-ATF4 Pathway and Stimulates IRG1-AChE to Induce Necroptosis in Bovine Granulosa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERS | endoplasmic reticulum stress |
| Tm | tunicamycin |
| AChE | acetylcholinesterase |
| UPR | unfolded protein response |
| IRG1 | immune-responsive gene 1 |
| 4-OI | 4-Octyl itaconate |
| CHOP | C/EBP homologous protein |
References
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Bagnjuk, K.; Lawson, M.S.; Xu, J.; Mayerhofer, A. Acetylcholine and Necroptosis Are Players in Follicular Development in Primates. Sci. Rep. 2018, 8, 6166. [Google Scholar] [CrossRef]
- Blohberger, J.; Kunz, L.; Einwang, D.; Berg, U.; Berg, D.; Ojeda, S.R.; Dissen, G.A.; Fröhlich, T.; Arnold, G.J.; Soreq, H.; et al. Readthrough Acetylcholinesterase (Ache-R) and Regulated Necrosis: Pharmacological Targets for the Regulation of Ovarian Functions? Cell Death Dis. 2015, 6, e1685. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Alesi, L.R.; Winship, A.L.; Hutt, K.J. Beyond Apoptosis: Evidence of Other Regulated Cell Death Pathways in the Ovary Throughout Development and Life. Hum. Reprod. Update 2023, 29, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, L.; Tsai, H.; Wen, Z.; Tsui, K. Phosphoglycerate Mutase Family Member 5 Maintains Oocyte Quality Via Mitochondrial Dynamic Rearrangement During Aging. Aging Cell 2022, 21, e13546. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-Microplastics and Dehp Co-Exposure Induced DNA Damage, Cell Cycle Arrest and Necroptosis of Ovarian Granulosa Cells in Mice by Promoting Ros Production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef]
- Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kunitomi, C.; Fujii, T.; Osuga, Y. Endoplasmic Reticulum Stress: A Key Regulator of the Follicular Microenvironment in the Ovary. Mol. Hum. Reprod. 2021, 27, gaaa088. [Google Scholar] [CrossRef]
- Huang, N.; Yu, Y.; Qiao, J. Dual Role for the Unfolded Protein Response in the Ovary: Adaption and Apoptosis. Protein Cell 2016, 8, 14–24. [Google Scholar] [CrossRef]
- Yan, T.; Ma, X.; Guo, L.; Lu, R. Targeting Endoplasmic Reticulum Stress Signaling in Ovarian Cancer Therapy. Cancer Biol. Med. 2023, 20, 748–764. [Google Scholar] [CrossRef]
- Yuan, S.; She, D.; Jiang, S.; Deng, N.; Peng, J.; Ma, L. Endoplasmic Reticulum Stress and Therapeutic Strategies in Metabolic, Neurodegenerative Diseases and Cancer. Mol. Med. 2024, 30, 40. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping Endoplasmic Reticulum Quality Control through the Unfolded Protein Response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, Q.; Zhang, L.; Zhuan, Q.; Meng, L.; Fu, X.; Hou, Y. Dihydroartemisinin Exposure Impairs Porcine Ovarian Granulosa Cells by Activating Perk-Eif2α-Atf4 through Endoplasmic Reticulum Stress. Toxicol. Appl. Pharmacol. 2020, 403, 115159. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, Y.; Liu, L.; Pang, B.; Sun, Z.; Guan, S.; Yan, Q.; Mo, T.; Chen, R.; Xu, M.; et al. Bushen Jieyu Tiaochong Formula Reduces Apoptosis of Granulosa Cells Via the Perk-Atf4-Chop Signaling Pathway in a Rat Model of Polycystic Ovary Syndrome with Chronic Stress. J. Ethnopharmacol. 2022, 292, 114923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Sun, M.; Hao, X.; Li, M. Analysis of Serum Insulin-Like Growth Factor-1, Fibroblast Growth Factor 23, and Klotho Levels in Girls with Rapidly Progressive Central Precocious Puberty. Eur. J. Pediatr. 2023, 182, 5007–5013. [Google Scholar] [CrossRef]
- Kishino, A.; Hayashi, K.; Maeda, M.; Jike, T.; Hidai, C.; Nomura, Y.; Oshima, T. Caspase-8 Regulates Endoplasmic Reticulum Stress-Induced Necroptosis Independent of the Apoptosis Pathway in Auditory Cells. Int. J. Mol. Sci. 2019, 20, 5896. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Luo, X.; Bao, X.; Weng, X.; Chen, Y.; Zhang, S.; Lv, Y.; Dai, X.; Zeng, M.; et al. Cigarette Tar Accelerates Atherosclerosis Progression Via Ripk3-Dependent Necroptosis Mediated by Endoplasmic Reticulum Stress in Vascular Smooth Muscle Cells. Cell Commun. Signal. 2024, 22, 41. [Google Scholar] [CrossRef]
- Koike, H.; Harada, M.; Kusamoto, A.; Xu, Z.; Tanaka, T.; Sakaguchi, N.; Kunitomi, C.; Azhary, J.M.K.; Takahashi, N.; Urata, Y.; et al. Roles of Endoplasmic Reticulum Stress in the Pathophysiology of Polycystic Ovary Syndrome. Front. Endocrinol. 2023, 14, 1124405. [Google Scholar] [CrossRef]
- Yang, A.Y.; Kim, K.; Kwon, H.H.; Leem, J.; Song, J.E. 6-Shogaol Ameliorates Liver Inflammation and Fibrosis in Mice on a Methionine- and Choline-Deficient Diet by Inhibiting Oxidative Stress, Cell Death, and Endoplasmic Reticulum Stress. Molecules 2024, 29, 419. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakano, Y.; Iwata, N.; Soejima, Y.; Suyama, A.; Hasegawa, T.; Otsuka, F. Oxytocin Enhances Progesterone Production with Upregulation of Bmp-15 Activity by Granulosa Cells. Biochem. Biophys. Res. Commun. 2023, 646, 103–109. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Bi, Y.; Wu, Q.; Xu, S. Baicalin Suppressed Necroptosis and Inflammation against Chlorpyrifos Toxicity; Involving in Er Stress and Oxidative Stress in Carp Gills. Fish Shellfish Immunol. 2023, 139, 108883. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Gao, S.; Jiang, J.-M.; Zhang, P.; Zou, W.; Tang, X.-Q.; Tang, Y.-Y. Itaconate Inhibits Corticosterone-Induced Necroptosis and Neuroinflammation Via up-Regulating Menin in Ht22 Cells. J. Physiol. Biochem. 2024, 80, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.-T.; Li, Q.; Chen, Y.; Shi, F.-L.; Wong, T.-S.; Yuan, L.-S.; Xu, R.; Gan, Y.-Q.; Lu, N.; Li, Y.-P.; et al. Anti-Necroptotic Effects of Itaconate and Its Derivatives. Inflammation 2024, 47, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, H.; Wei, J.; Mo, W.; Li, Q.; Lv, X. The Signaling Pathways and Therapeutic Potential of Itaconate to Alleviate Inflammation and Oxidative Stress in Inflammatory Diseases. Redox Biol. 2022, 58, 102553. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Michel, P.; Postel, M.; Clément, F. Multi-Scale Modelling of Ovarian Follicular Development: From Follicular Morphogenesis to Selection for Ovulation. Biol. Cell 2016, 108, 149–160. [Google Scholar] [CrossRef]
- Harada, M.; Nose, E.; Takahashi, N.; Hirota, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Fujii, T.; Osuga, Y. Evidence of the Activation of Unfolded Protein Response in Granulosa and Cumulus Cells During Follicular Growth and Maturation. Gynecol. Endocrinol. 2015, 31, 783–787. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.-A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of Folliculogenesis and the Determination of Ovulation Rate in Ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef]
- Lin, P.; Yang, Y.; Li, X.; Chen, F.; Cui, C.; Hu, L.; Li, Q.; Liu, W.; Jin, Y. Endoplasmic Reticulum Stress Is Involved in Granulosa Cell Apoptosis During Follicular Atresia in Goat Ovaries. Mol. Reprod. Dev. 2012, 79, 423–432. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Xu, X.; Wang, D.; He, J.; Zhou, H.; Lu, Y.; Zeng, J.; Du, F.; Gong, A.; et al. Yth Domain Family 2 Orchestrates Epithelial-Mesenchymal Transition/Proliferation Dichotomy in Pancreatic Cancer Cells. Cell Cycle 2017, 16, 2259–2271. [Google Scholar] [CrossRef]
- Foufelle, F.; Fromenty, B. Role of Endoplasmic Reticulum Stress in Drug-Induced Toxicity. Pharmacol. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. Grp78: A Cell’s Response to Stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jing, Y.; Qu, X.; Yang, J.; Pan, P.; Liu, X.; Gao, H.; Pei, X.; Zhang, C.; Yang, Y. The Activation of Reticulophagy by Er Stress through the Atf4-Map1lc3a-Ccpg1 Pathway in Ovarian Granulosa Cells Is Linked to Apoptosis and Necroptosis. Int. J. Mol. Sci. 2023, 24, 2749. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, J.P.; Carlin, S.; Ju, T.; van der Veen, J.N.; Nelson, R.C.; Buteau, J.; Thiesen, A.; Richard, C.; Willing, B.P.; Jacobs, R.L. Intestinal Phospholipid Disequilibrium Initiates an Er Stress Response That Drives Goblet Cell Necroptosis and Spontaneous Colitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 999–1021. [Google Scholar] [CrossRef]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.-P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. Perk Is Required at the Er-Mitochondrial Contact Sites to Convey Apoptosis after Ros-Based Er Stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.-D.; Ning, J.; Sun, S.-L. Oleandrin, a Cardiac Glycoside, Induces Immunogenic Cell Death Via the Perk/Elf2α/Atf4/Chop Pathway in Breast Cancer. Cell Death Dis. 2021, 12, 314. [Google Scholar] [CrossRef]
- León-Annicchiarico, C.L.; Ramírez-Peinado, S.; Domínguez-Villanueva, D.; Gonsberg, A.; Lampidis, T.J.; Muñoz-Pinedo, C. Atf4 Mediates Necrosis Induced by Glucose Deprivation and Apoptosis Induced by 2-Deoxyglucose in the Same Cells. FEBS J. 2015, 282, 3647–3658. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Y.; Liu, Z.; Yang, J.; Tang, H.; Wang, Y. Itaconic Acid Exerts Anti-Inflammatory and Antibacterial Effects Via Promoting Pentose Phosphate Pathway to Produce Ros. Sci. Rep. 2021, 11, 18173. [Google Scholar] [CrossRef]
- Li, X.-K.; Yang, H.-J.; Du, S.-H.; Zhang, B.; Li, L.-Y.; Li, S.-N.; Liu, C.-C.; Ma, Y.; Yu, J.-B. 4-Octyl Itaconate Alleviates Renal Ischemia Reperfusion Injury by Ameliorating Endoplasmic Reticulum Stress Via Nrf2 Pathway. Exp. Biol. Med. 2023, 248, 2408–2420. [Google Scholar] [CrossRef]
- He, F.; Wang, F.; Yang, Y.; Yuan, Z.; Sun, C.; Zou, H.; Chen, H.; Yi, H.; Gao, S.H.; Zhang, S.; et al. The Effect of Growth Hormone on the Metabolome of Follicular Fluid in Patients with Diminished Ovarian Reserve. Reprod. Biol. Endocrinol. 2023, 21, 21. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Jozaie, S.; Ghotbedin, Z.; Gorani, S. Dimethyl Itaconic Acid Improves Viability and Steroidogenesis and Suppresses Cytokine Production in Lps-Treated Bovine Ovarian Granulosa Cells by Regulating Tlr4/Nfkβ, Nlrp3, Jnk Signaling Pathways. Res. Veter. Sci. 2022, 152, 89–98. [Google Scholar] [CrossRef]
- Urra, J.; Blohberger, J.; Tiszavari, M.; Mayerhofer, A.; Lara, H.E. In Vivo Blockade of Acetylcholinesterase Increases Intraovarian Acetylcholine and Enhances Follicular Development and Fertility in the Rat. Sci. Rep. 2016, 6, 30129. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Synthesis and Activities of Acetylcholinesterase Inhibitors. Chem. Biol. Drug Des. 2021, 98, 997–1006. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, Y.; Wang, X.; Xu, G.; Wang, H.; Shu, X.; Ding, H.; Ma, X.; Guo, J.; Wang, J.; et al. Ameliorative Effect of Itaconic Acid/IRG1 Against Endoplasmic Reticulum Stress-Induced Necroptosis in Granulosa Cells via PERK-ATF4-AChE Pathway in Bovine. Cells 2025, 14, 419. https://doi.org/10.3390/cells14060419
Yang X, Chen Y, Wang X, Xu G, Wang H, Shu X, Ding H, Ma X, Guo J, Wang J, et al. Ameliorative Effect of Itaconic Acid/IRG1 Against Endoplasmic Reticulum Stress-Induced Necroptosis in Granulosa Cells via PERK-ATF4-AChE Pathway in Bovine. Cells. 2025; 14(6):419. https://doi.org/10.3390/cells14060419
Chicago/Turabian StyleYang, Xiaorui, Yue Chen, Xinzi Wang, Gaoqing Xu, Hongjie Wang, Xinqi Shu, He Ding, Xin Ma, Jing Guo, Jun Wang, and et al. 2025. "Ameliorative Effect of Itaconic Acid/IRG1 Against Endoplasmic Reticulum Stress-Induced Necroptosis in Granulosa Cells via PERK-ATF4-AChE Pathway in Bovine" Cells 14, no. 6: 419. https://doi.org/10.3390/cells14060419
APA StyleYang, X., Chen, Y., Wang, X., Xu, G., Wang, H., Shu, X., Ding, H., Ma, X., Guo, J., Wang, J., Zhao, J., Fang, Y., Liu, H., & Lu, W. (2025). Ameliorative Effect of Itaconic Acid/IRG1 Against Endoplasmic Reticulum Stress-Induced Necroptosis in Granulosa Cells via PERK-ATF4-AChE Pathway in Bovine. Cells, 14(6), 419. https://doi.org/10.3390/cells14060419






