Microglia in ALS: Insights into Mechanisms and Therapeutic Potential
Abstract
1. Introduction
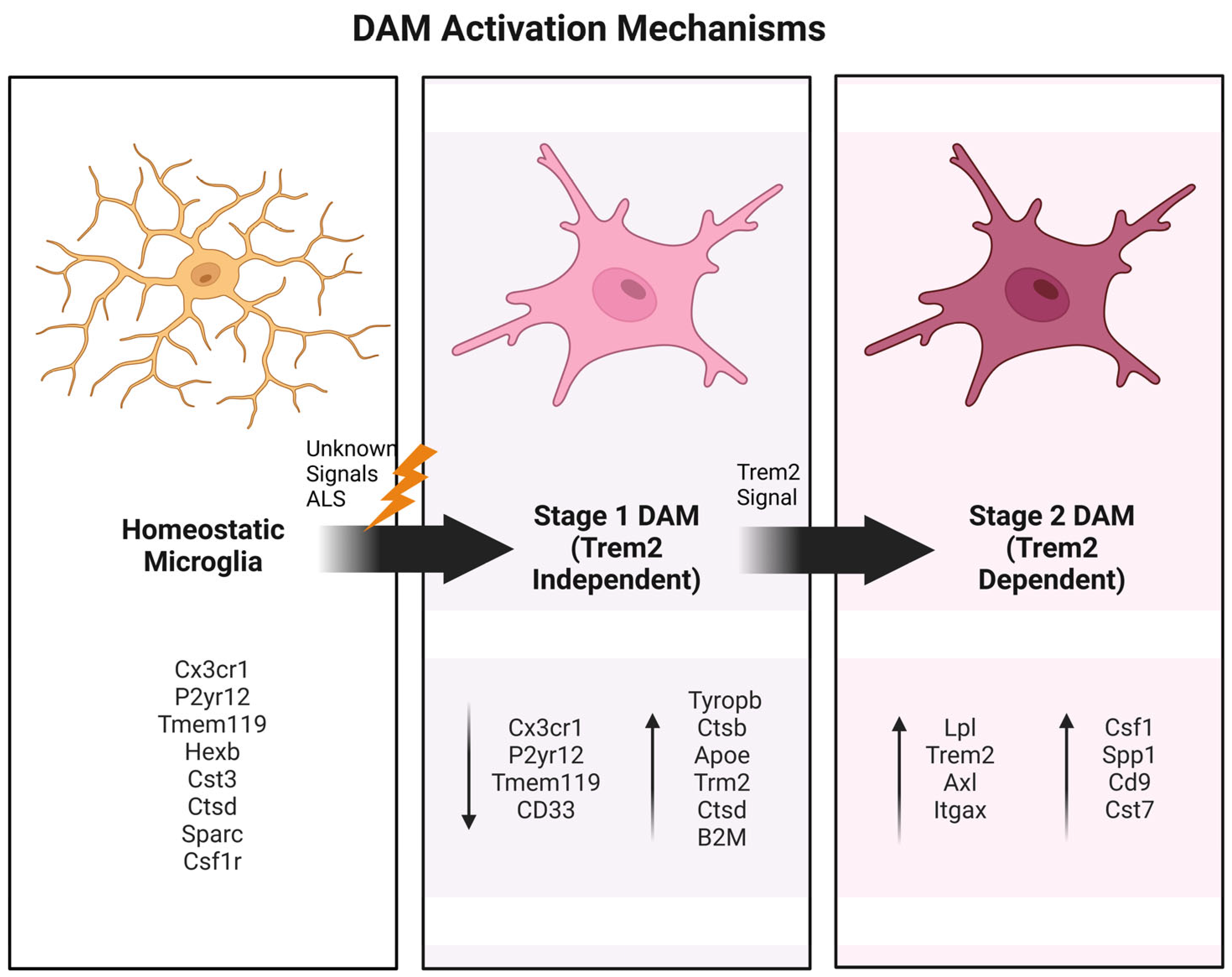
2. The Central Role of DAM Phenotypes
3. Microglia Alterations in Human Postmortem Tissue
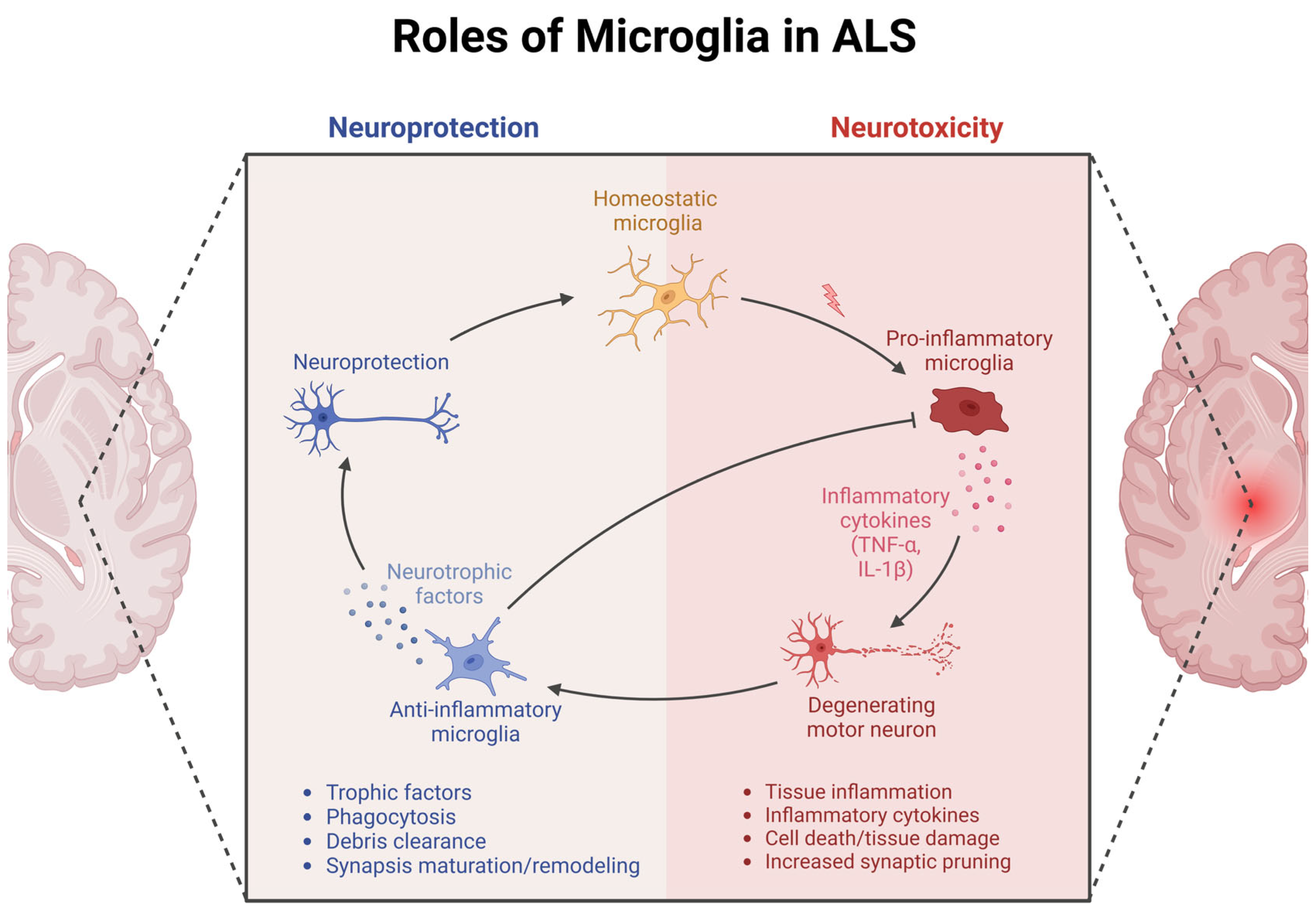
4. Insights from Mouse Models
4.1. Morphological Changes in Microglia in Mice
4.2. CX3CR1 Signaling and Microglia–Neuron Crosstalk
4.3. NF-κB Pathway and Microglial Activation in ALS Progression
4.4. The C9ORF72 Mouse Model
4.5. TREM2 Signaling in ALS
4.6. Role of TREM2 and CD14 in TDP-43-Driven ALS
5. Role of Microglia in Human iPSC in Vitro Systems
6. Therapeutic Targeting of Microglia in ALS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Raymond, J.; Nair, T.; Han, M.; Punjani, R.; Larson, T.; Berry, J.; Mohidul, S.; Horton, D.K. Prevalence of ALS in All 50 States in the United States, Data from the National ALS Registry, 2011–2018. Amyotroph Lateral Scler Front. Degener 2024, 25, 687–693. [Google Scholar] [CrossRef]
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016, 138, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ingre, C.; Roos, P.; Kamel, F.; Piehl, F. Risk Factors for Amyotrophic Lateral Sclerosis. Clin. Epidemiol. 2015, 7, 181. [Google Scholar] [CrossRef]
- Kaur, S.J.; McKeown, S.R.; Rashid, S. Mutant SOD1 Mediated Pathogenesis of Amyotrophic Lateral Sclerosis. Gene 2016, 577, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jiang, J.; Gendron, T.F.; McAlonis-Downes, M.; Jiang, L.; Taylor, A.; Diaz Garcia, S.; Ghosh Dastidar, S.; Rodriguez, M.J.; King, P.; et al. Reduced C9ORF72 Function Exacerbates Gain of Toxicity from ALS/FTD-Causing Repeat Expansion in C9orf72. Nat. Neurosci. 2020, 23, 615–624. [Google Scholar] [CrossRef]
- Källstig, E.; McCabe, B.D.; Schneider, B.L. The Links between ALS and NF-ΚB. Int. J. Mol. Sci. 2021, 22, 3875. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The Role of Excitotoxicity in the Pathogenesis of Amyotrophic Lateral Sclerosis. Biochimica et Biophysica Acta (BBA) Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Odierna, G.L.; Vucic, S.; Dyer, M.; Dickson, T.; Woodhouse, A.; Blizzard, C. How Do We Get from Hyperexcitability to Excitotoxicity in Amyotrophic Lateral Sclerosis? Brain 2024, 147, 1610–1621. [Google Scholar] [CrossRef]
- Spreux-Varoquaux, O.; Bensimon, G.; Lacomblez, L.; Salachas, F.; Pradat, P.F.; Le Forestier, N.; Marouan, A.; Dib, M.; Meininger, V. Glutamate Levels in Cerebrospinal Fluid in Amyotrophic Lateral Sclerosis: A Reappraisal Using a New HPLC Method with Coulometric Detection in a Large Cohort of Patients. J. Neurol. Sci. 2002, 193, 73–78. [Google Scholar] [CrossRef]
- Swarup, V.; Phaneuf, D.; Dupré, N.; Petri, S.; Strong, M.; Kriz, J.; Julien, J.-P. Deregulation of TDP-43 in Amyotrophic Lateral Sclerosis Triggers Nuclear Factor ΚB–Mediated Pathogenic Pathways. J. Exp. Med. 2011, 208, 2429–2447. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Akçimen, F.; Lopez, E.R.; Landers, J.E.; Nath, A.; Chiò, A.; Chia, R.; Traynor, B.J. Amyotrophic Lateral Sclerosis: Translating Genetic Discoveries into Therapies. Nat. Rev. Genet. 2023, 24, 642–658. [Google Scholar] [CrossRef]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Factors Regulating Microglia Activation. Front. Cell Neurosci. 2013, 7, 44. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP Mediates Rapid Microglial Response to Local Brain Injury in Vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Philips, T.; Robberecht, W. Neuroinflammation in Amyotrophic Lateral Sclerosis: Role of Glial Activation in Motor Neuron Disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Frick, L.R.; Williams, K.; Pittenger, C. Microglial Dysregulation in Psychiatric Disease. Clin. Dev. Immunol. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Jauregui, C.; Blanco-Luquin, I.; Macías, M.; Roldan, M.; Caballero, C.; Pagola, I.; Mendioroz, M.; Jericó, I. Exploring the Disease-Associated Microglia State in Amyotrophic Lateral Sclerosis. Biomedicines 2023, 11, 2994. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 Dependent and Independent Functions of Microglia in Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Takahashi, K. Microglial Heterogeneity in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2023, 82, 140–149. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K.; et al. SYK Coordinates Neuroprotective Microglial Responses in Neurodegenerative Disease. Cell 2022, 185, 4135–4152.e22. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Spiller, K.J.; Restrepo, C.R.; Khan, T.; Dominique, M.A.; Fang, T.C.; Canter, R.G.; Roberts, C.J.; Miller, K.R.; Ransohoff, R.M.; Trojanowski, J.Q.; et al. Microglia-Mediated Recovery from ALS-Relevant Motor Neuron Degeneration in a Mouse Model of TDP-43 Proteinopathy. Nat. Neurosci. 2018, 21, 329–340. [Google Scholar] [CrossRef]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; Fagegaltier, D.; Harris, B.T.; Ostrow, L.W.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e5. [Google Scholar] [CrossRef]
- Dols-Icardo, O.; Montal, V.; Sirisi, S.; López-Pernas, G.; Cervera-Carles, L.; Querol-Vilaseca, M.; Muñoz, L.; Belbin, O.; Alcolea, D.; Molina-Porcel, L.; et al. Motor Cortex Transcriptome Reveals Microglial Key Events in Amyotrophic Lateral Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e829. [Google Scholar] [CrossRef]
- D’Erchia, A.M.; Gallo, A.; Manzari, C.; Raho, S.; Horner, D.S.; Chiara, M.; Valletti, A.; Aiello, I.; Mastropasqua, F.; Ciaccia, L.; et al. Massive Transcriptome Sequencing of Human Spinal Cord Tissues Provides New Insights into Motor Neuron Degeneration in ALS. Sci. Rep. 2017, 7, 10046. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Toledo, J.B.; Van Deerlin, V.M.; Elman, L.; McCluskey, L.; Lee, V.M.-Y.; Trojanowski, J.Q. Microglial Activation Correlates with Disease Progression and Upper Motor Neuron Clinical Symptoms in Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e39216. [Google Scholar] [CrossRef]
- Corcia, P.; Tauber, C.; Vercoullie, J.; Arlicot, N.; Prunier, C.; Praline, J.; Nicolas, G.; Venel, Y.; Hommet, C.; Baulieu, J.-L.; et al. Molecular Imaging of Microglial Activation in Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e52941. [Google Scholar] [CrossRef] [PubMed]
- Alshikho, M.J.; Zürcher, N.R.; Loggia, M.L.; Cernasov, P.; Reynolds, B.; Pijanowski, O.; Chonde, D.B.; Izquierdo Garcia, D.; Mainero, C.; Catana, C.; et al. Integrated Magnetic Resonance Imaging and [ 11 C]-PBR28 Positron Emission Tomographic Imaging in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2018, 83, 1186–1197. [Google Scholar] [CrossRef]
- Turner, M.R.; Cagnin, A.; Turkheimer, F.E.; Miller, C.C.J.; Shaw, C.E.; Brooks, D.J.; Leigh, P.N.; Banati, R.B. Evidence of Widespread Cerebral Microglial Activation in Amyotrophic Lateral Sclerosis: An [11C](R)-PK11195 Positron Emission Tomography Study. Neurobiol. Dis. 2004, 15, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.M.; Sarlls, J.E.; Kwan, J.Y.; Bageac, D.; Gala, Z.S.; Danielian, L.E.; Ray-Chaudhury, A.; Wang, H.-W.; Miller, K.L.; Foxley, S.; et al. Pathology of Callosal Damage in ALS: An Ex-Vivo, 7 T Diffusion Tensor MRI Study. Neuroimage Clin. 2017, 15, 200–208. [Google Scholar] [CrossRef]
- Togawa, N.; Ayaki, T.; Yoshii, D.; Maki, T.; Sawamoto, N.; Takahashi, R. TMEM119-Positive Microglia Were Increased in the Brains of Patients with Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2024, 833, 137829. [Google Scholar] [CrossRef]
- Tuddenham, J.F.; Taga, M.; Haage, V.; Marshe, V.S.; Roostaei, T.; White, C.; Lee, A.J.; Fujita, M.; Khairallah, A.; Zhang, Y.; et al. A Cross-Disease Resource of Living Human Microglia Identifies Disease-Enriched Subsets and Tool Compounds Recapitulating Microglial States. Nat. Neurosci. 2024, 27, 2521–2537. [Google Scholar] [CrossRef]
- Shen, D.; Ji, Y.; Qiu, C.; Wang, K.; Gao, Z.; Liu, B.; Shen, Y.; Gong, L.; Yang, X.; Chen, X.; et al. Single-Cell RNA Sequencing Analysis of Microglia Dissected the Energy Metabolism and Revealed Potential Biomarkers in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2024, 61, 4473–4487. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, W.; Beers, D.R.; Yen, A.A.; Xie, W.; Henkel, J.S.; Appel, S.H. Mutant SOD1 G93A Microglia Are More Neurotoxic Relative to Wild-type Microglia. J. Neurochem. 2007, 102, 2008–2019. [Google Scholar] [CrossRef]
- Boilleée, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Henkel, J.S.; Xiao, Q.; Zhao, W.; Wang, J.; Yen, A.A.; Siklos, L.; McKercher, S.R.; Appel, S.H. Wild-Type Microglia Extend Survival in PU.1 Knockout Mice with Familial Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. 2006, 103, 16021–16026. [Google Scholar] [CrossRef]
- Liao, B.; Zhao, W.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Transformation from a Neuroprotective to a Neurotoxic Microglial Phenotype in a Mouse Model of ALS. Exp. Neurol. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Appel, S.H. CD4+ T Cells Support Glial Neuroprotection, Slow Disease Progression, and Modify Glial Morphology in an Animal Model of Inherited ALS. Proc. Natl. Acad. Sci. 2008, 105, 15558–15563. [Google Scholar] [CrossRef]
- Tu, P.H.; Raju, P.; Robinson, K.A.; Gurney, M.E.; Trojanowski, J.Q.; Lee, V.M. Transgenic Mice Carrying a Human Mutant Superoxide Dismutase Transgene Develop Neuronal Cytoskeletal Pathology Resembling Human Amyotrophic Lateral Sclerosis Lesions. Proc. Natl. Acad. Sci. 1996, 93, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.-X.; et al. Motor Neuron Degeneration in Mice That Express a Human Cu,Zn Superoxide Dismutase Mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Crane, J.; Xie, A.X. Modeling CNS Microglia: The Quest to Identify Predictive Models. Drug Discov. Today Dis. Models 2008, 5, 19–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henkel, J.S.; Beers, D.R.; Zhao, W.; Appel, S.H. Microglia in ALS: The Good, The Bad, and The Resting. J. Neuroimmune Pharmacol. 2009, 4, 389–398. [Google Scholar] [CrossRef]
- Cheung, S.W.; Bhavnani, E.; Simmons, D.G.; Bellingham, M.C.; Noakes, P.G. Perineuronal Nets Are Phagocytosed by MMP-9 Expressing Microglia and Astrocytes in the SOD1 G93A ALS Mouse Model. Neuropathol. Appl. Neurobiol. 2024, 50, e12982. [Google Scholar] [CrossRef]
- Crosio, C.; Valle, C.; Casciati, A.; Iaccarino, C.; Carrì, M.T. Astroglial Inhibition of NF-ΚB Does Not Ameliorate Disease Onset and Progression in a Mouse Model for Amyotrophic Lateral Sclerosis (ALS). PLoS ONE 2011, 6, e17187. [Google Scholar] [CrossRef]
- Nguyen, L.; Montrasio, F.; Pattamatta, A.; Tusi, S.K.; Bardhi, O.; Meyer, K.D.; Hayes, L.; Nakamura, K.; Banez-Coronel, M.; Coyne, A.; et al. Antibody Therapy Targeting RAN Proteins Rescues C9 ALS/FTD Phenotypes in C9orf72 Mouse Model. Neuron 2020, 105, 645–662.e11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Laboissonniere, L.A.; Guo, S.; Pilotto, F.; Scheidegger, O.; Oestmann, A.; Hammond, J.W.; Li, H.; Hyysalo, A.; Peltola, R.; et al. Survival and Motor Phenotypes in FVB C9-500 ALS/FTD BAC Transgenic Mice Reproduced by Multiple Labs. Neuron 2020, 108, 784–796.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, X.; Li, S.; Cheng, C.; Chen, S.; Le, W. Dynamic Changes of CX3CL1/CX3CR1 Axis during Microglial Activation and Motor Neuron Loss in the Spinal Cord of ALS Mouse Model. Transl. Neurodegener. 2018, 7, 35. [Google Scholar] [CrossRef]
- Liu, C.; Hong, K.; Chen, H.; Niu, Y.; Duan, W.; Liu, Y.; Ji, Y.; Deng, B.; Li, Y.; Li, Z.; et al. Evidence for a Protective Role of the CX3CL1/CX3CR1 Axis in a Model of Amyotrophic Lateral Sclerosis. Biol. Chem. 2019, 400, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Chen, L.-X.; Zhang, M.-D.; Xu, H.-F.; Ye, H.-Q.; Chen, D.-F.; Wang, P.-S.; Bao, Z.-W.; Zou, S.-M.; Lv, Y.-T.; Wu, Z.-Y.; et al. Single-Nucleus RNA Sequencing Reveals the Spatiotemporal Dynamics of Disease-Associated Microglia in Amyotrophic Lateral Sclerosis. Research 2024, 7, 0548. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell Transcriptomics Reveals Distinct Inflammation-induced Microglia Signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- MacLean, M.; Juranek, J.; Cuddapah, S.; López-Díez, R.; Ruiz, H.H.; Hu, J.; Frye, L.; Li, H.; Gugger, P.F.; Schmidt, A.M. Microglia RAGE Exacerbates the Progression of Neurodegeneration within the SOD1G93A Murine Model of Amyotrophic Lateral Sclerosis in a Sex-Dependent Manner. J. Neuroinflamm. 2021, 18, 139. [Google Scholar] [CrossRef]
- Barreto-Núñez, R.; Béland, L.; Boutej, H.; Picher-Martel, V.; Dupré, N.; Barbeito, L.; Kriz, J. Chronically Activated Microglia in <scp>ALS</Scp> Gradually Lose Their Immune Functions and Develop Unconventional Proteome. Glia 2024, 72, 1319–1339. [Google Scholar] [CrossRef]
- García-Revilla, J.; Boza-Serrano, A.; Espinosa-Oliva, A.M.; Soto, M.S.; Deierborg, T.; Ruiz, R.; de Pablos, R.M.; Burguillos, M.A.; Venero, J.L. Galectin-3, a Rising Star in Modulating Microglia Activation under Conditions of Neurodegeneration. Cell Death Dis. 2022, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Varvel, N.H.; Konerth, M.E.; Xu, G.; Cardona, A.E.; Ransohoff, R.M.; Lamb, B.T. CX3CR1 Deficiency Alters Microglial Activation and Reduces Beta-Amyloid Deposition in Two Alzheimer’s Disease Mouse Models. Am. J. Pathol. 2010, 177, 2549–2562. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, A.; Gamez, J.; Syriani, E.; Morales, M.; Salvado, M.; Rodríguez, M.J.; Mahy, N.; Vidal-Taboada, J.M. CX3CR1 Is a Modifying Gene of Survival and Progression in Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e96528. [Google Scholar] [CrossRef]
- Frakes, A.E.; Ferraiuolo, L.; Haidet-Phillips, A.M.; Schmelzer, L.; Braun, L.; Miranda, C.J.; Ladner, K.J.; Bevan, A.K.; Foust, K.D.; Godbout, J.P.; et al. Microglia Induce Motor Neuron Death via the Classical NF-ΚB Pathway in Amyotrophic Lateral Sclerosis. Neuron 2014, 81, 1009–1023. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Huang, A.; Wen, S.; Liao, B.; Appel, S.H. Endogenous Regulatory T Lymphocytes Ameliorate Amyotrophic Lateral Sclerosis in Mice and Correlate with Disease Progression in Patients with Amyotrophic Lateral Sclerosis. Brain 2011, 134, 1293–1314. [Google Scholar] [CrossRef]
- Uranishi, H.; Tetsuka, T.; Yamashita, M.; Asamitsu, K.; Shimizu, M.; Itoh, M.; Okamoto, T. Involvement of the Pro-Oncoprotein TLS (Translocated in Liposarcoma) in Nuclear Factor-ΚB P65-Mediated Transcription as a Coactivator. J. Biol. Chem. 2001, 276, 13395–13401. [Google Scholar] [CrossRef]
- O’Rourke, J.G.; Bogdanik, L.; Yáñez, A.; Lall, D.; Wolf, A.J.; Muhammad, A.K.M.G.; Ho, R.; Carmona, S.; Vit, J.P.; Zarrow, J.; et al. C9orf72 Is Required for Proper Macrophage and Microglial Function in Mice. Science 2016, 351, 1324–1329. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Finch, N.A.; Wang, X.; Gendron, T.F.; Bieniek, K.F.; Heckman, M.G.; Vasilevich, A.; Murray, M.E.; Rousseau, L.; Weesner, R.; et al. In-Depth Clinico-Pathological Examination of RNA Foci in a Large Cohort of C9ORF72 Expansion Carriers. Acta Neuropathol. 2017, 134, 255–269. [Google Scholar] [CrossRef]
- Limone, F.; Couto, A.; Wang, J.-Y.; Zhang, Y.; McCourt, B.; Huang, C.; Minkin, A.; Jani, M.; McNeer, S.; Keaney, J.; et al. Myeloid and Lymphoid Expression of C9orf72 Regulates IL-17A Signaling in Mice. Sci. Transl. Med. 2024, 16, eadg7895. [Google Scholar] [CrossRef]
- Lall, D.; Lorenzini, I.; Mota, T.A.; Bell, S.; Mahan, T.E.; Ulrich, J.D.; Davtyan, H.; Rexach, J.E.; Muhammad, A.K.M.G.; Shelest, O.; et al. C9orf72 Deficiency Promotes Microglial-Mediated Synaptic Loss in Aging and Amyloid Accumulation. Neuron 2021, 109, 2275–2291.e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pattamatta, A.; Zu, T.; Reid, T.; Bardhi, O.; Borchelt, D.R.; Yachnis, A.T.; Ranum, L.P.W. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron 2016, 90, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tagliatti, E.; Desiato, G.; Mancinelli, S.; Bizzotto, M.; Gagliani, M.C.; Faggiani, E.; Hernández-Soto, R.; Cugurra, A.; Poliseno, P.; Miotto, M.; et al. Trem2 Expression in Microglia Is Required to Maintain Normal Neuronal Bioenergetics during Development. Immunity 2024, 57, 86–105.e9. [Google Scholar] [CrossRef]
- Qin, Q.; Teng, Z.; Liu, C.; Li, Q.; Yin, Y.; Tang, Y. TREM2, Microglia, and Alzheimer’s Disease. Mech. Ageing Dev. 2021, 195, 111438. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, S.; Bosco, D.B.; Nguyen, A.; Wu, L. Microglial TREM2 in Amyotrophic Lateral Sclerosis. Dev. Neurobiol. 2022, 82, 125–137. [Google Scholar] [CrossRef]
- Liu, W.; Taso, O.; Wang, R.; Bayram, S.; Graham, A.C.; Garcia-Reitboeck, P.; Mallach, A.; Andrews, W.D.; Piers, T.M.; Botia, J.A.; et al. Trem2 Promotes Anti-Inflammatory Responses in Microglia and Is Suppressed under pro-Inflammatory Conditions. Hum. Mol. Genet. 2020, 29, 3224–3248. [Google Scholar] [CrossRef]
- Prater, K.E.; Latimer, C.S.; Jayadev, S. Glial TDP-43 and TDP-43 Induced Glial Pathology, Focus on Neurodegenerative Proteinopathy Syndromes. Glia 2022, 70, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, Y.U.; Zhao, S.; Zhang, L.; Bosco, D.B.; Pang, Y.-P.; Zhong, J.; Sheth, U.; Martens, Y.A.; Zhao, N.; et al. TREM2 Interacts with TDP-43 and Mediates Microglial Neuroprotection against TDP-43-Related Neurodegeneration. Nat. Neurosci. 2022, 25, 26–38. [Google Scholar] [CrossRef]
- Mills, W.A.; Eyo, U.B. TREMble Before TREM2: The Mighty Microglial Receptor Conferring Neuroprotective Properties in TDP-43 Mediated Neurodegeneration. Neurosci. Bull. 2023, 39, 163–166. [Google Scholar] [CrossRef]
- Zhao, W.; Beers, D.R.; Bell, S.; Wang, J.; Wen, S.; Baloh, R.H.; Appel, S.H. TDP-43 Activates Microglia through NF-ΚB and NLRP3 Inflammasome. Exp. Neurol. 2015, 273, 24–35. [Google Scholar] [CrossRef]
- Christoforidou, E.; Moody, L.; Joilin, G.; Simoes, F.A.; Gordon, D.; Talbot, K.; Hafezparast, M. An ALS-Associated Mutation Dysregulates Microglia-Derived Extracellular MicroRNAs in a Sex-Specific Manner. Dis. Model. Mech. 2024, 17, dmm050638. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Miller, A.S.; Pallegar, P.N.; Umpierre, A.; Liang, Y.; Wang, N.; Zhang, S.; Nagaraj, N.K.; Fogarty, Z.C.; Ghayal, N.B.; et al. Rod-Shaped Microglia Interact with Neuronal Dendrites to Regulate Cortical Excitability in TDP-43 Related. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and Temporal Heterogeneity of Mouse and Human Microglia at Single-Cell Resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.-A.; et al. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 2019, 179, 1609–1622.e16. [Google Scholar] [CrossRef] [PubMed]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. IPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293.e9. [Google Scholar] [CrossRef]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.-H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient Derivation of Microglia-like Cells from Human Pluripotent Stem Cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef]
- Lorenzini, I.; Alsop, E.; Levy, J.; Gittings, L.M.; Lall, D.; Rabichow, B.E.; Moore, S.; Pevey, R.; Bustos, L.M.; Burciu, C.; et al. Moderate Intrinsic Phenotypic Alterations in C9orf72 ALS/FTD IPSC-Microglia despite the Presence of C9orf72 Pathological Features. Front. Cell Neurosci. 2023, 17, 1179796. [Google Scholar] [CrossRef]
- Vahsen, B.F.; Nalluru, S.; Morgan, G.R.; Farrimond, L.; Carroll, E.; Xu, Y.; Cramb, K.M.L.; Amein, B.; Scaber, J.; Katsikoudi, A.; et al. C9orf72-ALS Human IPSC Microglia Are pro-Inflammatory and Toxic to Co-Cultured Motor Neurons via MMP9. Nat. Commun. 2023, 14, 5898. [Google Scholar] [CrossRef]
- Banerjee, P.; Mehta, A.R.; Nirujogi, R.S.; Cooper, J.; James, O.G.; Nanda, J.; Longden, J.; Burr, K.; McDade, K.; Salzinger, A.; et al. Cell-Autonomous Immune Dysfunction Driven by Disrupted Autophagy in C9orf72 -ALS IPSC-Derived Microglia Contributes to Neurodegeneration. Sci. Adv. 2023, 9, eabq0651. [Google Scholar] [CrossRef]
- Funes, S.; Jung, J.; Gadd, D.H.; Mosqueda, M.; Zhong, J.; Shankaracharya; Unger, M.; Stallworth, K.; Cameron, D.; Rotunno, M.S.; et al. Expression of ALS-PFN1 Impairs Vesicular Degradation in IPSC-Derived Microglia. Nat. Commun. 2024, 15, 2497. [Google Scholar] [CrossRef]
- Clarke, B.E.; Ziff, O.J.; Tyzack, G.; Petrić Howe, M.; Wang, Y.; Klein, P.; Smith, C.A.; Hall, C.A.; Helmy, A.; Howell, M.; et al. Human VCP Mutant ALS/FTD Microglia Display Immune and Lysosomal Phenotypes Independently of GPNMB. Mol. Neurodegener. 2024, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Kerk, S.Y.; Bai, Y.; Smith, J.; Lalgudi, P.; Hunt, C.; Kuno, J.; Nuara, J.; Yang, T.; Lanza, K.; Chan, N.; et al. Homozygous ALS-Linked FUS P525L Mutations Cell- Autonomously Perturb Transcriptome Profile and Chemoreceptor Signaling in Human IPSC Microglia. Stem Cell Reports 2022, 17, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.-Y.; Kwon, M.-S.; Oh, K.-W.; Nahm, M.; Park, J.; Kim, Y.-E.; Ki, C.-S.; Jin, H.K.; Bae, J.; Kim, S.H. Role of NCKAP1 in the Defective Phagocytic Function of Microglia-Like Cells Derived from Rapidly Progressing Sporadic ALS. Mol. Neurobiol. 2023, 60, 4761–4777. [Google Scholar] [CrossRef] [PubMed]
- Quek, H.; Cuní-López, C.; Stewart, R.; Colletti, T.; Notaro, A.; Nguyen, T.H.; Sun, Y.; Guo, C.C.; Lupton, M.K.; Roberts, T.L.; et al. ALS Monocyte-Derived Microglia-like Cells Reveal Cytoplasmic TDP-43 Accumulation, DNA Damage, and Cell-Specific Impairment of Phagocytosis Associated with Disease Progression. J. Neuroinflamm. 2022, 19, 58. [Google Scholar] [CrossRef]
- Salomon-Zimri, S.; Pushett, A.; Russek-Blum, N.; Van Eijk, R.P.A.; Birman, N.; Abramovich, B.; Eitan, E.; Elgrart, K.; Beaulieu, D.; Ennist, D.L.; et al. Combination of Ciprofloxacin/Celecoxib as a Novel Therapeutic Strategy for ALS. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 263–271. [Google Scholar] [CrossRef]
- Lam, L.; Halder, R.C.; Montoya, D.J.; Rubbi, L.; Rinaldi, A.; Sagong, B.; Weitzman, S.; Rubattino, R.; Singh, R.R.; Pellegrini, M.; et al. Anti-Inflammatory Therapies of Amyotrophic Lateral Sclerosis Guided by Immune Pathways. Am. J. Neurodegener. Dis. 2015, 4, 28–39. [Google Scholar]
- Mueller, C.; Berry, J.D.; McKenna-Yasek, D.M.; Gernoux, G.; Owegi, M.A.; Pothier, L.M.; Douthwright, C.L.; Gelevski, D.; Luppino, S.D.; Blackwood, M.; et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. New Engl. J. Med. 2020, 383, 151–158. [Google Scholar] [CrossRef]
- Chen, Y.A.; Kankel, M.W.; Hana, S.; Lau, S.K.; Zavodszky, M.I.; McKissick, O.; Mastrangelo, N.; Dion, J.; Wang, B.; Ferretti, D.; et al. In Vivo Genome Editing Using Novel AAV-PHP Variants Rescues Motor Function Deficits and Extends Survival in a SOD1-ALS Mouse Model. Gene Ther. 2023, 30, 443–454. [Google Scholar] [CrossRef]
- McCallister, T.X.; Lim, C.K.W.; Singh, M.; Zhang, S.; Ahsan, N.S.; Terpstra, W.M.; Xiong, A.Y.; Zeballos Castro, M.A.; Powell, J.E.; Drnevich, J.; et al. A High-Fidelity CRISPR-Cas13 System Improves Abnormalities Associated with C9ORF72-Linked ALS/FTD. Nat. Commun. 2025, 16, 460. [Google Scholar] [CrossRef]
- Baird, M.C.; Likhite, S.B.; Vetter, T.A.; Caporale, J.R.; Girard, H.B.; Roussel, F.S.; Howard, A.E.; Schwartz, M.K.; Reed, A.R.; Kaleem, A.; et al. Combination AAV Therapy with Galectin-1 and SOD1 Downregulation Demonstrates Superior Therapeutic Effect in a Severe ALS Mouse Model. Mol. Ther. Methods Clin. Dev. 2024, 32, 101312. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, D.; Du, X.; Chen, L.; Guo, Y. Microglial Upregulation of CD109 Expression in Spinal Cord of Amyotrophic Lateral Sclerosis Mouse Model and Its Role in Modulating Inflammation and TGFβ/SMAD Pathway. Neuroscience 2025, 564, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, Z.; Wang, J. MicroRNA-124: A Key Player in Microglia-Mediated Inflammation in Neurological Diseases. Front. Cell Neurosci. 2021, 15, 771898. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 Promotes Microglia Quiescence and Suppresses EAE by Deactivating Macrophages via the C/EBP-α–PU.1 Pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal Stem Cell-Derived Extracellular Vesicles Prevent the Development of Osteoarthritis via the CircHIPK3/MiR-124-3p/MYH9 Axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef]
| Mouse Model | Genetic Modification | Key Findings |
|---|---|---|
| SOD1G93A transgenic mouse [40,43] | Mouse expresses mutant human SOD1 with G93A mutation | - Early activation of microglia before clinical symptoms - Microglia shift from neuroprotective to neurotoxic phenotype as disease progresses - Upregulation of pro-inflammatory cytokines (TNF-α, IL-1β) - Impaired phagocytic capacity of microglia - Distinct proteomic signatures on chronically activated microglia - Depletion or resting of microglia delays disease onset and extends lifespan - Loss of perineuronal nets (PNNs) |
| SOD1G37R mouse [42] | Mouse expresses mutant human SOD1 with G37R mutation | - Slow-progressing model of ALS - Used to study disease progression and microglial contributions over time |
| TDP-43Q331K mouse [50] | Mouse expresses mutant TDP-43 with Q331K mutation | - Loss of perineuronal nets (PNNs) exacerbates α-motor neuron degeneration and ALS symptoms - Highlights the role of extracellular matrix in neuronal protection |
| SOD1G93A; IKKβf/wt; CSF1R-Cre+ mice [49] | Mice express SOD1G93A mutation with conditional NF-κB inhibition in microglia | - Reduced expression of inflammatory markers (CD68, CD86, iNOS) - Demonstrates that pro-inflammatory microglia contribute to motor neuron death - NF-κB inhibition in microglia delays disease progression |
| CSF1R-Cre mice [51] | Mice express Cre recombinase under the CSF1R promoter for microglia-specific gene manipulation | - Show reduced microglial activation and astrogliosis compared to controls - NF-κB inhibition in astrocytes does not improve ALS symptoms and may be detrimental - Suggests distinct roles of microglia and astrocytes in ALS progression |
| C9ORF72 mouse models (e.g., C9-500) [52,53] | Mice with hexanucleotide G4C2 repeat expansions in the C9ORF72 gene | - Microglia exhibit abnormal activation with increased pro-inflammatory cytokines - Impaired phagocytic function and autophagy - Accumulation of toxic protein aggregates - Contributes to motor neuron degeneration and disease progression - Indicates both gain-of-function toxicity and loss-of-function effects from C9ORF72 mutations |
| CX3CR1-deficient SOD1G93A mice [54,55] | Mice express SOD1G93A mutation with CX3CR1 receptor deficiency in microglia | - CX3CR1 deficiency exacerbates motor neuron loss - Accelerated disease progression - Highlights the importance of neuron–microglia communication via fractalkine–CX3CR1 signaling in neuroprotection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, S.; Saxena, S.; Sierra-Delgado, J.A. Microglia in ALS: Insights into Mechanisms and Therapeutic Potential. Cells 2025, 14, 421. https://doi.org/10.3390/cells14060421
Bond S, Saxena S, Sierra-Delgado JA. Microglia in ALS: Insights into Mechanisms and Therapeutic Potential. Cells. 2025; 14(6):421. https://doi.org/10.3390/cells14060421
Chicago/Turabian StyleBond, Silvano, Smita Saxena, and Julieth A. Sierra-Delgado. 2025. "Microglia in ALS: Insights into Mechanisms and Therapeutic Potential" Cells 14, no. 6: 421. https://doi.org/10.3390/cells14060421
APA StyleBond, S., Saxena, S., & Sierra-Delgado, J. A. (2025). Microglia in ALS: Insights into Mechanisms and Therapeutic Potential. Cells, 14(6), 421. https://doi.org/10.3390/cells14060421






