Selinexor’s Immunomodulatory Impact in Advancing Multiple Myeloma Treatment
Abstract
1. Introduction
2. Immunological Channeling of the TME in Myeloma
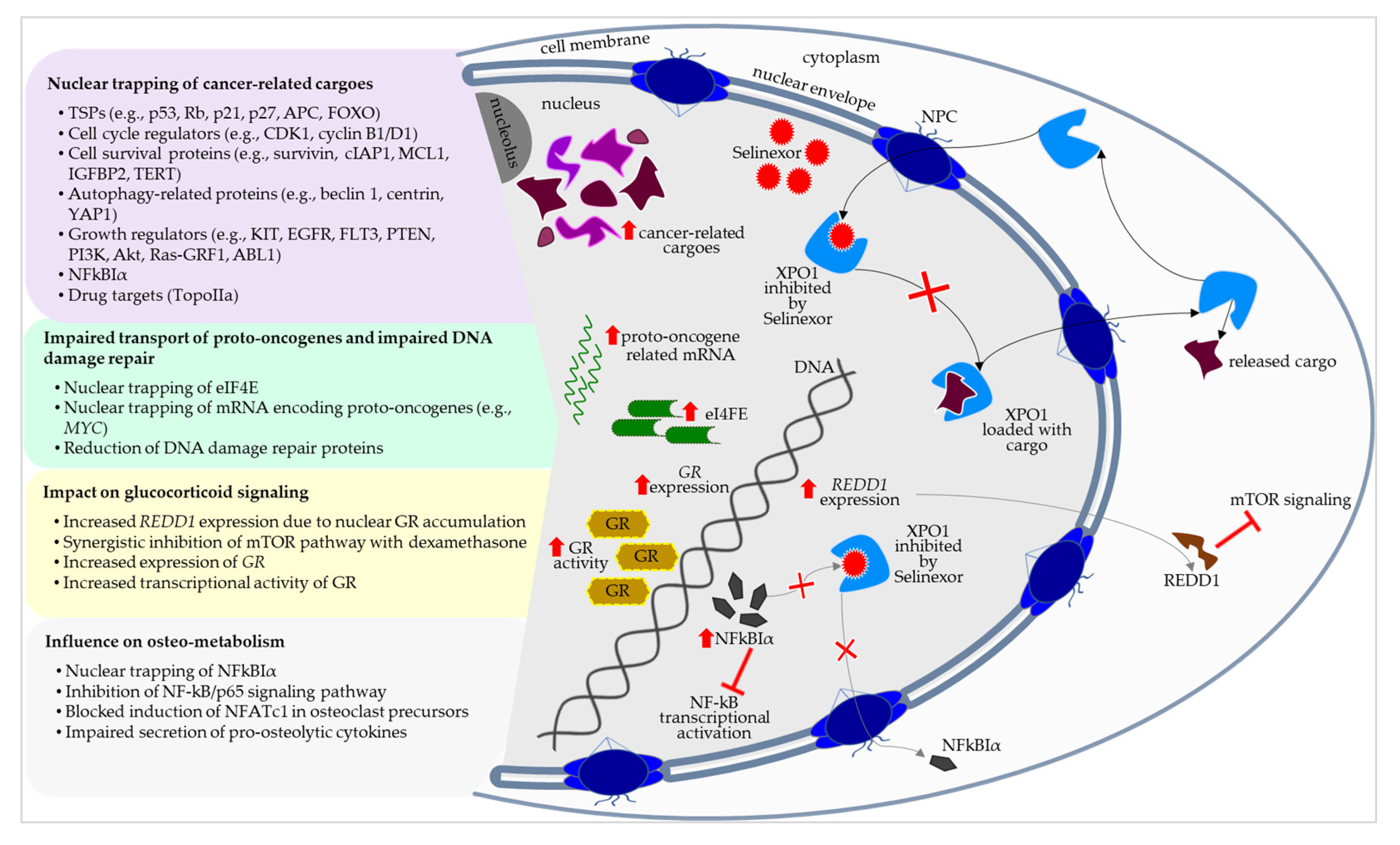
3. Selinexor’s Molecular–Biological Positioning in the Treatment of Myeloma
4. Selinexor’s Impact on the Immunological TME
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Hevroni, G.; Vattigunta, M.; Kazandjian, D.; Coffey, D.; Diamond, B.; Maura, F.; Hoffman, J.; Landgren, O. From MGUS to multiple myeloma: Unraveling the unknown of precursor states. Blood Rev. 2024, 68, 101242. [Google Scholar] [CrossRef] [PubMed]
- Bawek, S.; Joseph, J.; Hutson, A.; Parker, S.; Tario, J.; Hillengass, J. Prognostic factors for progression of MGUS including immune phenotype. J. Clin. Oncol. 2024, 42, 16. [Google Scholar] [CrossRef]
- Kyle, R.A.; Durie, B.G.M.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kröger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef]
- Patel, R.; Hill, E.; Dhodapkar, M. Smoldering Multiple Myeloma: Integrating Biology and Risk into Management. Semin. Hematol. 2024, in press. [Google Scholar] [CrossRef]
- Abdallah, N.H.; Lakshman, A.; Kumar, S.K.; Cook, J.; Binder, M.; Kapoor, P.; Dispenzieri, A.; Gertz, M.A.; Lacy, M.Q.; Hayman, S.R. Mode of progression in smoldering multiple myeloma: A study of 406 patients. Blood Cancer J. 2024, 14, 9. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S.; Lonial, S.; Mateos, M.V. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022, 12, 129. [Google Scholar] [CrossRef]
- Fernández de Larrea, C.; Kyle, R.; Rosiñol, L.; Paiva, B.; Engelhardt, M.; Usmani, S.; Caers, J.; Gonsalves, W.; Schjesvold, F.; Merlini, G. Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021, 11, 192. [Google Scholar] [CrossRef]
- Fernandez, N.; Perumal, D.; Rahman, A.; Kim-Schulze, S.; Yesil, J.; Auclair, D.; Adams, H., III; Parekh, S.; Gnjatic, S.; Cho, H.J. High dimensional immune profiling of smoldering multiple myeloma distinguishes distinct tumor microenvironments. Clin. Lymphoma Myeloma Leuk. 2022, 22, 853–862. [Google Scholar] [CrossRef]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef]
- Michels, T.C.; Petersen, K.E. Multiple myeloma: Diagnosis and treatment. Am. Fam. Physician 2017, 95, 373–383A. [Google Scholar] [PubMed]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; García-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of multiple myeloma-related bone disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021, 22, 119–130. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Z.; Cheng, H.; Mao, X.; Sui, W.; Deng, S.; Wei, X.; Lv, J.; Du, C.; Xu, J. Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Sci. Rep. 2020, 10, 20508. [Google Scholar] [CrossRef]
- Bridoux, F.; Leung, N.; Belmouaz, M.; Royal, V.; Ronco, P.; Nasr, S.H.; Fermand, J.P.; Kidney, I.; Group, M.G.R. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. 2021, 99, 570–580. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Han, X.; He, J.; Zheng, G.; Cai, Z. Baseline peripheral neuropathy was associated with age and a prognostic factor in newly diagnosed multiple myeloma patients. Sci. Rep. 2022, 12, 10061. [Google Scholar] [CrossRef]
- Raje, N.S.; Anaissie, E.; Kumar, S.K.; Lonial, S.; Martin, T.; Gertz, M.A.; Krishnan, A.; Hari, P.; Ludwig, H.; O’Donnell, E. Consensus guidelines and recommendations for infection prevention in multiple myeloma: A report from the International Myeloma Working Group. Lancet Haematol. 2022, 9, 143–161. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Musolino, C.; Pioggia, G.; Gangemi, S. Secondary immunodeficiency in hematological malignancies: Focus on multiple myeloma and chronic lymphocytic leukemia. Front. Immunol. 2021, 12, 738915. [Google Scholar] [CrossRef]
- Rasche, L.; Kortüm, K.M.; Raab, M.S.; Weinhold, N. The impact of tumor heterogeneity on diagnostics and novel therapeutic strategies in multiple myeloma. Int. J. Mol. Sci. 2019, 20, 1248. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: Real-world experience from the myeloma CAR T consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef]
- Martin, T.G.; Madduri, D.; Pacaud, L.; Usmani, S.Z. Cilta-cel, a BCMA-targeting CAR-T therapy for heavily pretreated patients with relapsed/refractory multiple myeloma. Future Oncol. 2023, 19, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Patel, K.K.; Peres, L.C.; Bansal, R.; Kocoglu, M.H.; Shune, L.; Atrash, S.; Smith, K.; Midha, S.; Ferreri, C. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood 2025, 145, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Tomasson, M.H.; Iida, S.; Niesvizky, R.; Mohty, M.; Bahlis, N.J.; Martinez-Lopez, J.; Koehne, G.; Rodriguez-Otero, P.; Prince, H.M.; Viqueira, A. Long-term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM-3 study. HemaSphere 2024, 8, e136. [Google Scholar] [CrossRef]
- Baines, A.C.; Kanapuru, B.; Zhao, J.; Price, L.S.; Zheng, N.; Konicki, R.; Manning, M.L.; Gehrke, B.J.; Theoret, M.R.; Gormley, N.J. FDA Approval Summary: Teclistamab–A Bispecific CD3 T-Cell Engager for Patients with Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2024, 30, 5515–5520. [Google Scholar] [CrossRef]
- Einsele, H.; Moreau, P.; Bahlis, N.; Bhutani, M.; Vincent, L.; Karlin, L.; Perrot, A.; Goldschmidt, H.; van de Donk, N.W.; Ocio, E.M. Comparative Efficacy of Talquetamab vs. Current Treatments in the LocoMMotion and MoMMent Studies in Patients with Triple-Class-Exposed Relapsed/Refractory Multiple Myeloma. Adv. Ther. 2024, 41, 1576–1593. [Google Scholar] [CrossRef]
- Costa, B.A.; Costa, T.A.; Chagas, G.C.L.; Mouhieddine, T.H.; Richter, J.; Usmani, S.Z.; Mailankody, S.; Rajeeve, S.; Hashmi, H. Addition of elotuzumab to backbone treatment regimens for multiple myeloma: An updated meta-analysis of randomized clinical trials. Clin. Lymphoma Myeloma Leuk. 2024, 25, 32–44. [Google Scholar] [CrossRef]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, staging, and management of multiple myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; Wong, M.C.S. The epidemiological landscape of multiple myeloma: A global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022, 9, 670–677. [Google Scholar] [CrossRef]
- IARC. Global Cancer Observatory. Available online: https://gco.iarc.who.int/en (accessed on 28 February 2025).
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Kengelbach-Weigand, A.; Tasbihi, K.; Strissel, P.L.; Schmid, R.; Marques, J.M.; Beier, J.P.; Beckmann, M.W.; Strick, R.; Horch, R.E.; Boos, A.M. Plasticity of patient-matched normal mammary epithelial cells is dependent on autologous adipose-derived stem cells. Sci. Rep. 2019, 9, 10722. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Nadorp, B.; Fornerod, M.; Nicolet, D.; Wu, H.; Walker, C.J.; Sun, Z.; Witkowski, M.T.; Tikhonova, A.N.; Guillamot-Ruano, M. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat. Cancer 2023, 4, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Battista, R.A.; Pini, G.M.; Finco, A.; Corso, F.; Galli, A.; Arrigoni, G.; Doglioni, C.; Callea, M.; Paccagnella, M.; Porcu, L. From Tumor Macroenvironment to Tumor Microenvironment: The Prognostic Role of the Immune System in Oral and Lung Squamous Cell Carcinoma. Cancers 2024, 16, 2759. [Google Scholar] [CrossRef] [PubMed]
- Bonato, A.; Chakraborty, S.; Bomben, R.; Canarutto, G.; Felician, G.; Martines, C.; Zucchetto, A.; Pozzo, F.; Vujovikj, M.; Polesel, J. NFKBIE mutations are selected by the tumor microenvironment and contribute to immune escape in chronic lymphocytic leukemia. Leukemia 2024, 38, 1511–1521. [Google Scholar] [CrossRef]
- Unal, B.; Kuzu, O.F.; Jin, Y.; Osorio, D.; Kildal, W.; Pradhan, M.; Kung, S.H.; Oo, H.Z.; Daugaard, M.; Vendelbo, M. Targeting IRE1α reprograms the tumor microenvironment and enhances anti-tumor immunity in prostate cancer. Nat. Commun. 2024, 15, 8895. [Google Scholar] [CrossRef]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.A.; João, C. The Immune Microenvironment in Multiple Myeloma: Friend or Foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef]
- Wang, S.S.Y.; Chng, W.J.; Liu, H.; de Mel, S. Tumor-Associated Macrophages and Related Myelomonocytic Cells in the Tumor Microenvironment of Multiple Myeloma. Cancers 2022, 14, 5654. [Google Scholar] [CrossRef]
- Sadaf, H.; Hong, H.; Maqbool, M.; Emhoff, K.; Lin, J.; Yan, S.; Anwer, F.; Zhao, J. Multiple myeloma etiology and treatment. J. Transl. Genet. Genom. 2022, 6, 63–83. [Google Scholar] [CrossRef]
- Dhodapkar, M.V. The immune system in multiple myeloma and precursor states: Lessons and implications for immunotherapy and interception. Am. J. Hematol. 2023, 98, S4–S12. [Google Scholar] [CrossRef]
- Verheye, E.; Kancheva, D.; Satilmis, H.; Vandewalle, N.; Fan, R.; Bardet, P.M.; Clappaert, E.J.; Verstaen, K.; De Becker, A.; Vanderkerken, K. A single-cell transcriptomic map of the murine and human multiple myeloma immune microenvironment across disease stages. J. Hematol. Oncol. 2024, 17, 107. [Google Scholar] [CrossRef]
- Rasche, L.; Schinke, C.; Touzeau, C.; Minnema, M.C.; van de Donk, N.W.; Rodríguez-Otero, P.; Mateos, M.-V.; Ye, J.C.; Vishwamitra, D.; Singh, I. MM-492 long-term efficacy and safety results from the phase 1/2 MonumenTAL-1 study of Talquetamab, a GPRC5D× CD3 Bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2024, 24, S561–S562. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Mateos, M.V.; Nooka, A.; Banerjee, A.; Kobos, R.; Pei, L.; Qi, M.; Verona, R.; Doyle, M.; Smit, J. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer 2023, 129, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Mo, C.C.; Yee, A.J.; Midha, S.; Hartley-Brown, M.A.; Nadeem, O.; O’Donnell, E.K.; Bianchi, G.; Sperling, A.S.; Laubach, J.P.; Richardson, P.G. Selinexor: Targeting a novel pathway in multiple myeloma. EJHaem 2023, 4, 792–810. [Google Scholar] [CrossRef]
- Binder, A.F.; Walker, C.J.; Mark, T.M.; Baljevic, M. Impacting T-cell fitness in multiple myeloma: Potential roles for selinexor and XPO1 inhibitors. Front. Immunol. 2023, 14, 1275329. [Google Scholar] [CrossRef]
- Camilli, S.; Lockey, R.; Kolliputi, N. Nuclear Export Inhibitors Selinexor (KPT-330) and Eltanexor (KPT-8602) Provide a Novel Therapy to Reduce Tumor Growth by Induction of PANoptosis. Cell Biochem. Biophys. 2023, 81, 421–426. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, F.; Alapat, D.V.; Wanchai, V.; Mery, D.; Siegel, E.R.; Xu, H.; Johnson, S.; Guo, W.; Bailey, C. Multi-omics reveal immune microenvironment alterations in multiple myeloma and its precursor stages. Blood Cancer J. 2024, 14, 194. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 421–426. [Google Scholar] [CrossRef]
- Kaur, S.; Raggatt, L.J.; Batoon, L.; Hume, D.A.; Levesque, J.-P.; Pettit, A.R. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin. Cell Dev. Biol. 2017, 61, 12–21. [Google Scholar] [CrossRef]
- Smith, T.D.; Tse, M.J.; Read, E.L.; Liu, W.F. Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. 2016, 8, 946–955. [Google Scholar] [CrossRef]
- De Beule, N.; De Veirman, K.; Maes, K.; De Bruyne, E.; Menu, E.; Breckpot, K.; De Raeve, H.; Van Rampelbergh, R.; Van Ginderachter, J.A.; Schots, R. Tumour-associated macrophage-mediated survival of myeloma cells through STAT3 activation. J. Pathol. 2017, 241, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, H.; Gu, H.; Zhang, E.; He, J.; Cao, W.; Qu, J.; Xu, R.; Cao, L.; He, D.; et al. Myeloma-derived IL-32γ induced PD-L1 expression in macrophages facilitates immune escape via the PFKFB3-JAK1 axis. Oncoimmunology 2022, 11, 2057837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, D.; Luo, Y.; Sun, Y.; Duan, C.; Yang, J.; Wei, J.; Li, X.; Lu, Y.; Lai, X. miR-34a promotes the immunosuppressive function of multiple myeloma-associated macrophages by dampening the TLR-9 signaling. Cancer Med. 2024, 13, e7387. [Google Scholar] [CrossRef]
- Sun, J.; Corradini, S.; Azab, F.; Shokeen, M.; Muz, B.; Miari, K.E.; Maksimos, M.; Diedrich, C.; Asare, O.; Alhallak, K.; et al. IL-10R inhibition reprograms tumor-associated macrophages and reverses drug resistance in multiple myeloma. Leukemia 2024, 38, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Tang, Q.; Zhao, J.; Wang, Z.; Wei, L.; Wei, Q.; Yin, L.; Luo, S.; Song, J. S100A9 Derived from Myeloma Associated Myeloid Cells Promotes TNFSF13B/TNFRSF13B-Dependent Proliferation and Survival of Myeloma Cells. Front. Oncol. 2021, 11, 691705. [Google Scholar] [CrossRef]
- Akhmetzyanova, I.; Aaron, T.; Galbo, P.; Tikhonova, A.; Dolgalev, I.; Tanaka, M.; Aifantis, I.; Zheng, D.; Zang, X.; Fooksman, D. Tissue-resident macrophages promote early dissemination of multiple myeloma via IL-6 and TNFα. Blood Adv. 2021, 5, 3592–3608. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of natural killer cell cytotoxicity by interleukin-6: Implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015, 67, 3037–3046. [Google Scholar] [CrossRef]
- Jurišić, V.; Čolović, M. Correlation of sera TNF-α with percentage of bone marrow plasma cells, LDH, β 2-microglobulin, and clinical stage in multiple myeloma. Med. Oncol. 2002, 19, 133–139. [Google Scholar] [CrossRef]
- Gu, J.; Huang, X.; Zhang, Y.; Bao, C.; Zhou, Z.; Jin, J. Cytokine profiles in patients with newly diagnosed multiple myeloma: Survival is associated with IL-6 and IL-17A levels. Cytokine 2021, 138, 155358. [Google Scholar] [CrossRef]
- Pop, V.S.; Iancu, M.; Țigu, A.B.; Adam, A.; Tomoaia, G.; Farcas, A.D.; Bojan, A.S.; Parvu, A. The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment. Curr. Issues Mol. Biol. 2024, 46, 9330–9341. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, M.; Wu, H.; Chen, C.; Li, H.; Qiu, S.; Wang, T.; Han, J.; Xiao, Q.; Chen, K. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma by polarizing M2 macrophages in the bone marrow microenvironment. Leuk. Res. 2021, 105, 106566. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xiao, Q.; Wang, X.; Yang, C.; Chen, C.; Tian, X.; Wang, S.; Li, H.; Qiu, S.; Shu, J. Tumor-associated macrophages modulate angiogenesis and tumor growth in a xenograft mouse model of multiple myeloma. Leuk. Res. 2021, 110, 106709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, J.; Wang, F.; Ding, H.; Cui, Y.; Yang, Y.; Xu, J.; Luo, H.; Gao, Y.; Pan, L. BMI1 regulates multiple myeloma-associated macrophage’s pro-myeloma functions. Cell Death Dis. 2021, 12, 495. [Google Scholar] [CrossRef]
- Hofbauer, D.; Mougiakakos, D.; Broggini, L.; Zaiss, M.; Büttner-Herold, M.; Bach, C.; Spriewald, B.; Neumann, F.; Bisht, S.; Nolting, J. β2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity 2021, 54, 1772–1787. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Cao, P.; Wang, H.; Liu, H.; Hua, L.; Xue, H.; Fu, R. Tumor-associated macrophages regulate the function of cytotoxic T lymphocyte through PD-1/PD-L1 pathway in multiple myeloma. Cancer Med. 2022, 11, 4838–4848. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, W.; Sun, R.; Liu, T.; Zheng, Y.; Wu, Y. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget 2017, 8, 112685–112696. [Google Scholar] [CrossRef]
- Wang, H.; Hu, W.M.; Xia, Z.J.; Liang, Y.; Lu, Y.; Lin, S.X.; Tang, H. High numbers of CD163+ tumor-associated macrophages correlate with poor prognosis in multiple myeloma patients receiving bortezomib-based regimens. J. Cancer 2019, 10, 3239–3245. [Google Scholar] [CrossRef]
- Niblock, A.; Rajendran, S.; Laverty, C.; Logue, P.; Alexander, H.D. Assessing the frequency of CD163+ tumor-associated macrophages and CD3+ T lymphocytes between MGUS and plasma cell myeloma. Exp. Hematol. 2023, 119–120, 3–7. [Google Scholar] [CrossRef]
- Katoh, H.; Okamoto, R.; Yokota, M.; Naito, K.; Kikuchi, M.; Tokito, T.; Sangai, T.; Yamashita, K. CD163(+) Tumor-Associated Macrophage Recruitment Predicts Papillary Thyroid Cancer Recurrence. J. Surg. Res. 2024, 303, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, M.M.; Tumelius, R.; Hämäläinen, K.; Rilla, K.; Oikari, S.; Rönkä, A.; Selander, T.; Mannermaa, A.; Tiainen, S.; Auvinen, P. High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer. Cancers 2024, 16, 634. [Google Scholar] [CrossRef] [PubMed]
- De Matos Rodrigues, J.; Lokhande, L.; Olsson, L.M.; Hassan, M.; Johansson, A.; Janská, A.; Kumar, D.; Schmidt, L.; Nikkarinen, A.; Hollander, P.; et al. CD163+ macrophages in mantle cell lymphoma induce activation of prosurvival pathways and immune suppression. Blood Adv. 2024, 8, 4370–4385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, D.; Wang, Y.; Pan, Q.; Zhao, J.; Chen, H.; Yang, X.; Tang, Y.; Wang, Q.; Li, Y.; et al. EBV promotes TCR-T-cell therapy resistance by inducing CD163+M2 macrophage polarization and MMP9 secretion. J. Immunother. Cancer 2024, 12, e008375. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Andersen, M.N.; Andersen, N.F.; Lauridsen, K.L.; Etzerodt, A.; Sorensen, B.S.; Abildgaard, N.; Plesner, T.; Hokland, M.; Møller, H.J. STAT3 is over-activated within CD163(pos) bone marrow macrophages in both Multiple Myeloma and the benign pre-condition MGUS. Cancer Immunol. Immunother. 2022, 71, 177–187. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Bach, C.; Bottcher, M.; Beier, F.; Rohner, L.; Stoll, A.; Rehli, M.; Gebhard, C.; Lischer, C.; Eberhardt, M.; et al. The IKZF1-IRF4/IRF5 Axis Controls Polarization of Myeloma-Associated Macrophages. Cancer Immunol. Res. 2021, 9, 265–278. [Google Scholar] [CrossRef]
- Coulton, A.; Murai, J.; Qian, D.; Thakkar, K.; Lewis, C.E.; Litchfield, K. Using a pan-cancer atlas to investigate tumour associated macrophages as regulators of immunotherapy response. Nat. Commun. 2024, 15, 5665. [Google Scholar] [CrossRef]
- Yang, T.; Deng, Z.; Xu, L.; Li, X.; Yang, T.; Qian, Y.; Lu, Y.; Tian, L.; Yao, W.; Wang, J. Macrophages-aPKCɩ-CCL5 feedback loop modulates the progression and chemoresistance in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 23. [Google Scholar] [CrossRef]
- Guan, W.; Li, F.; Zhao, Z.; Zhang, Z.; Hu, J.; Zhang, Y. Tumor-associated macrophage promotes the survival of cancer cells upon docetaxel chemotherapy via the CSF1/CSF1R–CXCL12/CXCR4 axis in castration-resistant prostate cancer. Genes 2021, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Cheney, E.M.; Hartl, C.A.; Pantelidou, C.; Oliwa, M.; Castrillon, J.A.; Lin, J.-R.; Hurst, K.E.; de Oliveira Taveira, M.; Johnson, N.T. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nat. Cancer 2021, 2, 66–82. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, D.; Wu, J.; Sui, C.; Deng, X.; Zhang, P.; Chen, Z.; Liu, D.; Yu, J.; Shi, J. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch. Biochem. Biophys. 2021, 702, 108838. [Google Scholar] [CrossRef]
- Beider, K.; Voevoda-Dimenshtein, V.; Zoabi, A.; Rosenberg, E.; Magen, H.; Ostrovsky, O.; Shimoni, A.; Weiss, L.; Abraham, M.; Peled, A. CXCL13 chemokine is a novel player in multiple myeloma osteolytic microenvironment, M2 macrophage polarization, and tumor progression. J. Hematol. Oncol. 2022, 15, 144. [Google Scholar] [CrossRef]
- Razi, B.; Soleimani, M.; Soufi-Zomorrod, M.; Adeli, A.; Davoudi, N. Multiple myeloma cell-derived exosomes promote favorable tumor functional performance by polarizing macrophages toward M2-like cells. Apmis 2023, 131, 381–393. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Urzì, O.; Moschetti, M.; Di Bella, M.A.; Conigliaro, A.; Caccamo, N.; La Manna, M.P.; Fontana, S.; Alessandro, R. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12118. [Google Scholar] [CrossRef]
- Jibril, A.; Hellmich, C.; Wojtowicz, E.E.; Hampton, K.; Maynard, R.; De Silva, R.; Fowler-Shorten, D.J.; Mistry, J.J.; Moore, J.A.; Bowles, K.M.; et al. Plasma cell-derived mtDAMPs activate the macrophage STING pathway, promoting myeloma progression. Blood 2023, 141, 3065–3077. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Li, B.; Chen, X.; Xiong, H.; Huang, C. Decreased STING predicts adverse efficacy in bortezomib regimens and poor survival in multiple myeloma. Clin. Exp. Med. 2025, 25, 1–12. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Giannotta, C.; Autino, F.; Massaia, M. The immune suppressive tumor microenvironment in multiple myeloma: The contribution of myeloid-derived suppressor cells. Front. Immunol. 2023, 13, 1102471. [Google Scholar] [CrossRef]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.R.; Shah, S.; Kang, K.-T. Dual roles of myeloid-derived suppressor cells in various diseases: A review. Arch. Pharmacal Res. 2024, 47, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Chakravarti, M.; Ganguly, N.; Saha, A.; Dasgupta, S.; Bera, S.; Sarkar, A.; Roy, K.; Das, J.; Bhuniya, A. High monocytic MDSC signature predicts multi-drug resistance and cancer relapse in non-Hodgkin lymphoma patients treated with R-CHOP. Front. Immunol. 2024, 14, 1303959. [Google Scholar] [CrossRef]
- Kuwahara-Ota, S.; Shimura, Y.; Steinebach, C.; Isa, R.; Yamaguchi, J.; Nishiyama, D.; Fujibayashi, Y.; Takimoto-Shimomura, T.; Mizuno, Y.; Matsumura-Kimoto, Y. Lenalidomide and pomalidomide potently interfere with induction of myeloid-derived suppressor cells in multiple myeloma. Br. J. Haematol. 2020, 191, 784–795. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Kim, T.-W.; Ryu, D.-B.; Park, S.-S.; Lee, S.-E.; Kim, B.-S.; Min, C.-K. Myeloma-secreted galectin-1 potently interacts with CD304 on monocytic myeloid-derived suppressor cells. Cancer Immunol. Res. 2021, 9, 503–513. [Google Scholar] [CrossRef]
- Bae, M.-H.; Park, C.-J.; Suh, C. Increased monocytic myeloid-derived suppressor cells in whole blood predict poor prognosis in patients with plasma cell myeloma. J. Clin. Med. 2021, 10, 4717. [Google Scholar] [CrossRef]
- Perez, C.; Botta, C.; Zabaleta, A.; Puig, N.; Cedena, M.-T.; Goicoechea, I.; Alameda, D.; San José-Eneriz, E.; Merino, J.; Rodríguez-Otero, P. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood J. Am. Soc. Hematol. 2020, 136, 199–209. [Google Scholar] [CrossRef]
- Mizuhara, K.; Shimura, Y.; Tsukamoto, T.; Kanai, A.; Kuwahara-Ota, S.; Yamaguchi, J.; Muramatsu, A.; Okamoto, H.; Taminishi-Katsuragawa, Y.; Kawaji-Kanayama, Y.; et al. Tumour-derived exosomes promote the induction of monocytic myeloid-derived suppressor cells from peripheral blood mononuclear cells by delivering miR-106a-5p and miR-146a-5p in multiple myeloma. Br. J. Haematol. 2023, 203, 426–438. [Google Scholar] [CrossRef]
- Xiong, W.; Xiao, L.; Duan, R.; Wang, Q.; Xian, M.; Zhang, C.; Su, P.; Yabo, L.; Zhong, L.; Qian, J. Depletion of myeloid-derived suppressor cells sensitizes murine multiple myeloma to PD-1 checkpoint inhibitors. J. ImmunoTherapy Cancer 2025, 13, 008979. [Google Scholar] [CrossRef]
- Aleman, A.; Zhao, R.; Grossman, L.; Sanawar, R.; Aluri, S.; Mohindru, M.; Dutta, R.P.; Reci, S.; Vieira, J.; Melnekoff, D. BCMA CART Therapy Failure in Myeloma Is Associated with Immunosuppressive S100A8/9 Overexpression and Can be Ameliorated with Novel Monoclonal Antibodies. Blood 2024, 144, 589. [Google Scholar] [CrossRef]
- Lubin, R.; Patel, A.A.; Mackerodt, J.; Zhang, Y.; Gvili, R.; Mulder, K.; Dutertre, C.-A.; Jalali, P.; Glanville, J.R.W.; Hazan, I.; et al. The lifespan and kinetics of human dendritic cell subsets and their precursors in health and inflammation. J. Exp. Med. 2024, 221, e20220867. [Google Scholar] [CrossRef] [PubMed]
- Hoefsmit, E.P.; van Royen, P.T.; Rao, D.; Stunnenberg, J.A.; Dimitriadis, P.; Lieftink, C.; Morris, B.; Rozeman, E.A.; Reijers, I.L.; Lacroix, R. Inhibitor of apoptosis proteins antagonist induces T-cell proliferation after cross-presentation by dendritic cells. Cancer Immunol. Res. 2023, 11, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Uto, T.; Fukaya, T.; Mitoma, S.; Riethmacher, D.; Umekita, K.; Yamashita, Y.; Sato, K. Crucial role of dendritic cells in the generation of anti-tumor T-cell responses and immunogenic tumor microenvironment to suppress tumor development. Front. Immunol. 2024, 15, 1200461. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Komiya, K.; Tsuda, S.; Yoshino, M.; Kaisho, T.; Bergsagel, P.L.; Kawamura, K.; Fukuda, T.; Tokoyoda, K. Type I-conventional dendritic cells support the progression of multiple myeloma in the bone marrow. Front. Immunol. 2024, 15, 1444821. [Google Scholar] [CrossRef]
- Knight, A.; Rihova, L.; Kralova, R.; Penka, M.; Adam, Z.; Pour, L.; Piskacek, M.; Hajek, R. Plasmacytoid Dendritic Cells in Patients with MGUS and Multiple Myeloma. J. Clin. Med. 2021, 10, 3717. [Google Scholar] [CrossRef]
- Ishibashi, M.; Sunakawa-Kii, M.; Imai, Y.; Yamaguchi, H.; Morita, R.; Tamura, H. Myeloma Cell-Derived Monoclonal Immunoglobulins Trigger IL-1β Secretion Via Inflammasome Activation in Tolerogenic Dendritic Cells. Blood 2024, 144, 1900. [Google Scholar] [CrossRef]
- Balasa, B.; Yun, R.; Belmar, N.A.; Fox, M.; Chao, D.T.; Robbins, M.D.; Starling, G.C.; Rice, A.G. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol. Immunother. 2015, 64, 61–73. [Google Scholar] [CrossRef]
- Pahl, J.H.; Koch, J.; Götz, J.-J.; Arnold, A.; Reusch, U.; Gantke, T.; Rajkovic, E.; Treder, M.; Cerwenka, A. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol. Res. 2018, 6, 517–527. [Google Scholar] [CrossRef]
- Muntasell, A.; Rojo, F.; Servitja, S.; Rubio-Perez, C.; Cabo, M.; Tamborero, D.; Costa-García, M.; Martínez-Garcia, M.; Menéndez, S.; Vazquez, I.; et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2+ Breast Cancer Predict and Uncouple Pathological Response and Disease-free Survival. Clin. Cancer Res. 2019, 25, 1535–1545. [Google Scholar] [CrossRef]
- Konjević, G.; Jović, V.; Jurišić, V.; Radulović, S.; Jelić, S.; Spužić, I. IL-2-mediated augmentation of NK-cell activity and activation antigen expression on NK-and T-cell subsets in patients with metastatic melanoma treated with interferon-α and DTIC. Clin. Exp. Metastasis 2003, 20, 647–655. [Google Scholar] [CrossRef]
- Sönmez, C.; Wölfer, J.; Holling, M.; Brokinkel, B.; Stummer, W.; Wiendl, H.; Thomas, C.; Schulte-Mecklenbeck, A.; Grauer, O.M. Blockade of inhibitory killer cell immunoglobulin-like receptors and IL-2 triggering reverses the functional hypoactivity of tumor-derived NK-cells in glioblastomas. Sci. Rep. 2022, 12, 6769. [Google Scholar] [CrossRef] [PubMed]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Young, A.; Mittal, D.; Martinet, L.; Bruedigam, C.; Takeda, K.; Andoniou, C.E.; Degli-Esposti, M.A.; Hill, G.R.; Smyth, M.J. NK cells require IL-28R for optimal in vivo activity. Proc. Natl. Acad. Sci. USA 2015, 112, 2376–2384. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; Van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Guan, X.; Guo, H.; Guo, Y.; Han, Q.; Li, Z.; Zhang, C. Perforin 1 in cancer: Mechanisms, therapy, and outlook. Biomolecules 2024, 14, 910. [Google Scholar] [CrossRef]
- Konjević, G.; Vuletić, A.; Martinović, K.M.; Colović, N.; Čolović, M.; Jurišić, V. Decreased CD161 activating and increased CD158a inhibitory receptor expression on NK cells underlies impaired NK cell cytotoxicity in patients with multiple myeloma. J. Clin. Pathol. 2016, 69, 1009–1016. [Google Scholar] [CrossRef]
- Pazina, T.; MacFarlane IV, A.W.; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.A.; Robbins, M.D.; Ross, E.A.; Campbell, K.S. Alterations of NK cell phenotype in the disease course of multiple myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef]
- D’Souza, C.; Keam, S.P.; Yeang, H.X.A.; Neeson, M.; Richardson, K.; Hsu, A.K.; Canfield, R.; Bezman, N.; Robbins, M.; Quach, H. Myeloma natural killer cells are exhausted and have impaired regulation of activation. Haematologica 2021, 106, 2522. [Google Scholar] [CrossRef]
- Seymour, F.; Cavenagh, J.D.; Mathews, J.; Gribben, J.G. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv. 2022, 6, 5152–5159. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Bernal, M.; Garrido, P.; Jiménez, P.; Carretero, R.; Almagro, M.; López, P.; Navarro, P.; Garrido, F.; Ruiz-Cabello, F. Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: Implications for tumor evasion of T and NK cells. Hum. Immunol. 2009, 70, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, E.; Ekren, R.; Rigaud, B.; Joubert, M.-V.; Baylot, V.; Daunes, H.; Cuisinier, M.; Villard, M.; Carrié, N.; Mazzotti, C. NK cells with adhesion defects and reduced cytotoxic functions are associated with a poor prognosis in multiple myeloma. Blood 2024, 144, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bergman, D.R.; Trujillo, E.; Pearson, A.T.; Sweis, R.F.; Jackson, T.L. Mathematical model predicts tumor control patterns induced by fast and slow cytotoxic T lymphocyte killing mechanisms. Sci. Rep. 2023, 13, 22541. [Google Scholar] [CrossRef]
- Lei, X.; de Groot, D.C.; Welters, M.J.; de Wit, T.; Schrama, E.; van Eenennaam, H.; Santegoets, S.J.; Oosenbrug, T.; van der Veen, A.; Vos, J.L. CD4+ T cells produce IFN-I to license cDC1s for induction of cytotoxic T-cell activity in human tumors. Cell. Mol. Immunol. 2024, 21, 374–392. [Google Scholar] [CrossRef]
- Glasner, A.; Rose, S.A.; Sharma, R.; Gudjonson, H.; Chu, T.; Green, J.A.; Rampersaud, S.; Valdez, I.K.; Andretta, E.S.; Dhillon, B.S. Conserved transcriptional connectivity of regulatory T cells in the tumor microenvironment informs new combination cancer therapy strategies. Nat. Immunol. 2023, 24, 1020–1035. [Google Scholar] [CrossRef]
- Beyer, M.; Kochanek, M.; Giese, T.; Endl, E.; Weihrauch, M.R.; Knolle, P.A.; Classen, S.; Schultze, J.L. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006, 107, 3940–3949. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Kaminska, W.; Hus, I.; Dmoszynska, A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: Detailed characterisation of immune status in multiple myeloma. Br. J. Cancer 2012, 106, 546–552. [Google Scholar] [CrossRef]
- Muthu Raja, K.R.; Kubiczkova, L.; Rihova, L.; Piskacek, M.; Vsianska, P.; Hezova, R.; Pour, L.; Hajek, R. Functionally Suppressive CD8 T Regulatory Cells Are Increased in Patients with Multiple Myeloma: A Cause for Immune Impairment. PLoS ONE 2012, 7, e49446. [Google Scholar] [CrossRef]
- Braga, W.M.T.; da Silva, B.R.; de Carvalho, A.C.; Maekawa, Y.H.; Bortoluzzo, A.B.; Rizzatti, E.G.; Atanackovic, D.; Colleoni, G.W.B. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4+ T regulatory cells. Cancer Immunol. Immunother. 2014, 63, 1189–1197. [Google Scholar] [CrossRef]
- Hadjiaggelidou, C.; Mandala, E.; Terpos, E.; Yiannaki, E.; Markala, D.; Triantafyllou, T.; Papatheodorou, A.; Gkastari, V.; Verrou, E.; Papanikolaou, A.; et al. Evaluation of regulatory T cells (Tregs) alterations in patients with multiple myeloma treated with bortezomib or lenalidomide plus dexamethasone: Correlations with treatment outcome. Ann. Hematol. 2019, 98, 1457–1466. [Google Scholar] [CrossRef]
- Kawano, Y.; Zavidij, O.; Park, J.; Moschetta, M.; Kokubun, K.; Mouhieddine, T.H.; Manier, S.; Mishima, Y.; Murakami, N.; Bustoros, M. Blocking IFNAR1 inhibits multiple myeloma–driven Treg expansion and immunosuppression. J. Clin. Investig. 2018, 128, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.K.; Allos, H.; Safadi, J.M.; Sulkaj, I.; Sanders, J.S.F.; Cravedi, P.; Ghobrial, I.M.; Berger, S.P.; Azzi, J.R. Type I interferons augment regulatory T cell polarization in concert with ancillary cytokine signals. Front. Transpl. 2023, 2, 1149334. [Google Scholar] [CrossRef] [PubMed]
- Alrasheed, N.; Lee, L.; Ghorani, E.; Henry, J.Y.; Conde, L.; Chin, M.; Galas-Filipowicz, D.; Furness, A.J.; Chavda, S.J.; Richards, H. Marrow-infiltrating regulatory T cells correlate with the presence of dysfunctional CD4+ PD-1+ cells and inferior survival in patients with newly diagnosed multiple myeloma. Clin. Cancer Res. 2020, 26, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhan, D.; Zhang, R.; Sun, Y.; Duan, C.; Yang, J.; Wei, J.; Li, X.; Lu, Y.; Lai, X. Treg-derived TGF-β1 dampens cGAS-STING signaling to downregulate the expression of class I MHC complex in multiple myeloma. Sci. Rep. 2024, 14, 11593. [Google Scholar] [CrossRef]
- Dahlhoff, J.; Manz, H.; Steinfatt, T.; Delgado-Tascon, J.; Seebacher, E.; Schneider, T.; Wilnit, A.; Mokhtari, Z.; Tabares, P.; Böckle, D. Transient regulatory T-cell targeting triggers immune control of multiple myeloma and prevents disease progression. Leukemia 2022, 36, 790–800. [Google Scholar] [CrossRef]
- Takahashi, S.; Minnie, S.A.; Ensbey, K.S.; Schmidt, C.R.; Sekiguchi, T.; Legg, S.R.; Zhang, P.; Koyama, M.; Olver, S.D.; Collinge, A.D. Regulatory T cells suppress myeloma-specific immunity during autologous stem cell mobilization and transplantation. Blood 2024, 143, 1656–1669. [Google Scholar] [CrossRef]
- Rossi, M.; Altomare, E.; Botta, C.; Gallo Cantafio, M.E.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, H.; Fan, J.; Mao, J.; Li, Y. Clinical significance of T helper cell subsets in the peripheral blood and bone marrow of patients with multiple myeloma. Front. Immunol. 2024, 15, 1445530. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, S.; Luo, T.; Guo, S.; Qu, J. Activated Tim-3/Galectin-9 participated in the development of multiple myeloma by negatively regulating Cd4 T cells. Hematology 2024, 29, 2288481. [Google Scholar] [CrossRef]
- Favaloro, J.; Bryant, C.E.; Abadir, E.; Gardiner, S.; Yang, S.; King, T.; Nassif, N.; Sedger, L.M.; Boyle, R.; Joshua, D.E.; et al. Single-cell analysis of the CD8(+) T-cell compartment in multiple myeloma reveals disease specific changes are chiefly restricted to a CD69(-) subset suggesting potent cytotoxic effectors exist within the tumor bed. Haematologica 2024, 109, 1220–1232. [Google Scholar] [CrossRef]
- Gardeney, H.; Narbeburu, E.; Barbarin, A.; Moya, N.; Bobin, A.; Levy, A.; Gruchet, C.; Leleu, X.; Herbelin, A.; Gombert, J.-M. NK Cells and KIR+/NKG2A+ Innate like CD8+ T Lymphocytes Features in the Bone Marrow of Multiple Myeloma Patients. Blood 2024, 144, 6865. [Google Scholar] [CrossRef]
- Larrayoz, M.; Garcia-Barchino, M.J.; Celay, J.; Etxebeste, A.; Jimenez, M.; Perez, C.; Ordoñez, R.; Cobaleda, C.; Botta, C.; Fresquet, V. Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma. Nat. Med. 2023, 29, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Awwad, M.H.; Mahmoud, A.; Bruns, H.; Echchannaoui, H.; Kriegsmann, K.; Lutz, R.; Raab, M.S.; Bertsch, U.; Munder, M.; Jauch, A. Selective elimination of immunosuppressive T cells in patients with multiple myeloma. Leukemia 2021, 35, 2602–2615. [Google Scholar] [CrossRef]
- Vishwamitra, D.; Skerget, S.; Cortes, D.; Lau, O.; Davis, C.; Renaud, T.; Tolbert, J.; Hilder, B.W.; Masterson, T.J.; Campagna, M. Mechanisms of resistance and relapse with talquetamab in patients with relapsed/refractory multiple myeloma from the phase 1/2 monumenTAL-1 study. Blood 2023, 142, 1933. [Google Scholar] [CrossRef]
- Casey, M.; Lee, C.; Kwok, W.Y.; Law, S.C.; Corvino, D.; Gandhi, M.K.; Harrison, S.J.; Nakamura, K. Regulatory T cells hamper the efficacy of T-cell-engaging bispecific antibody therapy. Haematologica 2023, 109, 787. [Google Scholar] [CrossRef]
- Firestone, R.S.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.; Wang, A.; Zhu, M.; Qin, W.G.; Patel, D.; Tan, C.R. CD8 effector T cells enhance teclistamab response in BCMA-exposed and-naïve multiple myeloma. Blood Adv. 2024, 8, 1600–1611. [Google Scholar] [CrossRef]
- Kriegsmann, K.; Ton, G.N.H.Q.; Awwad, M.H.; Benner, A.; Bertsch, U.; Besemer, B.; Hänel, M.; Fenk, R.; Munder, M.; Dürig, J. CD8+ CD28− regulatory T cells after induction therapy predict progression-free survival in myeloma patients: Results from the GMMG-HD6 multicenter phase III study. Leukemia 2024, 38, 1621–1625. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Steffen, J.M. The nuclear pore complex: A comprehensive review of structure and function. Int. J. Acad. Med. 2017, 3, 24–38. [Google Scholar] [CrossRef]
- Wing, C.E.; Fung, H.Y.J.; Chook, Y.M. Karyopherin-mediated nucleocytoplasmic transport. Nat. Rev. Mol. Cell Biol. 2022, 23, 307–328. [Google Scholar] [CrossRef]
- Ibarra, A.; Hetzer, M.W. Nuclear pore proteins and the control of genome functions. Genes Dev. 2015, 29, 337–349. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Holloway, M.P.; Altura, R.A. The CRM1 nuclear export protein in normal development and disease. Int. J. Biochem. Mol. Biol. 2012, 3, 137–151. [Google Scholar] [PubMed]
- Fung, H.Y.; Fu, S.C.; Chook, Y.M. Nuclear export receptor CRM1 recognizes diverse conformations in nuclear export signals. eLife 2017, 6, e23961. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, G.; He, R.-Q.; Huang, Z.-G.; Dang, Y.-W.; Luo, J.-Y.; Huang, W.-Y.; Huang, S.-N.; Liu, R.; Feng, Z.-B. Clinicopathological and prognostic significance of XPO1 in solid tumors: Meta-analysis and TCGA analysis. Expert Rev. Mol. Diagn. 2023, 23, 607–618. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, B.; Miao, Y.; Li, Y.; Qin, S.; Liang, J.; Kong, Y.; Zhang, X.; Tang, J.; Xia, Y. Prognostic value and therapeutic targeting of XPO1 in chronic lymphocytic leukemia. Clin. Exp. Med. 2023, 23, 2651–2662. [Google Scholar] [CrossRef]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef]
- Wolff, B.; Sanglier, J.-J.; Wang, Y. Leptomycin B is an inhibitor of nuclear export: Inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 1997, 4, 139–147. [Google Scholar] [CrossRef]
- Etchin, J.; Sun, Q.; Kentsis, A.; Farmer, A.; Zhang, Z.C.; Sanda, T.; Mansour, M.R.; Barcelo, C.; McCauley, D.; Kauffman, M.; et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 2013, 27, 66–74. [Google Scholar] [CrossRef]
- Razak, A.R.A.; Mau-Soerensen, M.; Gabrail, N.Y.; Gerecitano, J.F.; Shields, A.F.; Unger, T.J.; Saint-Martin, J.R.; Carlson, R.; Landesman, Y.; McCauley, D.; et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2016, 34, 4142–4150. [Google Scholar] [CrossRef]
- Karyopharm Therapeutics. Karyopharm and Menarini Group Receive Positive CHMP Opinion for NEXPOVIO® (selinexor) for the Treatment of Patients with Refractory Multiple Myeloma. Available online: https://investors.karyopharm.com/2022-05-20-Karyopharm-and-Menarini-Group-Receive-Positive-CHMP-Opinion-for-NEXPOVIO-R-selinexor-for-the-Treatment-of-Patients-with-Refractory-Multiple-Myeloma (accessed on 16 January 2025).
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. New Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- FDA. Prescribing Information for XPOVIO. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212306s005lbl.pdf (accessed on 16 January 2025).
- Bahlis, N.J.; Sutherland, H.; White, D.; Sebag, M.; Lentzsch, S.; Kotb, R.; Venner, C.P.; Gasparetto, C.; Del Col, A.; Neri, P. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood J. Am. Soc. Hematol. 2018, 132, 2546–2554. [Google Scholar] [CrossRef]
- Jakubowiak, A.J.; Jasielec, J.K.; Rosenbaum, C.A.; Cole, C.E.; Chari, A.; Mikhael, J.; Nam, J.; McIver, A.; Severson, E.; Stephens, L.A. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br. J. Haematol. 2019, 186, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Derman, B.A.; Chari, A.; Zonder, J.; Major, A.; Stefka, A.T.; Jiang, K.; Karrison, T.; Jasielec, J.; Jakubowiak, A. A phase I study of selinexor combined with weekly carfilzomib and dexamethasone in relapsed/refractory multiple myeloma. Eur. J. Haematol. 2023, 110, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Baljevic, M.; Bahlis, N.J.; Kotb, R.; Schiller, G.J.; Lipe, B.; Madan, S.; Sutherland, H.J.; Lentzsch, S.; Callander, N.; Biran, N. Efficacy and Safety of Selinexor, Pomalidomide, and Dexamethasone (SPd) for Treatment of Patients with Relapsed or Refractory Multiple Myeloma (RRMM). Blood 2024, 144, 1996. [Google Scholar] [CrossRef]
- Fu, S.-C.; Huang, H.-C.; Horton, P.; Juan, H.F. ValidNESs: A database of validated leucine-rich nuclear export signals. Nucleic Acids Res. 2012, 41, D338–D343. [Google Scholar] [CrossRef]
- Turner, J.G.; Dawson, J.; Emmons, M.F.; Cubitt, C.L.; Kauffman, M.; Shacham, S.; Hazlehurst, L.A.; Sullivan, D.M. CRM1 Inhibition Sensitizes Drug Resistant Human Myeloma Cells to Topoisomerase II and Proteasome Inhibitors both In Vitro and Ex Vivo. J. Cancer 2013, 4, 614–625. [Google Scholar] [CrossRef]
- Tai, Y.T.; Landesman, Y.; Acharya, C.; Calle, Y.; Zhong, M.Y.; Cea, M.; Tannenbaum, D.; Cagnetta, A.; Reagan, M.; Munshi, A.A.; et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: Molecular mechanisms and therapeutic implications. Leukemia 2014, 28, 155–165. [Google Scholar] [CrossRef]
- Fung, H.Y.J.; Chook, Y.M. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin. Cancer Biol. 2014, 27, 52–61. [Google Scholar] [CrossRef]
- Herrero, A.B.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutiérrez, N.C. Molecular Mechanisms of p53 Deregulation in Cancer: An Overview in Multiple Myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef]
- Crespo, M.; Carabia, J.; Jiménez, I.; Bobillo, S.; Abrisqueta, P.; Palacio, C.; Nieto, J.C.; Boix, J.; Carpio, C.; Castellvi, J. Xpo-1 inhibition synergizes with BCR inhibition, blocks tumor growth and tumor-associated macrophages infiltration and prolongs survival in a bioluminescent animal model of primary central nervous system lymphoma. Blood 2017, 130, 2808. [Google Scholar]
- Kalakonda, N.; Maerevoet, M.; Cavallo, F.; Follows, G.; Goy, A.; Vermaat, J.S.; Casasnovas, O.; Hamad, N.; Zijlstra, J.M.; Bakhshi, S. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020, 7, 511–ez522. [Google Scholar] [CrossRef]
- Boons, E.; Nogueira, T.C.; Dierckx, T.; Menezes, S.M.; Jacquemyn, M.; Tamir, S.; Landesman, Y.; Farré, L.; Bittencourt, A.; Kataoka, K. XPO1 inhibitors represent a novel therapeutic option in Adult T-cell Leukemia, triggering p53-mediated caspase-dependent apoptosis. Blood Cancer J. 2021, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, B.; Yang, Z.Y.; Li, O.; Liu, S.L.; Pan, L.J.; Gong, W.; Dong, P.; Shu, Y.J. Inhibition of XPO1 impairs cholangiocarcinoma cell proliferation by triggering p53 intranuclear accumulation. Cancer Med. 2023, 12, 5751–5763. [Google Scholar] [CrossRef] [PubMed]
- Hardas, T.; Ra, H.-J.; Sheridan, J.; Kovalenko, M.; Tribouley, C. Quantification of cell surface HLA-A2 and intracellular Survivin protein levels for tumor blasts and non-blast immune cells in multiple myeloma bone marrow aspirates using a rapid sample preservation methodology followed by 10-color FACS assay. Cancer Res. 2021, 81, 408. [Google Scholar] [CrossRef]
- Galinski, B.; Luxemburg, M.; Landesman, Y.; Pawel, B.; Johnson, K.J.; Master, S.R.; Freeman, K.W.; Loeb, D.M.; Hébert, J.M.; Weiser, D.A. XPO1 inhibition with selinexor synergizes with proteasome inhibition in neuroblastoma by targeting nuclear export of IkB. Transl. Oncol. 2021, 14, 101114. [Google Scholar] [CrossRef]
- Wang, Q.; Bode, A.M.; Zhang, T. Targeting CDK1 in cancer: Mechanisms and implications. NPJ Precis. Oncol. 2023, 7, 58. [Google Scholar] [CrossRef]
- Zhai, M.; Miao, J.; Zhang, R.; Liu, R.; Li, F.; Shen, Y.; Wang, T.; Xu, X.; Gao, G.; Hu, J. KIF22 promotes multiple myeloma progression by regulating the CDC25C/CDK1/cyclinB1 pathway. J. Cancer Res. Clin. Oncol. 2024, 150, 239. [Google Scholar] [CrossRef]
- Chakravarti, N.; Boles, A.; Burzinski, R.; Sindaco, P.; Isabelle, C.; McConnell, K.; Mishra, A.; Porcu, P. XPO1 blockade with KPT-330 promotes apoptosis in cutaneous T-cell lymphoma by activating the p53–p21 and p27 pathways. Sci. Rep. 2024, 14, 9305. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Rahimzadeh, P.; Khorrami, R.; Bonyadi, M.; Daneshi, S.; Nabavi, N.; Raesi, R.; Farani, M.R.; Dehkhoda, F.; Taheriazam, A.; et al. A comprehensive review of the PTEN/PI3K/Akt axis in multiple myeloma: From molecular interactions to potential therapeutic targets. Pathol. Res. Pract. 2024, 260, 155401. [Google Scholar] [CrossRef]
- Li, S.; Fu, J.; Lu, C.; Mapara, M.Y.; Raza, S.; Hengst, U.; Lentzsch, S. Elevated Translation Initiation Factor eIF4E Is an Attractive Therapeutic Target in Multiple Myeloma. Mol. Cancer Ther. 2016, 15, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic, B.; Topisirovic, I.; Skrabanek, L.; Ruiz-Gutierrez, M.; Borden, K.L. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J. Cell Biol. 2006, 175, 415–426. [Google Scholar] [CrossRef]
- Culjkovic-Kraljacic, B.; Baguet, A.; Volpon, L.; Amri, A.; Borden, K.L. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012, 2, 207–215. [Google Scholar] [CrossRef]
- Volpon, L.; Culjkovic-Kraljacic, B.; Sohn, H.S.; Blanchet-Cohen, A.; Osborne, M.J.; Borden, K.L.B. A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery. Rna 2017, 23, 927–937. [Google Scholar] [CrossRef]
- Zhu, Z.C.; Liu, J.W.; Yang, C.; Zhao, M.; Xiong, Z.Q. XPO1 inhibitor KPT-330 synergizes with Bcl-xL inhibitor to induce cancer cell apoptosis by perturbing rRNA processing and Mcl-1 protein synthesis. Cell Death Dis. 2019, 10, 395. [Google Scholar] [CrossRef]
- Marullo, R.; Rutherford, S.C.; Revuelta, M.V.; Zamponi, N.; Culjkovic-Kraljacic, B.; Kotlov, N.; Di Siervi, N.; Lara-Garcia, J.; Allan, J.N.; Ruan, J.; et al. XPO1 Enables Adaptive Regulation of mRNA Export Required for Genotoxic Stress Tolerance in Cancer Cells. Cancer Res. 2024, 84, 101–117. [Google Scholar] [CrossRef]
- Kashyap, T.; Crochiere, M.L.; Friedlander, S.; Klebanov, B.; Senapedis, W.; Baloglu, E.; del Alamo, D.; Tamir, S.; McCauley, D.; Rashal, T. Selective inhibitors of nuclear export (SINETM) block the expression of DNA damage repair proteins and sensitize cancer cells to DNA damage inducing therapeutic agents. In Proceedings of the 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Barcelona, Spain, 18–21 November 2014; pp. 18–21. [Google Scholar]
- Kashyap, T.; Argueta, C.; Unger, T.; Klebanov, B.; Debler, S.; Senapedis, W.; Crochiere, M.L.; Lee, M.S.; Kauffman, M.; Shacham, S.; et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget 2018, 9, 30773–30786. [Google Scholar] [CrossRef]
- Vogl, D.T.; Dingli, D.; Cornell, R.F.; Huff, C.A.; Jagannath, S.; Bhutani, D.; Zonder, J.; Baz, R.; Nooka, A.; Richter, J.; et al. Selective Inhibition of Nuclear Export with Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 859–866. [Google Scholar] [CrossRef]
- Argueta, C.; Kashyap, T.; Klebanov, B.; Unger, T.J.; Guo, C.; Harrington, S.; Baloglu, E.; Lee, M.; Senapedis, W.; Shacham, S.; et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget 2018, 9, 25529–25544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kwon, Y.-G.; Kim, Y.-M. The stress-responsive protein REDD1 and its pathophysiological functions. Exp. Mol. Med. 2023, 55, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Tortoreto, M.; Mancini, A.; Addis, A.; Di Cesare, E.; Lenzi, A.; Landesman, Y.; McCauley, D.; Kauffman, M.; Shacham, S.; et al. XPO1/CRM1-selective inhibitors of nuclear export (SINE) reduce tumor spreading and improve overall survival in preclinical models of prostate cancer (PCa). J. Hematol. Oncol. 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, D.; Xu, Y.; Wu, L.; Tang, L.; Su, Y.; Xie, X.; Zhao, J.; Xu, J.; Liu, Q. Anti-Osteoclast Effect of Exportin-1 Inhibitor Eltanexor on Osteoporosis Depends on Nuclear Accumulation of IκBα-NF-κB p65 Complex. Front. Pharmacol. 2022, 13, 896108. [Google Scholar] [CrossRef]
- Lv, J.-T.; Jiao, Y.-T.; Han, X.-L.; Cao, Y.-J.; Lv, X.-K.; Du, J.; Hou, J. Integrating p53-associated genes and infiltrating immune cell characterization as a prognostic biomarker in multiple myeloma. Heliyon 2024, 10, e30123. [Google Scholar] [CrossRef]
- Wen, Z.; Bissonnette, A.; Moat, L.F.; Wang, Y.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A.; Kim, S.H.; Shour, A.; Onitilo, A.A. Nuclear Receptor NR2E3 Activates Both Wild-Type and Mutated p53 in Multiple Myeloma Cells. Blood 2024, 144, 6840. [Google Scholar] [CrossRef]
- Ang, D.A.; Carter, J.-M.; Deka, K.; Tan, J.H.; Zhou, J.; Chen, Q.; Chng, W.J.; Harmston, N.; Li, Y. Aberrant non-canonical NF-κB signalling reprograms the epigenome landscape to drive oncogenic transcriptomes in multiple myeloma. Nat. Commun. 2024, 15, 2513. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Lee, H.; Chikhale, S.; Poorebrahim, M.; Ahn, S.; Benaoudia, S.; Leblay, N.; Maura, F.; Bahlis, N.J.; Neri, P. NF-Kb Pathway Activation Driven by TRAF3 Loss Mediates Resistance to Anti-BCMA T-Cell Based Therapies in Multiple Myeloma. Blood 2024, 144, 591. [Google Scholar] [CrossRef]
- Jiménez, I.; Carabia, J.; Bobillo, S.; Palacio, C.; Abrisqueta, P.; Pagès, C.; Nieto, J.C.; Castellví, J.; Martínez-Ricarte, F.; Escoda, L. Repolarization of tumor infiltrating macrophages and increased survival in mouse primary CNS lymphomas after XPO1 and BTK inhibition. J. Neuro Oncol. 2020, 149, 13–25. [Google Scholar] [CrossRef]
- Luo, W.; Xu, J.; Li, C.; Tang, L.; Li, Y.; Wang, X.; Zhuolin, W.; Zhang, Y.; Hu, Y.; Mei, H. Selinexor Reduces the Immunosuppressive Properties of Macrophages and Synergizes with CD19 CAR-T Cells Against B-Cell Lymphoma. Blood 2024, 144, 3420. [Google Scholar] [CrossRef]
- Daneshmandi, S.; Yan, Q.; Choi, J.E.; Katsuta, E.; MacDonald, C.R.; Goruganthu, M.; Roberts, N.; Repasky, E.A.; Singh, P.K.; Attwood, K. Exportin 1 governs the immunosuppressive functions of myeloid-derived suppressor cells in tumors through ERK1/2 nuclear export. Cell. Mol. Immunol. 2024, 21, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.G.; Walker, C.J.; Doyle, A.D.; Johnson, P.W.; Forconi, F.; Cragg, M.S.; Landesman, Y.; Khakoo, S.I.; Blunt, M.D. Selinexor Enhances NK Cell Activation Against Malignant B Cells via Downregulation of HLA-E. Front. Oncol. 2021, 11, 785635. [Google Scholar] [CrossRef] [PubMed]
- Kaulfuss, M.; Mietz, J.; Fabri, A.; vom Berg, J.; Münz, C.; Chijioke, O. The NK cell checkpoint NKG2A maintains expansion capacity of human NK cells. Sci. Rep. 2023, 13, 10555. [Google Scholar] [CrossRef] [PubMed]
- Tyler, P.M.; Servos, M.M.; de Vries, R.C.; Klebanov, B.; Kashyap, T.; Sacham, S.; Landesman, Y.; Dougan, M.; Dougan, S.K. Clinical Dosing Regimen of Selinexor Maintains Normal Immune Homeostasis and T-cell Effector Function in Mice: Implications for Combination with Immunotherapy. Mol. Cancer Ther. 2017, 16, 428–439. [Google Scholar] [CrossRef]
- Tong, X.; Zhu, X.; Shuai, S.; Zhu, Y.; Xu, B.; Peng, Y.; Zhang, S.; Jie, J.; Mao, X.; Wei, J. Impact of Selinexor on AML Cell Lines and Healthy Donor T Cell Function: Enhanced Treg Cell Proportion. Blood 2024, 144, 5817. [Google Scholar] [CrossRef]
- Wang, S.; Sellner, L.; Wang, L.; Sauer, T.; Neuber, B.; Gong, W.; Stock, S.; Ni, M.; Yao, H.; Kleist, C.; et al. Combining selective inhibitors of nuclear export (SINEs) with chimeric antigen receptor (CAR) T cells for CD19-positive malignancies. Oncol. Rep. 2021, 46, 170. [Google Scholar] [CrossRef]
- Martini, S.; Figini, M.; Croce, A.; Frigerio, B.; Pennati, M.; Gianni, A.M.; De Marco, C.; Daidone, M.G.; Argueta, C.; Landesman, Y. Selinexor sensitizes TRAIL-R2-positive TNBC cells to the activity of TRAIL-R2xCD3 bispecific antibody. Cells 2020, 9, 2231. [Google Scholar] [CrossRef]
- Baljevic, M.; Gasparetto, C.; Schiller, G.J.; Tuchman, S.A.; Callander, N.S.; Lentzsch, S.; Monge, J.; Kotb, R.; Bahlis, N.J.; White, D. Selinexor-based regimens in patients with multiple myeloma after prior anti-B-cell maturation antigen treatment. EJHaem 2022, 3, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Baljevic, M.; Moreau, P.; Tuchman, S.A.; Callander, N.S.; Lentzsch, S.; Van Domelen, D.R.; Bentur, O.S.; Monge, J.; Biran, N. Effectiveness of anti-B-cell maturation antigen (BCMA)-targeting therapy after selinexor treatment. J. Clin. Oncol. 2023, 41, 16. [Google Scholar] [CrossRef]
- Tao, R.; Liu, C.; Zhang, W.; Zhu, Y.; Ma, Y.; Hao, S. Selinexor with anti-PD-1 antibody as a potentially effective regimen for patients with natural killer/T-cell lymphoma failing prior L-asparaginase and PD-1 blockade. Oncologist 2024, 29, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Jagannath, S.; Jasielec, J.K.; DeCastro, A.; Unger, T.J.; Shah, J.; Jakubowiak, A. Selinexor-Containing Regimens for the Treatment of Patients with Multiple Myeloma Refractory to Chimeric Antigen Receptor T-Cell (CAR-T) Therapy. Blood 2019, 134, 1854. [Google Scholar] [CrossRef]
- Van Oekelen, O.; Nath, K.; Mouhieddine, T.H.; Farzana, T.; Aleman, A.; Melnekoff, D.T.; Ghodke-Puranik, Y.; Shah, G.L.; Lesokhin, A.; Giralt, S. Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood 2023, 141, 756–765. [Google Scholar] [CrossRef]
- Wang, D.; Fu, H.; Que, Y.; Ruan, H.; Xu, M.; Long, X.; Yu, Q.; Li, C.; Li, Z.; Cai, S.; et al. A novel two-step administration of XPO-1 inhibitor may enhance the effect of anti-BCMA CAR-T in relapsed/refractory extramedullary multiple myeloma. J. Transl. Med. 2023, 21, 812. [Google Scholar] [CrossRef]
- Stadel, R.; Liu, R.; Landesman, Y.; Wald, D.; Hosahalli Vasanna, S.; de Lima, M.J.G. Sequential Administration of Selinexor then CD19 CAR-T Cells Exhibits Enhanced Efficacy in a Mouse Model of Human Non-Hodgkin’s Lymphoma. Blood 2022, 140, 7413–7414. [Google Scholar] [CrossRef]
- Cruz, L.S.; Stevenson, D.; Robinson, M.; Mathew, S.; Amador, I.; Jordan, G.; House, C.D. Role of macrophages in the development of ovarian cancer stem-like cells during chemotherapy. Cancer Res. 2024, 84, 163. [Google Scholar] [CrossRef]
- Ntostoglou, K.; Theodorou, S.D.; Proctor, T.; Nikas, I.P.; Awounvo, S.; Sepsa, A.; Georgoulias, V.; Ryu, H.S.; Pateras, I.S.; Kittas, C. Distinct profiles of proliferating CD8+/TCF1+ T cells and CD163+/PD-L1+ macrophages predict risk of relapse differently among treatment-naïve breast cancer subtypes. Cancer Immunol. Immunother. 2024, 73, 46. [Google Scholar] [CrossRef]
- Kuan, C.-H.; Chang, L.; Ho, C.-Y.; Tsai, C.-H.; Liu, Y.-C.; Huang, W.-Y.; Wang, Y.-N.; Wang, W.-H.; Wang, T.-W. Immunomodulatory hydrogel orchestrates pro-regenerative response of macrophages and angiogenesis for chronic wound healing. Biomaterials 2025, 314, 122848. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Zhang, L.; Shi, C.; Zhou, J.; Wang, P.; Wang, H.; Shi, X.; Wei, S.; Wang, Q. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation. Front. Immunol. 2021, 11, 609060. [Google Scholar] [CrossRef]
- Hu, F.; Chen, X.Q.; Li, X.P.; Lu, Y.X.; Chen, S.L.; Wang, D.W.; Liang, Y.; Dai, Y.J. Drug resistance biomarker ABCC4 of selinexor and immune feature in multiple myeloma. Int. Immunopharmacol. 2022, 108, 108722. [Google Scholar] [CrossRef]
- Farren, M.R.; Hennessey, R.C.; Shakya, R.; Elnaggar, O.; Young, G.; Kendra, K.; Landesman, Y.; Elloul, S.; Crochiere, M.; Klebanov, B.; et al. The Exportin-1 Inhibitor Selinexor Exerts Superior Antitumor Activity when Combined with T-Cell Checkpoint Inhibitors. Mol. Cancer Ther. 2017, 16, 417–427. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasbihi, K.; Bruns, H. Selinexor’s Immunomodulatory Impact in Advancing Multiple Myeloma Treatment. Cells 2025, 14, 430. https://doi.org/10.3390/cells14060430
Tasbihi K, Bruns H. Selinexor’s Immunomodulatory Impact in Advancing Multiple Myeloma Treatment. Cells. 2025; 14(6):430. https://doi.org/10.3390/cells14060430
Chicago/Turabian StyleTasbihi, Kereshmeh, and Heiko Bruns. 2025. "Selinexor’s Immunomodulatory Impact in Advancing Multiple Myeloma Treatment" Cells 14, no. 6: 430. https://doi.org/10.3390/cells14060430
APA StyleTasbihi, K., & Bruns, H. (2025). Selinexor’s Immunomodulatory Impact in Advancing Multiple Myeloma Treatment. Cells, 14(6), 430. https://doi.org/10.3390/cells14060430





