Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway
Abstract
:1. Introduction
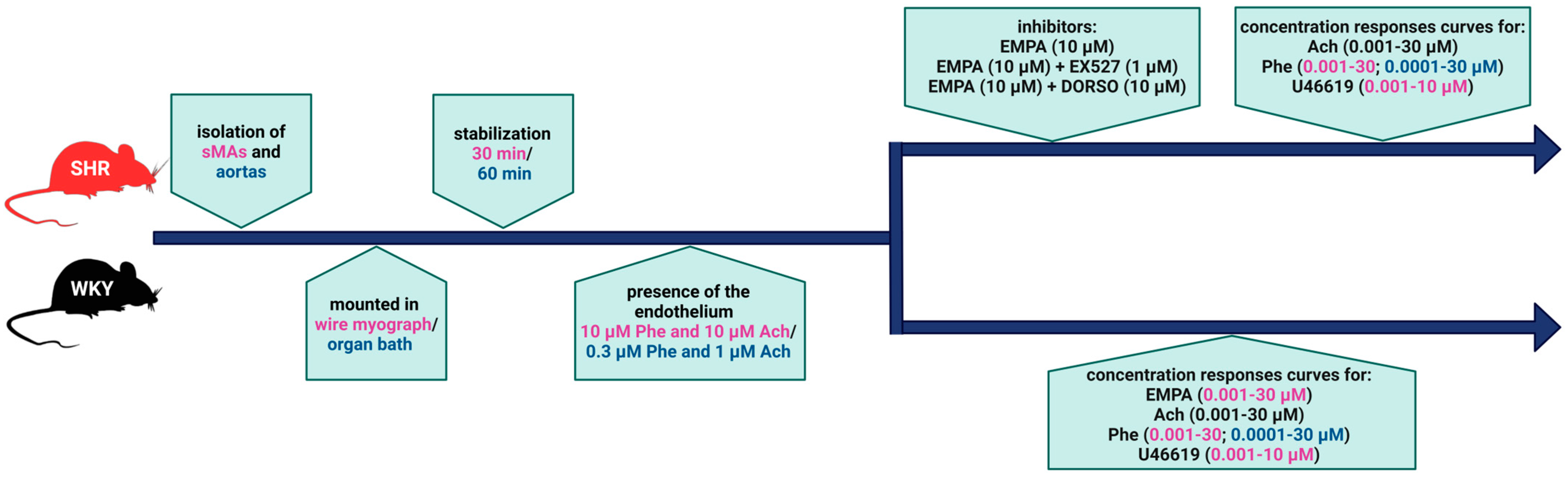
2. Materials and Methods
2.1. Animals
2.2. Measurements of Blood Pressure
2.3. Vessel Preparation
2.4. Western Blot
2.5. Drugs
2.6. Statistical Analysis
3. Results
3.1. Systolic Blood Pressure
3.2. General
3.3. Influence of Hypertension on the Vasorelaxant Effect of EMPA in sMAs
3.4. Influence of Hypertension on EMPA-Mediated Changes in Ach-Induced Relaxation in sMAs and Aortas
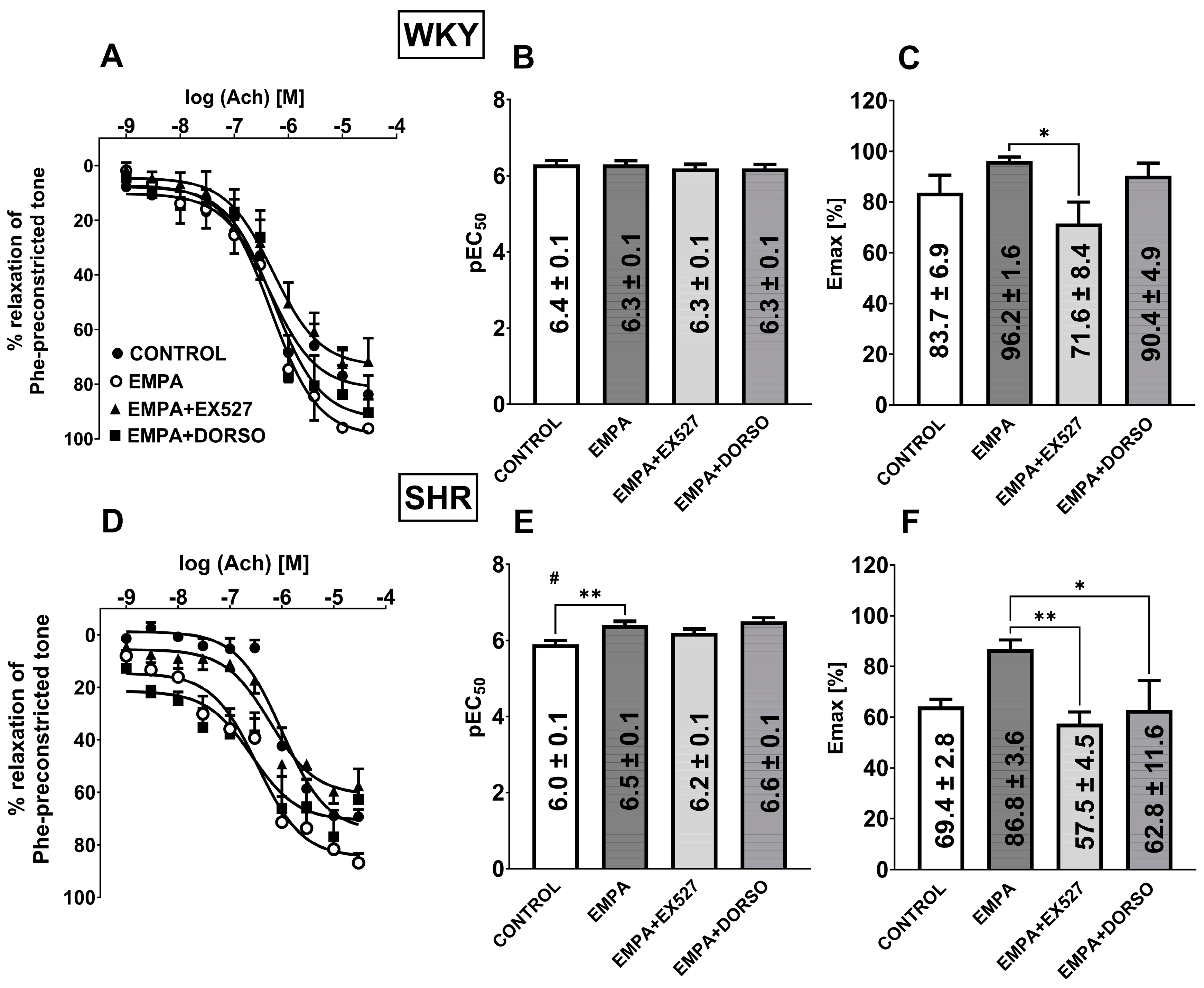
3.5. The Role of SIRT1 on EMPA-Mediated Changes in Ach-Induced Relaxation in sMAs and Aortas
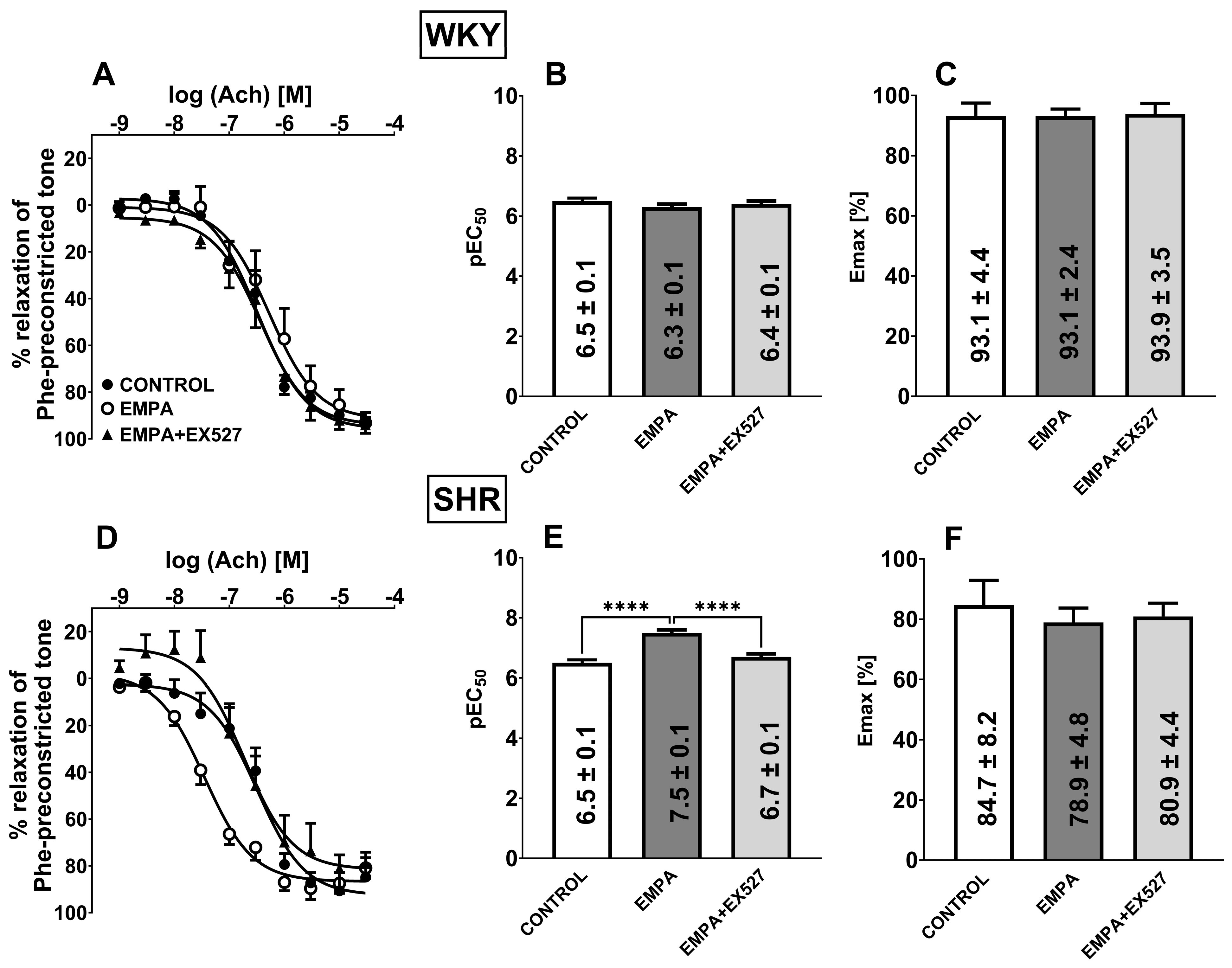
3.6. The Role of AMPK on EMPA-Mediated Changes in Ach-Induced Relaxation in sMAs
3.7. Influence of Hypertension on EMPA-Mediated Changes in Phe-Induced Vasoconstriction in sMAs and Aortas
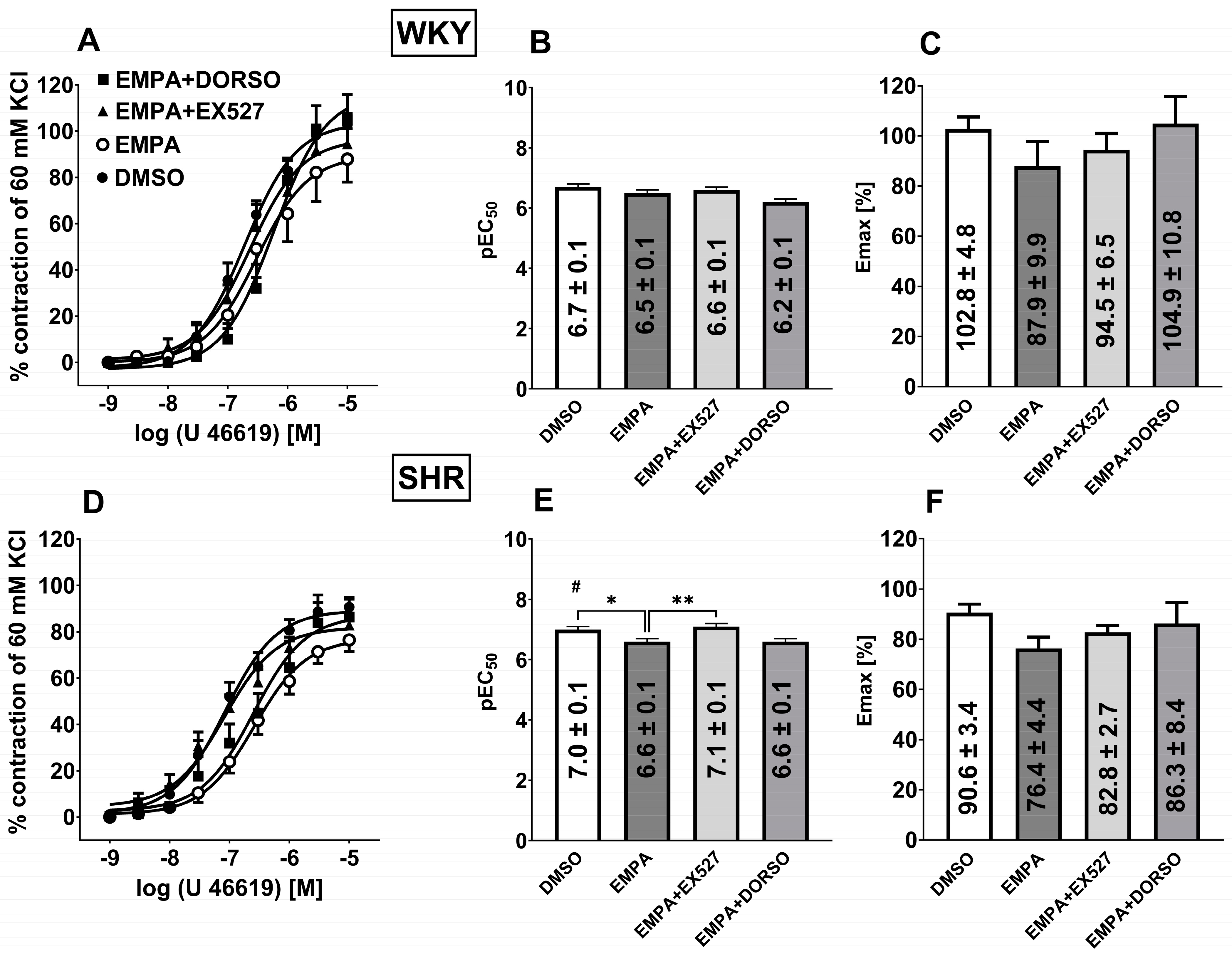
3.8. Influence of Hypertension on EMPA-Mediated Changes in Analog Thromboxane U46619-Induced Vasoconstriction in sMAs
3.9. Influence of Hypertension on the Expression of SGLT2, SIRT1, AMPK, and pAMPK in Isolated sMAs and Aortas
4. Discussion
4.1. EMPA Relaxed Endothelium-Intact sMAs
4.2. EMPA Improved Vascular Function in Hypertension
4.3. SIRT1 Is Involved in EMPA-Mediated Vascular Protection in Hypertension
4.4. AMPK Is Involved in EMPA-Mediated Vascular Protection in Hypertension
4.5. SIRT1/AMPK Crosstalk in Vascular Protection in Hypertension
5. Limitations
6. Conclusions
7. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| AMPK | AMP-activated protein kinase |
| CRCs | cumulative concentration-response curves |
| DORSO | dorsomorphin |
| EDH | endothelial-derived hyperpolarization |
| EMPA | empagliflozin |
| eNOS | endothelial nitric oxide synthase |
| NIBP | Non-Invasive Blood Pressure |
| NO | nitric oxide |
| pAMPK | phosphorylated form AMP-activated protein kinase |
| Phe | phenylephrine |
| SBP | systolic blood pressure |
| SGLT2 | a sodium-glucose co-transporter 2 |
| SHR | spontaneously hypertensive rats |
| SIRT1 | sirtuin 1 |
| sMAs | the third-order of the superior mesenteric artery |
| WKY | Wistar Kyoto rats |
References
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Mghaieth Zghal, F.; Abbassi, M.; Silini, A.; Ben Halima, M.; Jebberi, Z.; Daly, F.; Ouali, S.; Farhati, A.; Ben Mansour, N.; Boudiche, S.; et al. Impact of sodium-glucose cotransporter inhibitors in acute coronary syndrome patients on endothelial function and atherosclerosis related-biomarkers: ATH-SGLT2i pilot study. Medicine 2024, 103, e40536. [Google Scholar] [CrossRef]
- Komaniecka, N.; Maroszek, S.; Drozdzik, M.; Oswald, S.; Drozdzik, M. Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors. Int. J. Mol. Sci. 2024, 25, 6926. [Google Scholar] [CrossRef]
- Kurczyński, D.; Hudzik, B.; Jagosz, M.; Zabierowski, J.; Nowak, J.; Tomasik, A.; Badziński, A.; Rozentryt, P.; Gąsior, M. Sodium-Glucose Cotransporter-2 Inhibitors-from the Treatment of Diabetes to Therapy of Chronic Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 225. [Google Scholar] [CrossRef]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial function and dysfunction: Impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol. Ther. 2021, 224, 107832. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Forrester, E.A.; Benítez-Angeles, M.; Redford, K.E.; Rosenbaum, T.; Abbott, G.W.; Barrese, V.; Dora, K.; Albert, A.P.; Dannesboe, J.; Salles-Crawley, I.; et al. Crucial role for sensory nerves and Na/H exchanger inhibition in dapagliflozin and empagliflozin-induced arterial relaxation. Cardiovasc. Res. 2024, 26, cvae156. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Kalina, J.O.; Preckel, B.; Hollmann, M.W.; Albrecht, M.; Zuurbier, C.J.; Weber, N.C. Empagliflozin prevents oxidative stress in human coronary artery endothelial cells via the NHE/PKC/NOX axis. Redox Biol. 2024, 69, 102979. [Google Scholar] [CrossRef]
- Neutel, C.H.G.; Wesley, C.D.; Van Praet, M.; Civati, C.; Roth, L.; De Meyer, G.R.Y.; Martinet, W.; Guns, P.J. Empagliflozin decreases ageing-associated arterial stiffening and vascular fibrosis under normoglycemic conditions. Vasc. Pharmacol. 2023, 152, 107212. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Hasan, R. Empagliflozin Relaxes Resistance Mesenteric Arteries by Stimulating Multiple Smooth Muscle Cell Voltage-Gated K+ (KV) Channels. Int. J. Mol. Sci. 2021, 22, 10842. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Menon, S.N.; Zerin, F.; Hasan, R. Dapagliflozin induces vasodilation in resistance-size mesenteric arteries by stimulating smooth muscle cell KV7 ion channels. Heliyon 2022, 8, e09503. [Google Scholar] [CrossRef]
- Kong, Q.; Qian, L.L.; Zhang, L.; Liu, H.H.; Yang, F.; Zhang, X.L.; Wang, C.; Zhao, X.X.; Li, K.L.; Wang, R.X. Empagliflozin Induces Vascular Relaxation in Rat Coronary Artery Due to Activation of BK Channels. Diabetes Metab. Syndr. Obes. 2024, 17, 247–257. [Google Scholar] [CrossRef]
- Seo, M.S.; Jung, H.S.; An, J.R.; Kang, M.; Heo, R.; Li, H.; Han, E.T.; Yang, S.R.; Cho, E.H.; Bae, Y.M.; et al. Empagliflozin dilates the rabbit aorta by activating PKG and voltage-dependent K+ channels. Toxicol. Appl. Pharmacol. 2020, 403, 115153. [Google Scholar] [CrossRef]
- Wicik, Z.; Nowak, A.; Jarosz-Popek, J.; Wolska, M.; Eyileten, C.; Siller-Matula, J.M.; von Lewinski, D.; Sourij, H.; Filipiak, K.J.; Postuła, M. Characterization of the SGLT2 Interaction Network and Its Regulation by SGLT2 Inhibitors: A Bioinformatic Analysis. Front. Pharmacol. 2020, 13, 901340. [Google Scholar] [CrossRef]
- Zhou, Y.; Tai, S.; Zhang, N.; Fu, L.; Wang, Y. Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation. Biomed. Pharmacother. 2023, 165, 115213. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Yu, Y.; Deng, H.; Yang, L.; Shi, X.; Yu, B. Empagliflozin alleviates ethanol-induced cardiomyocyte injury through inhibition of mitochondrial apoptosis via a SIRT1/PTEN/Akt pathway. Clin. Exp. Pharmacol. Physiol. 2021, 48, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef]
- Vahtola, E.; Louhelainen, M.; Forstén, H.; Merasto, S.; Raivio, J.; Kaheinen, P.; Kytö, V.; Tikkanen, I.; Levijoki, J.; Mervaala, E. Sirtuin1-p53, forkhead box O3a, p38 and post-infarct cardiac remodeling in the spontaneously diabetic Goto-Kakizaki rat. Cardiovasc. Diabetol. 2010, 9, 5. [Google Scholar] [CrossRef]
- Packer, M. Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: A novel conceptual framework. Diabetes Obes. Metab. 2020, 22, 734–742. [Google Scholar] [CrossRef]
- Chen, H.; Vanhoutte, P.M.; Leung, S.W.S. Acute activation of endothelial AMPK surprisingly inhibits endothelium-dependent hyperpolarization-like relaxations in rat mesenteric arteries. Br. J. Pharmacol. 2019, 176, 2905–2921. [Google Scholar] [CrossRef] [PubMed]
- Seidu, S.; Alabraba, V.; Davies, S.; Newland-Jones, P.; Fernando, K.; Bain, S.C.; Diggle, J.; Evans, M.; James, J.; Kanumilli, N.; et al. SGLT2 Inhibitors—The New Standard of Care for Cardiovascular, Renal and Metabolic Protection in Type 2 Diabetes: A Narrative Review. Diabetes Ther. 2024, 15, 1099–1124. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef]
- Kong, B.W.; Man, R.Y.; Gao, Y.; Vanhoutte, P.M.; Leung, S.W. Reduced activity of SKC a and Na-K ATPase underlies the accelerated impairment of EDH-type relaxations in mesenteric arteries of aging spontaneously hypertensive rats. Pharmacol. Res. Perspect. 2015, 3, e00150. [Google Scholar] [CrossRef] [PubMed]
- Krzyżewska, A.; Baranowska-Kuczko, M.; Kasacka, I.; Kozłowska, H. Cannabidiol alleviates right ventricular fibrosis by inhibiting the transforming growth factor β pathway in monocrotaline-induced pulmonary hypertension in rats. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166753. [Google Scholar] [CrossRef]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 34, 432–441. [Google Scholar] [CrossRef]
- Díaz-Pérez, A.; Lope-Piedrafita, S.; Pérez, B.; Vázquez-Sufuentes, P.; Rodriguez-Garcia, M.; Briones, A.M.; Navarro, X.; Penas, C.; Jiménez-Altayó, F. Transient cerebral ischaemia alters mesenteric arteries in hypertensive rats: Limited reversal despite suberoylanilide hydroxamic acid cerebroprotection. Life Sci. 2024, 359, 123247. [Google Scholar] [CrossRef]
- Ortega, R.; Collado, A.; Selles, F.; Gonzalez-Navarro, H.; Sanz, M.J.; Real, J.T.; Piqueras, L. SGLT-2 (Sodium-Glucose Cotransporter 2) Inhibition Reduces Ang II (Angiotensin II)-Induced Dissecting Abdominal Aortic Aneurysm in ApoE (Apolipoprotein E) Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1614–1628. [Google Scholar] [CrossRef]
- Bruckert, C.; Matsushita, K.; Mroueh, A.; Amissi, S.; Auger, C.; Houngue, U.; Remila, L.; Chaker, A.B.; Park, S.H.; Algara-Suarez, P.; et al. Empagliflozin prevents angiotensin II-induced hypertension related micro and macrovascular endothelial cell activation and diastolic dysfunction in rats despite persistent hypertension: Role of endothelial SGLT1 and 2. Vasc. Pharmacol. 2022, 146, 107095. [Google Scholar] [CrossRef] [PubMed]
- Seman, L.; Macha, S.; Nehmiz, G.; Simons, G.; Ren, B.; Pinnetti, S.; Woerle, H.J.; Dugi, K. Empagliflozin (BI 10773), a Potent and Selective SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2013, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shin, S.E.; Seo, M.S.; An, J.R.; Choi, I.W.; Jung, W.K.; Firth, A.L.; Lee, D.S.; Yim, M.J.; Choi, G.; et al. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018, 197, 46–55. [Google Scholar] [CrossRef]
- Moustafa Ahmed, Y.; Shehata Messiha, B.A.; El-Sayed El-Daly, M.; Abo-Saif, A.A. Effects of ticagrelor, empagliflozin and tamoxifen against experimentally-induced vascular reactivity defects in rats in vivo and in vitro. Pharmacol. Rep. 2019, 71, 1034–1043. [Google Scholar] [CrossRef]
- Lascu, A.; Ionică, L.N.; Buriman, D.G.; Merce, A.P.; Deaconu, L.; Borza, C.; Crețu, O.M.; Sturza, A.; Muntean, D.M.; Feier, H.B. Metformin and empagliflozin modulate monoamine oxidase-related oxidative stress and improve vascular function in human mammary arteries. Mol. Cell. Biochem. 2023, 478, 1939–1947. [Google Scholar] [CrossRef]
- Sayour, A.A.; Korkmaz-Icöz, S.; Loganathan, S.; Ruppert, M.; Sayour, V.N.; Oláh, A.; Benke, K.; Brune, M.; Benkő, R.; Horváth, E.M.; et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J. Transl. Med. 2019, 17, 127. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Kocer, C.; Sayour, A.A.; Kraft, P.; Benker, M.I.; Abulizi, S.; Georgevici, A.I.; Brlecic, P.; Radovits, T.; Loganathan, S.; et al. The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 7774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, L.; Lu, Z.; Hu, Y.; Zhang, H.; Sun, F.; Li, Q.; He, C.; Shu, W.; Wang, L.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Canagliflozin Antagonizes Salt-Sensitive Hypertension Through Modifying Transient Receptor Potential Channels 3 Mediated Vascular Calcium Handling. J. Am. Heart Assoc. 2022, 11, e025328. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, B.; Fukuda, D.; Shinohara, M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Hirata, K.I.; Sata, M. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharmacol. 2020, 875, 173040. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Zarzuelo, M.J.; López-Sepúlveda, R.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Ungvary, Z.; Pérez-Vizcaíno, F.; Jiménez, R.; Duarte, J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: Implications for vascular aging. Biochem. Pharmacol. 2013, 85, 1288–1296. [Google Scholar] [CrossRef]
- Donato, A.J.; Magerko, K.A.; Lawson, B.R.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011, 589, 4545–4554. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.Z. Histone Deacetylase SIRT1, Smooth Muscle Cell Function, and Vascular Diseases. Front. Pharmacol. 2020, 11, 537519. [Google Scholar] [CrossRef]
- Jansen, T.; Kvandová, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Münzel, T.; Kröller-Schön, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525. [Google Scholar] [CrossRef]
- Moral-Sanz, J.; Lewis, S.A.; MacMillan, S.; Meloni, M.; McClafferty, H.; Viollet, B.; Foretz, M.; Del-Pozo, J.; Mark Evans, A. AMPK deficiency in smooth muscles causes persistent pulmonary hypertension of the new-born and premature death. Nat. Commun. 2022, 13, 5034. [Google Scholar] [CrossRef]
- Li, Q.; Cao, H.; Xu, X.; Chen, Y.; Zhang, Y.; Mi, Y.; Zhu, X.; Shi, Y.; Liu, J.; Wang, B.; et al. Resveratrol attenuates cyclosporin A-induced upregulation of the thromboxane A2 receptor and hypertension via the AMPK/SIRT1 and MAPK/NF-κB pathways in the rat mesenteric artery. Eur. J. Pharmacol. 2024, 972, 176543. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Bianchi, F.; Busceti, C.L.; Cotugno, M.; Stanzione, R.; Marchitti, S.; Di Castro, S.; Madonna, M.; Nicoletti, F.; Volpe, M. Differential modulation of AMPK/PPARα/UCP2 axis in relation to hypertension and aging in the brain, kidneys and heart of two closely related spontaneously hypertensive rat strains. Oncotarget 2015, 6, 18800–18818. [Google Scholar] [CrossRef]
- Vogt, J.; Traynor, R.; Sapkota, G.P. The specificities of small molecule inhibitors of the TGFß and BMP pathways. Cell Signal. 2011, 23, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.L.; Menéndez, S.G.; Inserra, F.; Ferder, L.; Manucha, W. Cellular and Mitochondrial Pathways Contribute to SGLT2 Inhibitors-mediated Tissue Protection: Experimental and Clinical Data. Curr. Pharm. Des. 2024, 30, 969–974. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, Y.; Tong, J.; Yu, Y.; Yang, L.; Gu, Y.; Niu, J. Empagliflozin Ameliorates Preeclampsia and Reduces Postpartum Susceptibility to Adriamycin in a Mouse Model Induced by Angiotensin Receptor Agonistic Autoantibodies. Front. Pharmacol. 2020, 13, 826792. [Google Scholar] [CrossRef]
- Chen, M. Empagliflozin attenuates doxorubicin-induced cardiotoxicity by activating AMPK/SIRT-1/PGC-1α-mediated mitochondrial biogenesis. Toxicol. Res. 2023, 12, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.N.; Tadros, M.G.; George, M.Y. Empagliflozin repurposing in Parkinson’s disease; modulation of oxidative stress, neuroinflammation, AMPK/SIRT-1/PGC-1α, and wnt/β-catenin pathways. Inflammopharmacology 2024, 32, 777–794. [Google Scholar] [CrossRef]
- ElBaset, M.A.; Salem, R.S.; Ayman, F.; Ayman, N.; Shaban, N.; Afifi, S.M.; Esatbeyoglu, T.; Abdelaziz, M.; Elalfy, Z.S. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants 2022, 11, 2152. [Google Scholar] [CrossRef]
- Yin, B.; Wang, Y.B.; Li, X.; Hou, X.W. β-aminoisobutyric acid ameliorates hypertensive vascular remodeling via activating the AMPK/SIRT1 pathway in VSMCs. Bioengineered 2022, 13, 14382–14401. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Przewłocki, T. Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. J. Clin. Med. 2022, 11, 6849. [Google Scholar] [CrossRef]
- Maiese, K. Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr. Neurovasc. Res. 2017, 14, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ni, Y.Q.; Zhan, J.K.; Liu, Y.S. The Role of SGLT2 Inhibitors in Vascular Aging. Aging Dis. 2021, 12, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.L.; Afonso, M.B.; Rodrigues, P.M.; Gama-Carvalho, M.; Machado, M.V.; Cortez-Pinto, H.; Rodrigues, C.M.P.; Castro, R.E. Skeletal muscle miR-34a/SIRT1:AMPK axis is activated in experimental and human non-alcoholic steatohepatitis. J. Mol. Med. 2019, 97, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.C.; Santos-Rios, T.M.; Martins, F.L.; Crajoinas, R.O.; Caetano, M.V.; Lessa, L.M.A.; Luchi, W.M.; McCormick, J.A.; Girardi, A.C.C. Renal upregulation of NCC counteracts empagliflozin-mediated NHE3 inhibition in normotensive but not in hypertensive male rat. Am. J. Physiol. Cell Physiol. 2024, 326, C1573–C1589. [Google Scholar] [CrossRef]
- Ahwin, P.; Martinez, D. The relationship between SGLT2 and systemic blood pressure regulation. Hypertens. Res. 2024, 47, 2094–2103. [Google Scholar] [CrossRef]
- Mira Hernandez, J.; Shen, E.Y.; Ko, C.Y.; Hourani, Z.; Spencer, E.R.; Smoliarchuk, D.; Bossuyt, J.; Granzier, H.; Bers, D.M.; Hegyi, B. Differential sex-dependent susceptibility to diastolic dysfunction and arrhythmia in cardiomyocytes from obese diabetic HFpEF model. Cardiovasc. Res. 2024, cvae070, epub ahead of print. [Google Scholar] [CrossRef]
- Braunwald, E. SGLT2 inhibitors: The statins of the 21st century. Eur. Heart J. 2022, 43, 1029–1030. [Google Scholar] [CrossRef]
- Piechocki, M.; Przewłocki, T.; Pieniążek, P.; Trystuła, M.; Podolec, J.; Kabłak-Ziembicka, A. A Non-Coronary, Peripheral Arterial Atherosclerotic Disease (Carotid, Renal, Lower Limb) in Elderly Patients—A Review PART II-Pharmacological Approach for Management of Elderly Patients with Peripheral Atherosclerotic Lesions outside Coronary Territory. J. Clin. Med. 2024, 13, 1508. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloza, M.; Krzyżewska, A.; Kozłowska, H.; Budziak, S.; Baranowska-Kuczko, M. Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway. Cells 2025, 14, 507. https://doi.org/10.3390/cells14070507
Kloza M, Krzyżewska A, Kozłowska H, Budziak S, Baranowska-Kuczko M. Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway. Cells. 2025; 14(7):507. https://doi.org/10.3390/cells14070507
Chicago/Turabian StyleKloza, Monika, Anna Krzyżewska, Hanna Kozłowska, Sandra Budziak, and Marta Baranowska-Kuczko. 2025. "Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway" Cells 14, no. 7: 507. https://doi.org/10.3390/cells14070507
APA StyleKloza, M., Krzyżewska, A., Kozłowska, H., Budziak, S., & Baranowska-Kuczko, M. (2025). Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway. Cells, 14(7), 507. https://doi.org/10.3390/cells14070507





