Role of T Follicular Helper Cells in Viral Infections and Vaccine Design
Abstract
1. Introduction
2. T Follicular Helper Cells
2.1. Tfh Cell Characteristics and Function
2.2. Tfh Cell Differentiation
3. Role of Tfh Cells in Viral Infections
3.1. Tfh Cells in Acute Viral Infections
3.2. Tfh Cells in Chronic Viral Infections
4. Targeting Tfh Cells Through Vaccine Design
4.1. Tfh Cells and Vaccines
4.2. RNA Vaccines: Immunity and Tfh Cell Responses in Humans and Animals
| Vaccine Type | Virus(es) (Antigen) | Species | Adjuvant | Tfh-Related Immune Responses | Ref(s). |
|---|---|---|---|---|---|
| mRNA | SARS-CoV-2 (Spike Protein) | Human | - | Induces strong neutralizing-antibody response and persistent cTfh cells for six months, with potent GC B and Tfh responses, including increase in Tfh1 cells after the first dose | [15,110] |
| mRNA | HIV-1 (Env), ZIKV (prM-E); influenza virus (HA) | Mice and rhesus macaques | - | Potent T follicular helper and germinal center B cell responses | [94] |
| mRNA | RVFV (Gn and Gc) | Mice and rhesus macaques | Induces potent GC B and Tfh cell responses 10 days after booster | [103] | |
| saRNA | SARS-CoV-2 (RBD-TM) | Mouse, NHP, and hamster models | Alum | Robustly activated Tfh cells; cross-reactive responses against heterologous variants and VOCs, lasting at least 12 months in NHPs (Cynomolgus macaques) | [93] |
| Ferritin–mRNA | HIV-1 (Env) | Mice | - | Induced specific GC B cells and memory B cells in spleen. Tfh cells and GC Tfh cells were also observed in vaccinated mice | [88] |
| Ferritin–mRNA | SARS-CoV-2 (RBD) | Mice | - | Elicited robust titers of specific antibodies, including neutralizing antibodies with increased expression of IFN-γ and IL-4 | [111] |
| LuS–mRNA | Rotavirus (P2-VP8*) | Mice and guinea pigs | Alum | Induced the highest specific IgG titers compared to conventional mRNA and subunit vaccines, which remained constant over time | [112] |
| LuS–mRNA | SARS-CoV-2 (RBD) | Mice | - | Strong neutralizing-antibody responses and protection against the Delta variant | [113] |
| LuS–saRNA | HIV (gp120) | Mice | ISCOM-like | Elicited GC B and Tfh cell responses with a balanced IgG1/IgG2 humoral immune response | [22] |
| Ferritin | Influenza virus (HA) | Mice and pigtail macaques | AddaVax | High antibody titers and directly activated germinal centers through a B cell-intrinsic mechanism | [114] |
| Ferritin | HBV (preS1 domain) | Mice | CpG-1826 | SIGNR1+ dendritic and macrophage cells activated Tfh and B cells, driving strong and lasting antibody responses | [16] |
| Ferritin | SARS-CoV-2 (Spike Protein) | Mice | ALFQ/Alhydrogel | One dose of vaccine induced IL-21-producing spike-specific Tfh and GC B cells, S-2P-specific IgM/IgG, and robust cross-neutralizing antibodies by day 7 | [10] |
| Encapsulin | Rotavirus (VP8*) | Mice | Alhydrogel | Induced specific IgG1, IgG2a, and neutralizing antibodies with superior immunogenicity over subunit vaccine | [115] |
| LuS and E2 | RVFV(Gn) | Mice and lambs | TS6 | Protection correlated with the induction of robust neutralizing antibodies in both species | [116] |
| sHSP | Antigen ovalbumin (OVA) | Mice | Alum | OVA-specific IgG1 was detectable by day 5, with strong mucosal sIgA and germinal center B and Tfh cell responses | [18] |
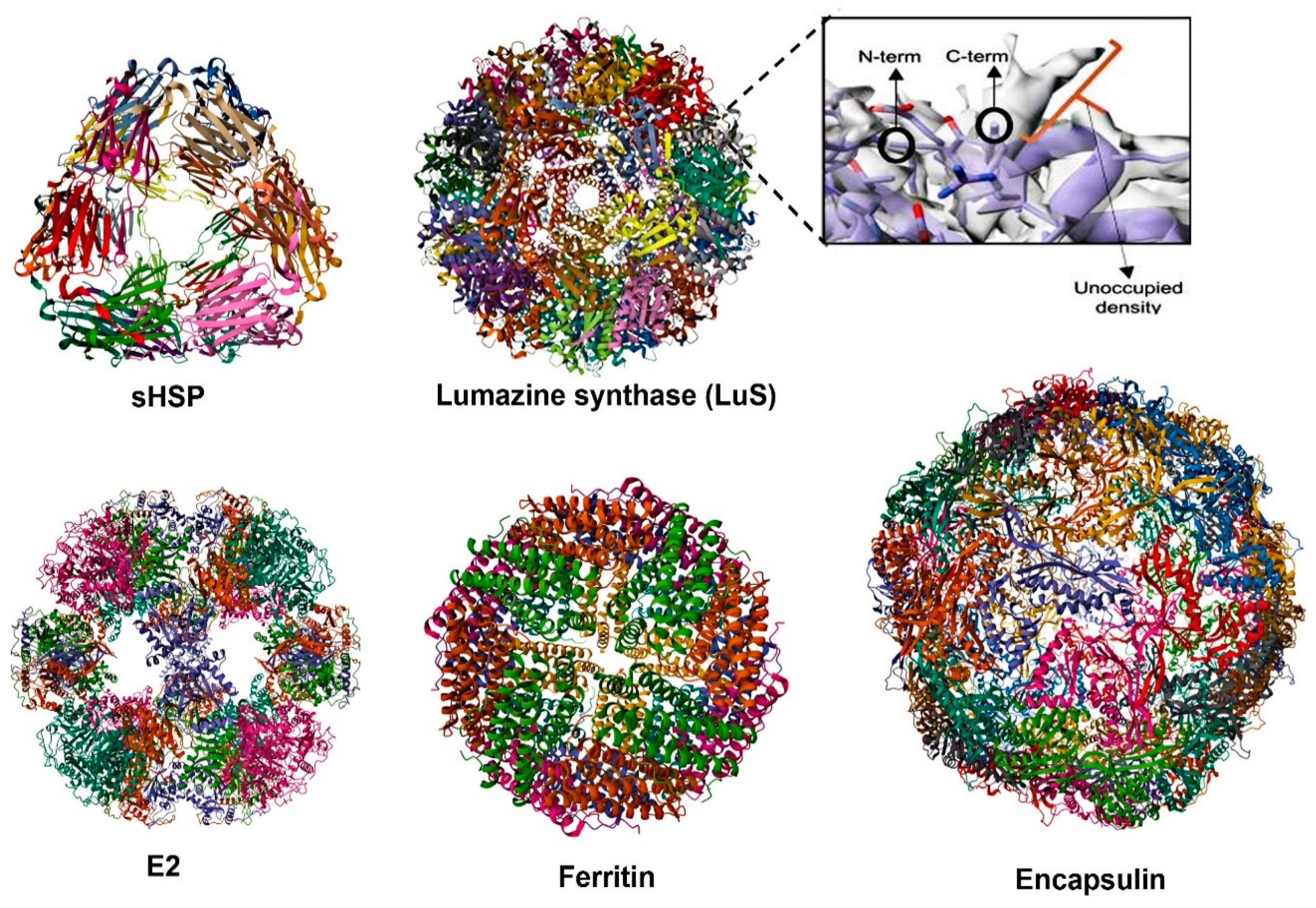
4.3. Self-Assembling Protein Nanocages (SAPNs) as Vaccine Platforms: Immunity and Tfh Cell Responses
4.4. Self-Assembling Protein Nanocages in RNA Vaccine Design (SAPN-RNA Vaccines)
4.5. Adjuvants Promote Tfh Cell Differentiation and Responses
5. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, F.A. Epidemiology of Human and Animal Viral Diseases. Encycl. Virol. 2008, 140–148. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Weidmann, M.; El-Matbouli, M.; Rahmati-Holasoo, H. Low pathogenic strain of infectious pancreatic necrosis virus (IPNV) associated with recent outbreaks in Iranian trout farms. Pathogens 2020, 9, 782. [Google Scholar] [CrossRef]
- Boyd, M.A.A.; Hoppe, A.C.; Kelleher, A.D.; Munier, C.M.L. T follicular helper cell responses to SARS-CoV-2 vaccination among healthy and immunocompromised adults. Immunol. Cell Biol. 2023, 101, 504–513. [Google Scholar] [CrossRef]
- Crotty, S. T follicular helper cell biology: A decade of discovery and diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [PubMed]
- Vella, L.A.; Herati, R.S.; Wherry, E.J. CD4+ T Cell Differentiation in Chronic Viral Infections: The Tfh Perspective. Trends Mol. Med. 2017, 23, 1072–1087. [Google Scholar] [PubMed]
- Koutsakos, M.; Nguyen, T.H.O.; Kedzierska, K. With a Little Help from T Follicular Helper Friends: Humoral Immunity to Influenza Vaccination. J. Immunol. 2019, 202, 360–367. [Google Scholar] [CrossRef]
- He, R.; Zheng, X.; Zhang, J.; Liu, B.; Wang, Q.; Wu, Q.; Liu, Z.; Chang, F.; Hu, Y.; Xie, T.; et al. SARS-CoV-2 spike-specific TFH cells exhibit unique responses in infected and vaccinated individuals. Sig. Transduct. Target. Ther. 2023, 8, 393. [Google Scholar]
- Ueno, H.; Banchereau, J.; Vinuesa, C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 2015, 16, 142–152. [Google Scholar]
- Webb, L.M.C.; Fra-Bido, S.; Innocentin, S.; Matheson, L.S.; Attaf, N.; Bignon, A.; Novarino, J.; Fazilleau, N.; Linterman, M.A. Ageing promotes early T follicular helper cell differentiation by modulating expression of RBPJ. Aging Cell 2021, 20, e13295. [Google Scholar] [CrossRef]
- Shrivastava, S.; Carmen, J.M.; Lu, Z.; Basu, S.; Sankhala, R.S.; Chen, W.H.; Nguyen, P.; Chang, W.C.; King, J.; Corbitt, C.; et al. SARS-CoV-2 spike-ferritin-nanoparticle adjuvanted with ALFQ induces long-lived plasma cells and cross-neutralizing antibodies. NPJ Vaccines 2023, 8, 43. [Google Scholar]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [PubMed]
- Long, J.R.; Yu, C.X.; Cao, Y.M.; Miao, Y.Q.; Sun, H.S.; Zhang, Z.; Mai, J.; Wang, X.; Mao, Y.; Li, H.; et al. A rabies mRNA vaccine provides a rapid and long-term immune response in mice. Nano Today 2023, 53, 102038. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451. [Google Scholar] [CrossRef] [PubMed]
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2022, 185, 603–613.e15. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Yang, J.X.; Tseng, J.C.; Tien, C.F.; Lee, C.Y.; Liu, Y.L.; Lin, J.J.; Tsai, P.J.; Liao, H.C.; Liu, S.J.; Su, Y.W.; et al. TLR9 and STING agonists cooperatively boost the immune response to SARS-CoV-2 RBD vaccine through an increased germinal center B cell response and reshaped T helper responses. Int. J. Biol. Sci. 2023, 19, 2897–2913. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Richert, L.E.; Servid, A.E.; Harmsen, A.L.; Rynda-Apple, A.; Han, S.; Wiley, J.A.; Douglas, T.; Harmsen, A.G. A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine 2012, 30, 3653–3665. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palić, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef]
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Archambault, D. Vaccination Strategies Based on Bacterial Self-Assembling Proteins as Antigen Delivery Nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liguori, A.; Skog, P.; Landais, E.; Menis, S.; et al. Immunogenicity of RNA Replicons Encoding HIV Env Immunogens Designed for Self-Assembly into Nanoparticles. Mol. Ther. 2019, 27, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Klarquist, J.; Cross, E.W.; Thompson, S.B.; Willett, B.; Aldridge, D.L.; Caffrey-Carr, A.K.; Xu, Z.; Hunter, C.A.; Getahun, A.; Kedl, R.M. B cells promote CD8 T cell primary and memory responses to subunit vaccines. Cell Rep. 2021, 36, 36. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C.M. Follicular helper T cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Walker, L.S.K.; Liu, Z.; Linterman, M.A.; Li, Z. Targeting TFH cells in human diseases and vaccination: Rationale and practice. Nat. Immunol. 2022, 23, 1157–1168. [Google Scholar] [CrossRef]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 controls follicular T helper cell positioning and function. Immunity 2018, 49, 264–274. [Google Scholar] [CrossRef]
- Goenka, R.; Barnett, L.G.; Silver, J.S.; O’Neill, P.J.; Hunter, C.A.; Cancro, M.P.; Laufer, T.M. Cutting edge: Dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 2011, 187, 1091–1095. [Google Scholar] [CrossRef]
- Choi, J.; Crotty, S. Bcl6-Mediated transcriptional regulation of follicular helper T cells (TFH). Trends Immunol. 2021, 42, 336–349. [Google Scholar]
- Ma, L.; Zhang, L.; Zhuang, Y.; Ding, Y.; Chen, J. Lactobacillus Improves the Effects of Prednisone on Autoimmune Hepatitis via Gut Microbiota-Mediated Follicular Helper T Cells. Cell Commun. Signal. 2022, 20, 83. [Google Scholar]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+) CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef]
- Wallin, E.F.; Jolly, E.C.; Suchánek, O.; Bradley, J.A.; Espéli, M.; Jayne, D.R.; Linterman, M.A.; Smith, K.G. Human T-follicular helper and T-follicular regulatory cell maintenance is independent of germinal centers. Blood 2014, 124, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Farooq, F.; Beck, K.; Paolino, K.M.; Phillips, R.; Waters, N.C.; Regules, J.A.; Bergmann-Leitner, E.S. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci. Rep. 2016, 6, 27944. [Google Scholar]
- Olatunde, A.C.; Hale, J.S.; Lamb, T.J. Cytokine-skewed Tfh cells: Functional consequences for B cell help. Trends Immunol. 2021, 42, 536–550. [Google Scholar] [PubMed]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar]
- Yao, Y.; Wang, Z.-C.; Wang, N.; Zhou, P.-C.; Chen, C.-L.; Song, J.; Sun, B. Allergen Immunotherapy Improves Defective Follicular Regulatory T Cells in Patients with Allergic Rhinitis. J. Allergy Clin. Immunol. 2019, 144, 118–128. [Google Scholar]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar]
- King, I.L.; Fortier, A.; Tighe, M.; Dibble, J.; Watts, G.F.M.; Veerapen, N.; Haberman, A.M.; Besra, G.S.; Mohrs, M.; Brenner, M.B.; et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 2011, 13, 44–50. [Google Scholar]
- Gaya, M.; Barral, P.; Burbage, M.; Aggarwal, S.; Montaner, B.; Warren Navia, A.; Aid, M.; Tsui, C.; Maldonado, P.; Nair, U.; et al. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 2018, 172, 517–533.e20. [Google Scholar] [CrossRef]
- Abos, B.; Wang, T.; Secombes, C.J.; Tafalla, C. Distinct modes of action of CD40L and adaptive cytokines IL-2, IL-4/13, IL-10, and IL-21 on rainbow trout IgM+ B cells. Dev. Comp. Immunol. 2020, 111, 103752. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 2011, 6, e17739. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Finney, J.; Okada, T.; Kurosaki, T.; Kelsoe, G. Primary germinal center-resident T follicular helper cells are a physiologically distinct subset of CXCR5hiPD-1hi T follicular helper cells. Immunity 2022, 55, 272–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Zhang, Y.; Zeng, W.; Wang, S.; Ji, P.; Pan, M.; Zhu, C.; Wang, Y. Distinct roles of ICOS and CD40L in human T-B cell adhesion and antibody production. Cell. Immunol. 2021, 368, 104420. [Google Scholar] [CrossRef]
- Reynaud, C.A.; Weill, J.C. Predicting AID off-targets: A step forward. J. Exp. Med. 2018, 215, 721–722. [Google Scholar] [CrossRef]
- Turner, J.S.; Ke, F.; Grigorova, I.L. B Cell Receptor Crosslinking Augments Germinal Center B Cell Selection when T Cell Help Is Limiting. Cell Rep. 2018, 25, 1395–1403.e4. [Google Scholar] [CrossRef]
- Xie, M.M.; Dent, A.L. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front. Immunol. 2018, 9, 1536. [Google Scholar] [CrossRef]
- Chen, J.S.; Grassmann, J.D.S.; Gowthaman, U.; Olyha, S.J.; Simoneau, T.; Berin, M.C.; Eisenbarth, S.C.; Williams, A. Flow Cytometric Identification of Tfh13 Cells in Mouse and Human. J. Allergy Clin. Immunol. 2021, 147, 470–483. [Google Scholar] [CrossRef]
- Qin, L.; Waseem, T.C.; Sahoo, A.; Bieerkehazhi, S.; Zhou, H.; Galkina, E.V.; Nurieva, R. Insights Into the Molecular Mechanisms of T Follicular Helper-Mediated Immunity and Pathology. Front. Immunol. 2018, 9, 1884. [Google Scholar] [CrossRef]
- Hoog, A.; Villanueva-Hernández, S.; Razavi, M.A.; van Dongen, K.; Eder, T.; Piney, L.; Chapat, L.; de Luca, K.; Grebien, F.; Mair, K.H.; et al. Identification of CD4+ T cells with T follicular helper cell characteristics in the pig. Dev. Comp. Immunol. 2022, 134, 104462. [Google Scholar] [CrossRef] [PubMed]
- Von La Roche, D.; Schumacher, M.; Kohn, M.; Trapp, J.; Schusser, B.; Rautenschlein, S.; Härtle, S. Characterization of class-switched B cells in chickens. Front. Immunol. 2024, 15, 1484288. [Google Scholar]
- Xiao, N.; Eto, D.; Elly, C.; Peng, G.; Crotty, S.; Liu, Y.-C. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 2014, 15, 657–666. [Google Scholar] [PubMed]
- Velu, V.; Mylvaganam, G.H.; Gangadhara, S.; Hong, J.J.; Iyer, S.S.; Gumber, S.; Ibegbu, C.C.; Villinger, F.; Amara, R.R. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J. Immunol. 2016, 197, 1832–1842. [Google Scholar]
- Juno, J.A.; Hill, D.L. T follicular helper cells and their impact on humoral responses during pathogen and vaccine challenge. Curr. Opin. Immunol. 2022, 74, 112–117. [Google Scholar] [PubMed]
- Yusuf, I.; Kageyama, R.; Monticelli, L.; Johnston, R.J.; Ditoro, D.; Hansen, K.; Barnett, B.; Crotty, S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol. 2010, 185, 190–202. [Google Scholar]
- Carpio, V.H.; Aussenac, F.; Puebla-Clark, L.; Wilson, K.D.; Villarino, A.V.; Dent, A.L.; Stephens, R. T Helper Plasticity Is Orchestrated by STAT3, Bcl6, and Blimp-1 Balancing Pathology and Protection in Malaria. iScience 2020, 23, 101310. [Google Scholar]
- Borrow, P.; Tishon, A.; Lee, S.; Xu, J.; Grewal, I.S.; Oldstone, M.B.; A Flavell, R. CD40L-Deficient Mice Show Deficits in Antiviral Immunity and Have an Impaired Memory CD8+ CTL Response. J. Exp. Med. 1996, 183, 2129–2142. [Google Scholar] [CrossRef]
- Bertram, E.M.; Tafuri, A.; Shahinian, A.; Chan, V.S.; Hunziker, L.; Recher, M.; Ohashi, P.S.; Mak, T.W.; Watts, T.H. Role of ICOS versus CD28 in Antiviral Immunity. Eur. J. Immunol. 2002, 32, 3376–3385. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Tang, J.; Xu, L.; Ye, L. Molecular Basis of the Differentiation and Function of Virus-Specific Follicular Helper CD4+ T Cells. Front. Immunol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Liang, H.; Tang, J.; Liu, Z.; Liu, Y.; Huang, Y.; Xu, Y.; Hao, P.; Yin, Z.; Zhong, J.; Ye, L.; et al. ZIKV infection induces robust Th1-like Tfh cell and long-term protective antibody responses in immunocompetent mice. Nat. Commun. 2019, 10, 3859. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Davydov, A.N.; Ladell, K.; McLaren, J.E.; Bonaiuti, P.; Metsger, M.; Ramsden, J.D.; Gilbert, S.C.; Lambe, T.; Price, D.A.; et al. CD4+ T Follicular Helper Cells in Human Tonsils and Blood Are Clonally Convergent but Divergent from Non-Tfh CD4+ Cells. Cell Rep. 2020, 30, 137–152.e5. [Google Scholar] [CrossRef]
- Devarajan, P.; Vong, A.M.; Castonguay, C.H.; Kugler-Umana, O.; Bautista, B.L.; Jones, M.C.; Kelly, K.A.; Xia, J.; Swain, S.L. Strong Influenza-Induced Tfh Generation Requires CD4 Effectors to Recognize Antigen Locally and Receive Signals from Continuing Infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2111064119. [Google Scholar] [CrossRef]
- Harker, J.A.; Dolgoter, A.; Zuniga, E.I. Cell-Intrinsic IL-27 and gp130 Cytokine Receptor Signaling Regulates Virus-Specific CD4+ T Cell Responses and Viral Control during Chronic Infection. Immunity 2013, 39, 548–559. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Wen, B.; Xie, T.; Tang, P.; Hu, Y.; Huang, L.; Jin, K.; Zhang, P.; Liu, Z.; et al. Circulating CXCR3+ Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci. Rep. 2019, 9, 10090. [Google Scholar] [CrossRef] [PubMed]
- Moysi, E.; Petrovas, C.; Koup, R.A. The role of follicular helper CD4 T cells in the development of HIV-1 specific broadly neutralizing antibody responses. Retrovirology 2018, 15, 54. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.L.; De Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Soltani, M.; Ahmadivand, S.; Behdani, M.; Hassanzadeh, R.; Rahmati-Holasoo, H.; Taheri-Mirghaed, A. Transcription of adaptive-immune genes upon challenge with infectious pancreatic necrosis virus (IPNV) in DNA vaccinated rainbow trout. Int. J. Aquat. Biol. 2016, 4, 353–359. [Google Scholar]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [PubMed]
- Ahmadivand, S.; Soltani, M.; Shokrpoor, S.; Rahmati-Holasoo, H.; El-Matbouli, M.; Taheri-Mirghaed, A. Cyprinid Herpesvirus 3 (CyHV-3) Transmission and Outbreaks in Iran: Detection and Characterization in Farmed Common Carp. Microb. Pathog. 2020, 149, 104321. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Yang, Z.; He, Q.; Bao, C.; Xie, Y.; Sun, Y.; Li, S.; Quan, Y.; Yang, H.; et al. Heterologous booster vaccination enhances antibody responses to SARS-CoV-2 by improving Tfh function and increasing B-cell clonotype SHM frequency. Front. Immunol. 2024, 15, 1406138. [Google Scholar]
- Dillard, J.A.; Taft-Benz, S.A.; Knight, A.C.; Anderson, E.J.; Pressey, K.D.; Parotti, B.; Martinez, S.A.; Diaz, J.L.; Sarkar, S.; Madden, E.A.; et al. Adjuvant-Dependent Impact of Inactivated SARS-CoV-2 Vaccines during Heterologous Infection by a SARS-Related Coronavirus. Nat. Commun. 2024, 15, 3738. [Google Scholar]
- Zhao, H.; Yang, J.; Qian, Q.; Wu, M.; Li, M.; Xu, W. Mesenteric CD103+DCs Initiate Switched Coxsackievirus B3 VP1-Specific IgA Response to Intranasal Chitosan-DNA Vaccine Through Secreting BAFF/IL-6 and Promoting Th17/Tfh Differentiation. Front. Immunol. 2018, 9, 2986. [Google Scholar]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Soltani, E.; Hassanzadeh, R.; Ashrafi-Helan, J. VP2 (PTA motif) encoding DNA vaccine confers protection against lethal challenge with infectious pancreatic necrosis virus (IPNV) in trout. Mol. Immunol. 2018, 94, 61–67. [Google Scholar] [CrossRef]
- Lee, J.H.; Hu, J.K.; Georgeson, E.; Nakao, C.; Groschel, B.; Dileepan, T.; Jenkins, M.K.; Seumois, G.; Vijayanand, P.; Schief, W.R.; et al. Modulating the Quantity of HIV Env-Specific CD4 T Cell Help Promotes Rare B Cell Responses in Germinal Centers. J. Exp. Med. 2021, 218, e20201254. [Google Scholar]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Hasanzadeh, R.; Soltani, E. Oral DNA vaccines based on CS-TPP nanoparticles and alginate microparticles confer high protection against infectious pancreatic necrosis virus (IPNV) infection in trout. Dev. Comp. Immunol. 2017, 74, 178–189. [Google Scholar] [CrossRef]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent Antigen and Germinal Center B Cells Sustain T Follicular Helper Cell Responses and Phenotype. Immunity 2013, 38, 596–605. [Google Scholar]
- Havenar-Daughton, C.; Newton, I.G.; Zare, S.Y.; Reiss, S.M.; Schwan, B.; Suh, M.J.; Hasteh, F.; Levi, G.; Crotty, S. Normal Human Lymph Node T Follicular Helper Cells and Germinal Center B Cells Accessed via Fine Needle Aspirations. J. Immunol. Methods 2020, 479, 112746. [Google Scholar]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human Circulating PD-1+CXCR3-CXCR5+ Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 2013, 39, 758–769. [Google Scholar] [PubMed]
- Hill, D.L.; Whyte, C.E.; Innocentin, S.; Lee, J.; Dooley, J.; Wang, J.; James, E.A.; Lee, J.C.; Kwok, W.W.; Zand, M.S.; et al. Impaired HA-Specific T Follicular Helper Cell and Antibody Responses to Influenza Vaccination Are Linked to Inflammation in Humans. eLife 2021, 10, e70554. [Google Scholar]
- Havenar-Daughton, C.; Carnathan, D.G.; Torrents de la Peña, A.; Pauthner, M.; Briney, B.; Reiss, S.M.; Wood, J.S.; Kaushik, K.; van Gils, M.J.; Rosales, S.L.; et al. Direct Probing of Germinal Center Responses Reveals Immunological Features and Bottlenecks for Neutralizing Antibody Responses to HIV Env Trimer. Cell Rep. 2016, 17, 2195–2209. [Google Scholar]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid Induction of Antigen-Specific CD4+ T Cells Is Associated with Coordinated Humoral and Cellular Immunity to SARS-CoV-2 mRNA Vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Yin, M.; Xiong, Y.; Liang, D.; Tang, H.; Hong, Q.; Liu, G.; Zeng, J.; Lian, T.; Huang, J.; Ni, J. Circulating Tfh Cell and Subsets Distribution Are Associated with Low-Responsiveness to Hepatitis B Vaccination. Mol. Med. 2021, 27, 32. [Google Scholar]
- Aljurayyan, A.; Puksuriwong, S.; Ahmed, M.; Sharma, R.; Krishnan, M.; Sood, S.; Davies, K.; Rajashekar, D.; Leong, S.; McNamara, P.S.; et al. Activation and Induction of Antigen-Specific T Follicular Helper Cells Play a Critical Role in Live-Attenuated Influenza Vaccine-Induced Human Mucosal Anti-Influenza Antibody Response. J. Virol. 2018, 92, e00114-18. [Google Scholar] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [PubMed]
- Mu, Z.; Wiehe, K.; Saunders, K.O.; Henderson, R.; Cain, D.W.; Parks, R.; Martik, D.; Mansouri, K.; Edwards, R.J.; Newman, A.; et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 2022, 38, 110514. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Son, S.; Lee, K. Development of mRNA Vaccines/Therapeutics and Their Delivery System. Mol. Cells 2023, 46, 41–47. [Google Scholar]
- Schmidt, C.; Schnierle, B.S. Self-Amplifying RNA Vaccine Candidates: Alternative Platforms for mRNA Vaccine Development. Pathogens 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Komori, M.; Nogimori, T.; Morey, A.L.; Sekida, T.; Ishimoto, K.; Hassett, M.R.; Masuta, Y.; Ode, H.; Tamura, T.; Suzuki, R.; et al. saRNA vaccine expressing membrane-anchored RBD elicits broad and durable immunity against SARS-CoV-2 variants of concern. Nat. Commun. 2023, 14, 2810. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines-A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar]
- Shim, M.S.; Kwon, Y.J. Stimuli-Responsive Polymers and Nanomaterials for Gene Delivery and Imaging Applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A.; Loré, K. Immune Responses Induced by mRNA Vaccination in Mice, Monkeys, and Humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate Immune Mechanisms of mRNA Vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Lindgren, G.; Ols, S.; Liang, F.; Thompson, E.A.; Lin, A.; Hellgren, F.; Bahl, K.; John, S.; Yuzhakov, O.; Hassett, K.J.; et al. Induction of Robust B Cell Responses after Influenza mRNA Vaccination Is Accompanied by Circulating Hemagglutinin-Specific ICOS+ PD-1+ CXCR3+ T Follicular Helper Cells. Front. Immunol. 2017, 8, 1539. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [PubMed]
- Fazel, F.; Doost, J.S.; Raj, S.; Boodhoo, N.; Karimi, K.; Sharif, S. The mRNA Vaccine Platform for Veterinary Species. Vet. Immunol. Immunopathol. 2024, 274, 110803. [Google Scholar] [CrossRef]
- Bian, T.; Hao, M.; Zhao, X.; Zhao, C.; Luo, G.; Zhang, Z.; Fu, G.; Yang, L.; Chen, Y.; Wang, Y.; et al. A Rift Valley fever mRNA vaccine elicits strong immune responses in mice and rhesus macaques. npj Vaccines 2023, 8, 164. [Google Scholar]
- Pulido, M.R.; Sobrino, F.; Borrego, B.; Sáiz, M. RNA immunization can protect mice against foot-and-mouth disease virus. Antivir. Res. 2010, 85, 556–558. [Google Scholar] [PubMed]
- Hajam, I.A.; Senevirathne, A.; Hewawaduge, C.; Kim, J.; Lee, J.H. Intranasally administered protein-coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 2020, 51, 37. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, Y.; Niu, Z.; Hao, W.; Ketema, H.; Wang, Z.; Xu, J.; Sheng, L.; Cai, Y.; Yu, Z.; et al. Preclinical Efficacy of Cap-Dependent and Independent mRNA Vaccines against Bovine Viral Diarrhea Virus-1. Vet. Sci. 2024, 11, 373. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Wang, L.; Zhao, X.; Wang, L.; Qiu, X.; Yang, X.; Zhu, W.; Lv, L.; Kang, Y.; et al. Advancing vaccine development: Evaluation of a mannose-modified lipid nanoparticle-based candidate for African swine fever p30 mRNA vaccine eliciting robust immune response in mice. Int. J. Biol. Macromol. 2024, 270 Pt 1, 132432. [Google Scholar]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.; Kamrud, K.I. Safety, Immunogenicity, and Efficacy of an Alphavirus Replicon-Based Swine Influenza Virus Hemagglutinin Vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar]
- Zhou, L.; Wubshet, A.K.; Zhang, J.; Hou, S.; Yao, K.; Zhao, Q.; Dai, J.; Liu, Y.; Ding, Y.; Zhang, J.; et al. The mRNA Vaccine Expressing Single and Fused Structural Proteins of Porcine Reproductive and Respiratory Syndrome Induces Strong Cellular and Humoral Immune Responses in BalB/C Mice. Viruses 2024, 16, 544. [Google Scholar] [CrossRef]
- Gupta, S.; Su, H.; Agrawal, S. Immune Response to SARS-CoV-2 Vaccine in 2 Men. Int. Arch. Allergy Immunol. 2022, 183, 350–359. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, C.; Xing, J.; Zhang, T.; Xu, Z.; Di, Y.; Yang, S.; Jiang, R.; Tang, J.; Zhuang, X.; et al. Ferritin-binding and ubiquitination-modified mRNA vaccines induce potent immune responses and protective efficacy against SARS-CoV-2. Int. Immunopharmacol. 2024, 129, 111630. [Google Scholar]
- Roier, S.; Mangala Prasad, V.; McNeal, M.M.; Lee, K.K.; Petsch, B.; Rauch, S. mRNA-based VP8* nanoparticle vaccines against rotavirus are highly immunogenic in rodents. npj Vaccines 2023, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Brandys, P.; Montagutelli, X.; Merenkova, I.; Barut, G.T.; Thiel, V.; Schork, N.J.; Trüeb, B.; Conquet, L.; Deng, A.; Antanasijevic, A.; et al. A mRNA Vaccine Encoding for a RBD 60-mer Nanoparticle Elicits Neutralizing Antibodies and Protective Immunity Against the SARS-CoV-2 Delta Variant in Transgenic K18-hACE2 Mice. Front. Immunol. 2022, 13, 912898. [Google Scholar]
- Kelly, H.G.; Tan, H.X.; Juno, J.A.; Esterbauer, R.; Ju, Y.; Jiang, W.; Wimmer, V.C.; Duckworth, B.C.; Groom, J.R.; Caruso, F.; et al. Self-Assembling Influenza Nanoparticle Vaccines Drive Extended Germinal Center Activity and Memory B Cell Maturation. JCI Insight 2020, 5, e136653. [Google Scholar] [PubMed]
- Jung, H.-G.; Jeong, S.; Kang, M.-J.; Hong, I.; Park, Y.-S.; Ko, E.; Kim, J.-O.; Choi, D.-Y. Molecular Design of Encapsulin Protein Nanoparticles to Display Rotavirus Antigens for Enhancing Immunogenicity. Vaccines 2024, 12, 1020. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Tacken, M.; Gutjahr, B.; Keller, M.; van Keulen, L.; Kant, J.; van de Water, S.; Lin, Y.; Eiden, M.; Rissmann, M.; et al. Vaccine Efficacy of Self-Assembled Multimeric Protein Scaffold Particles Displaying the Glycoprotein Gn Head Domain of Rift Valley Fever Virus. Vaccines 2021, 9, 301. [Google Scholar] [CrossRef]
- Soltani, M.; Zamani, M.; Taheri-Mirghaed, A.; Ahmadivand, S.; Mohamdian, S.; Abdi, K.; Soltani, E. Incidence and Genetic Analysis of White Spot Syndrome Virus (WSSV) in Farmed Shrimps (P. indicus and L. vannamei) in Iran. Bull. Eur. Assoc. Fish. Pathol. 2018, 38, 24–34. [Google Scholar]
- Ahmadivand, S.; Palić, D.; Weidmann, M. Molecular Epidemiology of Novirhabdoviruses Emerging in Iranian Trout Farms. Viruses 2021, 13, 448. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Ahmadivand, S.; Marandi, A.; Shokrpoor, S.; Palić, D.; Jahangard, A. Identification and Characterization of Lymphocystis Disease Virus (LCDV) from Indian Glassy Fish (Parambassis ranga Hamilton, 1822) in Iran. Aquacult. Int. 2022, 30, 2593–2602. [Google Scholar]
- Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA-Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines 2024, 12, 788. [Google Scholar] [CrossRef]
- See, S.A.; Bhassu, S.; Tang, S.S.; Yusoff, K. Newly Developed mRNA Vaccines Induce Immune Responses in Litopenaeus vannamei Shrimps During Primary Vaccination. Dev. Comp. Immunol. 2024, 162, 105264. [Google Scholar]
- Soltani, M.; Ahmadivand, S.; Ringø, E. Chapter 11—Bacillus as Probiotics in Shellfish Culture. In Bacillus Probiotics for Sustainable Aquaculture; Soltani, M., Elumalai, P., Ghosh, K., Ringø, E., Eds.; Taylor & Francis’: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2024; p. 280. [Google Scholar]
- Duhen, R.; Beymer, M.; Jensen, S.M.; Abbina, S.; Abraham, S.; Jain, N.; Thomas, A.; Geall, A.J.; Hu, H.M.; Fox, B.A.; et al. OX40 Agonist Stimulation Increases and Sustains Humoral and Cell-Mediated Responses to SARS-CoV-2 Protein and saRNA Vaccines. Front. Immunol. 2022, 13, 896310. [Google Scholar] [CrossRef]
- Brook, B.; Duval, V.; Barman, S.; Speciner, L.; Sweitzer, C.; Khanmohammed, A.; Menon, M.; Foster, K.; Ghosh, P.; Abedi, K.; et al. Adjuvantation of a SARS-CoV-2 mRNA Vaccine with Controlled Tissue-Specific Expression of an mRNA Encoding IL-12p70. Sci. Transl. Med. 2024, 16, eadm8451. [Google Scholar] [PubMed]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) from Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar]
- Ahmadivand, S.; Krpetic, Z.; Martínez, M.M.; García-Ordoñez, M.; Roher, N.; Palić, D. Self-assembling ferritin nanoplatform for the development of infectious hematopoietic necrosis virus vaccine. Front. Immunol. 2024, 15, 1346512. [Google Scholar]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines 2021, 6, 70. [Google Scholar]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef]
- Brune, K.D.; Howarth, M. New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue. Front. Immunol. 2018, 9, 1432. [Google Scholar]
- Inoue, T.; Baba, Y.; Kurosaki, T. BCR Signaling in Germinal Center B Cell Selection. Trends Immunol. 2024, 45, 693–704. [Google Scholar]
- Lainšček, D.; Fink, T.; Forstnerič, V.; Hafner-Bratkovič, I.; Orehek, S.; Strmšek, Ž.; Manček-Keber, M.; Pečan, P.; Esih, H.; Malenšek, Š.; et al. A Nanoscaffolded Spike-RBD Vaccine Provides Protection against SARS-CoV-2 with Minimal Anti-Scaffold Response. Vaccines 2021, 9, 431. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.R.; Whittle, J.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-Assembling Influenza Nanoparticle Vaccines Elicit Broadly Neutralizing H1N1 Antibodies. Nature 2013, 499, 102–106. [Google Scholar]
- Carmen, J.M.; Shrivastava, S.; Lu, Z.; Anderson, A.; Morrison, E.B.; Sankhala, R.S.; Chen, W.H.; Chang, W.C.; Bolton, J.S.; Matyas, G.R.; et al. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spike-specific T cell responses. Npj Vaccines 2021, 6, 151. [Google Scholar]
- Aebischer, A.; Wernike, K.; König, P.; Franzke, K.; Wichgers Schreur, P.J.; Kortekaas, J.; Vitikainen, M.; Wiebe, M.; Saloheimo, M.; Tchelet, R.; et al. Development of a Modular Vaccine Platform for Multimeric Antigen Display Using an Orthobunyavirus Model. Vaccines 2021, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chao, C.W.; Tsybovsky, Y.; Abiona, O.M.; Hutchinson, G.B.; Moliva, J.I.; Olia, A.S.; Pegu, A.; Phung, E.; Stewart-Jones, G.B.E.; et al. A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci. Rep. 2020, 10, 18149. [Google Scholar] [CrossRef]
- He, L.; de Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [PubMed]
- Kar, U.; Khaleeq, S.; Garg, P.; Bhat, M.; Reddy, P.; Vignesh, V.S.; Upadhyaya, A.; Das, M.; Chakshusmathi, G.; Pandey, S.; et al. Comparative Immunogenicity of Bacterially Expressed Soluble Trimers and Nanoparticle Displayed Influenza Hemagglutinin Stem Immunogens. Front. Immunol. 2022, 13, 890622. [Google Scholar]
- Khaleeq, S.; Sengupta, N.; Kumar, S.; Patel, U.R.; Rajmani, R.S.; Reddy, P.; Pandey, S.; Singh, R.; Dutta, S.; Ringe, R.P.; et al. Neutralizing Efficacy of Encapsulin Nanoparticles against SARS-CoV2 Variants of Concern. Viruses 2023, 15, 346. [Google Scholar] [CrossRef]
- Sheng, Y.; Chen, Z.; Cherrier, M.V.; Martin, L.; Bui, T.T.T.; Li, W.; Lynham, S.; Nicolet, Y.; Ebrahimi, K.H. A Versatile Virus-Mimetic Engineering Approach for Concurrent Protein Nanocage Surface-Functionalization and Cargo Encapsulation. Small 2024, 20, e2310913. [Google Scholar]
- Langenmayer, M.C.; Luelf-Averhoff, A.T.; Marr, L.; Jany, S.; Freudenstein, A.; Adam-Neumair, S.; Tscherne, A.; Fux, R.; Rojas, J.J.; Blutke, A.; et al. Newly Designed Poxviral Promoters to Improve Immunogenicity and Efficacy of MVA-NP Candidate Vaccines against Lethal Influenza Virus Infection in Mice. Pathogens 2023, 12, 867. [Google Scholar] [CrossRef]
- Riteau, N.; Radtke, A.J.; Shenderov, K.; Mittereder, L.; Oland, S.D.; Hieny, S.; Jankovic, D.; Sher, A. Water-in-Oil-Only Adjuvants Selectively Promote T Follicular Helper Cell Polarization through a Type I IFN and IL-6-Dependent Pathway. J. Immunol. 2016, 197, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Serre, K.; Mohr, E.; Benezech, C.; Bird, R.; Khan, M.; Caamano, J.H.; Cunningham, A.F.; Maclennan, I.C. Selective effects of NF-κB1 deficiency in CD4(+) T cells on Th2 and Tfh induction by alum-precipitated protein vaccines. Eur. J. Immunol. 2011, 41, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Baehr, C.; Schmiel, S.E.; Accetturo, C.; Mueller, D.L.; Pravetoni, M. Alum adjuvant is more effective than MF59 at prompting early germinal center formation in response to peptide-protein conjugates and enhancing efficacy of a vaccine against opioid use disorders. Hum. Vaccin. Immunother. 2019, 15, 909–917. [Google Scholar] [CrossRef]
- Hill, D.L.; Pierson, W.; Bolland, D.J.; Mkindi, C.; Carr, E.J.; Wang, J.; Houard, S.; Wingett, S.W.; Audran, R.; Wallin, E.F.; et al. The Adjuvant GLA-SE Promotes Human Tfh Cell Expansion and Emergence of Public TCRβ Clonotypes. J. Exp. Med. 2019, 216, 1857–1873. [Google Scholar] [CrossRef] [PubMed]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; Siegrist, C.A. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and germinal center responses in adult and early life. J. Immunol. 2015, 194, 4836–4846. [Google Scholar]
- Gómez, E.A.; Rodríguez-Cariño, C.; García-Blanco, M.; Martínez-Lobo, F.J.; Mateu, E. Enhanced germinal center responses and antibody production with Montanide ISA 206 in PRRSV vaccines. Vet. Immunol. Immunopathol. 2023, 254, 110550. [Google Scholar]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. Immunohorizons 2018, 2, 185–197. [Google Scholar]
- Chen, C.; Zhang, C.; Li, R.; Wang, Z.; Yuan, Y.; Li, H.; Fu, Z.; Zhou, M.; Zhao, L. Monophosphoryl-Lipid A (MPLA) is an efficacious adjuvant for inactivated rabies vaccines. Viruses 2019, 11, 1118. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-year efficacy of RTS, S/AS01 malaria vaccine among young African children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef]
- Garçon, N.; Morel, S.; Didierlaurent, A.; Descamps, D.; Wettendorff, M.; Van Mechelen, M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011, 25, 217–226. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Ogbe, A.; Pedroza-Pacheco, I.; Doeleman, S.E.; Chen, Y.; Silk, S.E.; Barrett, J.R.; Elias, S.C.; Miura, K.; Diouf, A.; et al. Protein/AS01B Vaccination Elicits Stronger, More Th2-Skewed Antigen-Specific Human T Follicular Helper Cell Responses than Heterologous Viral Vectors. Cell Rep. Med. 2021, 2, 100207. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Kato, Y.; Melo, M.B.; Phung, I.; Freeman, B.L.; Li, Z.; Roh, K.; Van Wijnbergen, J.W.; Watkins, H.; Enemuo, C.A.; et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci. Immunol. 2021, 6, eabf1152. [Google Scholar] [CrossRef] [PubMed]
- Burn, O.K.; Pankhurst, T.E.; Painter, G.F.; Connor, L.M.; Hermans, I.F. Harnessing NKT Cells for Vaccination. Oxf. Open Immunol. 2021, 2, iqab013. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.P.; de Carvalho Madrid, D.M.; Gu, W.; Artiaga, B.L.; Richt, J.A. Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells. Front. Immunol. 2020, 11, 2172. [Google Scholar] [CrossRef]
- Pankhurst, T.E.; Buick, K.H.; Lange, J.L.; Marshall, A.J.; Button, K.R.; Palmer, O.R.; Farrand, K.J.; Montgomerie, I.; Bird, T.W.; Mason, N.C.; et al. MAIT cells activate dendritic cells to promote TFH cell differentiation and induce humoral immunity. Cell Rep. 2023, 42, 112310. [Google Scholar] [CrossRef]
- Naderi-Samani, M.; Soltani, M.; Dadar, M.; Taheri-Mirghaed, A.; Zargar, A.; Ahmadivand, S.; Hassanzadeh, R.; Moazami Goudarzi, L. Oral Immunization of Trout Fry with Recombinant Lactococcus lactis NZ3900 Expressing G Gene of Viral Hemorrhagic Septicemia Virus (VHSV). Fish. Shellfish. Immunol. 2020, 105, 62–70. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadivand, S.; Fux, R.; Palić, D. Role of T Follicular Helper Cells in Viral Infections and Vaccine Design. Cells 2025, 14, 508. https://doi.org/10.3390/cells14070508
Ahmadivand S, Fux R, Palić D. Role of T Follicular Helper Cells in Viral Infections and Vaccine Design. Cells. 2025; 14(7):508. https://doi.org/10.3390/cells14070508
Chicago/Turabian StyleAhmadivand, Sohrab, Robert Fux, and Dušan Palić. 2025. "Role of T Follicular Helper Cells in Viral Infections and Vaccine Design" Cells 14, no. 7: 508. https://doi.org/10.3390/cells14070508
APA StyleAhmadivand, S., Fux, R., & Palić, D. (2025). Role of T Follicular Helper Cells in Viral Infections and Vaccine Design. Cells, 14(7), 508. https://doi.org/10.3390/cells14070508









