Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter
Abstract
1. Introduction
2. Part I
2.1. Heart Development in Zebrafish: An Overview
2.2. Contributions of the First and Second Heart Fields in Vertebrate and Zebrafish Heart Development
2.2.1. Early Blastula and Gastrula
2.2.2. Transcription Factors in Zebrafishes’ Early Cardiac Development
Nkx2.5
Gata5
Hand2
| Transcription Factor | Role in Zebrafish Heart | Role in Mammalian Heart | Expression in Heart |
|---|---|---|---|
| GATA4/GATA5 /GATA6 | Mesoderm-to-cardiac fate transition [77,78] | Anterior lateral plate mesoderm (ALPM) at early segmentation phases [78], outflow tract development [79,80] | Yes, mesoderm stage |
| NKX2.5 | Chamber formation and differentiation [47,55,81,82,83] | Differentiation of outflow tract and right ventricle from progenitors of the second heart field (SHF) [55,84] | Yes, gastrulation stage |
| TBX (T-box transcription factor family) | Cardiac progenitor formation, chamber formation and differentiation [85,86] | Cardiovascular development, homeostasis, and cardiac remodeling [85,87,88,89] | Yes, mesoderm phase |
| SRF (Serum response factor) | Cardiac crescent formation in embryos [90] | Mesoderm formation and cardiac crescent formation [91] | Yes, mesoderm phase |
| HAND2 | Promotes cardiomyocyte formation and cardiac fusion [65,92] | Formation of the right ventricle and the outflow tract in the second heart field [93] | |
| MEF2 | Drives cardiomyocyte differentiation [94,95] | Cardiac differentiation and cardiac organogenesis [95,96] | |
| Wt1a and Wt1b (Wilm’s tumor) | Help in transdifferentiation in cardiomyocytes, yielding epicardial-like cells [97] | Formation of the vasculature of the heart [98,99,100] | Expressed in the pericardium |
| TCF21 | Epicardium development [101] | Cellular differentiation and cellular fate specificity [102] | Mesoderm |
2.3. Cellular Signaling in Cardiac Development
2.3.1. Hedgehog (Hh) Signaling
2.3.2. Fibroblast Growth Factor (FGF) Signaling
2.3.3. Bone Morphogenetic Protein (BMP) Signaling
2.3.4. Retinoic Acid (RA) Signaling
2.3.5. Wnt Signaling
2.3.6. Heart Looping: Chamber Formation
3. Part II
3.1. Zebrafish Cardiovascular Toolkit
3.1.1. Transgenesis to Study Zebrafish Cardiac Development
3.1.2. Cardiovascular Imaging in Zebrafish
3.1.3. Electrophysiology: Measuring the Zebrafish Cardiac Function
3.2. Revisiting Genetic Tools in Zebrafish Cardiovascular Research
3.2.1. Forward and Reverse Genetics in Zebrafish Models
3.2.2. Zinc Finger Nucleases (ZFNs), TALENs, and TILLING: Revisiting Traditional Approaches
3.2.3. Clustered Regularly Interspaced Short Palindromic Repeats/Cas (CRISPR)
3.2.4. CRISPR/Cas9 and Beyond: The Frontier of Genetic Engineering in Zebrafish
3.2.5. Drug Screening in Zebrafish
4. Part III
4.1. Recent Advancements in Zebrafish Research Toolkits
4.1.1. CRISPR/Cas9-Mediated Gene Editing for Cardiac Research
4.1.2. Lineage Tracing and Cellular Origins of Cardiac Repair
4.1.3. High-Throughput Lineage Tracing with GESTALT
4.1.4. The LINNAEUS System for Simultaneous Lineage Tracing and Transcriptome Profiling
4.1.5. TEMPO for Real-Time Imaging
4.1.6. FRaeppli Toolbox for Multi-Spectral Imaging and Cellular Tracking
4.1.7. Cre/Lox System for Spatial and Temporal Control in Cardiac Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendis, S.; Puska, P.; Norrving, B.; World Health Organization; World Heart Federation; World Stroke Organization. Global Atlas on Cardiovascular Disease Prevention and Control/Edited by: Shanthi Mendis … [et al.]; World Health Organization: Geneva, Switzerland, 2011.
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W., Jr.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation 2007, 115, 3015–3038. [Google Scholar] [CrossRef]
- Bruneau, B.G.; Srivastava, D. Congenital Heart Disease. Circ. Res. 2014, 114, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Scuccimarri, R. Kawasaki disease. Pediatr. Clin. N. Am. 2012, 59, 425–445. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primers 2016, 2, 15084. [Google Scholar] [CrossRef]
- Lehrman, M.A.; Goldstein, J.L.; Brown, M.S.; Russell, D.W.; Schneider, W.J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell 1985, 41, 735–743. [Google Scholar] [CrossRef]
- Geisterfer-Lowrance, A.A.; Kass, S.; Tanigawa, G.; Vosberg, H.P.; McKenna, W.; Seidman, C.E.; Seidman, J.G. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell 1990, 62, 999–1006. [Google Scholar] [CrossRef]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef]
- Rohringer, S.; Bergmeister, H.; Hamza, O.; Kiss, A.; Nagel, F.; Pilz, P.M.; Plasenzotti, R.; Podesser, B.K. Animal Models in Cardiovascular Biology. In Fundamentals of Vascular Biology; Geiger, M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 351–383. [Google Scholar]
- Angom, R.S.; Nakka, N.M.R. Zebrafish as a Model for Cardiovascular and Metabolic Disease: The Future of Precision Medicine. Biomedicines 2024, 12, 693. [Google Scholar] [CrossRef]
- Tsang, H.G.; Rashdan, N.A.; Whitelaw, C.B.; Corcoran, B.M.; Summers, K.M.; MacRae, V.E. Large animal models of cardiovascular disease. Cell Biochem. Funct. 2016, 34, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Kooij, V.; Venkatraman, V.; Tra, J.; Kirk, J.A.; Rowell, J.; Blice-Baum, A.; Cammarato, A.; Van Eyk, J.E. Sizing up models of heart failure: Proteomics from flies to humans. Proteom. Clin. Appl. 2014, 8, 653–664. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Cao, J.; Wei, Z.; Nie, Y.; Chen, H.Z. Therapeutic potential of alternative splicing in cardiovascular diseases. EBioMedicine 2024, 101, 104995. [Google Scholar] [CrossRef]
- Yang, D.; Jian, Z.; Tang, C.; Chen, Z.; Zhou, Z.; Zheng, L.; Peng, X. Zebrafish Congenital Heart Disease Models: Opportunities and Challenges. Int. J. Mol. Sci. 2024, 25, 5943. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef]
- Milan, D.J.; MacRae, C.A. Zebrafish genetic models for arrhythmia. Progress. Biophys. Mol. Biol. 2008, 98, 301–308. [Google Scholar] [CrossRef]
- Arnaout, R.; Ferrer, T.; Huisken, J.; Spitzer, K.; Stainier, D.Y.R.; Tristani-Firouzi, M.; Chi, N.C. Zebrafish model for human long QT syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 11316–11321. [Google Scholar] [CrossRef] [PubMed]
- Nemtsas, P.; Wettwer, E.; Christ, T.; Weidinger, G.; Ravens, U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J. Mol. Cell. Cardiol. 2010, 48, 161–171. [Google Scholar] [CrossRef]
- Forouhar, A.S.; Hove, J.R.; Calvert, C.; Flores, J.; Jadvar, H.; Gharib, M. Electrocardiographic characterization of embryonic zebrafish. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 2004, 3615–3617. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 2007, 18, 36–45. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Gemberling, M.; Wang, J.; Holdway, J.E.; Shen, M.-C.; Karlstrom, R.O.; Poss, K.D. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 2013, 140, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, J.J.; Keegan, B.R.; Yelon, D. Vessel and Blood Specification Override Cardiac Potential in Anterior Mesoderm. Dev. Cell 2007, 13, 254–267. [Google Scholar] [CrossRef]
- Glickman, N.S.; Yelon, D. Cardiac development in zebrafish: Coordination of form and function. Semin. Cell Dev. Biol. 2002, 13, 507–513. [Google Scholar] [CrossRef]
- Stainier, D.Y.R.; Fishman, M.C. Patterning the zebrafish heart tube: Acquisition of anteroposterior polarity. Dev. Biol. 1992, 153, 91–101. [Google Scholar] [CrossRef]
- Stainier, D.Y.; Lee, R.K.; Fishman, M.C. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 1993, 119, 31–40. [Google Scholar] [CrossRef]
- Fishman, M.C.; Chien, K.R. Fashioning the vertebrate heart: Earliest embryonic decisions. Development 1997, 124, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- de Pater, E.; Clijsters, L.; Marques, S.R.; Lin, Y.-F.; Garavito-Aguilar, Z.V.; Yelon, D.; Bakkers, J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 2009, 136, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Yelon, D.; Conlon, F.L.; Kirby, M.L. Myocardial Lineage Development. Circ. Res. 2010, 107, 1428–1444. [Google Scholar] [CrossRef]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Yelon, D.; Ticho, B.; Halpern, M.E.; Ruvinsky, I.; Ho, R.K.; Silver, L.M.; Stainier, D.Y. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 2000, 127, 2573–2582. [Google Scholar] [CrossRef]
- Kidokoro, H.; Saijoh, Y.; Schoenwolf, G.C. Nodal signaling regulates asymmetric cellular behaviors, driving clockwise rotation of the heart tube in zebrafish. Commun. Biol. 2022, 5, 996. [Google Scholar] [CrossRef]
- Franco, D.; Sedmera, D.; Lozano-Velasco, E. Multiple Roles of Pitx2 in Cardiac Development and Disease. J. Cardiovasc. Dev. Dis. 2017, 4, 16. [Google Scholar] [CrossRef]
- Hoogaars, W.M.; Barnett, P.; Moorman, A.F.; Christoffels, V.M. T-box factors determine cardiac design. Cell Mol. Life Sci. 2007, 64, 646–660. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Zhu, D.; Fang, Y.; Gao, K.; Shen, J.; Zhong, T.P.; Li, F. Vegfa Impacts Early Myocardium Development in Zebrafish. Int. J. Mol. Sci. 2017, 18, 444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bressan, M.; Hassel, D.; Huisken, J.; Staudt, D.; Kikuchi, K.; Poss, K.D.; Mikawa, T.; Stainier, D.Y. A dual role for ErbB2 signaling in cardiac trabeculation. Development 2010, 137, 3867–3875. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; de la Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef]
- Lavine, K.J.; Long, F.; Choi, K.; Smith, C.; Ornitz, D.M. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 2008, 135, 3161–3171. [Google Scholar] [CrossRef]
- Guner-Ataman, B.; Paffett-Lugassy, N.; Adams, M.S.; Nevis, K.R.; Jahangiri, L.; Obregon, P.; Kikuchi, K.; Poss, K.D.; Burns, C.E.; Burns, C.G. Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function. Development 2013, 140, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.K.; Stainier, D.Y.; Weinstein, B.M.; Fishman, M.C. Cardiovascular development in the zebrafish: II. Endocardial progenitors are sequestered within the heart field. Development 1994, 120, 3361–3366. [Google Scholar] [CrossRef]
- Warga, R.M.; Nusslein-Volhard, C. Origin and development of the zebrafish endoderm. Development 1999, 126, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Keegan, B.R.; Meyer, D.; Yelon, D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development 2004, 131, 3081–3091. [Google Scholar] [CrossRef]
- Kathiriya, I.S.; Nora, E.P.; Bruneau, B.G. Investigating the transcriptional control of cardiovascular development. Circ. Res. 2015, 116, 700–714. [Google Scholar] [CrossRef]
- Yelon, D.; Horne, S.A.; Stainier, D.Y.R. Restricted Expression of Cardiac Myosin Genes Reveals Regulated Aspects of Heart Tube Assembly in Zebrafish. Dev. Biol. 1999, 214, 23–37. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Fishman, M.C. Notochord Regulates Cardiac Lineage in Zebrafish Embryos. Dev. Biol. 1998, 201, 247–252. [Google Scholar] [CrossRef]
- Harrington, J.K.; Sorabella, R.; Tercek, A.; Isler, J.R.; Targoff, K.L. Nkx2.5 is essential to establish normal heart rate variability in the zebrafish embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R265–R271. [Google Scholar] [CrossRef]
- Cao, C.; Li, L.; Zhang, Q.; Li, H.; Wang, Z.; Wang, A.; Liu, J. Nkx2.5: A crucial regulator of cardiac development, regeneration and diseases. Front. Cardiovasc. Med. 2023, 10, 1270951. [Google Scholar] [CrossRef] [PubMed]
- Serbedzija, G.N.; Chen, J.-N.; Fishman, M.C. Regulation in the heart field of zebrafish. Development 1998, 125, 1095–1101. [Google Scholar] [CrossRef]
- Lee, K.-H.; Xu, Q.; Breitbart, R.E. A Newtinman-Related Gene, nkx2.7,Anticipates the Expression ofnkx2.5andnkx2.3in Zebrafish Heart and Pharyngeal Endoderm. Dev. Biol. 1996, 180, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Targoff, K.L.; Schell, T.; Yelon, D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev. Biol. 2008, 322, 314–321. [Google Scholar] [CrossRef]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes. Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Ya, J.; de Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef]
- Patient, R.K.; McGhee, J.D. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 2002, 12, 416–422. [Google Scholar] [CrossRef]
- Maves, L.; Tyler, A.; Moens, C.B.; Tapscott, S.J. Pbx acts with Hand2 in early myocardial differentiation. Dev. Biol. 2009, 333, 409–418. [Google Scholar] [CrossRef]
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Schindler, Y.L.; Garske, K.M.; Wang, J.; Firulli, B.A.; Firulli, A.B.; Poss, K.D.; Yelon, D. Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development 2014, 141, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, X.F.; Shen, A.D.; Wang, Q.; Liu, C.X.; Guo, Y.J.; Song, Z.J.; Li, Z.Z. Transcription factor HAND2 mutations in sporadic Chinese patients with congenital heart disease. Chin. Med. J. 2010, 123, 1623–1627. [Google Scholar]
- Xu, W.; Ahmad, A.; Dagenais, S.; Iyer, R.K.; Innis, J.W. Chromosome 4q deletion syndrome: Narrowing the cardiovascular critical region to 4q32.2–q34.3. Am. J. Med. Genet. Part A 2012, 158A, 635–640. [Google Scholar] [CrossRef]
- Tamura, M.; Amano, T.; Shiroishi, T. Chapter Three—The Hand2 Gene Dosage Effect in Developmental Defects and Human Congenital Disorders. In Current Topics in Developmental Biology; Taneja, R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 110, pp. 129–152. [Google Scholar]
- Ranganayakulu, G.; Elliott, D.A.; Harvey, R.P.; Olson, E.N. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development 1998, 125, 3037–3048. [Google Scholar] [CrossRef]
- Park, M.; Lewis, C.; Turbay, D.; Chung, A.; Chen, J.-N.; Evans, S.; Breitbart, R.E.; Fishman, M.C.; Izumo, S.; Bodmer, R. Differential rescue of visceral and cardiac defects in Drosophila by vertebrate tinman-related genes. Proc. Natl. Acad. Sci. USA 1998, 95, 9366–9371. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, Z.; Bartunkova, S.; Yamasaki, N.; Izumo, S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 1999, 126, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Targoff, K.L.; Colombo, S.; George, V.; Schell, T.; Kim, S.-H.; Solnica-Krezel, L.; Yelon, D. Nkx genes are essential for maintenance of ventricular identity. Development 2013, 140, 4203–4213. [Google Scholar] [CrossRef]
- Peterkin, T.; Gibson, A.; Patient, R. Redundancy and evolution of GATA factor requirements in development of the myocardium. Dev. Biol. 2007, 311, 623–635. [Google Scholar] [CrossRef]
- Holtzinger, A.; Evans, T. Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Dev. Biol. 2007, 312, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-T.; Yang, T.-C.; Tsai, H.-J. Nkx2.7 and Nkx2.5 Function Redundantly and Are Required for Cardiac Morphogenesis of Zebrafish Embryos. PLoS ONE 2009, 4, e4249. [Google Scholar] [CrossRef]
- Hatcher, C.J.; Diman, N.Y.S.G.; McDermott, D.A.; Basson, C.T. Transcription factor cascades in congenital heart malformation. Trends Mol. Med. 2003, 9, 512–515. [Google Scholar] [CrossRef]
- Song, M.; Yuan, X.; Racioppi, C.; Leslie, M.; Stutt, N.; Aleksandrova, A.; Christiaen, L.; Wilson, M.D.; Scott, I.C. GATA4/5/6 family transcription factors are conserved determinants of cardiac versus pharyngeal mesoderm fate. Sci. Adv. 2022, 8, eabg0834. [Google Scholar] [CrossRef]
- Afouda, B.A. Towards Understanding the Gene-Specific Roles of GATA Factors in Heart Development: Does GATA4 Lead the Way? Int. J. Mol. Sci. 2022, 23, 5255. [Google Scholar] [CrossRef]
- Laforest, B.; Nemer, M. GATA5 interacts with GATA4 and GATA6 in outflow tract development. Dev. Biol. 2011, 358, 368–378. [Google Scholar] [CrossRef]
- Gharibeh, L.; Komati, H.; Bossé, Y.; Boodhwani, M.; Heydarpour, M.; Fortier, M.; Hassanzadeh, R.; Ngu, J.; Mathieu, P.; Body, S.; et al. GATA6 Regulates Aortic Valve Remodeling, and Its Haploinsufficiency Leads to Right-Left Type Bicuspid Aortic Valve. Circulation 2018, 138, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- George, V.; Colombo, S.; Targoff, K.L. An early requirement for nkx2.5 ensures the first and second heart field ventricular identity and cardiac function into adulthood. Dev. Biol. 2015, 400, 10–22. [Google Scholar] [CrossRef]
- Lu, F.; Langenbacher, A.D.; Chen, J.N. Transcriptional Regulation of Heart Development in Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 14. [Google Scholar] [CrossRef]
- Martin, K.E.; Ravisankar, P.; Beerens, M.; MacRae, C.A.; Waxman, J.S. Nr2f1a maintains atrial nkx2.5 expression to repress pacemaker identity within venous atrial cardiomyocytes of zebrafish. Elife 2023, 12, e77408. [Google Scholar] [CrossRef]
- Anderson, D.J.; Kaplan, D.I.; Bell, K.M.; Koutsis, K.; Haynes, J.M.; Mills, R.J.; Phelan, D.G.; Qian, E.L.; Leitoguinho, A.R.; Arasaratnam, D.; et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat. Commun. 2018, 9, 1373. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, D.; Zhang, L.; Cai, C.L.; Li, B.Y.; Liu, Y. The Role of Tbx20 in Cardiovascular Development and Function. Front. Cell Dev. Biol. 2021, 9, 638542. [Google Scholar] [CrossRef]
- Lu, F.; Langenbacher, A.; Chen, J.N. Tbx20 drives cardiac progenitor formation and cardiomyocyte proliferation in zebrafish. Dev. Biol. 2017, 421, 139–148. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Zhu, X.; Aneas, I.; Sakabe, N.; Zhang, L.; Sobreira, D.R.; Montefiori, L.; Bogomolovas, J.; Joslin, A.C.; Zhou, B.; et al. Tbx20 Is Required in Mid-Gestation Cardiomyocytes and Plays a Central Role in Atrial Development. Circ. Res. 2018, 123, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Toenjes, M.; Lange, M.; Fischer, J.J.; Dunkel, I.; Mebus, S.; Grimm, C.H.; Hetzer, R.; Berger, F.; Sperling, S. Characterization of TBX20 in human hearts and its regulation by TFAP2. J. Cell Biochem. 2008, 104, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.B.; Costa, M.W.; Pranoto, E.A.; Salimova, E.; Pinto, A.R.; Lam, N.T.; Park, A.; Snider, P.; Chandran, A.; Harvey, R.P.; et al. Cardiogenic Genes Expressed in Cardiac Fibroblasts Contribute to Heart Development and Repair. Circ. Res. 2014, 114, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Shetty, P.M.V.; Frey, N.; Rangrez, A.Y. SRF: A seriously responsible factor in cardiac development and disease. J. Biomed. Sci. 2022, 29, 38. [Google Scholar] [CrossRef]
- Niu, Z.; Li, A.; Zhang, S.X.; Schwartz, R.J. Serum response factor micromanaging cardiogenesis. Curr. Opin. Cell Biol. 2007, 19, 618–627. [Google Scholar] [CrossRef]
- Garavito-Aguilar, Z.V.; Riley, H.E.; Yelon, D. Hand2 ensures an appropriate environment for cardiac fusion by limiting Fibronectin function. Development 2010, 137, 3215–3220. [Google Scholar] [CrossRef]
- Morikawa, Y.; Cserjesi, P. Cardiac neural crest expression of Hand2 regulates outflow and second heart field development. Circ. Res. 2008, 103, 1422–1429. [Google Scholar] [CrossRef]
- Hinits, Y.; Pan, L.; Walker, C.; Dowd, J.; Moens, C.B.; Hughes, S.M. Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev. Biol. 2012, 369, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, C.A.; Naya, F.J. The Function of the MEF2 Family of Transcription Factors in Cardiac Development, Cardiogenomics, and Direct Reprogramming. J. Cardiovasc. Dev. Dis. 2016, 3, 26. [Google Scholar] [CrossRef]
- Edmondson, D.G.; Lyons, G.E.; Martin, J.F.; Olson, E.N. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 1994, 120, 1251–1263. [Google Scholar] [CrossRef]
- Bollig, F.; Mehringer, R.; Perner, B.; Hartung, C.; Schäfer, M.; Schartl, M.; Volff, J.N.; Winkler, C.; Englert, C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev. Dyn. 2006, 235, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.W.; McInnes, L.; Kreidberg, J.; Hastie, N.D.; Schedl, A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 1999, 126, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 is required for early kidney development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef]
- Hu, H.; Lin, S.; Wang, S.; Chen, X. The Role of Transcription Factor 21 in Epicardial Cell Differentiation and the Development of Coronary Heart Disease. Front. Cell Dev. Biol. 2020, 8, 457. [Google Scholar] [CrossRef]
- Thomas, N.A.; Koudijs, M.; van Eeden, F.J.; Joyner, A.L.; Yelon, D. Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development 2008, 135, 3789–3799. [Google Scholar] [CrossRef]
- Wong, K.S.; Rehn, K.; Palencia-Desai, S.; Kohli, V.; Hunter, W.; Uhl, J.D.; Rost, M.S.; Sumanas, S. Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish. Dev. Biol. 2012, 361, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef]
- Zaffran, S.; Frasch, M. Early signals in cardiac development. Circ. Res. 2002, 91, 457–469. [Google Scholar] [CrossRef]
- Alsan, B.; Schultheiss, T.M. Regulation of avian cardiogenesis by Fgf8 signaling. Development 2002, 129, 1935–1943. [Google Scholar]
- García-García, M.a.J.; Anderson, K.V. Essential Role of Glycosaminoglycans in Fgf Signaling during Mouse Gastrulation. Cell 2003, 114, 727–737. [Google Scholar] [CrossRef]
- Deng, C.X.; Wynshaw-Boris, A.; Shen, M.M.; Daugherty, C.; Ornitz, D.M.; Leder, P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes. Dev. 1994, 8, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.R.; Lee, Y.; Poss, K.D.; Yelon, D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev. Biol. 2008, 321, 397–406. [Google Scholar] [CrossRef]
- Reifers, F.; Walsh, E.C.; Léger, S.; Stainier, D.Y.R.; Brand, M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar). Development 2000, 127, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Reifers, F.; Böhli, H.; Walsh, E.C.; Crossley, P.H.; Stainier, D.Y.R.; Brand, M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesisy. Development 1998, 125, 2381–2395. [Google Scholar] [CrossRef]
- Znosko, W.A.; Yu, S.; Thomas, K.; Molina, G.A.; Li, C.; Tsang, W.; Dawid, I.B.; Moon, A.M.; Tsang, M. Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev. Biol. 2010, 342, 11–25. [Google Scholar] [CrossRef]
- Molina, G.; Vogt, A.; Bakan, A.; Dai, W.; de Oliveira, P.Q.; Znosko, W.; Smithgall, T.E.; Bahar, I.; Lazo, J.S.; Day, B.W.; et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 2009, 5, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, Y.; Zhou, B.; Adam, R.M.; Li, X.; Pu, W.T.; Morrow, B.E.; Moon, A.; Li, X. A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J. Clin. Investig. 2011, 121, 1585–1595. [Google Scholar] [CrossRef]
- White, R.A.; Dowler, L.L.; Angeloni, S.V.; Pasztor, L.M.; MacArthur, C.A. Assignment of FGF8 to human chromosome 10q25–q26: Mutations in FGF8 may be responsible for some types of acrocephalosyndactyly linked to this region. Genomics 1995, 30, 109–111. [Google Scholar] [CrossRef]
- Zeidler, C.; Woelfle, J.; Draaken, M.; Mughal, S.S.; Große, G.; Hilger, A.C.; Dworschak, G.C.; Boemers, T.M.; Jenetzky, E.; Zwink, N.; et al. Heterozygous FGF8 mutations in patients presenting cryptorchidism and multiple VATER/VACTERL features without limb anomalies. Birth Defects Res. Part A Clin. Mol. Teratol. 2014, 100, 750–759. [Google Scholar] [CrossRef]
- Frank, D.U.; Fotheringham, L.K.; Brewer, J.A.; Muglia, L.J.; Tristani-Firouzi, M.; Capecchi, M.R.; Moon, A.M. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 2002, 129, 4591–4603. [Google Scholar] [CrossRef]
- Marques, S.R.; Yelon, D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev. Biol. 2009, 328, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Lee, K.-H.; Zon, L.; Hammerschmidt, M.; Schulte-Merker, S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 1997, 124, 4457–4466. [Google Scholar] [CrossRef]
- Mintzer, K.A.; Lee, M.A.; Runke, G.; Trout, J.; Whitman, M.; Mullins, M.C. lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development 2001, 128, 859–869. [Google Scholar] [CrossRef]
- Bauer, H.; Lele, Z.; Rauch, G.-J.; Geisler, R.; Hammerschmidt, M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development 2001, 128, 849–858. [Google Scholar] [CrossRef]
- de Pater, E.; Ciampricotti, M.; Priller, F.; Veerkamp, J.; Strate, I.; Smith, K.; Lagendijk, A.K.; Schilling, T.F.; Herzog, W.; Abdelilah-Seyfried, S.; et al. Bmp signaling exerts opposite effects on cardiac differentiation. Circ. Res. 2012, 110, 578–587. [Google Scholar] [CrossRef]
- Smith, K.A.; Lagendijk, A.K.; Courtney, A.D.; Chen, H.; Paterson, S.; Hogan, B.M.; Wicking, C.; Bakkers, J. Transmembrane protein 2 (Tmem2) is required to regionally restrict atrioventricular canal boundary and endocardial cushion development. Development 2011, 138, 4193–4198. [Google Scholar] [CrossRef]
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration. Cell 2006, 127, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Joziasse, I.C.; Smith, K.A.; Chocron, S.; van Dinther, M.; Guryev, V.; van de Smagt, J.J.; Cuppen, E.; ten Dijke, P.; Mulder, B.J.M.; Maslen, C.L.; et al. ALK2 mutation in a patient with Down’s syndrome and a congenital heart defect. Eur. J. Human. Genet. 2011, 19, 389–393. [Google Scholar] [CrossRef]
- Smith, K.A.; Joziasse, I.C.; Chocron, S.; Dinther, M.v.; Guryev, V.; Verhoeven, M.C.; Rehmann, H.; Smagt, J.J.v.d.; Doevendans, P.A.; Cuppen, E.; et al. Dominant-Negative ALK2 Allele Associates With Congenital Heart Defects. Circulation 2009, 119, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Keegan, B.R.; Feldman, J.L.; Begemann, G.; Ingham, P.W.; Yelon, D. Retinoic Acid Signaling Restricts the Cardiac Progenitor Pool. Science 2005, 307, 247–249. [Google Scholar] [CrossRef]
- Cui, C.; Cheuvront, T.J.; Lansford, R.D.; Moreno-Rodriguez, R.A.; Schultheiss, T.M.; Rongish, B.J. Dynamic positional fate map of the primary heart-forming region. Dev. Biol. 2009, 332, 212–222. [Google Scholar] [CrossRef]
- Sirbu, I.O.; Zhao, X.; Duester, G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008, 237, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.B.; Waxman, J.S. Patterning of vertebrate cardiac progenitor fields by retinoic acid signaling. Genesis 2021, 59, e23458. [Google Scholar] [CrossRef]
- Begemann, G.; Schilling, T.F.; Rauch, G.J.; Geisler, R.; Ingham, P.W. The zebrafish neckless mutation reveals a requirement forraldh2 in mesodermal signals that pattern the hindbrain. Development 2001, 128, 3081–3094. [Google Scholar] [CrossRef]
- Waxman, J.S.; Keegan, B.R.; Roberts, R.W.; Poss, K.D.; Yelon, D. Hoxb5b Acts Downstream of Retinoic Acid Signaling in the Forelimb Field to Restrict Heart Field Potential in Zebrafish. Dev. Cell 2008, 15, 923–934. [Google Scholar] [CrossRef]
- Sorrell, M.R.; Waxman, J.S. Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev. Biol. 2011, 358, 44–55. [Google Scholar] [CrossRef]
- Rydeen, A.; Voisin, N.; D’Aniello, E.; Ravisankar, P.; Devignes, C.S.; Waxman, J.S. Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling. Dev. Biol. 2015, 405, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, A.; Boink, G.J.J.; Christoffels, V.M.; Devalla, H.D. Retinoic acid signaling in heart development: Application in the differentiation of cardiovascular lineages from human pluripotent stem cells. Stem Cell Rep. 2021, 16, 2589–2606. [Google Scholar] [CrossRef]
- Missinato, M.A.; Tobita, K.; Romano, N.; Carroll, J.A.; Tsang, M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovasc. Res. 2015, 107, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Shiojima, I.; Akazawa, H.; Hidaka, K.; Morisaki, T.; Kikuchi, A.; Komuro, I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19812–19817. [Google Scholar] [CrossRef]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Riley, B.B. Mesendodermal signals required for otic induction: Bmp-antagonists cooperate with Fgf and can facilitate formation of ectopic otic tissue. Dev. Dyn. 2009, 238, 1582–1594. [Google Scholar] [CrossRef]
- Tzahor, E. Wnt/β-Catenin Signaling and Cardiogenesis: Timing Does Matter. Dev. Cell 2007, 13, 10–13. [Google Scholar] [CrossRef]
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous Wnt/β-Catenin Signaling Is Required for Cardiac Differentiation in Human Embryonic Stem Cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Dohn, T.E.; Waxman, J.S. Distinct phases of Wnt/β-catenin signaling direct cardiomyocyte formation in zebrafish. Dev. Biol. 2012, 361, 364–376. [Google Scholar] [CrossRef]
- Lee, H.; Evans, T. TMEM88 Inhibits Wnt Signaling by Promoting Wnt Signalosome Localization to Multivesicular Bodies. iScience 2019, 19, 267–280. [Google Scholar] [CrossRef]
- Novikov, N.; Evans, T. Tmem88a mediates GATA-dependent specification of cardiomyocyte progenitors by restricting WNT signaling. Development 2013, 140, 3787–3798. [Google Scholar] [CrossRef]
- Palpant, N.J.; Pabon, L.; Rabinowitz, J.S.; Hadland, B.K.; Stoick-Cooper, C.L.; Paige, S.L.; Bernstein, I.D.; Moon, R.T.; Murry, C.E. Transmembrane protein 88: A Wnt regulatory protein that specifies cardiomyocyte development. Development 2013, 140, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fan, S.; Tan, J.; Zeng, Z.; Su, M.; Zhang, Y.; Yang, M.; Xia, L.; Fan, X.; Cai, W.; et al. Wnt2bb Induces Cardiomyocyte Proliferation in Zebrafish Hearts via the jnk1/c-jun/creb1 Pathway. Front. Cell Dev. Biol. 2020, 8, 323. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Zhong, T.P. Wnt Signaling in Heart Development and Regeneration. Curr. Cardiol. Rep. 2022, 24, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, O.H.; Coskun, H.; Minguillón, C.; Murawala, P.; Tanaka, E.M.; Galcerán, J.; Muñoz-Chápuli, R.; Nieto, M.A. A right-handed signalling pathway drives heart looping in vertebrates. Nature 2017, 549, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.J.; Lewandowski, A.J. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2020, 47, 373–386. [Google Scholar] [CrossRef]
- Taber, L.A.; Voronov, D.A.; Ramasubramanian, A. The role of mechanical forces in the torsional component of cardiac looping. Ann. N. Y. Acad. Sci. 2010, 1188, 103–110. [Google Scholar] [CrossRef]
- Desgrange, A.; Le Garrec, J.-F.; Meilhac, S.M. Left-right asymmetry in heart development and disease: Forming the right loop. Development 2018, 145, dev162776. [Google Scholar] [CrossRef]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef]
- Grimes, D.T.; Patterson, V.L.; Luna-Arvizu, G.; Schottenfeld-Roames, J.; Irons, Z.H.; Burdine, R.D. Left-right asymmetric heart jogging increases the robustness of dextral heart looping in zebrafish. Dev. Biol. 2020, 459, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-N.; Eeden, F.J.M.v.; Warren, K.S.; Chin, A.; Nüsslein-Volhard, C.; Haffter, P.; Fishman, M.C. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development 1997, 124, 4373–4382. [Google Scholar] [CrossRef]
- Auman, H.J.; Coleman, H.; Riley, H.E.; Olale, F.; Tsai, H.-J.; Yelon, D. Functional Modulation of Cardiac Form through Regionally Confined Cell Shape Changes. PLoS Biol. 2007, 5, e53. [Google Scholar] [CrossRef]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef]
- Ahmad, N.; Long, S.; Rebagliati, M. A Southpaw Joins the Roster: The Role of the Zebrafish nodal-related Gene Southpaw in Cardiac LR Asymmetry. Trends Cardiovasc. Med. 2004, 14, 43–49. [Google Scholar] [CrossRef]
- Baker, K.; Holtzman, N.G.; Burdine, R.D. Direct and indirect roles for Nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc. Natl. Acad. Sci. USA 2008, 105, 13924–13929. [Google Scholar] [CrossRef]
- Putotto, C.; Pugnaloni, F.; Unolt, M.; Calcagni, G.; Versacci, P.; Marino, B. Laterality, heterotaxy, and isolated congenital heart defects: The genetic basis of the segmental nature of the heart. Genome Med. 2024, 16, 100. [Google Scholar] [CrossRef]
- Lenhart, K.F.; Holtzman, N.G.; Williams, J.R.; Burdine, R.D. Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry. PLoS Genet. 2013, 9, e1003109. [Google Scholar] [CrossRef]
- Männer, J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin. Anat. 2009, 22, 21–35. [Google Scholar] [CrossRef]
- Biben, C.; Harvey, R.P. Homeodomain factor Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand during murine heart development. Genes. Dev. 1997, 11, 1357–1369. [Google Scholar] [CrossRef]
- Choudhry, P.; Trede, N.S. DiGeorge Syndrome Gene tbx1 Functions through wnt11r to Regulate Heart Looping and Differentiation. PLoS ONE 2013, 8, e58145. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Zhu, J.; Zhu, C.; Zhu, S.; Liu, X.; Xu, J.; Han, S.; Yu, Z. Overexpression of miR-19b impairs cardiac development in zebrafish by targeting ctnnb1. Cell Physiol. Biochem. 2014, 33, 1988–2002. [Google Scholar] [CrossRef]
- Deacon, D.C.; Nevis, K.R.; Cashman, T.J.; Zhou, Y.; Zhao, L.; Washko, D.; Guner-Ataman, B.; Burns, C.G.; Burns, C.E. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development 2010, 137, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.C.; Yelon, D. Chapter 1.4—Cardiac Development in the Zebrafish. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 103–120. [Google Scholar]
- Liu, J.; Stainier, D.Y.R. Zebrafish in the Study of Early Cardiac Development. Circ. Res. 2012, 110, 870–874. [Google Scholar] [CrossRef]
- Francoeur, N.; Sen, R. Advances in Cardiac Development and Regeneration Using Zebrafish as a Model System for High-Throughput Research. J. Dev. Biol. 2021, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Petree, C.; Requena, T.; Varshney, P.; Varshney, G.K. Expanding the CRISPR Toolbox in Zebrafish for Studying Development and Disease. Front. Cell Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef]
- Angom, R.S.; Wang, Y.; Wang, E.; Dutta, S.K.; Mukhopadhyay, D. Conditional, Tissue-Specific CRISPR/Cas9 Vector System in Zebrafish Reveals the Role of Nrp1b in Heart Regeneration. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1921–1934. [Google Scholar] [CrossRef]
- Rajpurohit, S.K.; Gopal, A.; Mon, M.Y.; Patel, N.G.; Arora, V. Development of Tg(UAS:SEC-Hsa.ANXA5-YFP,myl7:RFP); Casper(roy(-/-),nacre(-/-)) Transparent Transgenic In Vivo Zebrafish Model to Study the Cardiomyocyte Function. Cells 2021, 10, 1963. [Google Scholar] [CrossRef]
- Vicente, M.; Salgado-Almario, J.; Collins, M.M.; Martínez-Sielva, A.; Minoshima, M.; Kikuchi, K.; Domingo, B.; Llopis, J. Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling. Biomedicines 2021, 9, 1294. [Google Scholar] [CrossRef]
- Salgado-Almario, J.; Vicente, M.; Vincent, P.; Domingo, B.; Llopis, J. Mapping Calcium Dynamics in the Heart of Zebrafish Embryos with Ratiometric Genetically Encoded Calcium Indicators. Int. J. Mol. Sci. 2020, 21, 6610. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; McCammon, J.M.; Miller, J.C.; Faraji, F.; Ngo, C.; Katibah, G.E.; Amora, R.; Hocking, T.D.; Zhang, L.; Rebar, E.J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Smith, T.; McNulty, J.; Rayla, A.L.; Lakshmanan, A.; Siekmann, A.F.; Buffardi, M.; Meng, X.; Shin, J.; Padmanabhan, A.; et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development 2011, 138, 4555–4564. [Google Scholar] [CrossRef]
- Moore, F.E.; Reyon, D.; Sander, J.D.; Martinez, S.A.; Blackburn, J.S.; Khayter, C.; Ramirez, C.L.; Joung, J.K.; Langenau, D.M. Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PLoS ONE 2012, 7, e37877. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Oikonomou, G.; Chiu, C.N.; Niles, B.J.; Liu, J.; Lee, D.A.; Antoshechkin, I.; Prober, D.A. A large-scale in vivo analysis reveals that TALENs are significantly more mutagenic than ZFNs generated using context-dependent assembly. Nucleic Acids Res. 2013, 41, 2769–2778. [Google Scholar] [CrossRef]
- Watkins, S.C.; Maniar, S.; Mosher, M.; Roman, B.L.; Tsang, M.; St Croix, C.M. High Resolution Imaging of Vascular Function in Zebrafish. PLoS ONE 2012, 7, e44018. [Google Scholar] [CrossRef]
- Sun, L.; Lien, C.L.; Xu, X.; Shung, K.K. In vivo cardiac imaging of adult zebrafish using high frequency ultrasound (45–75 MHz). Ultrasound Med. Biol. 2008, 34, 31–39. [Google Scholar] [CrossRef]
- Oralová, V.; Rosa, J.T.; Soenens, M.; Bek, J.W.; Willaert, A.; Witten, P.E.; Huysseune, A. Beyond the whole-mount phenotype: High-resolution imaging in fluorescence-based applications on zebrafish. Biol. Open 2019, 8, bio042374. [Google Scholar] [CrossRef]
- Salman, H.E.; Yalcin, H.C. Advanced blood flow assessment in Zebrafish via experimental digital particle image velocimetry and computational fluid dynamics modeling. Micron 2020, 130, 102801. [Google Scholar] [CrossRef]
- Rocke, J.; Lees, J.; Packham, I.; Chico, T. The zebrafish as a novel tool for cardiovascular drug discovery. Recent. Pat. Cardiovasc. Drug Discov. 2009, 4, 1–5. [Google Scholar] [CrossRef]
- Keßler, M.; Rottbauer, W.; Just, S. Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert. Opin. Drug Discov. 2015, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Chang, W.T.; Lai, Y.C.; Liau, I. Toward functional screening of cardioactive and cardiotoxic drugs with zebrafish in vivo using pseudodynamic three-dimensional imaging. Anal. Chem. 2014, 86, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Benslimane, F.M.; Nasrallah, G.K.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’as, S.I.; Yalcin, H.C. Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity. Biomed. Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Rand-Weaver, M.; Winter, M.J. Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs. Front. Pharmacol. 2019, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.W.; McMurray, J.V.; Westerfield, M. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development 1988, 103, 403–412. [Google Scholar] [CrossRef]
- Culp, P.; Nüsslein-Volhard, C.; Hopkins, N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc. Natl. Acad. Sci. USA 1991, 88, 7953–7957. [Google Scholar] [CrossRef]
- Lin, S.; Gaiano, N.; Culp, P.; Burns, J.C.; Friedmann, T.; Yee, J.-K.; Hopkins, N. Integration and Germ-Line Transmission of a Pseudotyped Retroviral Vector in Zebrafish. Science 1994, 265, 666–669. [Google Scholar] [CrossRef]
- Gaiano, N.; Amsterdam, A.; Kawakami, K.; Allende, M.; Becker, T.; Hopkins, N. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 1996, 383, 829–832. [Google Scholar] [CrossRef]
- Amsterdam, A. Insertional mutagenesis in zebrafish. Dev. Dyn. 2003, 228, 523–534. [Google Scholar] [CrossRef]
- Kawakami, K.; Koga, A.; Hori, H.; Shima, A. Excision of the Tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene 1998, 225, 17–22. [Google Scholar] [CrossRef]
- Koga, A.; Suzuki, M.; Inagaki, H.; Bessho, Y.; Hori, H. Transposable element in fish. Nature 1996, 383, 30. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Takeda, H.; Kawakami, N.; Kobayashi, M.; Matsuda, N.; Mishina, M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 2004, 7, 133–144. [Google Scholar] [CrossRef]
- Parinov, S.; Kondrichin, I.; Korzh, V.; Emelyanov, A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev. Dyn. 2004, 231, 449–459. [Google Scholar] [CrossRef]
- Balciunas, D.; Davidson, A.E.; Sivasubbu, S.; Hermanson, S.B.; Welle, Z.; Ekker, S.C. Enhancer trapping in zebrafish using the Sleeping Beauty transposon. BMC Genom. 2004, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Sivasubbu, S.; Balciunas, D.; Davidson, A.E.; Pickart, M.A.; Hermanson, S.B.; Wangensteen, K.J.; Wolbrink, D.C.; Ekker, S.C. Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech. Dev. 2006, 123, 513–529. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, W.; Deng, Y.; Jomok, B.; Yang, J.; Huang, W.; Clark, K.J.; Zhong, T.P.; Lin, X.; Ekker, S.C.; et al. Trapping Cardiac Recessive Mutants via Expression-Based Insertional Mutagenesis Screening. Circ. Res. 2013, 112, 606–617. [Google Scholar] [CrossRef]
- Clark, K.J.; Balciunas, D.; Pogoda, H.-M.; Ding, Y.; Westcot, S.E.; Bedell, V.M.; Greenwood, T.M.; Urban, M.D.; Skuster, K.J.; Petzold, A.M.; et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 2011, 8, 506–512. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307. [Google Scholar] [CrossRef] [PubMed]
- Motoike, T.; Loughna, S.; Perens, E.; Roman, B.L.; Liao, W.; Chau, T.C.; Richardson, C.D.; Kawate, T.; Kuno, J.; Weinstein, B.M.; et al. Universal GFP reporter for the study of vascular development. Genesis 2000, 28, 75–81. [Google Scholar] [CrossRef]
- Huang, C.-J.; Tu, C.-T.; Hsiao, C.-D.; Hsieh, F.-J.; Tsai, H.-J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003, 228, 30–40. [Google Scholar] [CrossRef]
- D’Amico, L.; Scott, I.C.; Jungblut, B.; Stainier, D.Y.R. A Mutation in Zebrafish hmgcr1b Reveals a Role for Isoprenoids in Vertebrate Heart-Tube Formation. Curr. Biol. 2007, 17, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.C.; Bussen, M.; Brand-Arzamendi, K.; Ding, C.; Olgin, J.E.; Shaw, R.M.; Martin, G.R.; Stainier, D.Y. Cardiac conduction is required to preserve cardiac chamber morphology. Proc. Natl. Acad. Sci. USA 2010, 107, 14662–14667. [Google Scholar] [CrossRef] [PubMed]
- Blum, Y.; Belting, H.-G.; Ellertsdottir, E.; Herwig, L.; Lüders, F.; Affolter, M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 2008, 316, 312–322. [Google Scholar] [CrossRef]
- Missinato, M.A.; Zuppo, D.A.; Watkins, S.C.; Bruchez, M.P.; Tsang, M. Zebrafish heart regenerates after chemoptogenetic cardiomyocyte depletion. Dev. Dyn. 2021, 250, 986–1000. [Google Scholar] [CrossRef]
- Poon, K.-L.; Liebling, M.; Kondrychyn, I.; Garcia-Lecea, M.; Korzh, V. Zebrafish cardiac enhancer trap lines: New tools for in vivo studies of cardiovascular development and disease. Dev. Dyn. 2010, 239, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Kawakami, K. Targeted gene expression by the Gal4-UAS system in zebrafish. DGD 2008, 50, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Kawakami, K. The Tol2-mediated Gal4-UAS method for gene and enhancer trapping in zebrafish. Methods 2009, 49, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.R.; Baier, H.; Huisken, J. Optogenetic Control of Cardiac Function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Balciuniene, J.; Nagelberg, D.; Walsh, K.T.; Camerota, D.; Georlette, D.; Biemar, F.; Bellipanni, G.; Balciunas, D. Efficient disruption of Zebrafish genes using a Gal4-containing gene trap. BMC Genom. 2013, 14, 619. [Google Scholar] [CrossRef]
- Karlsson, J.; von Hofsten, J.; Olsson, P.E. Generating transparent zebrafish: A refined method to improve detection of gene expression during embryonic development. Mar. Biotechnol. 2001, 3, 522–527. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A Simple ImageJ-Based Method to Measure Cardiac Rhythm in Zebrafish Embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Abu-Siniyeh, A.; Al-Zyoud, W. Highlights on selected microscopy techniques to study zebrafish developmental biology. Lab. Anim. Res. 2020, 36, 12. [Google Scholar] [CrossRef]
- Chi, N.C.; Shaw, R.M.; De Val, S.; Kang, G.; Jan, L.Y.; Black, B.L.; Stainier, D.Y. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes. Dev. 2008, 22, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Almario, J.; Vicente, M.; Molina, Y.; Martinez-Sielva, A.; Vincent, P.; Domingo, B.; Llopis, J. Simultaneous imaging of calcium and contraction in the beating heart of zebrafish larvae. Theranostics 2022, 12, 1012–1029. [Google Scholar] [CrossRef]
- Bloomekatz, J.; Diaz, J.T.; Yelon, D.; Chi, N.C. Cardiac Morphogenesis: Crowding and Tension Resolved through Social Distancing. Dev. Cell 2021, 56, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.W.; Liu, J.; Thorn, K.S.; Stuurman, N.; Liebling, M.; Stainier, D.Y.R. High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 2014, 141, 585–593. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lee, P.Y.; Huang, C.C.; Sun, L.; Shung, K.K. A study of the adult zebrafish ventricular function by retrospective Doppler-gated ultrahigh-frame-rate echocardiography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1827–1837. [Google Scholar] [CrossRef]
- Fritsche, R.; Schwerte, T.; Pelster, B. Nitric oxide and vascular reactivity in developing zebrafish, Danio rerio. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, R2200–R2207. [Google Scholar] [CrossRef]
- Lu, J.H.; Lu, J.K.; Choo, S.L.; Li, Y.-C.; Yeh, H.-W.; Shiue, J.-F.; Yeh, V.C. Cascade effect of cardiac myogenesis gene expression during cardiac looping in tbx5 knockdown zebrafish embryos. J. Biomed. Sci. 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Jamison, R.A.; Samarage, C.R.; Bryson-Richardson, R.J.; Fouras, A. In Vivo Wall Shear Measurements within the Developing Zebrafish Heart. PLoS ONE 2013, 8, e75722. [Google Scholar] [CrossRef]
- Craig, M.P.; Gilday, S.D.; Dabiri, D.; Hove, J.R. An Optimized Method for Delivering Flow Tracer Particles to Intravital Fluid Environments in the Developing Zebrafish. Zebrafish 2012, 9, 108–119. [Google Scholar] [CrossRef]
- Hove, J.R.; Craig, M.P. High-Speed Confocal Imaging of Zebrafish Heart Development. In Cardiovascular Development: Methods and Protocols; Peng, X., Antonyak, M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 309–328. [Google Scholar]
- Dempsey, W.P.; Qin, H.; Pantazis, P. In Vivo Cell Tracking Using PhOTO Zebrafish. In Photoswitching Proteins: Methods and Protocols; Cambridge, S., Ed.; Springer: New York, NY, USA, 2014; pp. 217–228. [Google Scholar]
- Dempsey, W.P.; Fraser, S.E.; Pantazis, P. PhOTO Zebrafish: A Transgenic Resource for In Vivo Lineage Tracing during Development and Regeneration. PLoS ONE 2012, 7, e32888. [Google Scholar] [CrossRef]
- Chen, C.-H.; Durand, E.; Wang, J.; Zon, L.I.; Poss, K.D. Zebraflash transgenic lines for in vivo bioluminescence imaging of stem cells and regeneration in adult zebrafish. Development 2013, 140, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, W.R.; Burggren, W.W. O2 consumption and heart rate in developing zebrafish (Danio rerio): Influence of temperature and ambient O2. Am. J. Physiol. 1999, 276, R505–R513. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Pan, H.; Hung, C.-M.; Hou, P.-T.; Li, Y.-C.; Lee, Y.-J.; Shen, Y.-T.; Wu, T.-T.; Li, C. The predominant protein arginine methyltransferase PRMT1 is critical for zebrafish convergence and extension during gastrulation. FEBS J. 2011, 278, 905–917. [Google Scholar] [CrossRef]
- Leong, I.U.S.; Skinner, J.R.; Shelling, A.N.; Love, D.R. Identification and expression analysis of kcnh2 genes in the zebrafish. Biochem. Biophys. Res. Commun. 2010, 396, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; MacRae, C.A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Wu, C.-K.; Chiang, F.-T.; Tseng, C.-D.; Lee, J.-K.; Yu, C.-C.; Wang, Y.-C.; Lai, L.-P.; Lin, J.-L.; Hwang, J.-J. In-vitro recording of adult zebrafish heart electrocardiogram—A platform for pharmacological testing. Clin. Chim. Acta 2011, 412, 1963–1967. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Remme, C.A. Zebrafish: A novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front. Physiol. 2012, 3, 255. [Google Scholar] [CrossRef]
- Duong, T.; Rose, R.; Blazeski, A.; Fine, N.; Woods, C.E.; Thole, J.F.; Sotoodehnia, N.; Soliman, E.Z.; Tung, L.; McCallion, A.S.; et al. Development and optimization of an in vivo electrocardiogram recording method and analysis program for adult zebrafish. Dis. Model. Mech. 2021, 14, dmm048827. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Dóró, É.; Magyary, I.; Egginton, S.; Sík, A.; Müller, F. Optimisation of Embryonic and Larval ECG Measurement in Zebrafish for Quantifying the Effect of QT Prolonging Drugs. PLoS ONE 2013, 8, e60552. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, L.; Lam, Y.W.; Siu, C.W.; Cheng, S.H. Improvement of surface ECG recording in adult zebrafish reveals that the value of this model exceeds our expectation. Sci. Rep. 2016, 6, 25073. [Google Scholar] [CrossRef]
- Denvir, M.A.; Tucker, C.S.; Mullins, J.J. Systolic and diastolic ventricular function in zebrafish embryos: Influence of norepenephrine, MS-222 and temperature. BMC Biotechnol. 2008, 8, 21. [Google Scholar] [CrossRef]
- Rider, S.A.; Tucker, C.S.; del-Pozo, J.; Rose, K.N.; MacRae, C.A.; Bailey, M.A.; Mullins, J.J. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J. Physiol. 2012, 590, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Belmonte, J.C.I.; Giles, W.; Bodmer, R.; Ocorr, K. A New Method for Detection and Quantification of Heartbeat Parameters in Drosophila, Zebrafish, and Embryonic Mouse Hearts. BioTechniques 2009, 46, 101–113. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Zakaria, Z.Z.; Shurbaji, S.; Abdelrasool, M.K.A.; Al-Badr, M.A.H.I.; Al Absi, E.S.K.; Yalcin, H.C. Cardiac function and blood flow hemodynamics assessment of zebrafish (Danio rerio) using high-speed video microscopy. Micron 2020, 136, 102876. [Google Scholar] [CrossRef]
- Ebert, A.M.; Hume, G.L.; Warren, K.S.; Cook, N.P.; Burns, C.G.; Mohideen, M.A.; Siegal, G.; Yelon, D.; Fishman, M.C.; Garrity, D.M. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc. Natl. Acad. Sci. USA 2005, 102, 17705–17710. [Google Scholar] [CrossRef]
- Langenbacher, A.D.; Dong, Y.; Shu, X.; Choi, J.; Nicoll, D.A.; Goldhaber, J.I.; Philipson, K.D.; Chen, J.-N. Mutation in sodium–calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc. Natl. Acad. Sci. USA 2005, 102, 17699–17704. [Google Scholar] [CrossRef]
- Shimizu, H.; Schredelseker, J.; Huang, J.; Lu, K.; Naghdi, S.; Lu, F.; Franklin, S.; Fiji, H.D.; Wang, K.; Zhu, H.; et al. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife 2015, 4, e04801. [Google Scholar] [CrossRef]
- Shimizu, H.; Langenbacher, A.D.; Huang, J.; Wang, K.; Otto, G.; Geisler, R.; Wang, Y.; Chen, J.N. The Calcineurin-FoxO-MuRF1 signaling pathway regulates myofibril integrity in cardiomyocytes. Elife 2017, 6, e27955. [Google Scholar] [CrossRef]
- Panáková, D.; Werdich, A.A.; MacRae, C.A. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca2+ channel. Nature 2010, 466, 874–878. [Google Scholar] [CrossRef]
- Jou, C.J.; Spitzer, K.W.; Tristani-Firouzi, M. Blebbistatin Effectively Uncouples the Excitation-Contraction Process in Zebrafish Embryonic Heart. Cell. Physiol. Biochem. 2010, 25, 419–424. [Google Scholar] [CrossRef]
- Wythe, J.D.; Jurynec, M.J.; Urness, L.D.; Jones, C.A.; Sabeh, M.K.; Werdich, A.A.; Sato, M.; Yost, H.J.; Grunwald, D.J.; MacRae, C.A.; et al. Hadp1, a newly identified pleckstrin homology domain protein, is required for cardiac contractility in zebrafish. Dis. Models Mech. 2011, 4, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Näbauer, M.; Kääb, S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998, 37, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Horváth, A.; Lemoine, M.D.; Löser, A.; Mannhardt, I.; Flenner, F.; Uzun, A.U.; Neuber, C.; Breckwoldt, K.; Hansen, A.; Girdauskas, E.; et al. Low Resting Membrane Potential and Low Inward Rectifier Potassium Currents Are Not Inherent Features of hiPSC-Derived Cardiomyocytes. Stem Cell Rep. 2018, 10, 822–833. [Google Scholar] [CrossRef]
- Brette, F.; Luxán, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Ramirez, R.J.; Kaprielian, R.; Backx, P.H. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J. Physiol. 2001, 533, 201–214. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Marchal, G.A.; Zegers, J.G.; Kawasaki, M.; Driessen, A.H.G.; Remme, C.A.; de Groot, J.R.; Wilders, R. Patch-Clamp Recordings of Action Potentials From Human Atrial Myocytes: Optimization Through Dynamic Clamp. Front. Pharmacol. 2021, 12, 649414. [Google Scholar] [CrossRef]
- Sah, R.; Ramirez, R.J.; Oudit, G.Y.; Gidrewicz, D.; Trivieri, M.G.; Zobel, C.; Backx, P.H. Regulation of cardiac excitation-contraction coupling by action potential repolarization: Role of the transient outward potassium current (Ito). J. Physiol. 2003, 546, 5–18. [Google Scholar] [CrossRef]
- Faber, G.M.; Rudy, Y. Action Potential and Contractility Changes in [Na+]i Overloaded Cardiac Myocytes: A Simulation Study. Biophys. J. 2000, 78, 2392–2404. [Google Scholar] [CrossRef]
- Lawson, N.D.; Wolfe, S.A. Forward and Reverse Genetic Approaches for the Analysis of Vertebrate Development in the Zebrafish. Dev. Cell 2011, 21, 48–64. [Google Scholar] [CrossRef]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; van Eeden, F.J.; Jiang, Y.J.; Heisenberg, C.P.; et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar] [CrossRef]
- Kegel, L.; Rubio, M.; Almeida, R.G.; Benito, S.; Klingseisen, A.; Lyons, D.A. Forward Genetic Screen Using Zebrafish to Identify New Genes Involved in Myelination. Methods Mol. Biol. 2019, 1936, 185–209. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I. The art and design of genetic screens: Zebrafish. Nat. Rev. Genet. 2001, 2, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Letelier, J.; Tajer, B.; Valdivia, L.E.; Mullins, M.C. Fishing forward and reverse: Advances in zebrafish phenomics. Mech. Dev. 2018, 154, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Nasevicius, A.; Ekker, S.C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Pickart, M.A.; Klee, E.W.; Nielsen, A.L.; Sivasubbu, S.; Mendenhall, E.M.; Bill, B.R.; Chen, E.; Eckfeldt, C.E.; Knowlton, M.; Robu, M.E.; et al. Genome-Wide Reverse Genetics Framework to Identify Novel Functions of the Vertebrate Secretome. PLoS ONE 2006, 1, e104. [Google Scholar] [CrossRef][Green Version]
- Baron, C.S.; Alemany, A. Paired Single-Cell Transcriptome and DNA Barcode Detection in Zebrafish Using ScarTrace. Methods Mol. Biol. 2025, 2886, 221–241. [Google Scholar] [CrossRef]
- Saunders, L.M.; Srivatsan, S.R.; Duran, M.; Dorrity, M.W.; Ewing, B.; Linbo, T.H.; Shendure, J.; Raible, D.W.; Moens, C.B.; Kimelman, D.; et al. Embryo-scale reverse genetics at single-cell resolution. Nature 2023, 623, 782–791. [Google Scholar] [CrossRef]
- Shah, A.N.; Davey, C.F.; Whitebirch, A.C.; Miller, A.C.; Moens, C.B. Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 2015, 12, 535–540. [Google Scholar] [CrossRef]
- Dixit, A.; Parnas, O.; Li, B.; Chen, J.; Fulco, C.P.; Jerby-Arnon, L.; Marjanovic, N.D.; Dionne, D.; Burks, T.; Raychowdhury, R.; et al. Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 2016, 167, 1853–1866.e17. [Google Scholar] [CrossRef]
- Angom, R.S.; Joshi, A.; Patowary, A.; Sivadas, A.; Ramasamy, S.; Vellarikkal, S.K.; Kaushik, K.; Sabarwal, A.; Lalwani, M.K.; K, S.; et al. Forward genetic screen using a gene-breaking trap approach identifies a novel role of grin2bb-associated RNA transcript (grin2bbART) in zebrafish heart function. Front. Cell Dev. Biol. 2024, 12, 1339292. [Google Scholar]
- Chen, Y.G.; Forsberg, M.H.; Khaja, S.; Ciecko, A.E.; Hessner, M.J.; Geurts, A.M. Gene targeting in NOD mouse embryos using zinc-finger nucleases. Diabetes 2014, 63, 68–74. [Google Scholar] [CrossRef]
- Anguela, X.M.; Sharma, R.; Doyon, Y.; Miller, J.C.; Li, H.; Haurigot, V.; Rohde, M.E.; Wong, S.Y.; Davidson, R.J.; Zhou, S.; et al. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood 2013, 122, 3283–3287. [Google Scholar] [CrossRef]
- Li, H.; Haurigot, V.; Doyon, Y.; Li, T.; Wong, S.Y.; Bhagwat, A.S.; Malani, N.; Anguela, X.M.; Sharma, R.; Ivanciu, L.; et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 2011, 475, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Maier, D.A.; Brennan, A.L.; Jiang, S.; Binder-Scholl, G.K.; Lee, G.; Plesa, G.; Zheng, Z.; Cotte, J.; Carpenito, C.; Wood, T.; et al. Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Hum. Gene Ther. 2013, 24, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; McGrew, M.J. Genome editing of avian species: Implications for animal use and welfare. Lab. Anim. 2022, 56, 50–59. [Google Scholar] [CrossRef]
- Wefers, B.; Panda, S.K.; Ortiz, O.; Brandl, C.; Hensler, S.; Hansen, J.; Wurst, W.; Kühn, R. Generation of targeted mouse mutants by embryo microinjection of TALEN mRNA. Nat. Protoc. 2013, 8, 2355–2379. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.-C.; Markoulaki, S.; Welstead, G.G.; Cheng, A.W.; Shivalila, C.S.; Pyntikova, T.; Dadon, D.B.; Voytas, D.F.; Bogdanove, A.J.; et al. TALEN-mediated editing of the mouse Y chromosome. Nat. Biotechnol. 2013, 31, 530–532. [Google Scholar] [CrossRef]
- Sood, R.; English, M.A.; Jones, M.; Mullikin, J.; Wang, D.-M.; Anderson, M.; Wu, D.; Chandrasekharappa, S.C.; Yu, J.; Zhang, J.; et al. Methods for reverse genetic screening in zebrafish by resequencing and TILLING. Methods 2006, 39, 220–227. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Schulte-Merker, S.; Walderich, B.; Plasterk, R.H.A. Target-Selected Inactivation of the Zebrafish rag1 Gene. Science 2002, 297, 99–102. [Google Scholar] [CrossRef]
- Wienholds, E.; van Eeden, F.; Kosters, M.; Mudde, J.; Plasterk, R.H.; Cuppen, E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003, 13, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Sun, C.; Gao, L.; Zhu, D.; Xu, X.; Zhu, X.; Xiong, J.-W.; Xi, J.J. Genome editing with RNA-guided Cas9 nuclease in Zebrafish embryos. Cell Res. 2013, 23, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, S.; Cai, C.; Yuan, P.; Li, C.; Huang, Y.; Wei, W. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 2014, 509, 487–491. [Google Scholar] [CrossRef]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef]
- Jao, L.E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tien, C.L.; Jain, M.K.; Zhang, L. Kruppel-Like Factor 15 Regulates the Circadian Susceptibility to Ischemia Reperfusion Injury in the Heart. Circulation 2020, 141, 1427–1429. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Apolínová, K.; Pérez, F.A.; Dyballa, S.; Coppe, B.; Mercader Huber, N.; Terriente, J.; Di Donato, V. ZebraReg-a novel platform for discovering regulators of cardiac regeneration using zebrafish. Front. Cell Dev. Biol. 2024, 12, 1384423. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Qin, W.; Zheng, S.; Xiao, J.; Xia, X.; Yuan, X.; Zeng, J.; Shi, Y.; Zhang, Y.; et al. Cytosine base editors with increased PAM and deaminase motif flexibility for gene editing in zebrafish. Nat. Commun. 2024, 15, 9526. [Google Scholar] [CrossRef]
- Kitambi, S.S.; Nilsson, E.S.; Sekyrova, P.; Ibarra, C.; Tekeoh, G.N.; Andäng, M.; Ernfors, P.; Uhlén, P. Small molecule screening platform for assessment of cardiovascular toxicity on adult zebrafish heart. BMC Physiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lin, G.; Jiao, K.; Hong, X.; Lin, X. Fully automated in vivo screening system for multi-organ imaging and pharmaceutical evaluation. Microsyst. Nanoeng. 2025, 11, 22. [Google Scholar] [CrossRef]
- Basu, S.; Sachidanandan, C. Zebrafish: A multifaceted tool for chemical biologists. Chem. Rev. 2013, 113, 7952–7980. [Google Scholar] [CrossRef]
- Tsang, M. Zebrafish: A tool for chemical screens. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 185–192. [Google Scholar] [CrossRef]
- Wittmann, C.; Reischl, M.; Shah, A.H.; Mikut, R.; Liebel, U.; Grabher, C. Facilitating Drug Discovery: An Automated High-content Inflammation Assay in Zebrafish. JoVE 2012, 16, e4203. [Google Scholar] [CrossRef]
- Antkiewicz, D.S.; Burns, C.G.; Carney, S.A.; Peterson, R.E.; Heideman, W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005, 84, 368. [Google Scholar] [CrossRef]
- Peterson, R.T.; Shaw, S.Y.; Peterson, T.A.; Milan, D.J.; Zhong, T.P.; Schreiber, S.L.; MacRae, C.A.; Fishman, M.C. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol. 2004, 22, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.C.; Sneed, B.; Haider, J.; Blavo, D.; White, A.; Aiyejorun, T.; Baranowski, T.C.; Rubinstein, A.L.; Doan, T.N.; Dingledine, R.; et al. Automated, Quantitative Screening Assay for Antiangiogenic Compounds Using Transgenic Zebrafish. Cancer Res. 2007, 67, 11386–11392. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Yang, C.-C.; Chen, I.H.; Liu, Y.-M.L.; Chang, S.-J.; Chuang, Y.-J. Treatment of Glucocorticoids Inhibited Early Immune Responses and Impaired Cardiac Repair in Adult Zebrafish. PLoS ONE 2013, 8, e66613. [Google Scholar] [CrossRef]
- Mittelstadt, S.W.; Hemenway, C.L.; Craig, M.P.; Hove, J.R. Evaluation of zebrafish embryos as a model for assessing inhibition of hERG. J. Pharmacol. Toxicol. Methods 2008, 57, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yozzo, K.L.; Isales, G.M.; Raftery, T.D.; Volz, D.C. High-Content Screening Assay for Identification of Chemicals Impacting Cardiovascular Function in Zebrafish Embryos. Environ. Sci. Technol. 2013, 47, 11302–11310. [Google Scholar] [CrossRef]
- Burczyk, M.S.; Burkhalter, M.D.; Tena, T.C.; Grisanti, L.A.; Kauk, M.; Matysik, S.; Donow, C.; Kustermann, M.; Rothe, M.; Cui, Y.; et al. Muscarinic receptors promote pacemaker fate at the expense of secondary conduction system tissue in zebrafish. JCI Insight 2019, 4, e121971. [Google Scholar] [CrossRef]
- Cui, S.; Hayashi, K.; Kobayashi, I.; Hosomichi, K.; Nomura, A.; Teramoto, R.; Usuda, K.; Okada, H.; Deng, Y.; Kobayashi-Sun, J.; et al. The utility of zebrafish cardiac arrhythmia model to predict the pathogenicity of KCNQ1 variants. J. Mol. Cell Cardiol. 2023, 177, 50–61. [Google Scholar] [CrossRef]
- Li, H.; Trager, L.E.; Liu, X.; Hastings, M.H.; Xiao, C.; Guerra, J.; To, S.; Li, G.; Yeri, A.; Rodosthenous, R.; et al. lncExACT1 and DCHS2 Regulate Physiological and Pathological Cardiac Growth. Circulation 2022, 145, 1218–1233. [Google Scholar] [CrossRef]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Izpisúa Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Kemmler, C.L.; Riemslagh, F.W.; Moran, H.R.; Mosimann, C. From Stripes to a Beating Heart: Early Cardiac Development in Zebrafish. J. Cardiovasc. Dev. Dis. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Findlay, G.M.; Gagnon, J.A.; Horwitz, M.S.; Schier, A.F.; Shendure, J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 2016, 353, aaf7907. [Google Scholar] [CrossRef]
- Raj, B. Single-Cell Profiling of Lineages and Cell Types in the Vertebrate Brain. In Lineage Tracing: Methods and Protocols; García-Marqués, J., Lee, T., Eds.; Springer: New York, NY, USA, 2025; pp. 299–310. [Google Scholar]
- Spanjaard, B.; Hu, B.; Mitic, N.; Olivares-Chauvet, P.; Janjuha, S.; Ninov, N.; Junker, J.P. Simultaneous lineage tracing and cell-type identification using CRISPR-Cas9-induced genetic scars. Nat. Biotechnol. 2018, 36, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Spanjaard, B.; Junker, J.P. LINNAEUS: Simultaneous Single-Cell Lineage Tracing and Cell Type Identification. In Lineage Tracing: Methods and Protocols; García-Marqués, J., Lee, T., Eds.; Springer: New York, NY, USA, 2025; pp. 243–263. [Google Scholar]
- Espinosa-Medina, I.; Feliciano, D.; Belmonte-Mateos, C.; Linda Miyares, R.; Garcia-Marques, J.; Foster, B.; Lindo, S.; Pujades, C.; Koyama, M.; Lee, T. TEMPO enables sequential genetic labeling and manipulation of vertebrate cell lineages. Neuron 2023, 111, 345–361.e10. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Medina, I. Design and Generation of TEMPO Reagents for Sequential Labeling and Manipulation of Vertebrate Cell Lineages. In Lineage Tracing: Methods and Protocols; García-Marqués, J., Lee, T., Eds.; Springer: New York, NY, USA, 2025; pp. 327–353. [Google Scholar]
- Caviglia, S.; Unterweger, I.A.; Gasiūnaitė, A.; Vanoosthuyse, A.E.; Cutrale, F.; Trinh, L.A.; Fraser, S.E.; Neuhauss, S.C.F.; Ober, E.A. FRaeppli: A multispectral imaging toolbox for cell tracing and dense tissue analysis in zebrafish. Development 2022, 149, dev199615. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kambakam, S.; Almeida, M.P.; Ming, Z.; Welker, J.M.; Wierson, W.A.; Schultz-Rogers, L.E.; Ekker, S.C.; Clark, K.J.; Essner, J.J.; et al. Cre/lox regulated conditional rescue and inactivation with zebrafish UFlip alleles generated by CRISPR-Cas9 targeted integration. eLife 2022, 11, e71478. [Google Scholar] [CrossRef]
- Almeida, M.P.; Welker, J.M.; Siddiqui, S.; Luiken, J.; Ekker, S.C.; Clark, K.J.; Essner, J.J.; McGrail, M. Endogenous zebrafish proneural Cre drivers generated by CRISPR/Cas9 short homology directed targeted integration. Sci. Rep. 2021, 11, 1732. [Google Scholar] [CrossRef]
- Kesavan, G.; Hammer, J.; Hans, S.; Brand, M. Targeted knock-in of CreERT2 in zebrafish using CRISPR/Cas9. Cell Tissue Res. 2018, 372, 41–50. [Google Scholar] [CrossRef]
- Wei, K.-H.; Lin, I.T.; Chowdhury, K.; Lim, K.L.; Liu, K.-T.; Ko, T.-M.; Chang, Y.-M.; Yang, K.-C.; Lai, S.-L. Comparative single-cell profiling reveals distinct cardiac resident macrophages essential for zebrafish heart regeneration. eLife 2023, 12, e84679. [Google Scholar] [CrossRef]
- Botos, M.A.; Arora, P.; Chouvardas, P.; Mercader, N. Transcriptomic data meta-analysis reveals common and injury model specific gene expression changes in the regenerating zebrafish heart. Sci. Rep. 2023, 13, 5418. [Google Scholar] [CrossRef]
- Carey, C.M.; Hollins, H.L.; Schmid, A.V.; Gagnon, J.A. Distinct features of the regenerating heart uncovered through comparative single-cell profiling. Biol. Open 2024, 13, bio060156. [Google Scholar] [CrossRef]
- Mu, X.; Chen, X.; Liu, J.; Yuan, L.; Wang, D.; Qian, L.; Qian, Y.; Shen, G.; Huang, Y.; Li, X.; et al. A multi-omics approach reveals molecular mechanisms by which phthalates induce cardiac defects in zebrafish (Danio rerio). Environ. Pollut. 2020, 265, 113876. [Google Scholar] [CrossRef]
- Hallab, J.C.; Nim, H.T.; Stolper, J.; Chahal, G.; Waylen, L.; Bolk, F.; Elliott, D.A.; Porrello, E.; Ramialison, M. Towards spatio-temporally resolved developmental cardiac gene regulatory networks in zebrafish. Brief. Funct. Genom. 2021, 20, 427–433. [Google Scholar] [CrossRef]


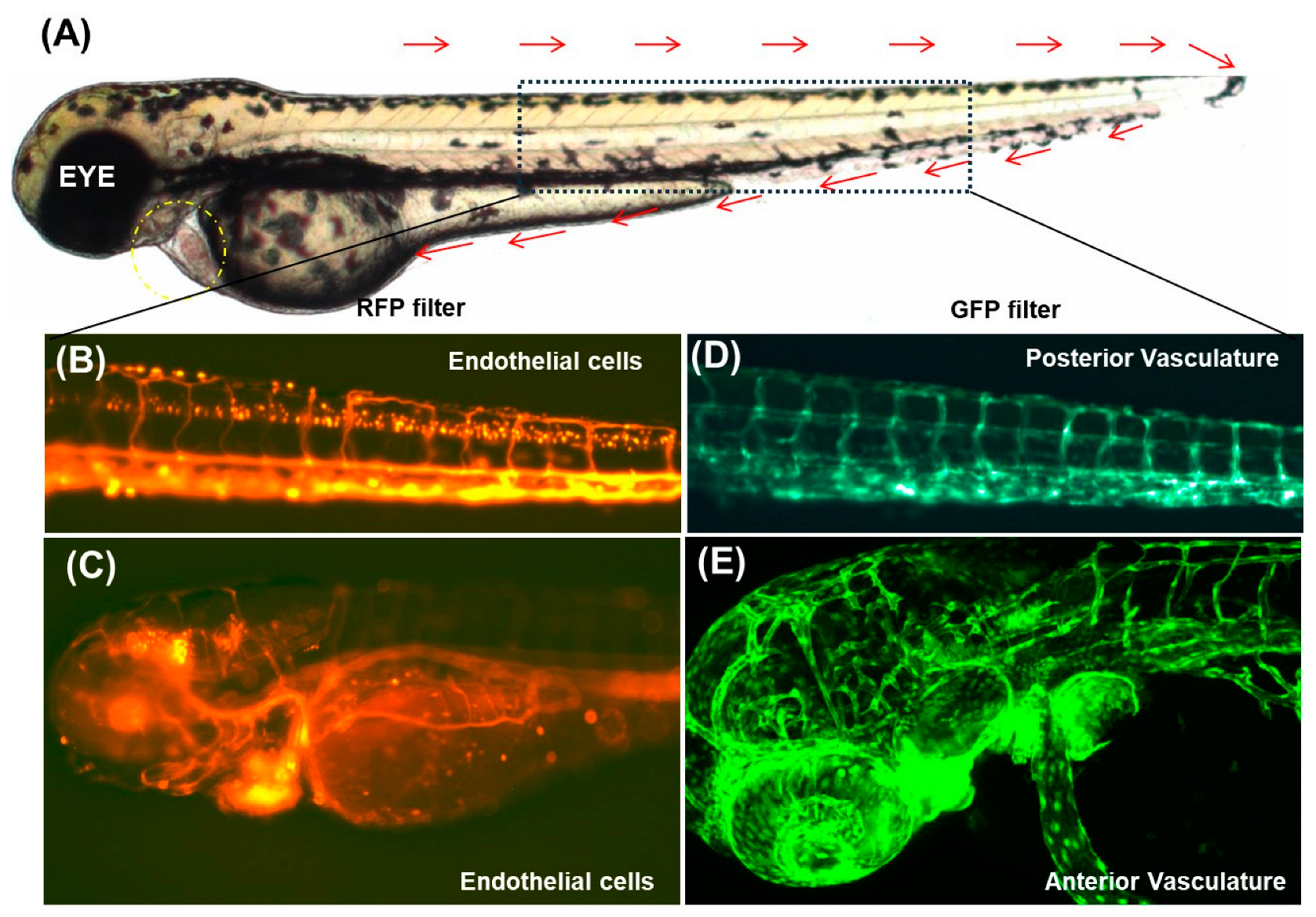
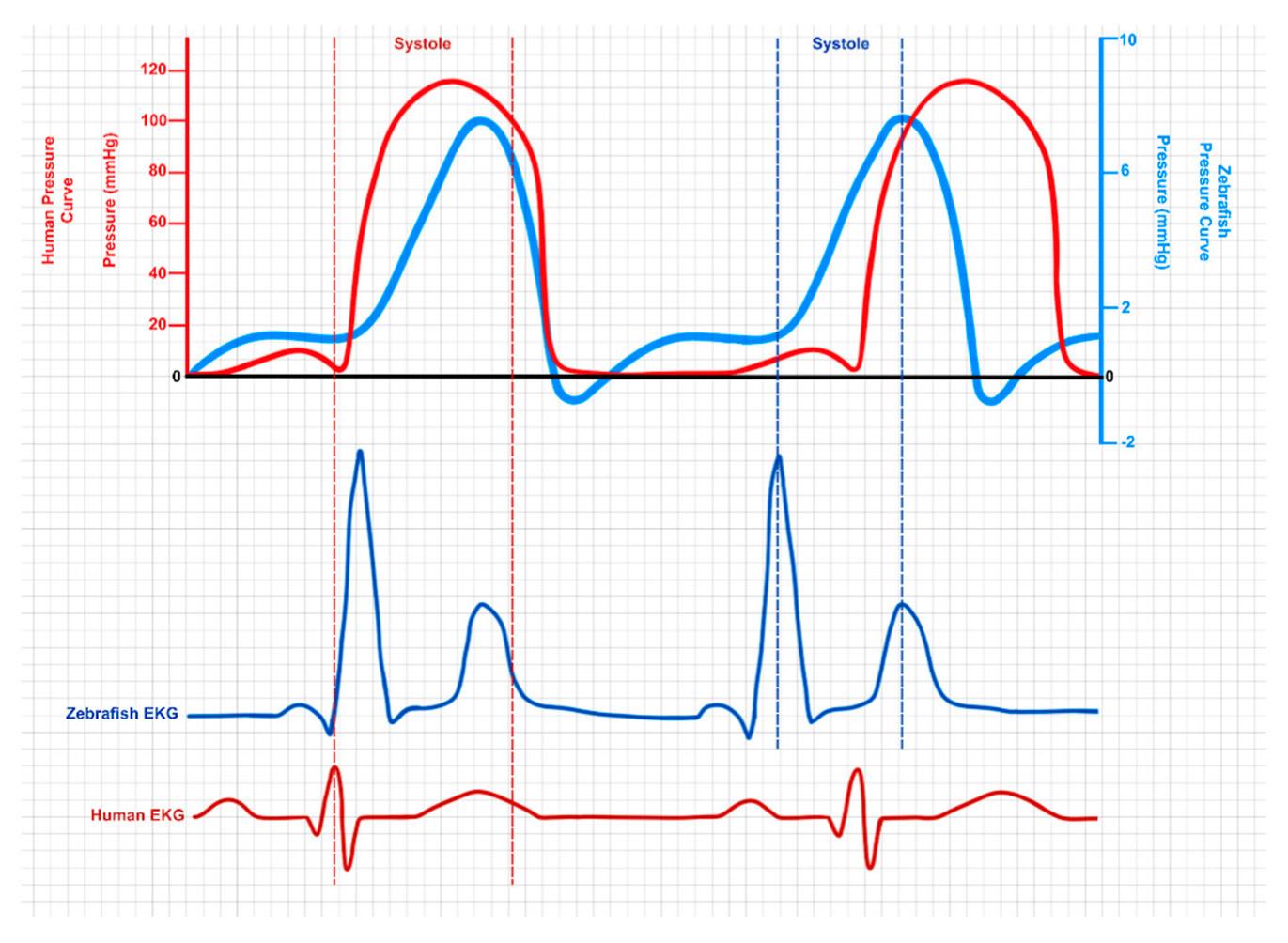
| 1 | Specification of Cardiac Progenitor Cells | |||
| Human | Zebrafish | Signaling Pathways | References | |
| Specification of mesodermal cells to become cardiac progenitors and migration to form the cardiac crescent (early heart field) | The lateral plate mesoderm gives rise to bilateral populations of cardiac progenitor cells that migrate toward the midline. | BMP, FGF, and Wnt signaling | [31,36] | |
| 2 | Migration and Formation of the Heart Tube | |||
| Migration of the progenitor cells to the midline and formation of a linear heart tube | The bilateral heart fields migrate and merge at the midline, creating a single-layered primitive heart tube. | Wnt, VEGF, and nodal signaling | [37,38] | |
| 3 | Cardiac Looping | |||
| The linear heart tube elongates and bends to the right (dextral looping), establishing the left–right asymmetry critical for chamber formation. | The primitive heart tube loops in a similar manner but occurs on a shorter timescale. | Pitx2 and nodal signaling | [39,40] | |
| 4 | Chamber Formation and Septation | |||
| The heart tube differentiates into atrial and ventricular chambers. Endocardial cushions contribute to septation, forming the four-chambered heart. | Zebrafish form two chambers (atrium and ventricle) without septation. The atrial and ventricular compartments are morphologically distinct. | Tbx, Nkx2.5, and hand transcription factors | [41,42,43] | |
| 5 | Trabeculation and Valvular Formation | |||
| The ventricles develop trabeculae (muscular ridges), enhancing contractility. Valves develop from endocardial cushions. | Trabeculation occurs in the ventricle, and valves develop at the atrioventricular junction. | Notch, Bmp, and ErbB pathways | [44,45] | |
| 6 | Maturation and Growth of the Heart | |||
| The heart continues to grow through cardiomyocyte proliferation and maturation, and coronary vessels form to supply the heart muscle. | Growth primarily occurs through cardiomyocyte proliferation, and coronary vasculature develops later. | IGF and VEGF | [26,43,46] | |
| Experimental Technique | Approach | Application | References |
|---|---|---|---|
| Genetic manipulation techniques | CRISPR/Cas9 | High-throughput screens in cardiomyocytes, multigene targeting, heat-shock-inducible CRISPR/Cas9 for cardiomyocyte-specific knockouts | [171,172] |
| Transgenic lines | Tg(myl7:GA): calcium dynamics and heart failure modeling, Casper (roy−/−, nacre−/−): cardiac injury and regeneration modeling, transposon-based transgenesis (Tol2 system): cardiac calcium dynamic mapping | [173,174,175] | |
| Zinc finger nucleases (ZFNs) | Targeted germline mutagenesis (heritable gene disruption (e.g., golden and no-tail genes), vascular development studies (e.g., gata2a mutation)) | [176,177] | |
| Transcription activator-like effector nucleases (TALENs) | High-efficiency gene knockout, expanded targeting capabilities (e.g., CpG-rich and start codon regions) | [178,179] | |
| Imaging techniques | Confocal microscopy and high-speed imaging | Real-time vascular dynamics and endothelial function imaging | [180] |
| Ultrasound bio-microscopy | Cardiac structure imaging and blood flow measurements | [181] | |
| Fluorescence imaging | Glycol methacrylate (GMA) embedding as a practical approach for studying deeper structures (e.g., vasculatures) | [182] | |
| Light-sheet fluorescence microscopy | High-resolution, real-time imaging of zebrafish cardiac development, blood flow, and biomechanical forces (e.g., wall shear stresses) | [183] | |
| Pharmacological utility | Small-molecule screening/small-compound screening | Drug discovery and vascular disease modeling | [184,185] |
| Retro-orbital microinjection, immersion-based drug delivery, and pseudodynamic 3D imaging of cardiac functions | Drug response evaluation (of cardiac functions to model cardiomyopathy), cardiotoxicity testing, and cardiac parameter quantification | [186] | |
| Drug exposure assays and high-throughput screening | Drug-induced toxicity screening and heart rate and rhythm analyses | [187] | |
| In vivo zebrafish cardiovascular assays | Drug effect evaluation, high-throughput screening, and mechanistic insights | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angom, R.S.; Singh, M.; Muhammad, H.; Varanasi, S.M.; Mukhopadhyay, D. Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter. Cells 2025, 14, 531. https://doi.org/10.3390/cells14070531
Angom RS, Singh M, Muhammad H, Varanasi SM, Mukhopadhyay D. Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter. Cells. 2025; 14(7):531. https://doi.org/10.3390/cells14070531
Chicago/Turabian StyleAngom, Ramcharan Singh, Meghna Singh, Huzaifa Muhammad, Sai Manasa Varanasi, and Debabrata Mukhopadhyay. 2025. "Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter" Cells 14, no. 7: 531. https://doi.org/10.3390/cells14070531
APA StyleAngom, R. S., Singh, M., Muhammad, H., Varanasi, S. M., & Mukhopadhyay, D. (2025). Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter. Cells, 14(7), 531. https://doi.org/10.3390/cells14070531










