Artesunate Inhibits Metastatic Potential in Cisplatin-Resistant Bladder Cancer Cells by Altering Integrins
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Resistance Induction and Drug Treatment
2.3. Tumor Cell Adhesion to Collagen and HUVECs
2.4. Tumor Cell Motility
2.5. Integrin Surface Expression
2.6. Western Blot
2.7. Integrin Signaling Blockade
2.8. Statistics
3. Results
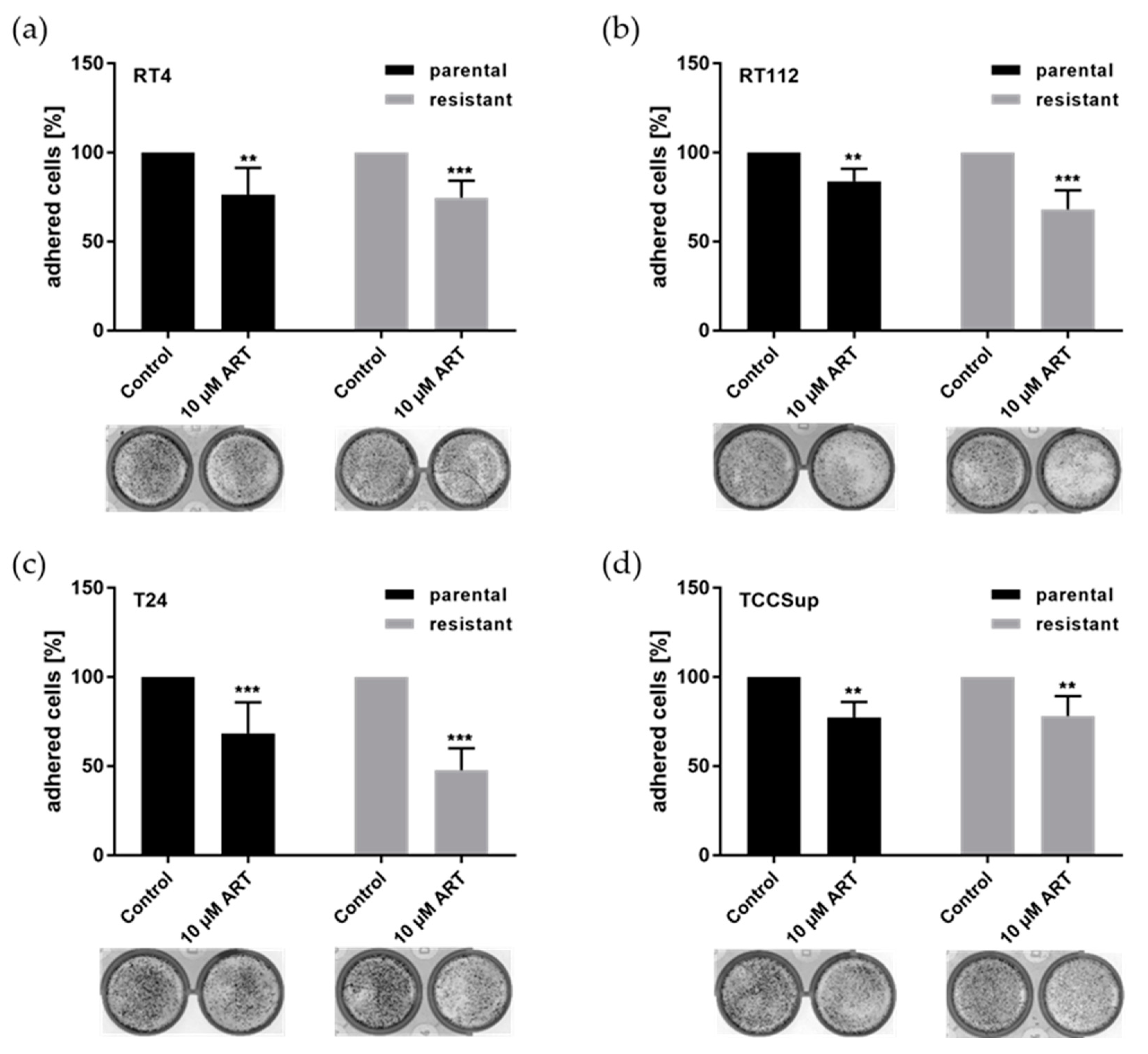
3.1. ART Significantly Inhibited Bladder Cancer Cell Adhesion
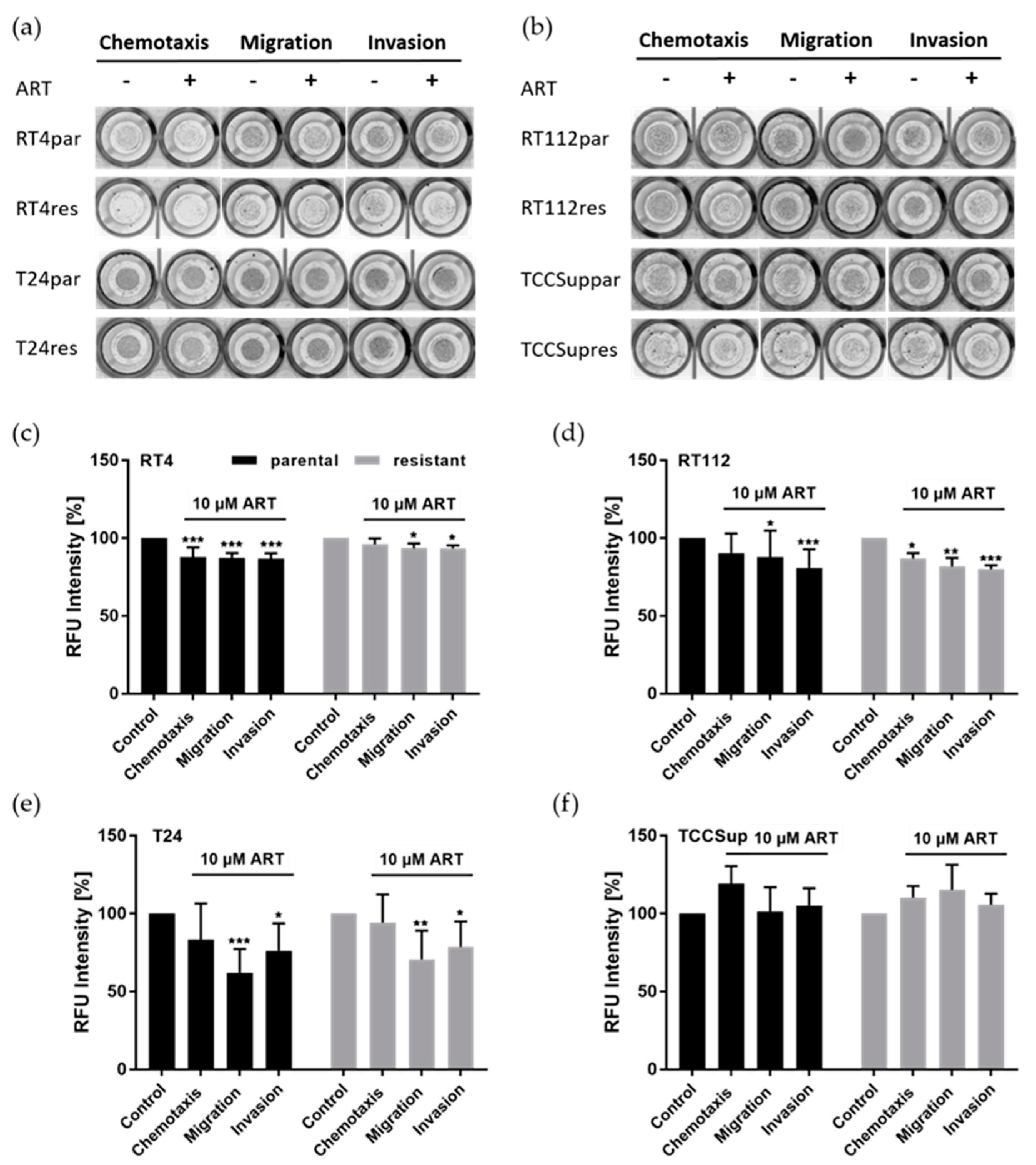
3.2. ART Impaired Chemotaxis, Migration, and Invasion of Bladder Cancer Cells
3.3. ART Suppressed the Cell Surface Expression of Integrins
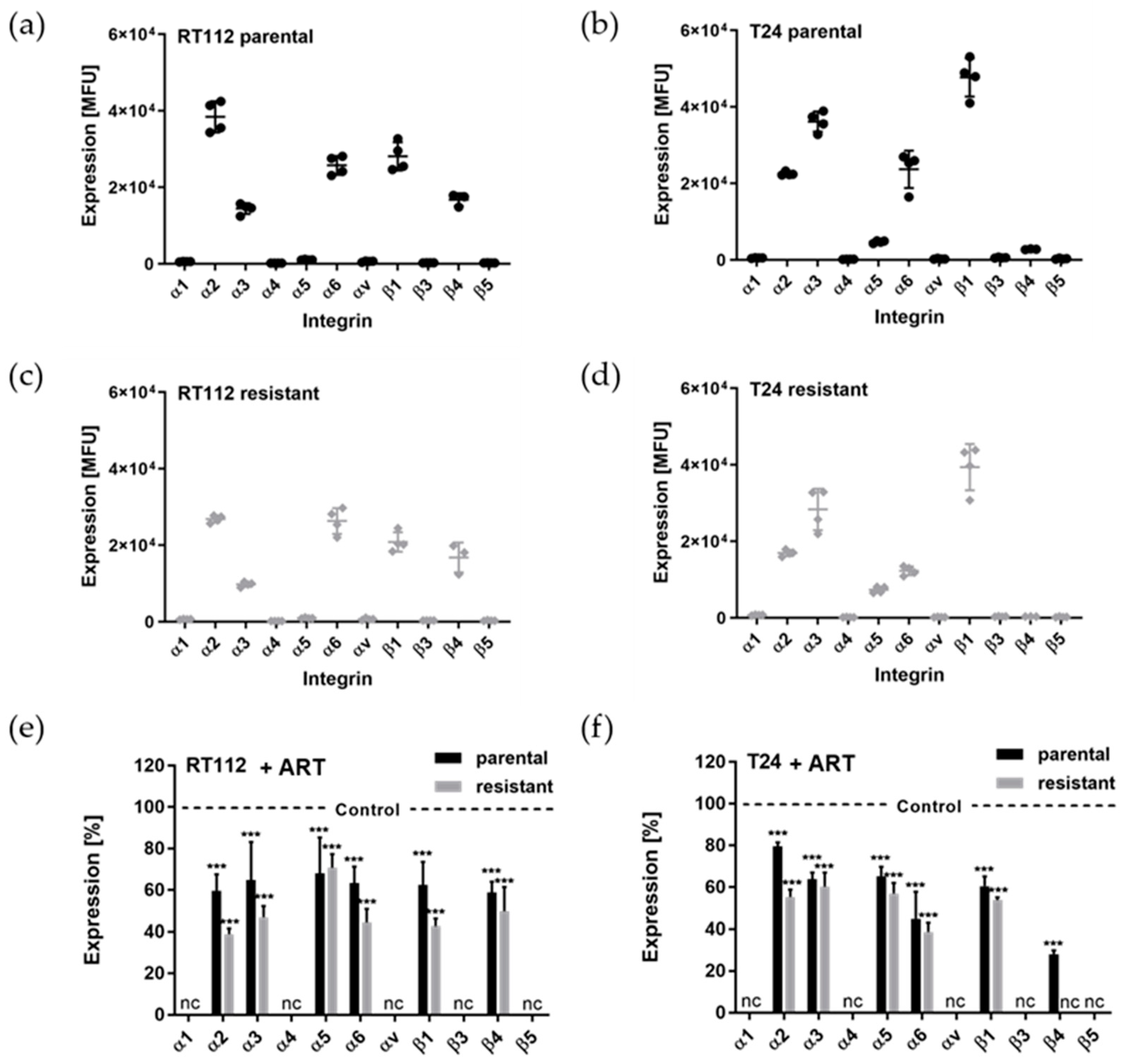
3.4. Modifications of the Total Integrin Content by ART

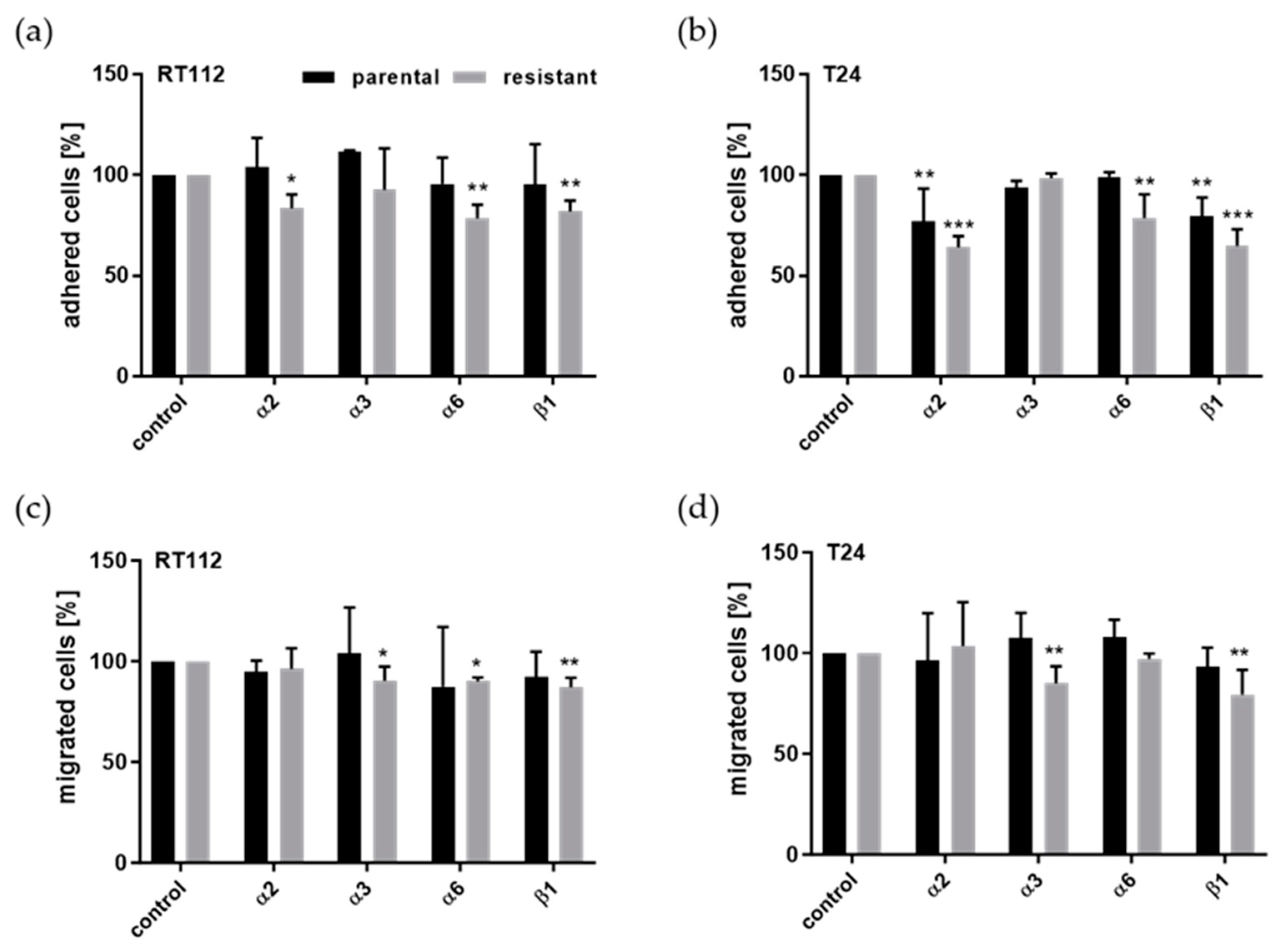
3.5. Integrin Blockade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCa | Bladder cancer |
| MIBC | Muscle-invasive bladder cancer |
| ART | Artesunate |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts. Bladder Cancer. NIH NCI: Surveillance, Epidemiology, and End Results Program. 2019. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 8 April 2025).
- Kamat, A.M.; Hegarty, P.K.; Gee, J.R.; Clark, P.E.; Svatek, R.S.; Hegarty, N.; Shariat, S.F.; Xylinas, E.; Schmitz-Drager, B.J.; Lotan, Y.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, diagnosis, and molecular markers. Eur. Urol. 2013, 63, 4–15. [Google Scholar] [CrossRef]
- Abufaraj, M.; Gust, K.; Moschini, M.; Foerster, B.; Soria, F.; Mathieu, R.; Shariat, S.F. Management of muscle invasive, locally advanced and metastatic urothelial carcinoma of the bladder: A literature review with emphasis on the role of surgery. Transl. Androl. Urol. 2016, 5, 735–744. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Demuru, E.; Baili, P.; Troussard, X.; Katalinic, A.; Chirlaque Lopez, M.D.; Innos, K.; Santaquilani, M.; Blum, M.; Ventura, L.; et al. Complete cancer prevalence in Europe in 2020 by disease duration and country (EUROCARE-6): A population-based study. Lancet Oncol. 2024, 25, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Cabiddu, M.; Ghilardi, M.; Vavassori, I.; Barni, S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur. Urol. 2014, 65, 350–357. [Google Scholar] [CrossRef]
- Arthur, K.; Belliard, J.C.; Hardin, S.B.; Knecht, K.; Chen, C.S.; Montgomery, S. Practices, attitudes, and beliefs associated with complementary and alternative medicine (CAM) use among cancer patients. Integr. Cancer Ther. 2012, 11, 232–242. [Google Scholar] [CrossRef]
- Harris, P.E.; Cooper, K.L.; Relton, C.; Thomas, K.J. Prevalence of complementary and alternative medicine (CAM) use by the general population: A systematic review and update. Int. J. Clin. Pract. 2012, 66, 924–939. [Google Scholar] [CrossRef]
- Molassiotis, A.; Fernandez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef]
- Horneber, M.; Bueschel, G.; Dennert, G.; Less, D.; Ritter, E.; Zwahlen, M. How many cancer patients use complementary and alternative medicine: A systematic review and metaanalysis. Integr. Cancer Ther. 2012, 11, 187–203. [Google Scholar] [CrossRef]
- Juengel, E.; Nowaz, S.; Makarevi, J.; Natsheh, I.; Werner, I.; Nelson, K.; Reiter, M.; Tsaur, I.; Mani, J.; Harder, S.; et al. HDAC-inhibition counteracts everolimus resistance in renal cell carcinoma in vitro by diminishing cdk2 and cyclin A. Mol. Cancer 2014, 13, 152. [Google Scholar] [CrossRef]
- Juengel, E.; Maxeiner, S.; Rutz, J.; Justin, S.; Roos, F.; Khoder, W.; Tsaur, I.; Nelson, K.; Bechstein, W.O.; Haferkamp, A.; et al. Sulforaphane inhibits proliferation and invasive activity of everolimus-resistant kidney cancer cells in vitro. Oncotarget 2016, 7, 85208–85219. [Google Scholar] [CrossRef]
- Justin, S.; Rutz, J.; Maxeiner, S.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro. Int. J. Mol. Sci. 2020, 21, 5582. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.; Benchellal, A.; Kassabra, W.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zoller, N.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. Int. J. Mol. Sci. 2021, 22, 9966. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.J.; Pandey, S.K.; Yadav, A.; Goel, S.; Ateeq, B. Targeting NF-kappa B Signaling by Artesunate Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 2017, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Q.; Mao, Z.; Fan, X.; Hu, B.; Wang, Z.; Chen, Z.; Jiang, X.; Wang, Z.; Lei, N.; et al. Combined treatment with artesunate and bromocriptine has synergistic anticancer effects in pituitary adenoma cell lines. Oncotarget 2017, 8, 45874–45887. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Barnes, K.I.; Mwenechanya, J.; Tembo, M.; McIlleron, H.; Folb, P.I.; Ribeiro, I.; Little, F.; Gomes, M.; Molyneux, M.E. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: A randomised study. Lancet 2004, 363, 1598–1605. [Google Scholar] [CrossRef]
- Karunajeewa, H.A.; Reeder, J.; Lorry, K.; Dabod, E.; Hamzah, J.; Page-Sharp, M.; Chiswell, G.M.; Ilett, K.F.; Davis, T.M. Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea. Antimicrob. Agents Chemother. 2006, 50, 968–974. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, F.; Vakhrusheva, O.; Markowitsch, S.D.; Slade, K.S.; Tsaur, I.; Cinatl, J., Jr.; Michaelis, M.; Efferth, T.; Haferkamp, A.; Juengel, E. Artesunate Impairs Growth in Cisplatin-Resistant Bladder Cancer Cells by Cell Cycle Arrest, Apoptosis and Autophagy Induction. Cells 2020, 9, 2643. [Google Scholar] [CrossRef]
- Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017, 139, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, X.; Johnson, S.; Garg, T.; Tian, E.; Tytarenko, R.; Zhang, Q.; Stein, C.; Barlogie, B.; Epstein, J.; Heuck, C. Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis. Oncotarget 2014, 5, 4118–4128. [Google Scholar] [CrossRef]
- Markowitsch, S.D.; Schupp, P.; Lauckner, J.; Vakhrusheva, O.; Slade, K.S.; Mager, R.; Efferth, T.; Haferkamp, A.; Juengel, E. Artesunate Inhibits Growth of Sunitinib-Resistant Renal Cell Carcinoma Cells through Cell Cycle Arrest and Induction of Ferroptosis. Cancers 2020, 12, 3150. [Google Scholar] [CrossRef]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Shi, R.; Cui, H.; Bi, Y.; Huang, X.; Song, B.; Cheng, C.; Zhang, L.; Liu, J.; He, C.; Wang, F.; et al. Artesunate altered cellular mechanical properties leading to deregulation of cell proliferation and migration in esophageal squamous cell carcinoma. Oncol. Lett. 2015, 9, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Song, H.J.; Lim, S.; Lee, S.J.; Lim, J.E.; Nam, D.H.; Joo, K.M.; Jeong, B.C.; Jeon, S.S.; Choi, H.Y.; et al. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 2015, 6, 33046–33064. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Zhang, J.; He, A.; Wang, Y.L.; Han, K.; Su, Y.; Yin, J.; Lv, X.; Hu, H. Artesunate suppresses the viability and mobility of prostate cancer cells through UCA1, the sponge of miR-184. Oncotarget 2017, 8, 18260–18270. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, Y.; Zheng, H.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Li, F.; Hu, M.; et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/beta-catenin signaling. Oncotarget 2016, 7, 31413–31428. [Google Scholar] [CrossRef]
- Michaelis, M.; Wass, M.N.; Cinatl, J. Drug-adapted cancer cell lines as preclinical models of acquired resistance. Cancer Drug Resist. 2019, 2, 447–456. [Google Scholar] [CrossRef]
- Michaelis, M.; Rothweiler, F.; Barth, S.; Cinatl, J.; van Rikxoort, M.; Loschmann, N.; Voges, Y.; Breitling, R.; von Deimling, A.; Rodel, F.; et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011, 2, e243. [Google Scholar] [CrossRef] [PubMed]
- Perabo, F.G.; Kamp, S.; Schmidt, D.; Lindner, H.; Steiner, G.; Mattes, R.H.; Wirger, A.; Pegelow, K.; Albers, P.; Kohn, E.C.; et al. Bladder cancer cells acquire competent mechanisms to escape Fas-mediated apoptosis and immune surveillance in the course of malignant transformation. Br. J. Cancer 2001, 84, 1330–1338. [Google Scholar] [CrossRef]
- Markowitsch, S.D.; Vakhrusheva, O.; Schupp, P.; Akele, Y.; Kitanovic, J.; Slade, K.S.; Efferth, T.; Thomas, A.; Tsaur, I.; Mager, R.; et al. Shikonin Inhibits Cell Growth of Sunitinib-Resistant Renal Cell Carcinoma by Activating the Necrosome Complex and Inhibiting the AKT/mTOR Signaling Pathway. Cancers 2022, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Rutz, J.; Maxeiner, S.; Grein, T.; Thomas, A.; Juengel, E.; Chun, F.K.; Cinatl, J.; Haferkamp, A.; Tsaur, I.; et al. Plant-Derived Sulforaphane Suppresses Growth and Proliferation of Drug-Sensitive and Drug-Resistant Bladder Cancer Cell Lines In Vitro. Cancers 2022, 14, 4682. [Google Scholar] [CrossRef]
- Gild, P.; Nguyen, D.D.; Fletcher, S.A.; Cole, A.P.; Lipsitz, S.R.; Kibel, A.S.; Fisch, M.; Preston, M.A.; Trinh, Q.D. Contemporary Survival Rates for Muscle-Invasive Bladder Cancer Treated With Definitive or Non-Definitive Therapy. Clin. Genitourin. Cancer 2019, 17, e488–e493. [Google Scholar] [CrossRef]
- Berkoz, M.; Ozkan-Yilmaz, F.; Ozluer-Hunt, A.; Krosniak, M.; Turkmen, O.; Korkmaz, D.; Keskin, S. Artesunate inhibits melanoma progression in vitro via suppressing STAT3 signaling pathway. Pharmacol. Rep. 2021, 73, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Dong, H.; Min, M.; Runpeng, Z.; Xuewei, X.; Ru, C.; Yingru, X.; Shengfa, N.; Baoxian, T.; Jinbo, Y.; et al. Dependence of artesunate on long noncoding RNA-RP11 to inhibit epithelial-mesenchymal transition of hepatocellular carcinoma. J. Cell. Biochem. 2019, 120, 6026–6034. [Google Scholar] [CrossRef]
- Souza, M.C.; Paixao, F.H.; Ferraris, F.K.; Ribeiro, I.; Henriques, M. Artesunate Exerts a Direct Effect on Endothelial Cell Activation and NF-kappaB Translocation in a Mechanism Independent of Plasmodium Killing. Malar. Res. Treat. 2012, 2012, 679090. [Google Scholar] [PubMed]
- Dormoi, J.; Briolant, S.; Pascual, A.; Desgrouas, C.; Travaille, C.; Pradines, B. Improvement of the efficacy of dihydroartemisinin with atorvastatin in an experimental cerebral malaria murine model. Malar. J. 2013, 12, 302. [Google Scholar] [CrossRef]
- Lian, S.; Shi, R.; Huang, X.; Hu, X.; Song, B.; Bai, Y.; Yang, B.; Dong, J.; Du, Z.; Zhang, Y.; et al. Artesunate attenuates glioma proliferation, migration and invasion by affecting cellular mechanical properties. Oncol. Rep. 2016, 36, 984–990. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Chen, H.; Teng, Y.; Ding, X.; Wu, H.; Jin, X. Artesunate inhibits proliferation and invasion of mouse hemangioendothelioma cells in vitro and of tumor growth in vivo. Oncol. Lett. 2017, 14, 6170–6176. [Google Scholar] [CrossRef]
- Su, H.; Karin, M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer 2023, 9, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Bravo, M.; Thorlacius-Ussing, J.; Nissen, N.I.; Pedersen, R.S.; Boisen, M.K.; Liljefors, M.; Johansen, A.Z.; Johansen, J.S.; Karsdal, M.A.; Willumsen, N. Levels of type XVII collagen (BP180) ectodomain are elevated in circulation from patients with multiple cancer types and is prognostic for patients with metastatic colorectal cancer. BMC Cancer 2023, 23, 949. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Angre, T.; Kumar, A.; Singh, A.K.; Thareja, S.; Kumar, P. Role of Collagen Regulators in Cancer Treatment: A Comprehensive Review. Anticancer Agents Med. Chem. 2022, 22, 2956–2984. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M.; Clark, A.G. Collective Cell Migration on Collagen-I Networks: The Impact of Matrix Viscoelasticity. Front. Cell Dev. Biol. 2022, 10, 901026. [Google Scholar] [CrossRef] [PubMed]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Tsaur, I.; Nelson, K.; Pfitzenmaier, J.; Haferkamp, A.; Blaheta, R.A. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS ONE 2014, 9, e110244. [Google Scholar] [CrossRef]
- Yamada, T.; Tsuda, M.; Wagatsuma, T.; Fujioka, Y.; Fujioka, M.; Satoh, A.O.; Horiuchi, K.; Nishide, S.; Nanbo, A.; Totsuka, Y.; et al. Receptor activator of NF-kappaB ligand induces cell adhesion and integrin alpha2 expression via NF-kappaB in head and neck cancers. Sci. Rep. 2016, 6, 23545. [Google Scholar] [CrossRef]
- Van Slambrouck, S.; Jenkins, A.R.; Romero, A.E.; Steelant, W.F. Reorganization of the integrin alpha2 subunit controls cell adhesion and cancer cell invasion in prostate cancer. Int. J. Oncol. 2009, 34, 1717–1726. [Google Scholar] [CrossRef]
- Tian, L.; Chen, M.; He, Q.; Yan, Q.; Zhai, C. MicroRNA-199a-5p suppresses cell proliferation, migration and invasion by targeting ITGA3 in colorectal cancer. Mol. Med. Rep. 2020, 22, 2307–2317. [Google Scholar] [CrossRef]
- Nakada, M.; Nambu, E.; Furuyama, N.; Yoshida, Y.; Takino, T.; Hayashi, Y.; Sato, H.; Sai, Y.; Tsuji, T.; Miyamoto, K.I.; et al. Integrin alpha3 is overexpressed in glioma stem-like cells and promotes invasion. Br. J. Cancer 2013, 108, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Hashida, H.; Takabayashi, A.; Adachi, M.; Imai, T.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. The novel monoclonal antibody MH8-4 inhibiting cell motility recognizes integrin alpha 3: Inverse of its expression withmetastases in colon cancer. Int. J. Oncol. 2001, 18, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sa, K.D.; Zhang, X.; Li, X.F.; Gu, Z.P.; Yang, A.G.; Zhang, R.; Li, J.P.; Sun, J.Y. A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem. Biophys. Res. Commun. 2018, 501, 758–764. [Google Scholar] [CrossRef]
- Lv, G.; Lv, T.; Qiao, S.; Li, W.; Gao, W.; Zhao, X.; Wang, J. RNA interference targeting human integrin alpha6 suppresses the metastasis potential of hepatocellular carcinoma cells. Eur. J. Med. Res. 2013, 18, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smadja, D.M.; Guerin, C.L.; Boscolo, E.; Bieche, I.; Mulliken, J.B.; Bischoff, J. alpha6-Integrin is required for the adhesion and vasculogenic potential of hemangioma stem cells. Stem Cells 2014, 32, 684–693. [Google Scholar] [CrossRef]
- Vallo, S.; Rutz, J.; Kautsch, M.; Winkelmann, R.; Michaelis, M.; Wezel, F.; Bartsch, G.; Haferkamp, A.; Rothweiler, F.; Blaheta, R.A.; et al. Blocking integrin beta1 decreases adhesion in chemoresistant urothelial cancer cell lines. Oncol. Lett. 2017, 14, 5513–5518. [Google Scholar]
- Yamasaki, M.; Iwase, M.; Kawano, K.; Sakakibara, Y.; Suiko, M.; Ikeda, M.; Nishiyama, K. alpha-Lipoic acid suppresses migration and invasion via downregulation of cell surface beta1-integrin expression in bladder cancer cells. J. Clin. Biochem. Nutr. 2014, 54, 18–25. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, A.; White, S.M.; Guha, S. Granulocyte colony-stimulating receptor promotes beta1-integrin-mediated adhesion and invasion of bladder cancer cells. Urology 2006, 68, 208–213. [Google Scholar] [CrossRef]
- Liu, Y.R.; Yin, P.N.; Silvers, C.R.; Lee, Y.F. Enhanced metastatic potential in the MB49 urothelial carcinoma model. Sci. Rep. 2019, 9, 7425. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Wei, H.; Zhang, D. Focal adhesion kinase (FAK) is associated with poor prognosis in urinary bladder carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 831–838. [Google Scholar]
- Ying, X.; Huang, Y.; Liu, B.; Hu, W.; Ji, D.; Chen, C.; Zhang, H.; Liang, Y.; Lv, Y.; Ji, W. Targeted m6A demethylation of ITGA6 mRNA by a multisite dCasRx-m6A editor inhibits bladder cancer development. J. Adv. Res. 2024, 56, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, P.; Li, J.; Lin, Z.; Zhang, Q.; Kwok, H.F. Inhibition of bladder cancer growth with homoharringtonine by inactivating integrin alpha5/beta1-FAK/Src axis: A novel strategy for drug application. Pharmacol. Res. 2023, 188, 106654. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lv, M.; Zhong, Z.; Zhang, L.; Jiang, R.; Chen, J. Interplay between intergrin-linked kinase and ribonuclease inhibitor affects growth and metastasis of bladder cancer through signaling ILK pathways. J. Exp. Clin. Cancer Res. 2016, 35, 130. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhrusheva, O.; Zhao, F.; Markowitsch, S.D.; Slade, K.S.; Brandt, M.P.; Tsaur, I.; Cinatl, J., Jr.; Michaelis, M.; Efferth, T.; Blaheta, R.A.; et al. Artesunate Inhibits Metastatic Potential in Cisplatin-Resistant Bladder Cancer Cells by Altering Integrins. Cells 2025, 14, 570. https://doi.org/10.3390/cells14080570
Vakhrusheva O, Zhao F, Markowitsch SD, Slade KS, Brandt MP, Tsaur I, Cinatl J Jr., Michaelis M, Efferth T, Blaheta RA, et al. Artesunate Inhibits Metastatic Potential in Cisplatin-Resistant Bladder Cancer Cells by Altering Integrins. Cells. 2025; 14(8):570. https://doi.org/10.3390/cells14080570
Chicago/Turabian StyleVakhrusheva, Olesya, Fuguang Zhao, Sascha Dennis Markowitsch, Kimberly Sue Slade, Maximilian Peter Brandt, Igor Tsaur, Jindrich Cinatl, Jr., Martin Michaelis, Thomas Efferth, Roman Alexander Blaheta, and et al. 2025. "Artesunate Inhibits Metastatic Potential in Cisplatin-Resistant Bladder Cancer Cells by Altering Integrins" Cells 14, no. 8: 570. https://doi.org/10.3390/cells14080570
APA StyleVakhrusheva, O., Zhao, F., Markowitsch, S. D., Slade, K. S., Brandt, M. P., Tsaur, I., Cinatl, J., Jr., Michaelis, M., Efferth, T., Blaheta, R. A., Haferkamp, A., & Juengel, E. (2025). Artesunate Inhibits Metastatic Potential in Cisplatin-Resistant Bladder Cancer Cells by Altering Integrins. Cells, 14(8), 570. https://doi.org/10.3390/cells14080570








