Transcription-Driven Repurposing of Cardiotonic Steroids for Lithium Treatment of Severe Depression
Abstract
:1. Background
2. Methods
2.1. Cell Culture Treatment
2.2. Microarray Data
2.3. Expression Profile Analysis
2.4. Publicly Available MDD and BPD Gene Expression Data
2.5. NorPD Prescription Data
3. Results
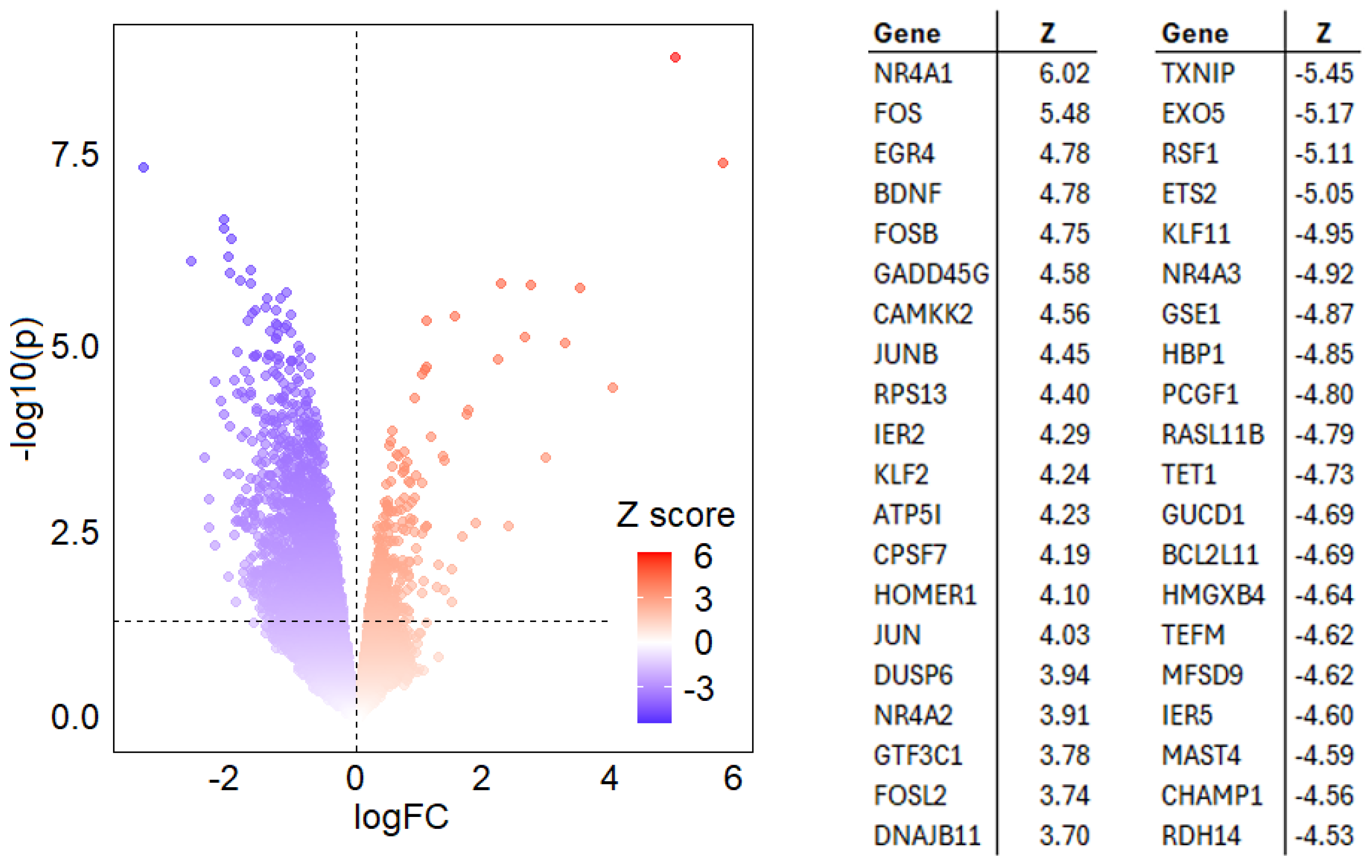
3.1. Transcriptional Changes Associated with Acute Li Treatment
3.2. The Li Expression Profile in the Context of Depression
3.3. Repurposing Candidates
3.4. Prescription Frequencies of CTS and Li in the NorPD Psychiatric Medication Cohort
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cade, J.F. Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 1949, 2, 349–352. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Nutt, D.J. Lithium treatment effect on serotonin function. Arch. Gen. Psychiatry 1990, 47, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Broderick, P.; Lynch, V. Behavioral and biochemical changes induced by lithium and L-tryptophan in muricidal rats. Neuropharmacology 1982, 21, 671–679. [Google Scholar] [CrossRef]
- Staunton, D.A.; Magistretti, P.J.; Shoemaker, W.J.; Bloom, F.E. Effects of chronic lithium treatment on dopamine receptors in the rat corpus striatum. I. Locomotor activity and behavioral supersensitivity. Brain Res. 1982, 232, 391–400. [Google Scholar] [CrossRef]
- Gottesfeld, Z.; Ebstein, B.S.; Samuel, D. Effect of lithium on concentrations of glutamate and GABA levels in amygdala and hypothalamus of rat. Nat. New Biol. 1971, 234, 124–125. [Google Scholar] [CrossRef]
- Stambolic, V.; Ruel, L.; Woodgett, J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996, 6, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.R.; Leavitt, A.L.; Honchar, M.P.; Hallcher, L.M.; Phillips, B.E. Evidence that lithium alters phosphoinositide metabolism: Chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J. Neurochem. 1981, 36, 1947–1951. [Google Scholar] [CrossRef]
- Whitworth, P.; Heal, D.J.; Kendall, D.A. Chronic lithium treatment enhances inositol polyphosphate formation in mouse brain in vivo. Br. J. Pharmacol. 1989, 98, 834P. [Google Scholar]
- Reiser, G.; Scholz, F.; Hamprecht, B. Pharmacological and electrophysiological characterization of lithium ion flux through the action potential sodium channel in neuroblastoma X glioma hybrid cells. J. Neurochem. 1982, 39, 228–234. [Google Scholar] [CrossRef]
- Kato, M.; Lledo, P.M.; Vincent, J.D. Blockade by lithium ions of potassium channels in rat anterior pituitary cells. Am. J. Physiol. 1991, 261 Pt 1, C218–C223. [Google Scholar] [CrossRef] [PubMed]
- Wood, K. The neurochemistry of mania. The effect of lithium on catecholamines, indoleamines and calcium mobilization. J. Affect. Disord. 1985, 8, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Butler-Munro, C.; Coddington, E.J.; Shirley, C.H.; Heyward, P.M. Lithium modulates cortical excitability in vitro. Brain Res. 2010, 1352, 50–60. [Google Scholar] [CrossRef]
- Ballard, C.; Aarsland, D.; Cummings, J.; O’Brien, J.; Mills, R.; Molinuevo, J.L.; Fladby, T.; Williams, G.; Doherty, P.; Corbett, A.; et al. Drug repositioning and repurposing for Alzheimer disease. Nat. Rev. Neurol. 2020, 16, 661–673. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Marton, M.J.; DeRisi, J.L.; Bennett, H.A.; Iyer, V.R.; Meyer, M.R.; Roberts, C.J.; Stoughton, R.; Burchard, J.; Slade, D.; Dai, H.; et al. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nat. Med. 1998, 4, 1293–1301. [Google Scholar] [CrossRef]
- Hughes, T.R.; Marton, M.J.; Jones, A.R.; Roberts, C.J.; Stoughton, R.; Armour, C.D.; Bennett, H.A.; Coffey, E.; Dai, H.; He, Y.D.; et al. Functional discovery via a compendium of expression profiles. Cell 2000, 102, 109–126. [Google Scholar] [CrossRef]
- Wei, G.; Twomey, D.; Lamb, J.; Schlis, K.; Agarwal, J.; Stam, R.W.; Opferman, J.T.; Sallan, S.E.; den Boer, M.L.; Pieters, R.; et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006, 10, 331–342. [Google Scholar] [CrossRef]
- Williams, G. A searchable cross-platform gene expression database reveals connections between drug treatments and disease. BMC Genom. 2012, 13, 12. [Google Scholar] [CrossRef]
- Walf-Vorderwulbecke, V.; Pearce, K.; Brooks, T.; Hubank, M.; van den Heuvel-Eibrink, M.M.; Zwaan, C.M.; Adams, S.; Edwards, D.; Bartram, J.; Samarasinghe, S.; et al. Targeting acute myeloid leukemia by drug-induced c-MYB degradation. Leukemia 2018, 32, 882–889. [Google Scholar] [CrossRef]
- Williams, G.; Gatt, A.; Clarke, E.; Corcoran, J.; Doherty, P.; Chambers, D.; Ballard, C. Drug repurposing for Alzheimer’s disease based on transcriptional profiling of human iPSC-derived cortical neurons. Transl. Psychiatry 2019, 9, 220. [Google Scholar] [CrossRef]
- Fletcher, E.J.R.; Jamieson, A.D.; Williams, G.; Doherty, P.; Duty, S. Targeted repositioning identifies drugs that increase fibroblast growth factor 20 production and protect against 6-hydroxydopamine-induced nigral cell loss in rats. Sci. Rep. 2019, 9, 8336. [Google Scholar] [CrossRef]
- Barrett, T.; Troup, D.B.; Wilhite, S.E.; Ledoux, P.; Rudnev, D.; Evangelista, C.; Kim, I.F.; Soboleva, A.; Tomashevsky, M.; Edgar, R. NCBI GEO: Mining tens of millions of expression profiles—Database and tools update. Nucleic Acids Res. 2007, 35, D760–D765. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Bjornevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B.; et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science 2017, 357, 891–898. [Google Scholar] [CrossRef]
- Osete, J.R.; Akkouh, I.A.; Ievglevskyi, O.; Vandenberghe, M.; de Assis, D.R.; Ueland, T.; Kondratskaya, E.; Holen, B.; Szabo, A.; Hughes, T.; et al. Transcriptional and functional effects of lithium in bipolar disorder iPSC-derived cortical spheroids. Mol. Psychiatry 2023, 28, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, I.A.; Skrede, S.; Holmgren, A.; Ersland, K.M.; Hansson, L.; Bahrami, S.; Andreassen, O.A.; Steen, V.M.; Djurovic, S.; Hughes, T. Exploring lithium’s transcriptional mechanisms of action in bipolar disorder: A multi-step study. Neuropsychopharmacology 2020, 45, 947–955. [Google Scholar] [CrossRef]
- Maloney, B.; Balaraman, Y.; Liu, Y.; Chopra, N.; Edenberg, H.J.; Kelsoe, J.; Nurnberger, J.I.; Lahiri, D.K. Lithium alters expression of RNAs in a type-specific manner in differentiated human neuroblastoma neuronal cultures, including specific genes involved in Alzheimer’s disease. Sci. Rep. 2019, 9, 18261. [Google Scholar] [CrossRef]
- Szczepankiewicz, D.; Celichowski, P.; Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Sassek, M.; Zakowicz, P.; Banach, E.; Langwiński, W.; Sakrajda, K.; Nowakowska, J.; et al. Transcriptome Changes in Three Brain Regions during Chronic Lithium Administration in the Rat Models of Mania and Depression. Int. J. Mol. Sci. 2021, 22, 1148. [Google Scholar] [CrossRef]
- Anand, A.; McClintick, J.N.; Murrell, J.; Karne, H.; Nurnberger, J.I.; Edenberg, H.J. Effects of Lithium Monotherapy for Bipolar Disorder on Gene Expression in Peripheral Lymphocytes. Mol. Neuropsychiatry 2016, 2, 115–123. [Google Scholar] [CrossRef]
- Anand, A.; Nakamura, K.; Spielberg, J.M.; Cha, J.; Karne, H.; Hu, B. Integrative analysis of lithium treatment associated effects on brain structure and peripheral gene expression reveals novel molecular insights into mechanism of action. Transl. Psychiatry 2020, 10, 103. [Google Scholar] [CrossRef]
- de Souza, I.D.; Fernandes, V.G.; Cavalcante, J.V.F.; Coelho, A.C.M.; Morais, D.A.; Cabral-Marques, O.; Pasquali, M.A.; Dalmolin, R.J. Sex-specific gene expression differences in the prefrontal cortex of major depressive disorder individuals. Neuroscience 2024, 559, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Labonte, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.E.; Cahill, M.; et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017, 23, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.A.; Reinhart, V.; Sheehan, M.J.; Rizzo, S.J.S.; Bove, S.E.; James, L.C.; Volfson, D.; Lewis, D.A.; Kleiman, R.J. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: A comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar]
- Chang, L.C.; Jamain, S.; Lin, C.W.; Rujescu, D.; Tseng, G.C.; Sibille, E. A conserved BDNF, glutamate- and GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies. PLoS ONE 2014, 9, e90980. [Google Scholar] [CrossRef]
- Ryan, M.M.; Lockstone, H.E.; Huffaker, S.J.; Wayland, M.T.; Webster, M.J.; Bahn, S. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol. Psychiatry 2006, 11, 965–978. [Google Scholar] [CrossRef]
- Akula, N.; Barb, J.; Jiang, X.; Wendland, J.R.; Choi, K.H.; Sen, S.K.; Hou, L.; Chen, D.T.; Laje, G.; Johnson, K.; et al. RNA-sequencing of the brain transcriptome implicates dysregulation of neuroplasticity, circadian rhythms and GTPase binding in bipolar disorder. Mol. Psychiatry 2014, 19, 1179–1185. [Google Scholar] [CrossRef]
- de Baumont, A.; Maschietto, M.; Lima, L.; Carraro, D.M.; Olivieri, E.H.; Fiorini, A.; Barreta, L.A.; Palha, J.A.; Belmonte-de-Abreu, P.; Moreira Filho, C.A.; et al. Innate immune response is differentially dysregulated between bipolar disease and schizophrenia. Schizophr. Res. 2015, 161, 215–221. [Google Scholar] [CrossRef]
- Pacifico, R.; Davis, R.L. Transcriptome sequencing implicates dorsal striatum-specific gene network, immune response and energy metabolism pathways in bipolar disorder. Mol. Psychiatry 2017, 22, 441–449. [Google Scholar] [CrossRef]
- MacMullen, C.M.; Fallahi, M.; Davis, R.L. Novel PDE10A transcript diversity in the human striatum: Insights into gene complexity, conservation and regulation. Gene 2017, 606, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chao, Y.L.; Chang, C.E.; Hsieh, M.H.; Liu, K.T.; Chen, H.C.; Lu, M.L.; Chen, W.Y.; Chen, C.H.; Tsai, M.H.; et al. Transcriptome Changes in Relation to Manic Episode. Front. Psychiatry 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, N.; Zhao, X.; Ross, C.A.; O’Shea, K.S.; McInnis, M.G. Gene expression alterations in bipolar disorder postmortem brains. Bipolar Disord. 2013, 15, 177–187. [Google Scholar] [CrossRef]
- Holmgren, A.; Akkouh, I.; O’Connell, K.S.; Osete, J.R.; Bjornstad, P.M.; Djurovic, S.; Hughes, T. Bipolar patients display stoichiometric imbalance of gene expression in post-mortem brain samples. Mol. Psychiatry 2024, 29, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.J. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 1982, 16, 965–968. [Google Scholar] [CrossRef]
- Hooper, C.; Killick, R.; Fernandes, C.; Cocks, G.; Sugden, D.; Lovestone, S. Transcriptomic profiles of Wnt3a and insulin in primary cultured rat cortical neurones. J. Neurochem. 2011, 118, 512–520. [Google Scholar] [CrossRef]
- Camacho, C.P.; Latini, F.R.; Oler, G.; Hojaij, F.C.; Maciel, R.M.; Riggins, G.J.; Cerutti, J.M. Down-regulation of NR4A1 in follicular thyroid carcinomas is restored following lithium treatment. Clin. Endocrinol. 2009, 70, 475–483. [Google Scholar] [CrossRef]
- Sun, A.; Shanmugam, I.; Song, J.; Terranova, P.F.; Thrasher, J.B.; Li, B. Lithium suppresses cell proliferation by interrupting E2F-DNA interaction and subsequently reducing S-phase gene expression in prostate cancer. Prostate 2007, 67, 976–988. [Google Scholar] [CrossRef]
- Rojek, A.; Nielsen, J.; Brooks, H.L.; Gong, H.; Kim, Y.H.; Kwon, T.H.; Frokiaer, J.; Nielsen, S. Altered expression of selected genes in kidney of rats with lithium-induced NDI. Am. J. Physiol.-Renal Physiol. 2005, 288, F1276–F1289. [Google Scholar] [CrossRef]
- Detera-Wadleigh, S.D. Lithium-related genetics of bipolar disorder. Ann. Med. 2001, 33, 272–285. [Google Scholar] [CrossRef]
- Yazlovitskaya, E.M.; Edwards, E.; Thotala, D.; Fu, A.; Osusky, K.L.; Whetsell, W.O., Jr.; Boone, B.; Shinohara, E.T.; Hallahan, D.E. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006, 66, 11179–11186. [Google Scholar] [CrossRef] [PubMed]
- McQuillin, A.; Rizig, M.; Gurling, H.M. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet. Genom. 2007, 17, 605–617. [Google Scholar] [CrossRef]
- Youngs, R.M.; Chu, M.S.; Meloni, E.G.; Naydenov, A.; Carlezon, W.A., Jr.; Konradi, C. Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J. Neurosci. 2006, 26, 6031–6039. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Garcia-Albeniz, X.; Logan, R.W.; Denaxas, S.; Hernan, M.A. Avoidable flaws in observational analyses: An application to statins and cancer. Nat. Med. 2019, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Searles Nielsen, S.; Gross, A.; Camacho-Soto, A.; Willis, A.W.; Racette, B.A. β2-adrenoreceptor medications and risk of Parkinson disease. Ann. Neurol. 2018, 84, 683–693. [Google Scholar] [CrossRef]
- Hernan, M.A.; Takkouche, B.; Caamano-Isorna, F.; Gestal-Otero, J.J. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann. Neurol. 2002, 52, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Hack, J.B.; Wingate, S.; Zolty, R.; Rich, M.W.; Hauptman, P.J. Expert Consensus on the Diagnosis and Management of Digoxin Toxicity. Am. J. Med. 2025, 138, 25–33.e14. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Udupa, K.; Sathyaprabha, T.N.; Thirthalli, J.; Kishore, K.R.; Lavekar, G.S.; Raju, T.R.; Gangadhar, B.N. Alteration of cardiac autonomic functions in patients with major depression: A study using heart rate variability measures. J. Affect. Disord. 2007, 100, 137–141. [Google Scholar] [CrossRef] [PubMed]
| GENE | ALP | MDD | GENE | ALP | MDD |
|---|---|---|---|---|---|
| NR4A1 | 6.02 | −2.72 | TXNIP | −5.45 | 2.24 |
| FOS | 5.48 | −2.95 | ETS2 | −5.05 | −4.04 |
| EGR4 | 4.78 | −4.53 | KLF11 | −4.95 | 2.51 |
| BDNF | 4.78 | −3.82 | NR4A3 | −4.92 | −2.46 |
| JUNB | 4.45 | −2.27 | GSE1 | −4.87 | −2.82 |
| RPS13 | 4.40 | 2.06 | BCL2L11 | −4.69 | 2.93 |
| IER2 | 4.29 | −2.16 | IER5 | −4.60 | −2.15 |
| HOMER1 | 4.10 | −2.71 | FBXL3 | −4.44 | 2.01 |
| DUSP6 | 3.94 | −4.97 | KLF15 | −4.29 | 2.70 |
| NR4A2 | 3.91 | −3.93 | CREBRF | −4.24 | 2.44 |
| FOSL2 | 3.74 | −4.11 | PLXNA2 | −4.20 | −4.06 |
| CREM | 3.68 | −3.21 | PIK3IP1 | −4.16 | 3.38 |
| SIK1 | 3.59 | −2.96 | ZKSCAN1 | −4.14 | 3.95 |
| SRF | 3.54 | −2.19 | DSTYK | −4.06 | 3.90 |
| RGS2 | 3.37 | −2.48 | ZEB2 | −4.01 | 2.72 |
| PROFILE | Lithium | MDD COMBO | BPD COMBO |
|---|---|---|---|
| MDD COMBO | −0.24 [−0.34 −0.13] 2.65 × 10−5 | ||
| BPD COMBO | −0.13 [−0.23 −0.03] 0.01133 | 0.21 [0.12 0.29] 8.18 × 10−6 | |
| CUS Mouse | −0.25 [−0.32 −0.17] 2.46 × 10−9 | 0.38 [0.30 0.46] < 2.2 × 10−16 | 0.13 [0.05 0.21] 0.001 |
| Lithium | MDD | |||
|---|---|---|---|---|
| Compound | Z | Rank | Z | Rank |
| digitoxigenin | 5.22 | 12 | −5.73 | 14 |
| digoxin | 5.2 | 13 | −6 | 9 |
| ouabain | 4.27 | 21 | −7.19 | 2 |
| proscillaridin | 4.26 | 22 | −5.27 | 24 |
| digoxigenin | 3.82 | 35 | −2.99 | 168 |
| lanatoside C | 3.43 | 46 | −6.19 | 6 |
| helveticoside | 2.74 | 94 | −4.95 | 31 |
| Cohort | LogODDS [95% CO] | Prob | Li and CTS | Li | CTS | TOT |
|---|---|---|---|---|---|---|
| ALL | −1.15 [−0.94 −1.37] | 6.24 × 10−27 | 88 | 17,488 | 33,234 | 1,926,252 |
| Pr > 10 | −0.96 [−0.63 −1.28] | 6.82 × 10−9 | 37 | 10,607 | 15,784 | 1,901,972 |
| >40 years | −0.96 [−0.75 −1.17] | 4.55 × 10−19 | 88 | 14,231 | 33,177 | 1,525,892 |
| >70 years | −1.03 [−0.78 −1.29] | 2.61 × 10−15 | 60 | 4355 | 30,290 | 652,988 |
| men | −1.18 [−0.88 −1.47] | 1.07 × 10−14 | 44 | 7663 | 15,155 | 802,285 |
| women | −1.14 [−0.84 −1.44] | 6.06 × 10−14 | 44 | 9825 | 18,079 | 1,123,967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Killick, R.; Hooper, C.; Fernandes, C.; Elliott, C.; Aarsland, D.; Kjosavik, S.R.; Østerhus, R.; Williams, G. Transcription-Driven Repurposing of Cardiotonic Steroids for Lithium Treatment of Severe Depression. Cells 2025, 14, 575. https://doi.org/10.3390/cells14080575
Killick R, Hooper C, Fernandes C, Elliott C, Aarsland D, Kjosavik SR, Østerhus R, Williams G. Transcription-Driven Repurposing of Cardiotonic Steroids for Lithium Treatment of Severe Depression. Cells. 2025; 14(8):575. https://doi.org/10.3390/cells14080575
Chicago/Turabian StyleKillick, Richard, Claudie Hooper, Cathy Fernandes, Christina Elliott, Dag Aarsland, Svein R. Kjosavik, Ragnhild Østerhus, and Gareth Williams. 2025. "Transcription-Driven Repurposing of Cardiotonic Steroids for Lithium Treatment of Severe Depression" Cells 14, no. 8: 575. https://doi.org/10.3390/cells14080575
APA StyleKillick, R., Hooper, C., Fernandes, C., Elliott, C., Aarsland, D., Kjosavik, S. R., Østerhus, R., & Williams, G. (2025). Transcription-Driven Repurposing of Cardiotonic Steroids for Lithium Treatment of Severe Depression. Cells, 14(8), 575. https://doi.org/10.3390/cells14080575





