Investigation on ABCC6-Deficient Human Hepatocytes Generated by CRISPR–Cas9 Genome Editing
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Hepatocyte Cell Culture
2.2. CRISPR–Cas9 Mediated hABCC6 Knock-Out
2.3. Nucleic Acid Isolation
2.4. Genomic DNA Amplification
2.5. T7 Endonuclease/Mutagenesis Assay
2.6. TA Cloning for Single Allele Sequencing
2.7. Sanger Sequencing
2.8. Gene Expression Analysis
2.9. Immunoassay for Protein Detection
2.10. Immunofluorescent Staining and Fluorescence Microscopy
2.11. SA-β-Galactosidase Assay
2.12. Bicinchoninic Acid Assay
2.13. ENPP1 Activity Assay
2.14. Statistical Analysis
3. Results
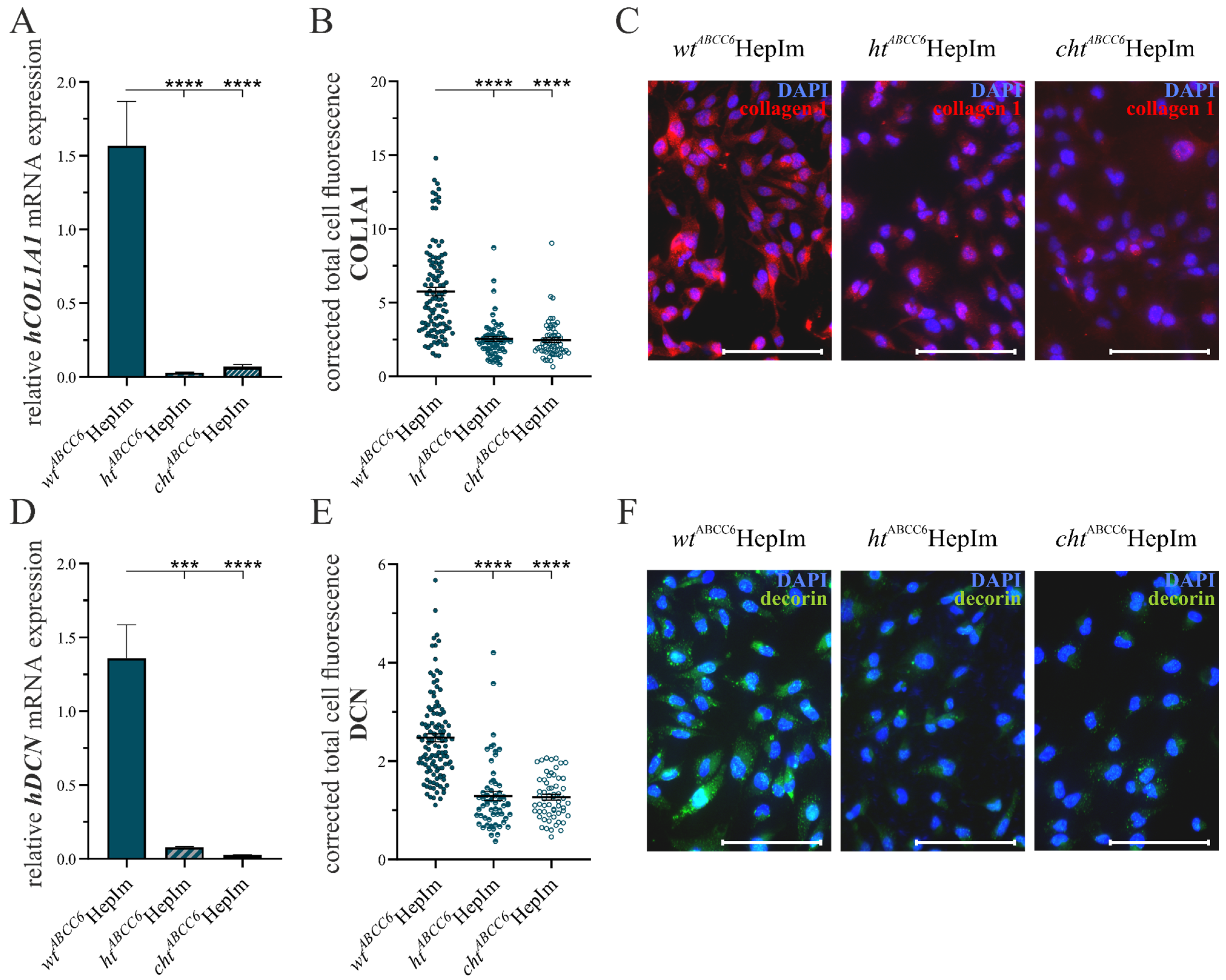
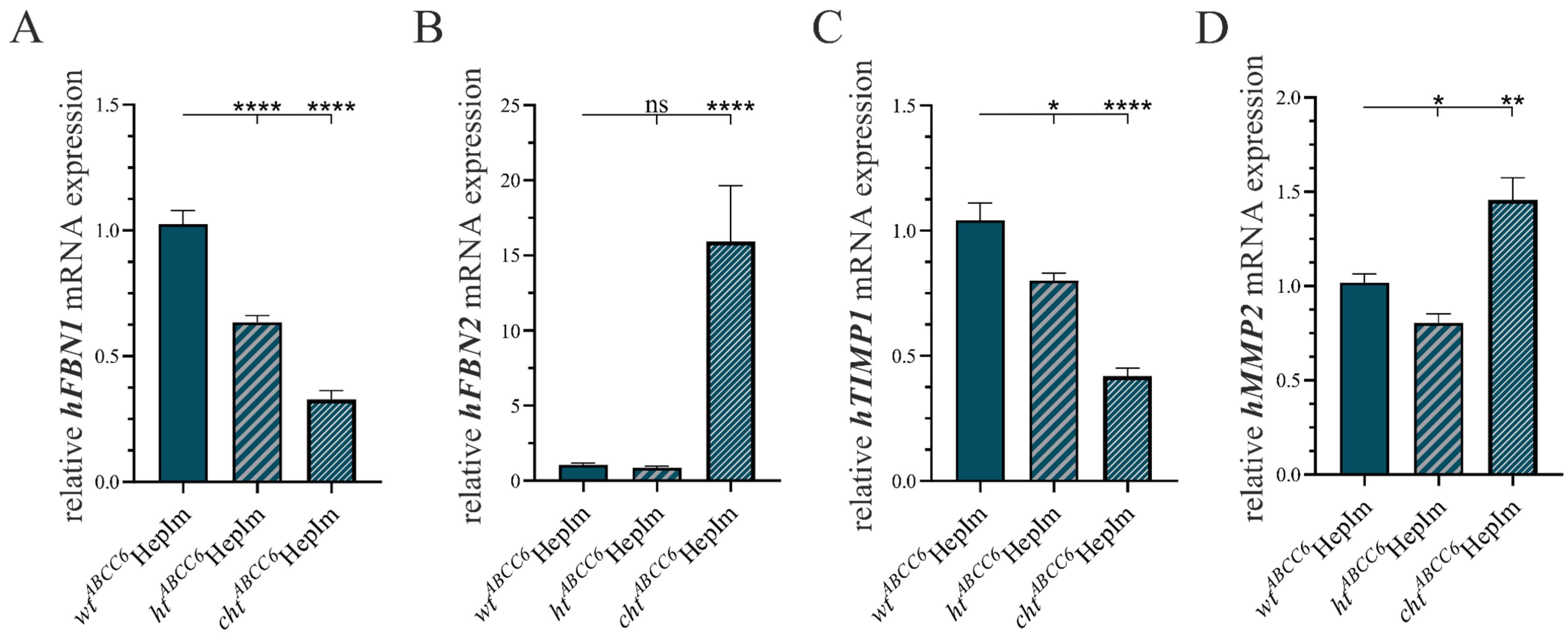
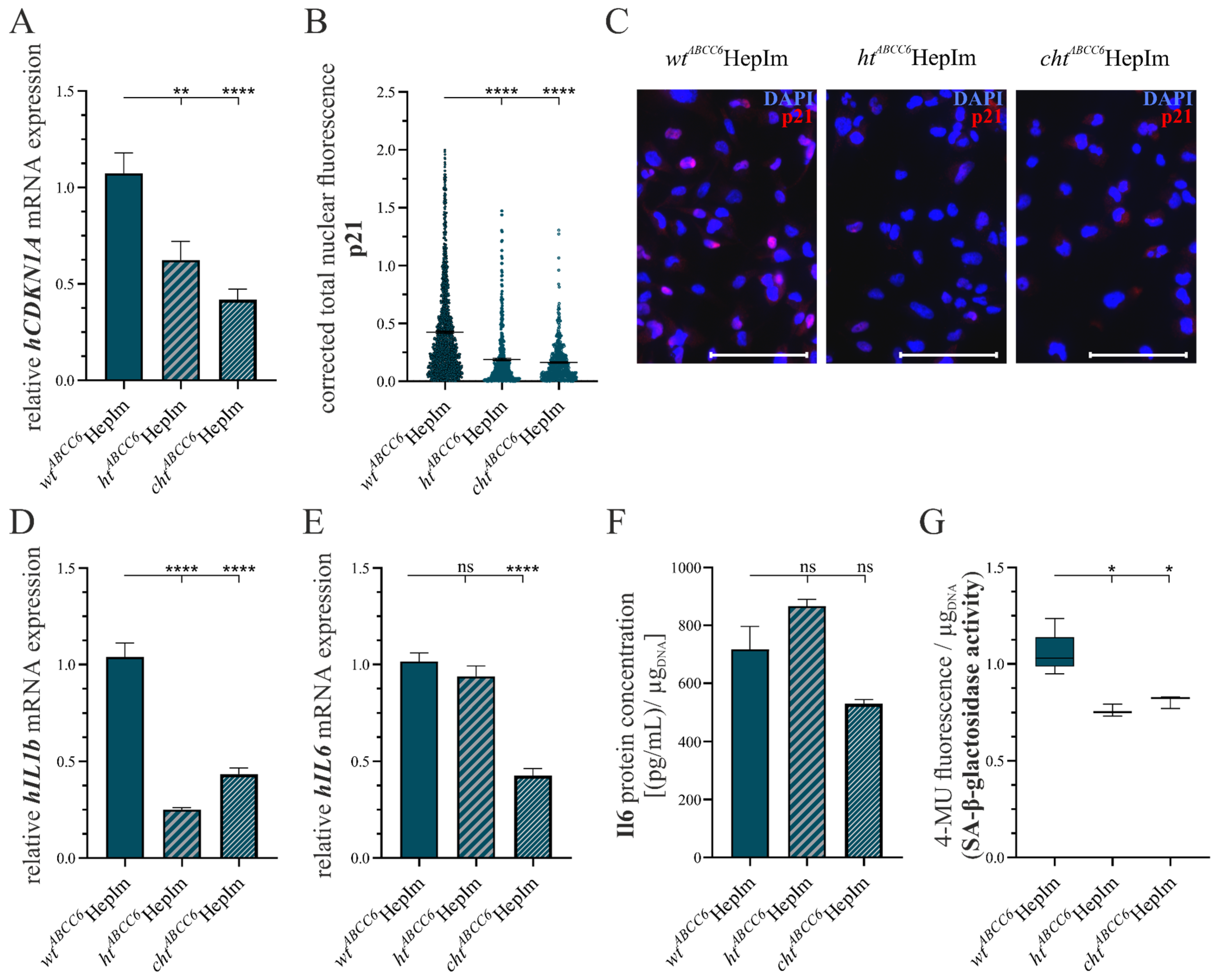
3.1. CRISPR–Cas9-Mediated Knock-Out in Immortalized Hepatocytes
3.2. First Insight into ABCC6-Deficient Hepatocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passi, A.; Albertini, R.; Contri, M.B.; de Luca, G.; de Paepe, A.; Pallavicini, G.; Pasquali Ronchetti, I.; Tiozzo, R. Proteoglycan alterations in skin fibroblast cultures from patients affected with pseudoxanthoma elasticum. Cell Biochem. Funct. 1996, 14, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.B.; van Dijk, M.R.; de Jong, P.T.V.M. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med. Genet. 2010, 152, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Gliem, M.; Birtel, J.; Müller, P.L.; Hendig, D.; Faust, I.; Herrmann, P.; Holz, F.G.; Adamus, G.; Issa, P.C. Acute Retinopathy in Pseudoxanthoma Elasticum. JAMA Ophthalmol. 2019, 137, 1165–1173. [Google Scholar] [CrossRef]
- Nolte, K.B. Sudden cardiac death owing to pseudoxanthoma elasticum: A case report. Hum. Pathol. 2000, 31, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Michael Pope, F.; Richards, A.; Terry, S.; et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef]
- Kuzaj, P.; Kuhn, J.; Michalek, R.D.; Karoly, E.D.; Faust, I.; Dabisch-Ruthe, M.; Knabbe, C.; Hendig, D. Large-Scaled Metabolic Profiling of Human Dermal Fibroblasts Derived from Pseudoxanthoma Elasticum Patients and Healthy Controls. PLoS ONE 2014, 9, e108336. [Google Scholar] [CrossRef]
- Azari, F.; Vali, H.; Guerquin-Kern, J.-L.; Wu, T.-D.; Croisy, A.; Sears, S.K.; Tabrizian, M.; McKee, M.D. Intracellular precipitation of hydroxyapatite mineral and implications for pathologic calcification. J. Struct. Biol. 2008, 162, 468–479. [Google Scholar] [CrossRef]
- Fleisch, H. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 1966, 26, 901–903. [Google Scholar] [CrossRef]
- Ziegler, S.G.; Ferreira, C.R.; MacFarlane, E.G.; Riddle, R.C.; Tomlinson, R.E.; Chew, E.Y.; Martin, L.; Ma, C.-T.; Sergienko, E.; Pinkerton, A.B.; et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci. Transl. Med. 2017, 9, eaal1669. [Google Scholar] [CrossRef]
- Gheduzzi, D.; Boraldi, F.; Annovi, G.; DeVincenzi, C.P.; Schurgers, L.J.; Vermeer, C.; Quaglino, D.; Ronchetti, I.P. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab. Invest. 2007, 87, 998–1008. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Gheduzzi, D.; Sammarco, R.; Quaglino, D.; Bercovitch, L.; Terry, S.; Taylor, W.; Ronchetti, I.P. Extracutaneous Ultrastructural Alterations in Pseudoxanthoma Elasticum. Ultrastruct. Pathol. 2003, 27, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Plümers, R.; Lindenkamp, C.; Osterhage, M.R.; Knabbe, C.; Hendig, D. Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum. Biomolecules 2023, 13, 672. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Kuzaj, P.; Kuhn, J.; Dabisch-Ruthe, M.; Faust, I.; Götting, C.; Knabbe, C.; Hendig, D. ABCC6- a new player in cellular cholesterol and lipoprotein metabolism? Lipids Health Dis. 2014, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Plümers, R.; Osterhage, M.R.; Lindenkamp, C.; Knabbe, C.; Hendig, D. Targeting ABCC6 in Mesenchymal Stem Cells: Impairment of Mature Adipocyte Lipid Homeostasis. Int. J. Mol. Sci. 2022, 23, 9218. [Google Scholar] [CrossRef]
- Lambert, G.; Charlton, F.; Rye, K.-A.; Piper, D.E. Molecular basis of PCSK9 function. Atherosclerosis 2009, 203, 1–7. [Google Scholar] [CrossRef]
- Tall, A.R. An overview of reverse cholesterol transport. Eur. Heart J. 1998, 19 (Suppl. A), A31–A35. [Google Scholar] [CrossRef]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Hofmann, A.F. Chemistry and Enterohepatic Circulation of Bile Acids. Hepatology 1984, 4, 4S–14S. [Google Scholar] [CrossRef]
- Ibold, B.; Faust, I.; Tiemann, J.; Gorgels, T.G.M.F.; Bergen, A.A.B.; Knabbe, C.; Hendig, D. Abcc6 deficiency in mice leads to altered ABC transporter gene expression in metabolic active tissues. Lipids Health Dis. 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Tiemann, J.; Wagner, T.; Lindenkamp, C.; Plümers, R.; Faust, I.; Knabbe, C.; Hendig, D. Linking ABCC6 Deficiency in Primary Human Dermal Fibroblasts of PXE Patients to p21-Mediated Premature Cellular Senescence and the Development of a Proinflammatory Secretory Phenotype. Int. J. Mol. Sci. 2020, 21, 9665. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef]
- Lindenkamp, C.; Plümers, R.; Osterhage, M.R.; Vanakker, O.M.; Van Wynsberghe, J.; Knabbe, C.; Hendig, D. The Activation of JAK/STAT3 Signaling and the Complement System Modulate Inflammation in the Primary Human Dermal Fibroblasts of PXE Patients. Biomedicines 2023, 11, 2673. [Google Scholar] [CrossRef]
- Tsuruga, Y.; Kiyono, T.; Matsushita, M.; Takahashi, T.; Kasai, H.; Todo, S. Establishment of Immortalized Human Hepatocytes by Introduction of HPV16 E6/E7 and hTERT as Cell Sources for Liver Cell-Based Therapy. Cell Transplant. 2008, 17, 1083–1094. [Google Scholar] [CrossRef]
- Grant, A.; Neuberger, J. Guidelines on the use of liver biopsy in clinical practice. Gut 1999, 45, IV1–IV11. [Google Scholar] [CrossRef]
- Zeilinger, K.; Freyer, N.; Damm, G.; Seehofer, D.; Knöspel, F. Cell sources for in vitro human liver cell culture models. Exp. Biol. Med. 2016, 241, 1684–1698. [Google Scholar] [CrossRef]
- Gupta, R.; Schrooders, Y.; Hauser, D.; van Herwijnen, M.; Albrecht, W.; ter Braak, B.; Brecklinghaus, T.; Castell, J.V.; Elenschneider, L.; Escher, S.; et al. Comparing in vitro human liver models to in vivo human liver using RNA-Seq. Arch. Toxicol. 2021, 95, 573–589. [Google Scholar] [CrossRef]
- Gibson, K.M.; Hoffmann, G.; Schwall, A.; Broock, R.L.; Aramaki, S.; Sweetman, L.; Nyhan, W.L.; Brandt, I.K.; Wappner, R.S.; Lehnert, W.; et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: Differential response to lipid supplied by fetal bovine serum in tissue culture medium. J. Lipid Res. 1990, 31, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef]
- Lau, W.M.; Doucet, M.; Stadel, R.; Huang, D.; Weber, K.L.; Kominsky, S.L. Enpp1: A Potential Facilitator of Breast Cancer Bone Metastasis. PLoS ONE 2013, 8, e66752. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Küçükosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; van de Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Prod. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Martin, L.; Aherrahrou, Z.; Leftheriotis, G.; Varadi, A.; Brampton, C. The molecular and physiological roles of ABCC6: More than meets the eye. Front. Genet. 2012, 3, 289. [Google Scholar] [CrossRef]
- Beck, K.; Hayashi, K.; Dang, K.; Hayashi, M.; Boyd, C.D. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem. Cell Biol. 2005, 123, 517–528. [Google Scholar] [CrossRef]
- Beck, K.; Hayashi, K.; Nishiguchi, B.; Saux, O.L.; Hayashi, M.; Boyd, C.D. The Distribution of Abcc6 in Normal Mouse Tissues Suggests Multiple Functions for this ABC Transporter. J. Histochem. Cytochem. 2003, 51, 887–902. [Google Scholar] [CrossRef]
- Le Saux, O.; Beck, K.; Sachsinger, C.; Silvestri, C.; Treiber, C.; Göring, H.H.H.; Johnson, E.W.; Paepe, A.D.; Pope, F.M.; Pasquali-Ronchetti, I.; et al. A Spectrum of ABCC6 Mutations Is Responsible for Pseudoxanthoma Elasticum. Am. J. Hum. Genetics. 2001, 69, 749–764. [Google Scholar] [CrossRef]
- Arias, I.M.; Alter, H.J.; Boyer, J.L.; Cohen, D.E.; Shafritz, D.A.; Thorgeirsson, S.S.; Thorgeirsson, S.S.; Wolkoff, A.W. The Liver: Biology and Pathobiology; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Schulze, R.J.; Schott, M.B.; Casey, C.A.; Tuma, P.L.; McNiven, M.A. The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. 2019, 218, 2096–2112. [Google Scholar] [CrossRef]
- Decaens, C.; Durand, M.; Grosse, B.; Cassio, D. Which in vitro models could be best used to study hepatocyte polarity? Biol. Cell 2008, 100, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.A.; Holman, N.S.; Green, A.M.; Andersen, M.E.; LeCluyse, E.L. Co-culture of Hepatocytes and Kupffer Cells as an In Vitro Model of Inflammation and Drug-Induced Hepatotoxicity. J. Pharm Sci. 2016, 105, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Ringpfeil, F.; McGuigan, K.; Fuchsel, L.; Kozic, H.; Larralde, M.; Lebwohl, M.; Uitto, J. Pseudoxanthoma Elasticum Is a Recessive Disease Characterized by Compound Heterozygosity. J. Investig. Dermatol. 2006, 126, 782–786. [Google Scholar] [CrossRef]
- Ibold, B.; Tiemann, J.; Faust, I.; Ceglarek, U.; Dittrich, J.; Gorgels, T.G.M.F.; Bergen, A.A.B.; Vanakker, O.; Gils, M.V.; Knabbe, C.; et al. Genetic deletion of Abcc6 disturbs cholesterol homeostasis in mice. Sci Rep. 2021, 11, 2137. [Google Scholar] [CrossRef]
- Brampton, C.; Pomozi, V.; Chen, L.-H.; Apana, A.; McCurdy, S.; Zoll, J.; Boisvert, W.A.; Lambert, G.; Henrion, D.; Blanchard, S.; et al. ABCC6 deficiency promotes dyslipidemia and atherosclerosis. Sci. Rep. 2021, 11, 3881. [Google Scholar] [CrossRef]
- Baghy, K.; Iozzo, R.V.; Kovalszky, I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem. 2012, 60, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Faust, I.; Donhauser, E.; Fischer, B.; Ibold, B.; Kuhn, J.; Knabbe, C.; Hendig, D. Characterization of dermal myofibroblast differentiation in pseudoxanthoma elasticum. Exp. Cell Res. 2017, 360, 153–162. [Google Scholar] [CrossRef]
- Zhang, H.; Apfelroth, S.; Hu, W.; Davis, E.; Sanguineti, C.; Bonadio, J.; Mecham, R.P.; Ramirez, F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J. Cell Biol. 1994, 124, 855–863. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Schurgers, L.J.; Uitto, J. Pseudoxanthoma elasticum: Reduced γ-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6−/−). Biochem. Biophys. Res. Commun. 2007, 364, 208–213. [Google Scholar] [CrossRef][Green Version]
- Dabisch-Ruthe, M.; Kuzaj, P.; Götting, C.; Knabbe, C.; Hendig, D. Pyrophosphates as a major inhibitor of matrix calcification in Pseudoxanthoma elasticum. J. Dermatol. Sci. 2014, 75, 109–120. [Google Scholar] [CrossRef]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Gils, M.V.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef] [PubMed]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Letters 2017, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]





| Gene | 5′-3′ Primer Sequences | PAM |
|---|---|---|
| hABCC6 (exon 12) | CACCGTCGAAGACCATCAAGTTCCA | TGG |
| AAACTGGAACTTGATGGTCTTCGAC |
| Gene | 5′-3′ Primer Sequences | TA | Product Size |
|---|---|---|---|
| hABCC6 | CTGTTCTCCGGGCATCAGAGGATGGACGGGGTGGTAGGAT | 59 | 554 |
| hGRIN1 | GCGCCGCTAACCATAAACAAGAATCTCCTGCGGAGGGACG | 56 | 213 |
| hHMGN5 | AGCAGATGCTTGTGCCAGTTCCCCACCCAAGGGGTTTAC | 56 | 218 |
| Gene | Primer Sequence (5′-3′) | TA | Efficiency |
|---|---|---|---|
| hABCA1 | ATCCCCAGCACAGCCTAT | 60 | 1.88 |
| TCTCCCCAAACCTTTCCA | |||
| hABCC2 | CACAAGCAACTGCTGAAC | 60 | 1.76 |
| TGCCAAGAGGAATGACGA | |||
| hABCC3 | GCTCCAGCTTCCTCATCA | 60 | 1.78 |
| TGGAGCACAGGAACATCA | |||
| hABCC6 | CCTGCTGATGTACGCCTT | 60 | 1.92 |
| ACGCGAGCATTGTTCTGA | |||
| hC1R | GAGGAGAATGCCCAGTGGTGG | 68 | 1.92 |
| GCTTCACCCTGTATCCCGTG | |||
| hC3 | GAGAAGACTGTGCTGACCCC | 68 | 1.91 |
| GATGCCTTCCGGGTTCTCAA | |||
| hCD36 | CCTGCTTATCCAGAAGAC | 59 | 2.00 |
| CACAGCCAGATTGAGAAC | |||
| hCDKN1A1 | GCAGACCAGCATGACAGATTTC | 66 | 1.81 |
| ACCTCCGGGAGAGAGGAAAA | |||
| hCOL1A1 | GATGTGCCACTCTGACT | 63 | 1.74 |
| GGGTTCTTGCTGATG | |||
| hCYP27A1 | CAAACTCCCGGATCATAG | 59 | 2.00 |
| GACCACCTTGTACTTCTG | |||
| hDCN | CCTTCCGCTGTCAATG | 63 | 1.76 |
| GCAGGTCTAGCAGAGTTG | |||
| hENPP1 | AATGCCCCTTTGGACATC | 59 | 1.72 |
| CCCGTAACTCACTTTGGT | |||
| hFBN1 | TCCCGTGGGATATGTGCTCAG | 61 | 1.97 |
| ACAGCCTTCTCCATCAGGTCTC | |||
| hFBN2 | AACACGCCAGGAAGTTACAG | 63 | 2.00 |
| ATCTAGTTCACACCGCTCAC | |||
| hGAPDH | AGGTCGGAGTCAACGGAT | 63 | 1.84 |
| TCCTGGAAGATGGTGATG | |||
| hHMBS | CTGCCAGAGAAGAGTGTG | 63 | 1.92 |
| AGCTGTTGCCAGGATGAT | |||
| hIL1B | ACAGATGAAGTGCTCCTTCCA | 63 | 1.94 |
| GTCGGAGATTCGTAGCTGGAT | |||
| hIL6 | ACAGCCACTCACCTCTTCAG | 63 | 1.91 |
| GTGCCTCTTTGCTGCTTTCAC | |||
| hLDLR | CGACTGCAAGGACAAATCTG | 61 | 2.00 |
| AGTCATATTCCCGGTCACAC | |||
| hMGP | AGCGGTAGTAACTTTGTG | 63 | 1.97 |
| GTGGACAGGCTTAGAG | |||
| hPCSK9 | CAGCCTGGTGGAGGTGTATC | 62 | 1.70 |
| CCTATGAGGGTGCCGCTAAC | |||
| hRPL13 | CGGAAGGTGGTGGTCGTA | 63 | 1.87 |
| CTCGGGAAGGGTTGGTGT | |||
| hSCARB1 | TGGGCTCTTCACGGTGTTC | 63 | 2.00 |
| CCTTCGTTGGGTGGGTAGATG | |||
| hSDHA | AACTCGCTCTTGGACCTG | 63 | 1.93 |
| GAGTCGCAGTTCCGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plümers, R.; Jelinek, S.; Lindenkamp, C.; Osterhage, M.R.; Knabbe, C.; Hendig, D. Investigation on ABCC6-Deficient Human Hepatocytes Generated by CRISPR–Cas9 Genome Editing. Cells 2025, 14, 576. https://doi.org/10.3390/cells14080576
Plümers R, Jelinek S, Lindenkamp C, Osterhage MR, Knabbe C, Hendig D. Investigation on ABCC6-Deficient Human Hepatocytes Generated by CRISPR–Cas9 Genome Editing. Cells. 2025; 14(8):576. https://doi.org/10.3390/cells14080576
Chicago/Turabian StylePlümers, Ricarda, Svenja Jelinek, Christopher Lindenkamp, Michel R. Osterhage, Cornelius Knabbe, and Doris Hendig. 2025. "Investigation on ABCC6-Deficient Human Hepatocytes Generated by CRISPR–Cas9 Genome Editing" Cells 14, no. 8: 576. https://doi.org/10.3390/cells14080576
APA StylePlümers, R., Jelinek, S., Lindenkamp, C., Osterhage, M. R., Knabbe, C., & Hendig, D. (2025). Investigation on ABCC6-Deficient Human Hepatocytes Generated by CRISPR–Cas9 Genome Editing. Cells, 14(8), 576. https://doi.org/10.3390/cells14080576






