The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Collective
2.2. Liposuction Technique
2.3. Mechanical Sample Processing
2.4. Macroscopic Sample Composition
2.5. Viability Assays
2.6. Histologic Sample Preparation and Evaluation
2.7. Statistical Analysis
3. Results
3.1. Differences Between CELTPLUS and Nanofat
3.2. Impact of Remaining Tumescent Solution on the Outcome of Mechanical Processing
3.3. Preventing a Re-Mixing of Phases After Sedimentation
3.4. Remaining Aqueous Phase After Sedimentation
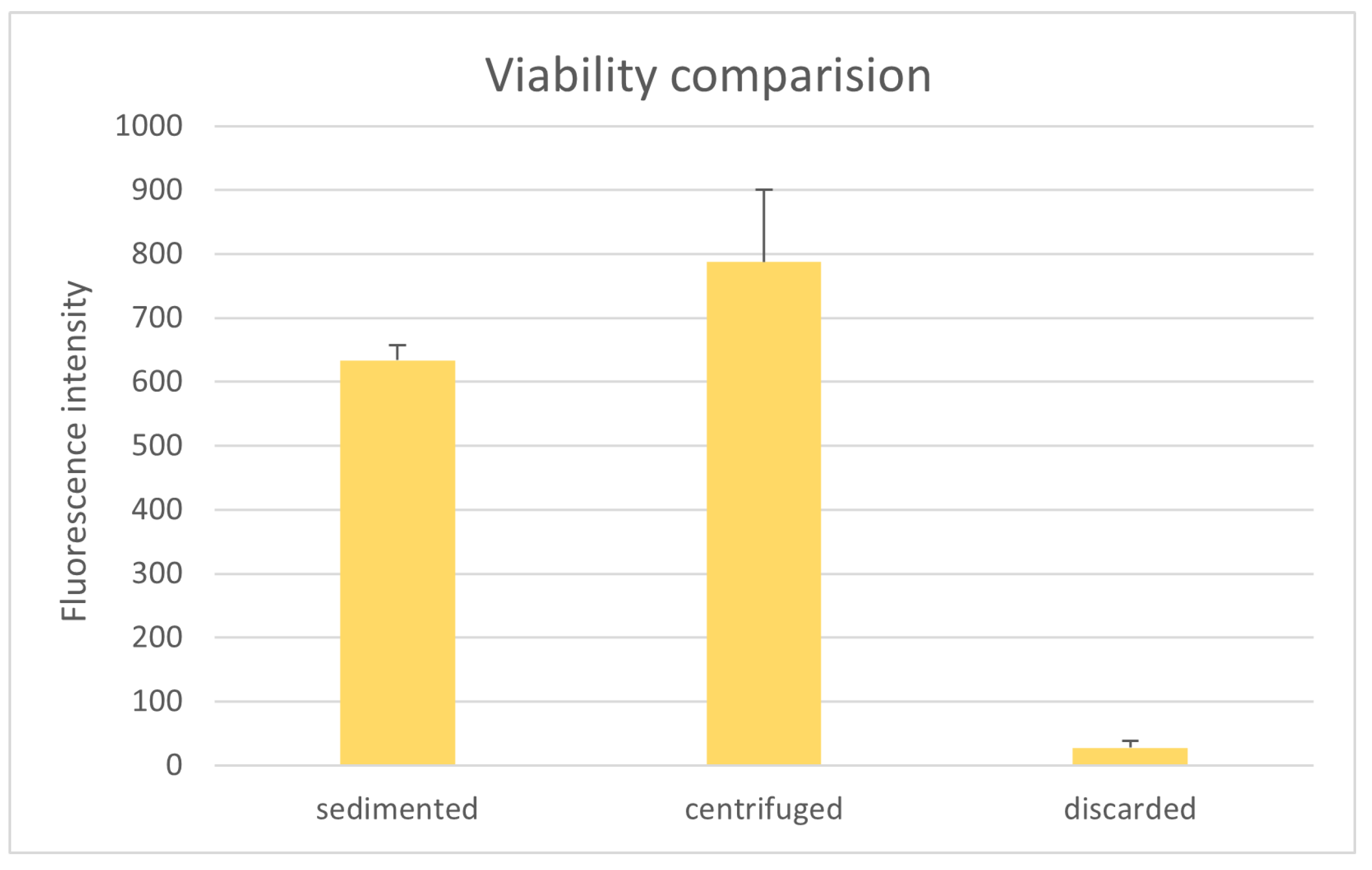
3.5. Comparison of Tissue Viability
3.6. Analysis of the Filtered Discard
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herold, C.; Lange, J.; Rennekampff, H.O.; Allert, S.; Meyer Marcotty, M. Eigenfettinjektion bei Rhizarthrose: 5 Jahresergebnisse bei 42 Patienten. Z. Orthop. Unfall 2023, 161, 511–515. [Google Scholar] [CrossRef]
- van den Boer, C.; Wiersma, A.L.; Marie, J.P.; van Lith-Bijl, J.T. Treatment of Unilateral Vocal Fold Paralysis with Ansa Cervicalis to Recurrent Nerve Anastomosis in a Young Adolescent: European Case Report. J. Laryngol. Otol. 2018, 132, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Eschborn, J.; Kruppa, P.; Georgiou, I.; Infanger, M.; Ghods, M. Long-Term Results After Autologous Fat Transfer for Treatment of Chronic Lower Extremity Wounds. Int. J. Low. Extrem. Wounds 2023, 22, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Krastev, T.K.; Schop, S.J.; Hommes, J.; Piatkowski, A.; Van Der Hulst, R.R.W.J. Autologous Fat Transfer to Treat Fibrosis and Scar-Related Conditions: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 2033–2048. [Google Scholar] [CrossRef] [PubMed]
- Haas-Lützenberger, E.M.; Emelianova, I.; Bader, M.C.; Mert, S.; Moellhoff, N.; Demmer, W.; Berger, U.; Giunta, R. Clinical Results of Autologous Fat Transfer for Basal Thumb Arthritis with a Minimum of Three Years’ Follow-Up. Bone Jt. Open 2024, 5, 1101–1107. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, C.; Jin, X.; Huo, H.; Cui, T.; Wang, J. Management of Severe Dysphonia and Dysphagia Following Lateral Skull Base Surgery. J. Voice 2024. [Google Scholar] [CrossRef]
- Doornaert, M.; Colle, J.; De Maere, E.; Declercq, H.; Blondeel, P. Autologous Fat Grafting: Latest Insights. Ann. Med. Surg. 2019, 37, 47–53. [Google Scholar] [CrossRef]
- Shih, L.; Davis, M.J.; Winocour, S.J. The Science of Fat Grafting. Semin. Plast. Surg. 2020, 34, 005–010. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, H.; Gao, Q.; Yuan, X.; Hu, Y.; Qi, Z. Enhancing Fat Transplantation Efficiency in a Mouse Model through Pretreatment of Adipose-Derived Stem Cells with RIP3 Inhibitors. Aesthetic Plast. Surg. 2024, 48, 3488–3499. [Google Scholar] [CrossRef]
- Nishimura, T.; Hashimoto, H.; Nakanishi, I.; Furukawa, M. Microvascular Angiogenesis and Apoptosis in the Survival of Free Fat Grafts. Laryngoscope 2000, 110, 1333–1338. [Google Scholar] [CrossRef]
- Leong, D.; Hutmacher, D.; Chew, F.; Lim, T. Viability and Adipogenic Potential of Human Adipose Tissue Processed Cell Population Obtained from Pump-Assisted and Syringe-Assisted Liposuction. J. Dermatol. Sci. 2005, 37, 169–176. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, J.E.; Yong, L.Y.; Goddard, N.V.; Wong, J.K. Does Secondary Mechanical Manipulation of Lipoaspirate Enhance the Vasculogenic Potential of Fat Grafts? A Systematic Review. Ann. Plast. Surg. 2024, 93, 389–396. [Google Scholar] [CrossRef]
- Bellini, E.; Grieco, M.P.; Raposio, E. The Science behind Autologous Fat Grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Laloze, J.; Varin, A.; Bertheuil, N.; Grolleau, J.L.; Vaysse, C.; Chaput, B. Cell-Assisted Lipotransfer: Current Concepts. Ann. Chir. Plast. Esthet. 2017, 62, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kølle, S.-F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.-L.M.; et al. Enrichment of Autologous Fat Grafts with Ex-Vivo Expanded Adipose Tissue-Derived Stem Cells for Graft Survival: A Randomised Placebo-Controlled Trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Ma, X.; Dong, M.; Chen, B.; Jiang, C.; Li, F. Innovative Mechanical Processing of Adipose Tissue: A Comparative Evaluation of Microfat and Concentrated Micronized Fat for Superficial Fat Grafting. Aesth. Plast. Surg. 2025, 1–14. [Google Scholar] [CrossRef]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and Rapid Non-Enzymatic Procedure Allows the Isolation of Structurally Preserved Connective Tissue Micro-Fragments Enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [CrossRef]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.; Kocak, P. A 3-Step Mechanical Digestion Method to Harvest Adipose-Derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef]
- Goulas, P.; Karakwta, M.; Zatagias, A.; Bakoutsi, M.; Zevgaridis, A.; Ioannidis, A.; Krokou, D.; Michalopoulos, A.; Zevgaridis, V.; Koliakos, G. A Simple and Effective Mechanical Method for Adipose-Derived Stromal Vascular Fraction Isolation. Cureus 2024, 16, 3. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Eigenberger, A.; Felthaus, O.; Schratzenstaller, T.; Haerteis, S.; Utpatel, K.; Prantl, L. The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells 2022, 11, 2543. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Reinhard, R.; Siegmund, A.; Heumann, K.; Felthaus, O. Cell-Enriched Lipotransfer (CELT) Improves Tissue Regeneration and Rejuvenation without Substantial Manipulation of the Adipose Tissue Graft. Cells 2022, 11, 3159. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Felthaus, O. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef]
- Shauly, O.; Gould, D.J.; Ghavami, A. Fat Grafting: Basic Science, Techniques, and Patient Management. Plast. Reconstr. Surg. Glob. Open 2022, 10, e3987. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, Applications, and Outcomes of Autologous Fat Grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, Z.; Han, X.; Li, F. Blood Impairs Viability of Fat Grafts and Adipose Stem Cells: Importance of Washing in Fat Processing. Aesthet. Surg. J. 2021, 41, 86–97. [Google Scholar] [CrossRef]
- Yin, S.; Luan, J.; Fu, S.; Wang, Q.; Zhuang, Q. Does Water-Jet Force Make a Difference in Fat Grafting? In Vitro and in Vivo Evidence of Improved Lipoaspirate Viability and Fat Graft Survival. Plast. Reconstr. Surg. 2015, 135, 127–138. [Google Scholar] [CrossRef]
- Patel, P.N.; Smith, C.K.; Patrick, C.W., Jr. Rheological and Recovery Properties of Poly(Ethylene Glycol) Diacrylate Hydrogels and Human Adipose Tissue. J. Biomed. Mater. Res. Part A 2005, 73, 313–319. [Google Scholar] [CrossRef]
- Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Available online: https://www.mdpi.com/2073-4409/10/3/594 (accessed on 29 October 2024).
- Osinga, R.; Menzi, N.R.; Tchang, L.A.H.; Caviezel, D.; Kalbermatten, D.F.; Martin, I.; Schaefer, D.J.; Scherberich, A.; Largo, R.D. Effects of Intersyringe Processing on Adipose Tissue and Its Cellular Components: Implications in Autologous Fat Grafting. Plast. Reconstr. Surg. 2015, 135, 1618. [Google Scholar] [CrossRef]
- Banyard, D.A.; Sarantopoulos, C.N.; Borovikova, A.A.; Qiu, X.; Wirth, G.A.; Paydar, K.Z.; Haun, J.B.; Evans, G.R.D.; Widgerow, A.D. Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations. Plast. Reconstr. Surg. 2016, 138, 237e. [Google Scholar] [CrossRef]
- Girard, P.; Dulong, J.; Duisit, J.; Mocquard, C.; Le Gallou, S.; Chaput, B.; Lupon, E.; Watier, E.; Varin, A.; Tarte, K.; et al. Modified Nanofat Grafting: Stromal Vascular Fraction Simple and Efficient Mechanical Isolation Technique and Perspectives in Clinical Recellularization Applications. Front. Bioeng. Biotechnol. 2022, 10, 895735. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Sharma, S.; Ganta, C.; Ranjan, R.; Jha, S.K. Nanofat: A Therapeutic Paradigm in Regenerative Medicine. World J. Stem Cells 2021, 13, 1733–1746. [Google Scholar] [CrossRef]
- Tran, V.V.T.; Hong, K.Y.; Chang, H. Letters in Reply: Histological Comparison of Nanofat and Lipoconcentrate: Enhanced Effects of Lipoconcentrate on Adipogenesis and Angiogenesis. Aesth. Plast. Surg. 2024, 48, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, Z.; Feng, J.; Chang, Q.; Lu, F.; Yuan, Y. Necroptosis in Macrophage Foam Cells Promotes Fat Graft Fibrosis in Mice. Front. Cell Dev. Biol. 2021, 9, 651360. [Google Scholar] [CrossRef]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The Fate of Adipocytes after Nonvascularized Fat Grafting: Evidence of Early Death and Replacement of Adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) Assay When Compared to Three Commonly Used Cell Enumeration Assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Muench, D.P. Breast Augmentation by Water-Jet Assisted Autologous Fat Grafting: A Report of 300 Operations. Surg. J. 2016, 2, e19–e30. [Google Scholar] [CrossRef]
- Kling, R.E.; Mehrara, B.J.; Pusic, A.L.; Young, V.L.; Hume, K.M.; Crotty, C.A.; Rubin, J.P. Trends in Autologous Fat Grafting to the Breast: A National Survey of the American Society of Plastic Surgeons. Plast. Reconstr. Surg. 2013, 132, 35–46. [Google Scholar] [CrossRef]
- Wang, G.-H.-E.; Zhao, J.-F.; Xue, H.-Y.; Li, D. Facial Aesthetic Fat Graft Retention Rates after Filtration, Centrifugation, or Sedimentation Processing Techniques Measured Using Three-Dimensional Surface Imaging Devices. Chin. Med. J. 2019, 132, 69–77. [Google Scholar] [CrossRef]
- Demirel, O.; Karakol, P.; Bozkurt, M.; Tuglu, M.I. The Effect of Centrifuge Duration on Fat Graft Survival. Indian. J. Plast. Surg. 2023, 56, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Heine, N.; Eigenberger, A.; Brebant, V.; Kempa, S.; Seitz, S.; Prantl, L.; Kuehlmann, B. The Effect of Radiotherapy on Fat Engraftment for Complete Breast Reconstruction Using Lipofilling Only. Arch. Gynecol. Obstet. 2022, 307, 549–555. [Google Scholar] [CrossRef] [PubMed]
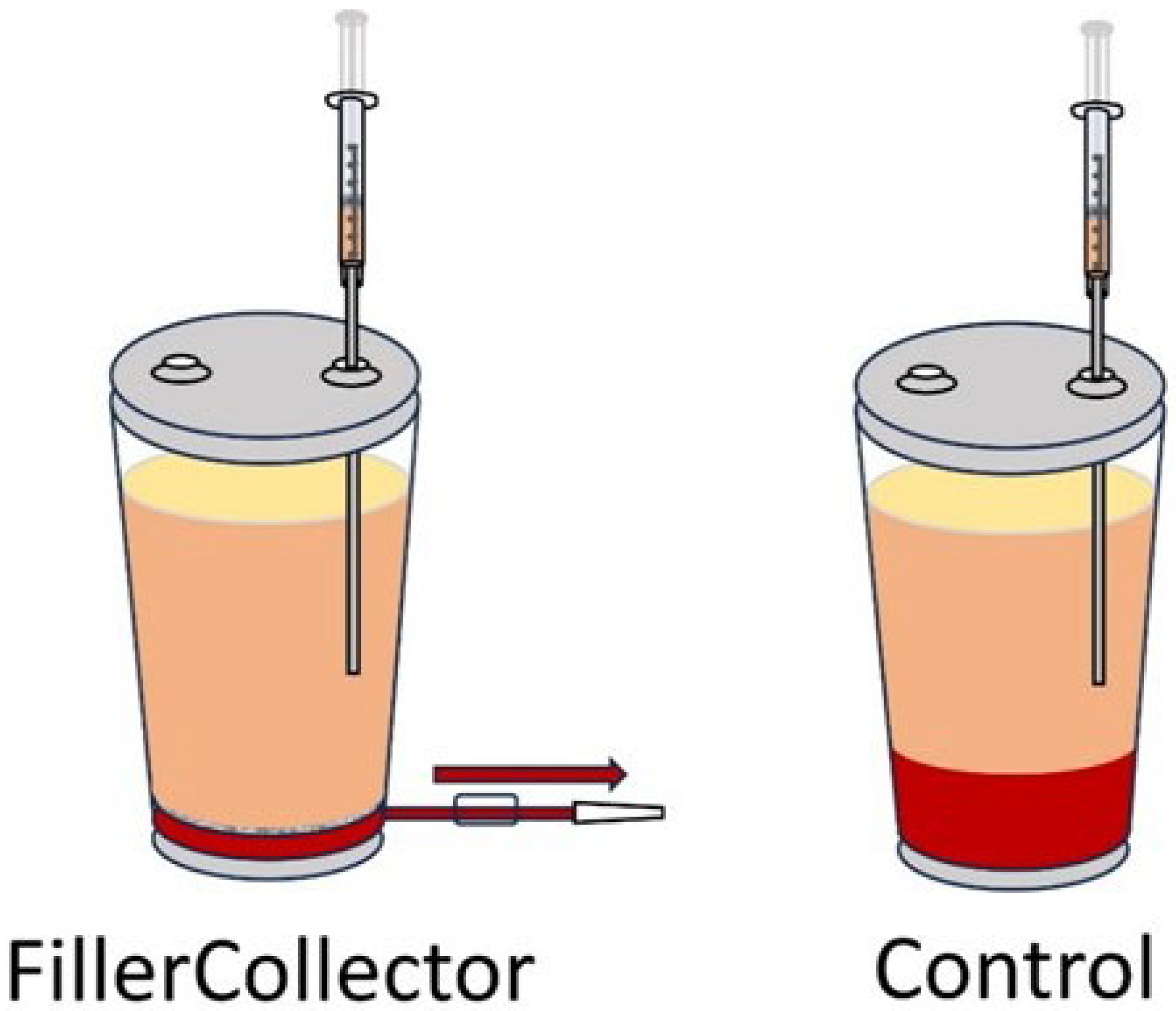







| Volume Percentage of Aqueous Contamination | ||||
|---|---|---|---|---|
| Replicate | 0% | 5% | 10% | 15% |
| 1 | CELTPLUS | CELTPLUS | Nanofat | Nanofat |
| 2 | CELTPLUS | Nanofat | Nanofat | Nanofat |
| 3 | CELTPLUS | Nanofat | Nanofat | Nanofat |
| 4 | CELTPLUS | Nanofat | Nanofat | Nanofat |
| FillerCollector® | Control | |||
|---|---|---|---|---|
| Patient | Sedimented | Centrifuged | Sedimented | Centrifuged |
| 1 | CELTPLUS | CELTPLUS | Nanofat | CELTPLUS |
| 2 | Nanofat | CELTPLUS | Nanofat | CELTPLUS |
| 3 | Nanofat | CELTPLUS | Nanofat | CELTPLUS |
| 4 | Nanofat | CELTPLUS | Nanofat | CELTPLUS |
| 5 | Nanofat | CELTPLUS | Nanofat | CELTPLUS |
| Fluorescence Intensity | FillerCollector® | Control |
|---|---|---|
| aqueous phase | 1.6 ± 1.6 | 5.0 ± 1.5 |
| fat phase | 1057.7 ± 290.9 | 772.7 ± 203.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eigenberger, A.; Felthaus, O.; Bartsch, A.; Schimanski, T.; Utpatel, K.; Prantl, L. The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing. Cells 2025, 14, 601. https://doi.org/10.3390/cells14080601
Eigenberger A, Felthaus O, Bartsch A, Schimanski T, Utpatel K, Prantl L. The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing. Cells. 2025; 14(8):601. https://doi.org/10.3390/cells14080601
Chicago/Turabian StyleEigenberger, Andreas, Oliver Felthaus, Alexander Bartsch, Tom Schimanski, Kirsten Utpatel, and Lukas Prantl. 2025. "The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing" Cells 14, no. 8: 601. https://doi.org/10.3390/cells14080601
APA StyleEigenberger, A., Felthaus, O., Bartsch, A., Schimanski, T., Utpatel, K., & Prantl, L. (2025). The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing. Cells, 14(8), 601. https://doi.org/10.3390/cells14080601







