LFA-1/ICAM-1 Interactions Between CD8+ and CD4+ T Cells Promote CD4+ Th1-Dominant Differentiation and CD8+ T Cell Cytotoxicity for Strong Antitumor Immunity After Cryo-Thermal Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Animal Model
2.2. Cryo-Thermal Therapy Procedures
2.3. CD4+ and CD8+ T Cell Depletion
2.4. Flow Cytometry Analysis
2.5. CD4+ and CD8+ T Cell Isolation
2.6. In Vitro Tumor Killing Assay
2.7. CD4+ and CD8+ T Cells Coculture System
2.8. In Vivo LFA-1 Blockade
2.9. RNA-Seq and Analysis
2.10. Immunofluorescence Staining
2.11. IL-2 ELISA
2.12. Western Blot
2.13. scRNA-Seq Analysis
2.14. Statistical Analysis
3. Results
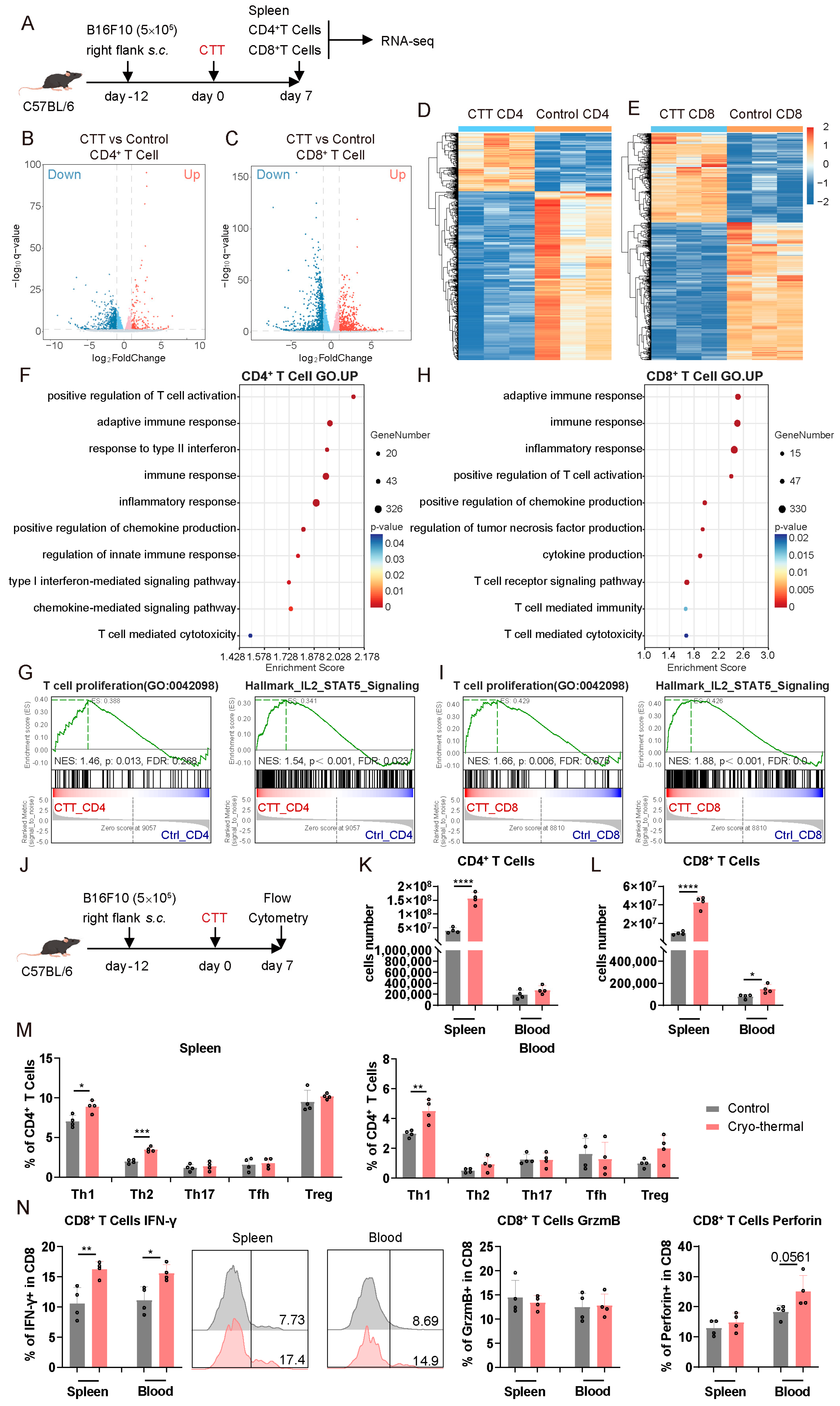
3.1. CD4+ and CD8+ T Cells Were Activated at an Early Stage After CTT
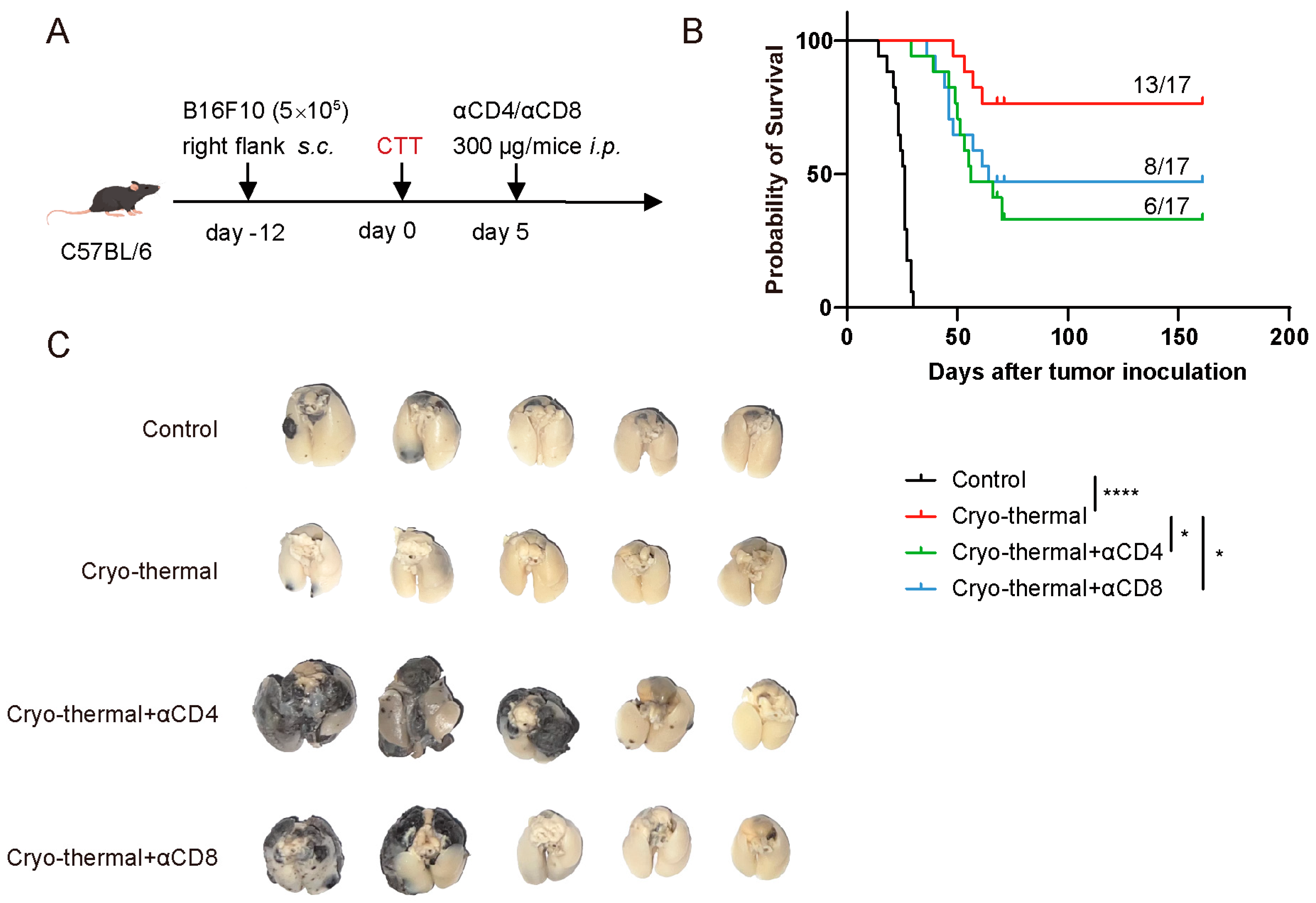
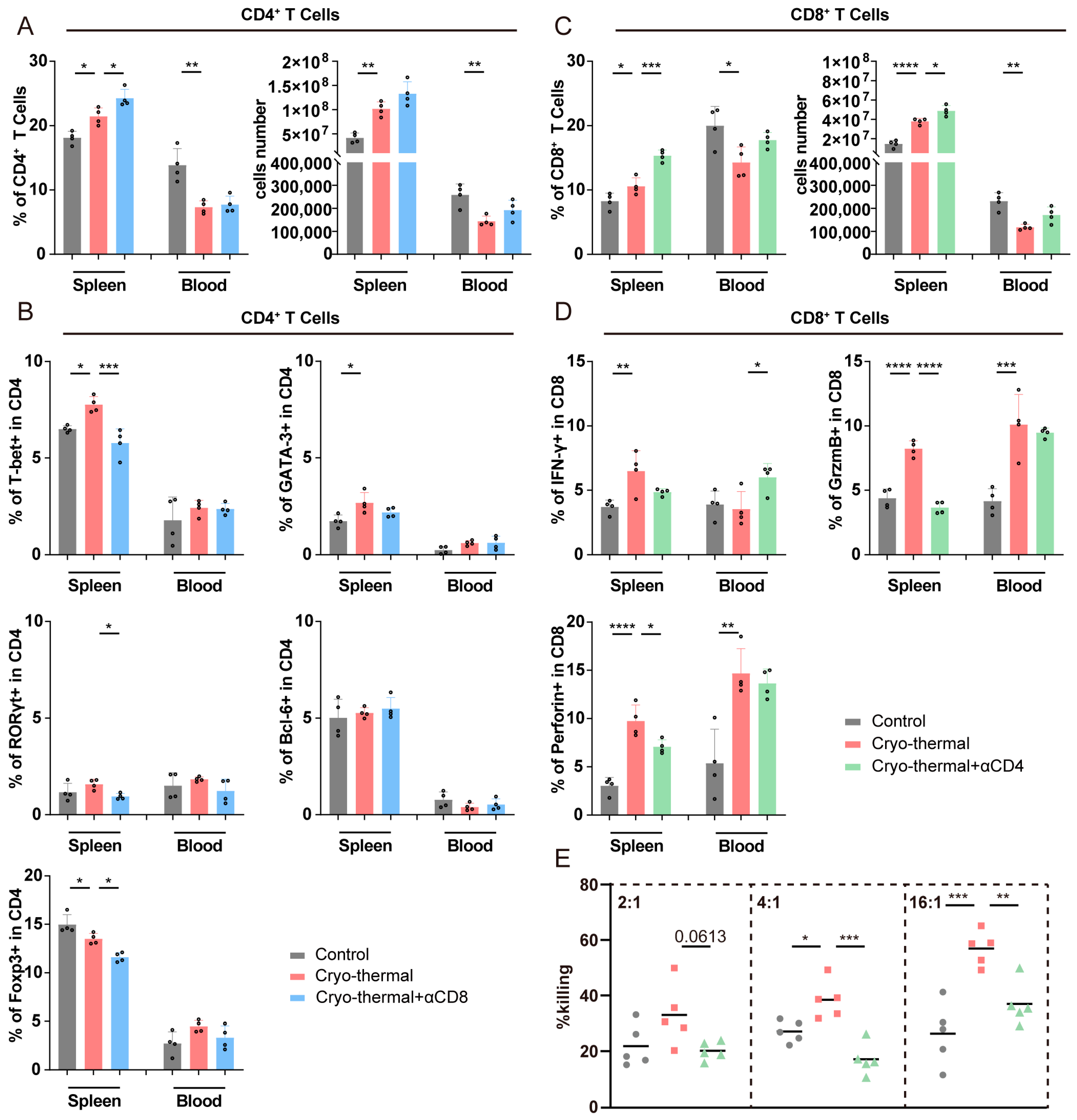
3.2. Activated CD4+ and CD8+ T Cells Mediated Persistent Antitumor Immunity at the Early Stage After CTT
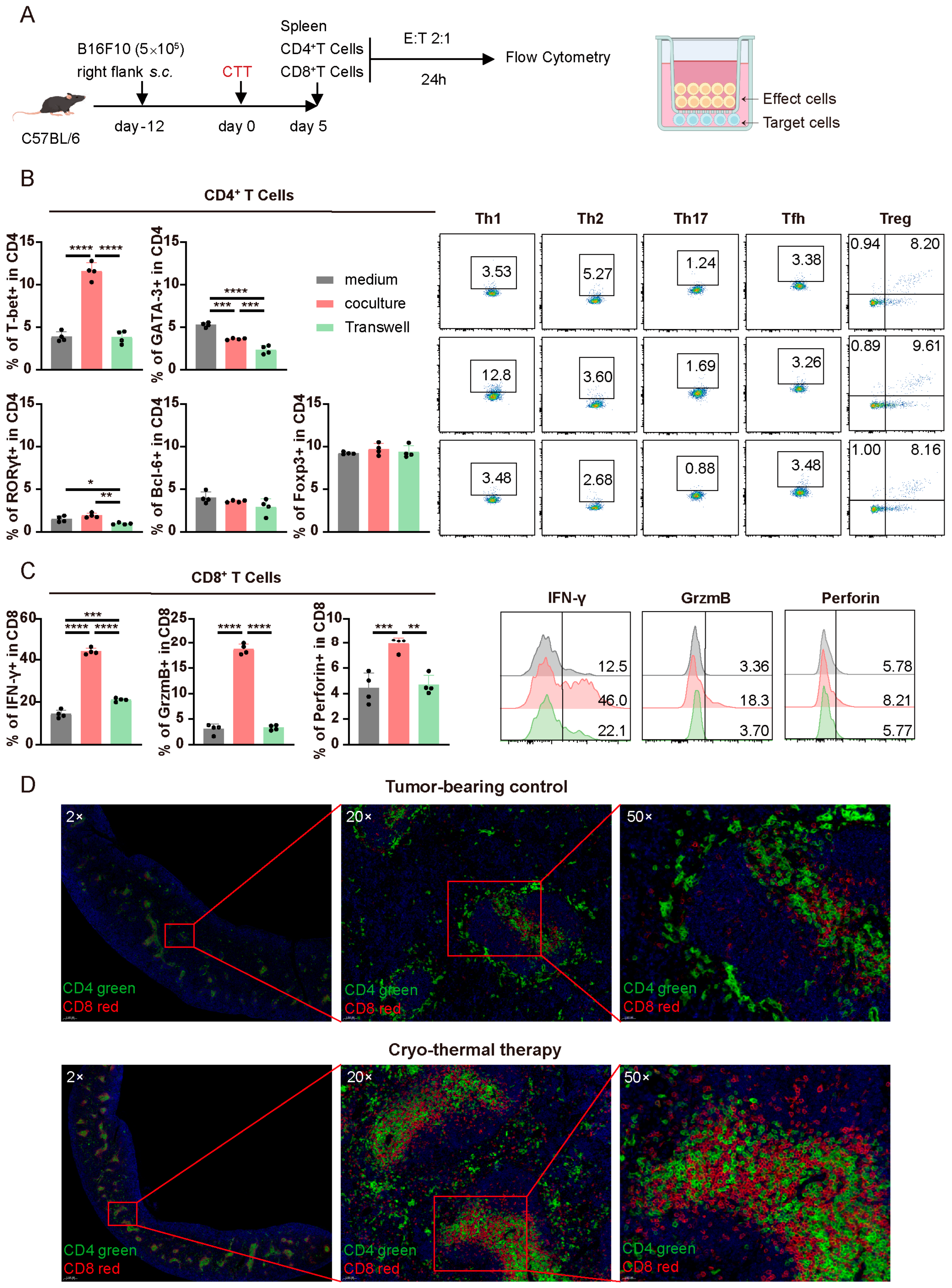
3.3. Crosstalk Occurred Between Activated CD4+ and CD8+ T Cells at the Early Stage After CTT
3.4. The Mutual Influence of CTT-Activated CD4+ and CD8+ T Cells in the Early Phase Was Dependent on Cell–Cell Contact
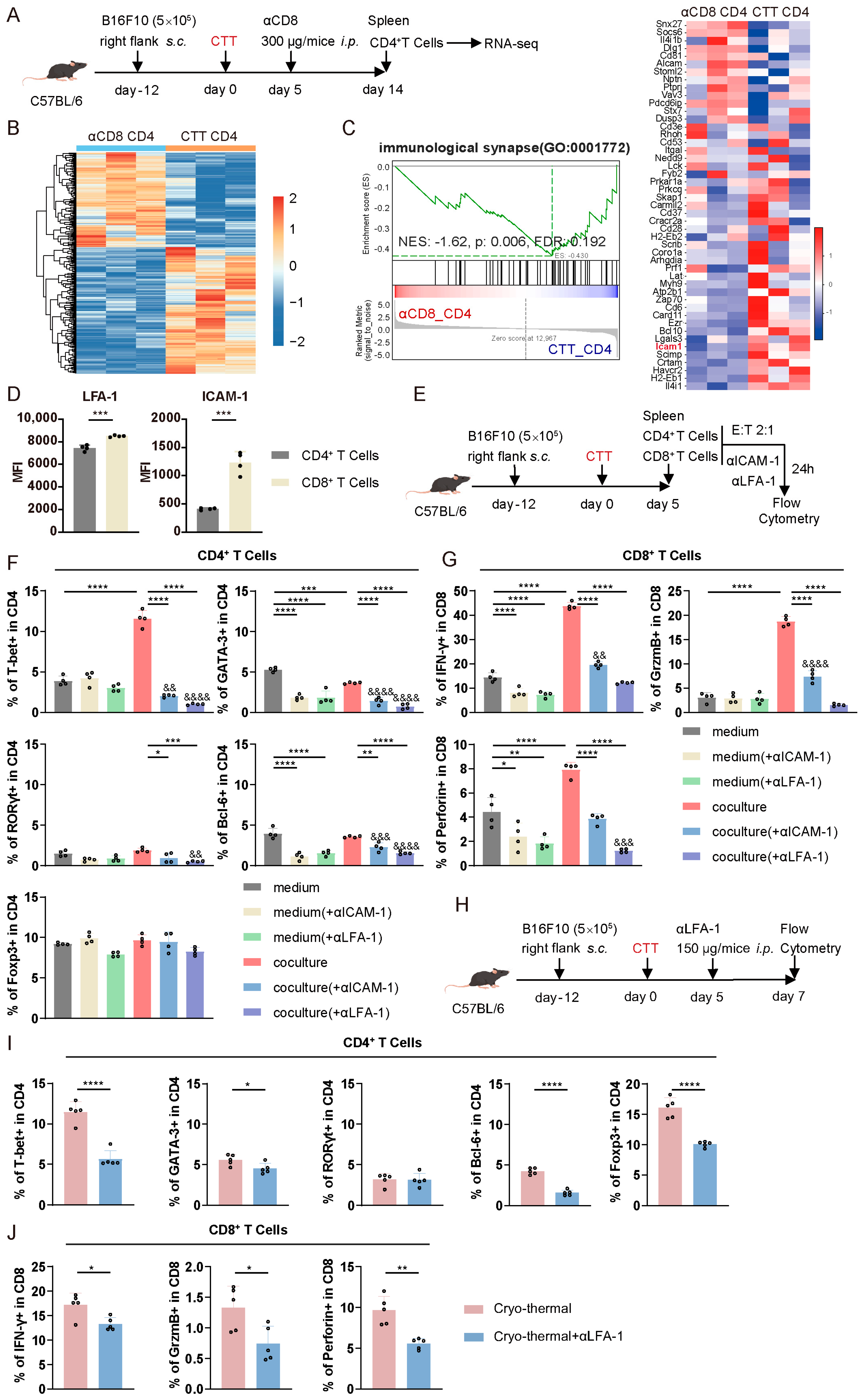
3.5. CTT-Activated CD4+ and CD8+ T Cell Crosstalk Depended on the LFA-1/ICAM-1 Interaction
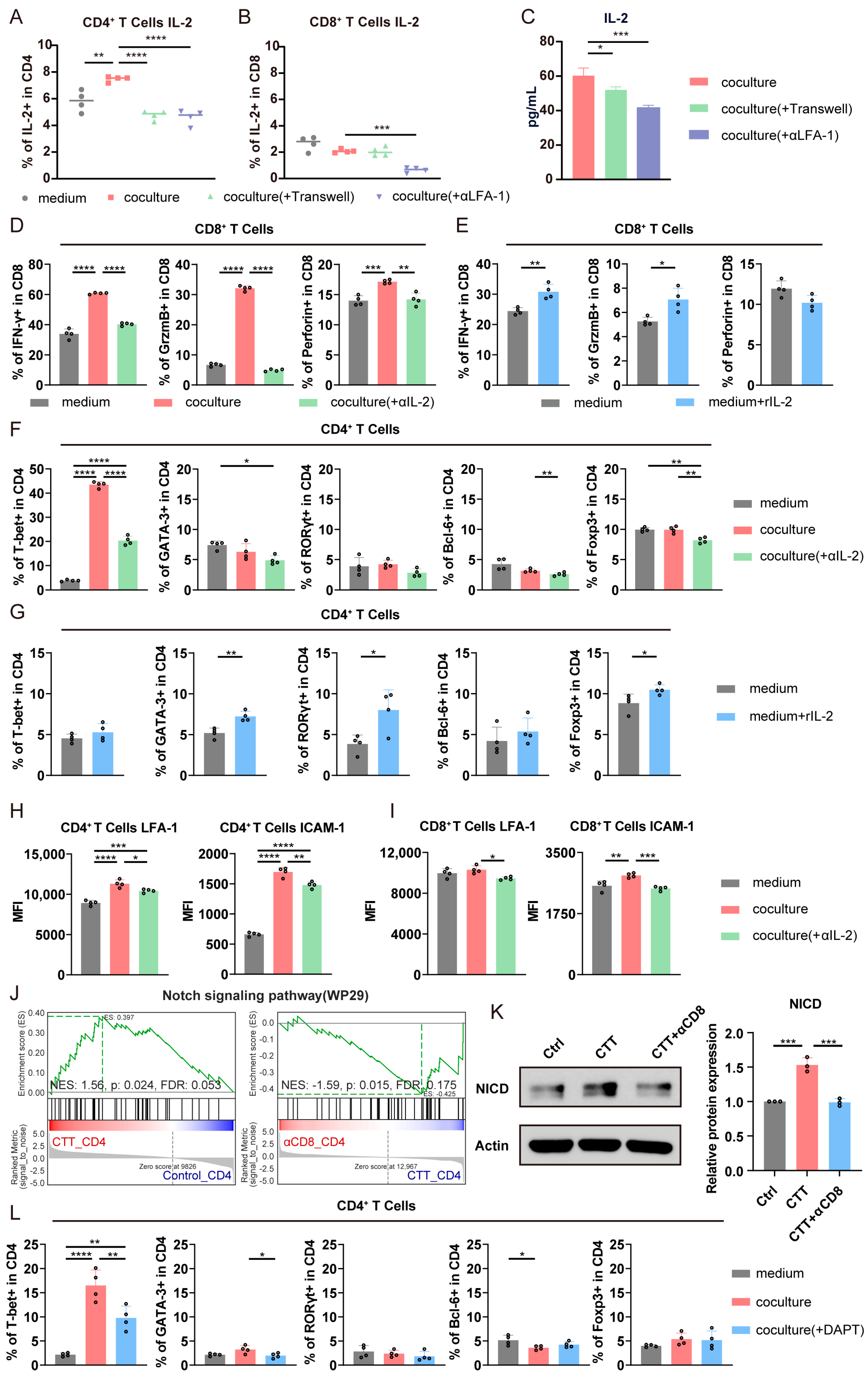
3.6. The LFA-1/ICAM-1 Interaction Between CD4+ and CD8+ T Cells Promoted CD4+ Th1-Dominant Differentiation via the Notch1 Signaling Pathway and the Cytotoxic Function of CD8+ T Cells via the IL-2–STAT5 Signaling Pathway
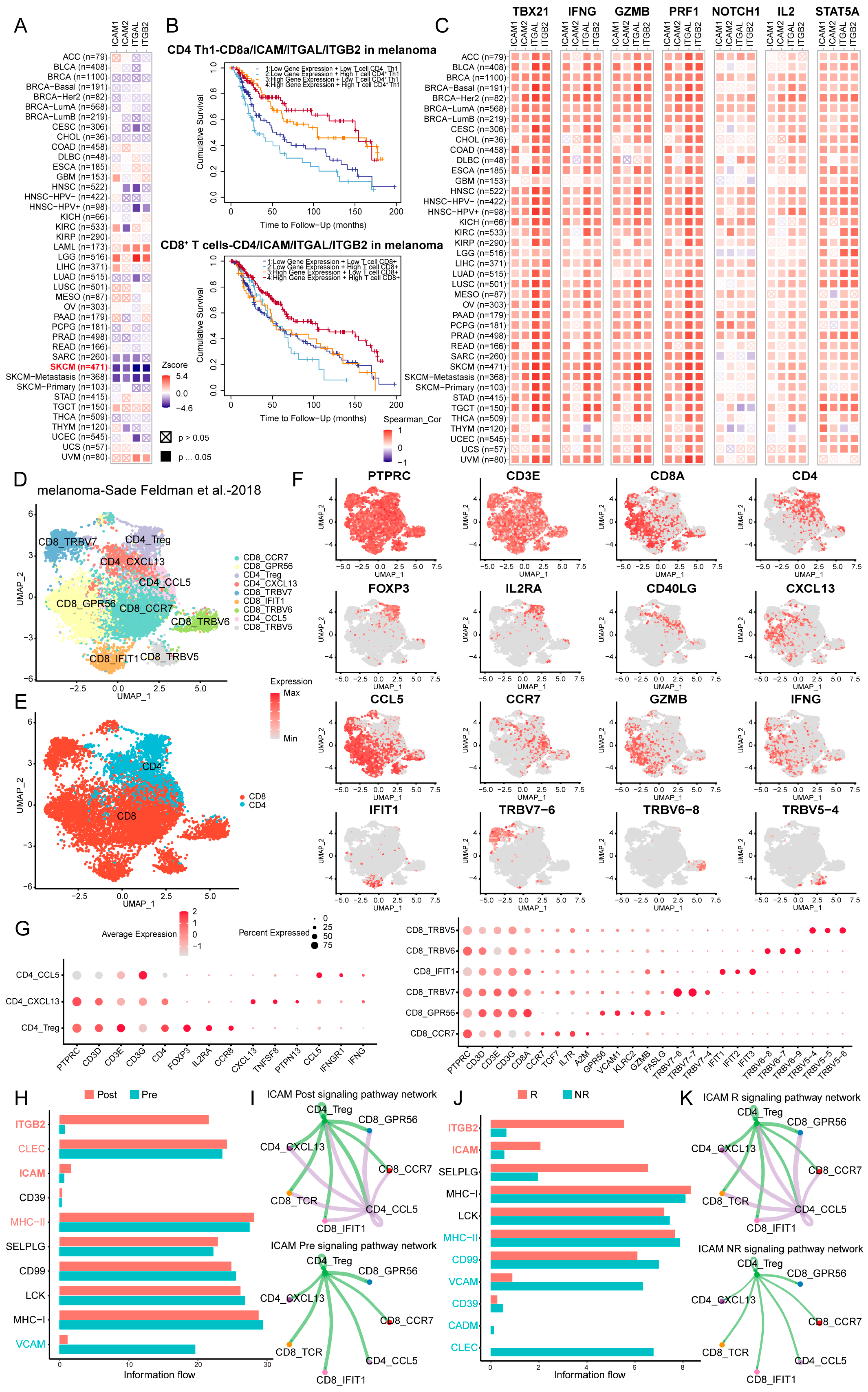
3.7. LFA-1/ICAM-Dependent Interactions Between CD4+ and CD8+ T Cells Were Correlated with Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTT | cryo-thermal therapy |
| Th1 | T helper type 1 |
| Th2 | T helper type 2 |
| Th17 | T helper type 17 |
| Tfh | T follicular helper |
| Treg | regulatory T cell |
| GrzmB | granzyme B |
| CTL | Cytotoxic T lymphocytes |
| APC | antigen-presenting cell |
| MDSC | myeloid-derived suppressor cell |
| DC | dendritic cell |
| MHC II | major histocompatibility complex class II |
| DAMP | damage-associated molecular pattern |
| IFN | interferon |
| IL | interleukin |
| LFA-1 | leukocyte function-associated antigen |
| ICAM-1 | intercellular adhesion molecule 1 |
| NICD | Notch1 intracellular domain |
| ICB | immune checkpoint blockade |
| DMEM | Dulbecco’s modified eagle’s medium |
| FBS | fetal bovine serum |
| ELISA | enzyme-linked immunosorbent assay |
| MACS | magnetic-activated cell sorting |
| GSEA | gene set enrichment analysis |
| PVDF | polyvinylidene fluoride |
| i.p | intraperitoneal injection |
| s.c | subcutaneous injection |
| SD | standard deviation |
References
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, D.; Qian, H.; Shi, Y.; Tao, Z. CD8(+) T cell-based cancer immunotherapy. J. Transl. Med. 2024, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Münz, C. CD4(+) T cells in cancer. Nat. Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, D.S.; Erbe, A.K.; Sondel, P.M.; Rakhmilevich, A.L. Roles of CD4+ T cells as mediators of antitumor immunity. Front. Immunol. 2022, 13, 972021. [Google Scholar] [CrossRef]
- Ferris, S.T.; Durai, V.; Wu, R.; Theisen, D.J.; Ward, J.P.; Bern, M.D.; Davidson, J.T., IV; Bagadia, P.; Liu, T.; Briseño, C.G.; et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature 2020, 584, 624–629. [Google Scholar] [CrossRef]
- Espinosa-Carrasco, G.; Chiu, E.; Scrivo, A.; Zumbo, P.; Dave, A.; Betel, D.; Kang, S.W.; Jang, H.-J.; Hellmann, M.D.; Burt, B.M.; et al. Intratumoral immune triads are required for immunotherapy-mediated elimination of solid tumors. Cancer Cell 2024, 42, 1202–1216.e8. [Google Scholar] [CrossRef]
- Magen, A.; Hamon, P.; Fiaschi, N.; Soong, B.Y.; Park, M.D.; Mattiuz, R.; Humblin, E.; Troncoso, L.; D’souza, D.; Dawson, T.; et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 2023, 29, 1389–1399. [Google Scholar] [CrossRef]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. CR 2022, 41, 127. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, P.; Xu, L.X. A novel thermal treatment modality for controlling breast tumor growth and progression. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, San Diego, CA, USA, 28 August–1 September 2012; pp. 5703–5706. [Google Scholar]
- Zhu, J.; Zhang, Y.; Zhang, A.; He, K.; Liu, P.; Xu, L.X. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci. Rep. 2016, 6, 27136. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Liu, P.; Xu, L.X. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int. J. Hyperthermia Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2020, 37, 843–853. [Google Scholar] [CrossRef]
- He, K.; Jia, S.; Lou, Y.; Liu, P.; Xu, L.X. Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis. 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Lou, Y.; Wang, J.; Wang, S.; Liu, P.; Xu, L.X. Th1-Dominant CD4(+) T Cells Orchestrate Endogenous Systematic Antitumor Immune Memory After Cryo-Thermal Therapy. Front. Immunol. 2022, 13, 944115. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Yizhak, K.; Bjorgaard, S.L.; Ray, J.P.; de Boer, C.G.; Jenkins, R.W.; Lieb, D.J.; Chen, J.H.; Frederick, D.T.; Barzily-Rokni, M.; et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 2018, 175, 998–1013.e20. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ogawa, S.; Wakamatsu, E.; Abe, R. Cell cycle arrest caused by MEK/ERK signaling is a mechanism for suppressing growth of antigen-hyperstimulated effector T cells. Int. Immunol. 2016, 28, 547–557. [Google Scholar] [CrossRef]
- Zumwalde, N.A.; Domae, E.; Mesher, M.F.; Shimizu, Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J. Immunol. 2013, 191, 3681–3693. [Google Scholar] [CrossRef]
- Zenke, S.; Palm, M.M.; Braun, J.; Gavrilov, A.; Meiser, P.; Böttcher, J.P.; Beyersdorf, N.; Ehl, S.; Gerard, A.; Lämmermann, T.; et al. Quorum Regulation via Nested Antagonistic Feedback Circuits Mediated by the Receptors CD28 and CTLA-4 Confers Robustness to T Cell Population Dynamics. Immunity. 2020, 52, 313–327.e317. [Google Scholar] [CrossRef]
- Shan, J.; Jing, W.; Ping, Y.; Shen, C.; Han, D.; Liu, F.; Liu, Y.; Li, C.; Zhang, Y. LFA-1 regulated by IL-2/STAT5 pathway boosts antitumor function of intratumoral CD8(+) T cells for improving anti-PD-1 antibody therapy. Oncoimmunology 2024, 13, 2293511. [Google Scholar] [CrossRef]
- Gazdik, T.R.; Crow, J.J.; Lawton, T.; Munroe, C.J.; Theriault, H.; Wood, T.M.; Albig, A.R. Notch intracellular domains form transcriptionally active heterodimeric complexes on sequence-paired sites. Sci. Rep. 2024, 14, 218. [Google Scholar] [CrossRef]
- Minter, L.M.; Turley, D.M.; Das, P.; Shin, H.M.; Joshi, I.; Lawlor, R.G.; Cho, O.H.; Palaga, T.; Gottipati, S.; Telfer, J.C.; et al. Inhibitors of γ-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 2005, 6, 680–688. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Ligocki, A.J.; Brown, J.R.; Niederkorn, J.Y. Role of interferon-γ and cytotoxic T lymphocytes in intraocular tumor rejection. J. Leukoc. Biol. 2016, 99, 735–747. [Google Scholar] [CrossRef]
- Zimmerman, M.; Yang, D.; Hu, X.; Liu, F.; Singh, N.; Browning, D.; Ganapathy, V.; Chandler, P.; Choubey, D.; Abrams, S.I.; et al. IFN-γ upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PLoS ONE 2010, 5, e14076. [Google Scholar] [CrossRef] [PubMed]
- Pipkin, M.E.; Sacks, J.A.; Cruz-Guilloty, F.; Lichtenheld, M.G.; Bevan, M.J.; Rao, A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 2010, 32, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Shouse, A.N.; LaPorte, K.M.; Malek, T.R. Interleukin-2 signaling in the regulation of T cell biology in autoimmunity and cancer. Immunity 2024, 57, 414–428. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Liu, Y.; Wang, X.; Yu, S.; Huang, H.; Shen, X.; Zhang, Q.; Hong, N.; Jin, W. Exploring the dynamics and influencing factors of CD4 T cell activation using single-cell RNA-seq. Science 2023, 26, 107588. [Google Scholar] [CrossRef]
- Mutascio, S.; Mota, T.; Franchitti, L.; Sharma, A.A.; Willemse, A.; Bergstresser, S.N.; Wang, H.; Statzu, M.; Tharp, G.K.; Weiler, J.; et al. CD8(+) T cells promote HIV latency by remodeling CD4(+) T cell metabolism to enhance their survival, quiescence, and stemness. Immunity 2023, 56, 1132–1147.e6. [Google Scholar] [CrossRef]
- Verma, N.K.; Fazil, M.H.; Ong, S.T.; Chalasani, M.L.; Low, J.H.; Kottaiswamy, A.; Kizhakeyil, A.; Kumar, S.; Panda, A.K.; Freeley, M.; et al. LFA-1/ICAM-1 Ligation in Human T Cells Promotes Th1 Polarization through a GSK3β Signaling-Dependent Notch Pathway. J. Immunol. 2016, 197, 108–118. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Shi, H.; Shao, B. LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation. Cells 2023, 12, 1136. [Google Scholar] [CrossRef]
- Gérard, A.; Cope, A.P.; Kemper, C.; Alon, R.; Köchl, R. LFA-1 in T cell priming, differentiation, and effector functions. Trends Immunol. 2021, 42, 706–722. [Google Scholar] [CrossRef]
- Sabatos, C.A.; Doh, J.; Chakravarti, S.; Friedman, R.S.; Pandurangi, P.G.; Tooley, A.J.; Krummel, M.F. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity 2008, 29, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.; Khan, O.; Beemiller, P.; Oswald, E.; Hu, J.; Matloubian, M.; Krummel, M.F. Secondary T cell-T cell synaptic interactions drive the differentiation of protective CD8+ T cells. Nat. Immunol. 2013, 14, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chirathaworn, C.; Kohlmeier, J.E.; Tibbetts, S.A.; Rumsey, L.M.; Chan, M.A.; Benedict, S.H. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J. Immunol. 2002, 168, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Edlund, K.; Madjar, K.; Mattsson, J.S.M.; Djureinovic, D.; Lindskog, C.; Brunnström, H.; Koyi, H.; Brandén, E.; Jirström, K.; Pontén, F.; et al. Prognostic Impact of Tumor Cell Programmed Death Ligand 1 Expression and Immune Cell Infiltration in NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 628–640. [Google Scholar] [CrossRef]
- Chen, C.; Zou, P.; Wu, X. Development and Validation of an Immune Prognostic Index Related to Infiltration of CD4+ and CD8+ T Cells in Colorectal Cancer. Mol. Biotechnol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Palomero, J.; Panisello, C.; Lozano-Rabella, M.; Tirtakasuma, R.; Díaz-Gómez, J.; Grases, D.; Pasamar, H.; Arregui, L.; Duch, E.D.; Fernández, E.G.; et al. Biomarkers of tumor-reactive CD4(+) and CD8(+) TILs associate with improved prognosis in endometrial cancer. J. Immunother. Cancer 2022, 10, e005443. [Google Scholar] [CrossRef]
- Peng, P.; Lou, Y.; Wang, S.; Wang, J.; Zhang, Z.; Du, P.; Zheng, J.; Liu, P.; Xu, L.X. Activated NK cells reprogram MDSCs via NKG2D-NKG2DL and IFN-γ to modulate antitumor T-cell response after cryo-thermal therapy. J. Immunother. Cancer 2022, 10, e005769. [Google Scholar] [CrossRef]
- Hickman, A.; Koetsier, J.; Kurtanich, T.; Nielsen, M.C.; Winn, G.; Wang, Y.; Bentebibel, S.-E.; Shi, L.; Punt, S.; Williams, L.; et al. LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. J. Clin. Investig. 2022, 132, e154152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zhang, Z.; Wang, S.; Wang, J.; Hao, Y.; Wang, K.; Liu, P. LFA-1/ICAM-1 Interactions Between CD8+ and CD4+ T Cells Promote CD4+ Th1-Dominant Differentiation and CD8+ T Cell Cytotoxicity for Strong Antitumor Immunity After Cryo-Thermal Therapy. Cells 2025, 14, 620. https://doi.org/10.3390/cells14080620
Yao Y, Zhang Z, Wang S, Wang J, Hao Y, Wang K, Liu P. LFA-1/ICAM-1 Interactions Between CD8+ and CD4+ T Cells Promote CD4+ Th1-Dominant Differentiation and CD8+ T Cell Cytotoxicity for Strong Antitumor Immunity After Cryo-Thermal Therapy. Cells. 2025; 14(8):620. https://doi.org/10.3390/cells14080620
Chicago/Turabian StyleYao, Yichen, Zelu Zhang, Shicheng Wang, Junjun Wang, Yuankai Hao, Ke Wang, and Ping Liu. 2025. "LFA-1/ICAM-1 Interactions Between CD8+ and CD4+ T Cells Promote CD4+ Th1-Dominant Differentiation and CD8+ T Cell Cytotoxicity for Strong Antitumor Immunity After Cryo-Thermal Therapy" Cells 14, no. 8: 620. https://doi.org/10.3390/cells14080620
APA StyleYao, Y., Zhang, Z., Wang, S., Wang, J., Hao, Y., Wang, K., & Liu, P. (2025). LFA-1/ICAM-1 Interactions Between CD8+ and CD4+ T Cells Promote CD4+ Th1-Dominant Differentiation and CD8+ T Cell Cytotoxicity for Strong Antitumor Immunity After Cryo-Thermal Therapy. Cells, 14(8), 620. https://doi.org/10.3390/cells14080620





