Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.1.1. Preparation of Hydrophilic Surfaces
2.1.2. Preparation of Hydrophobic Slides
2.1.3. Synthesis of Polyacrylamide Hydrogels (PAAs)
2.1.4. Coating of Functionalized Hydrogels with Proteins
2.2. Isolation and Culture of LSECs
2.3. Atomic Force Spectroscopy and Microscopy
2.3.1. Imaging of Hydrogel and LSEC Topography
2.3.2. Deformability of LSEC Fenestrations
2.3.3. Force Spectroscopy
2.4. Fluorescence Microscopy
2.4.1. Protein Staining of the Substrates Studied
2.4.2. Staining of LSECs
2.4.3. Fluorescence Microscopy and Structured Illumination Microscopy
2.5. Scanning Electron Microscopy (SEM)
2.6. Rheology of Hydrogels
2.7. Endocytosis Assay
2.8. Statistical Analysis
3. Results
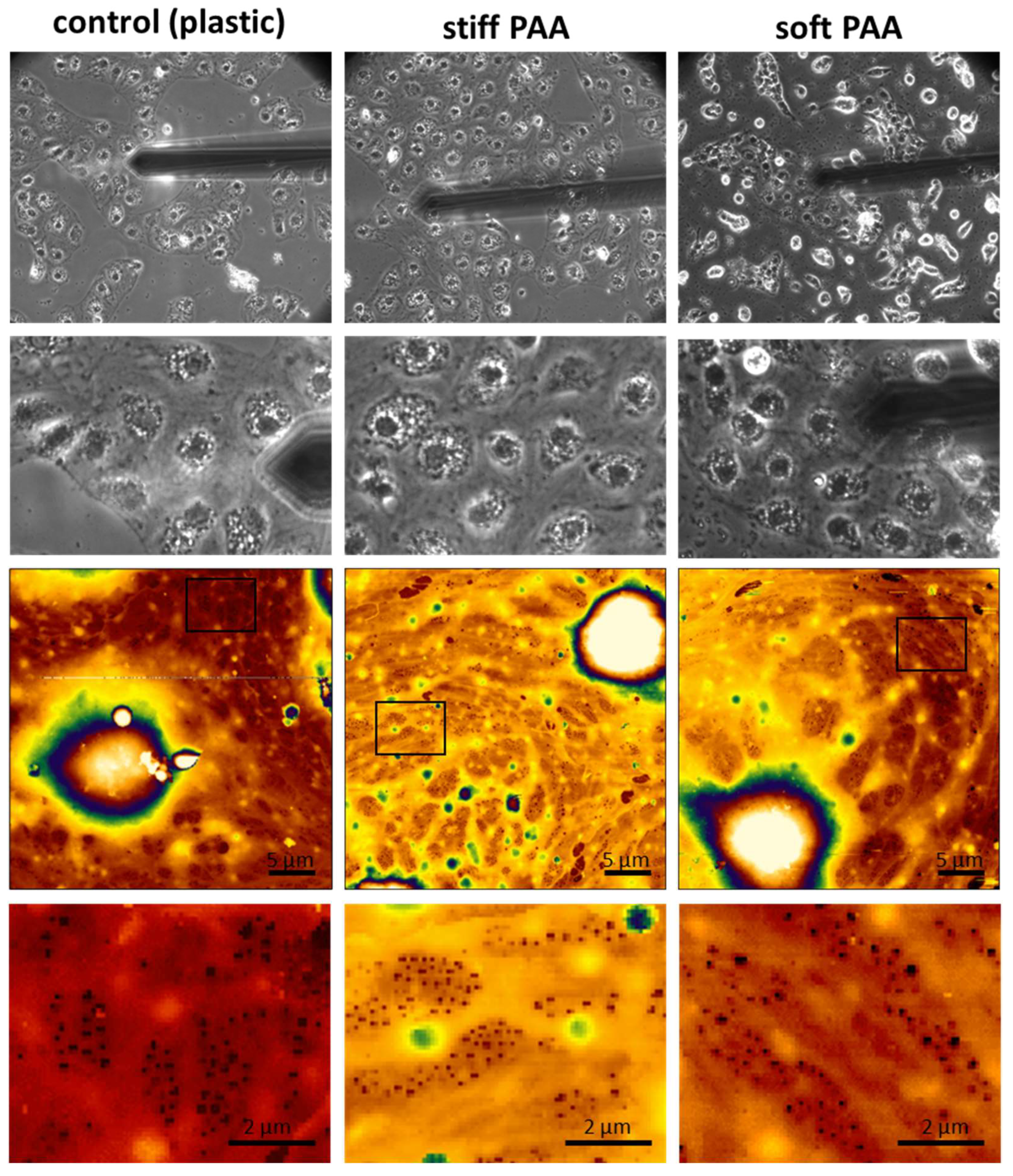
3.1. PAA Hydrogels Functionalized with Collagen III
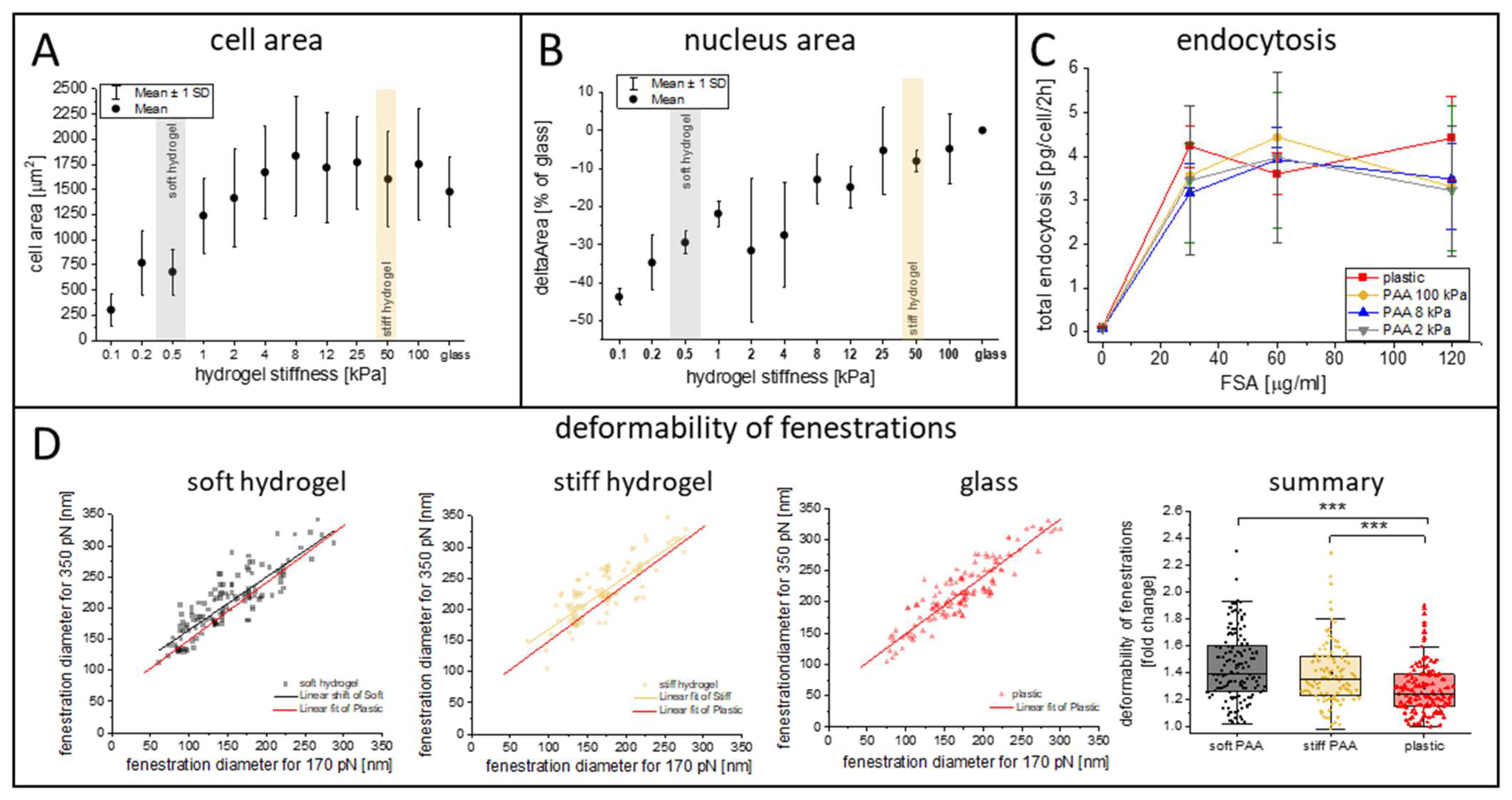
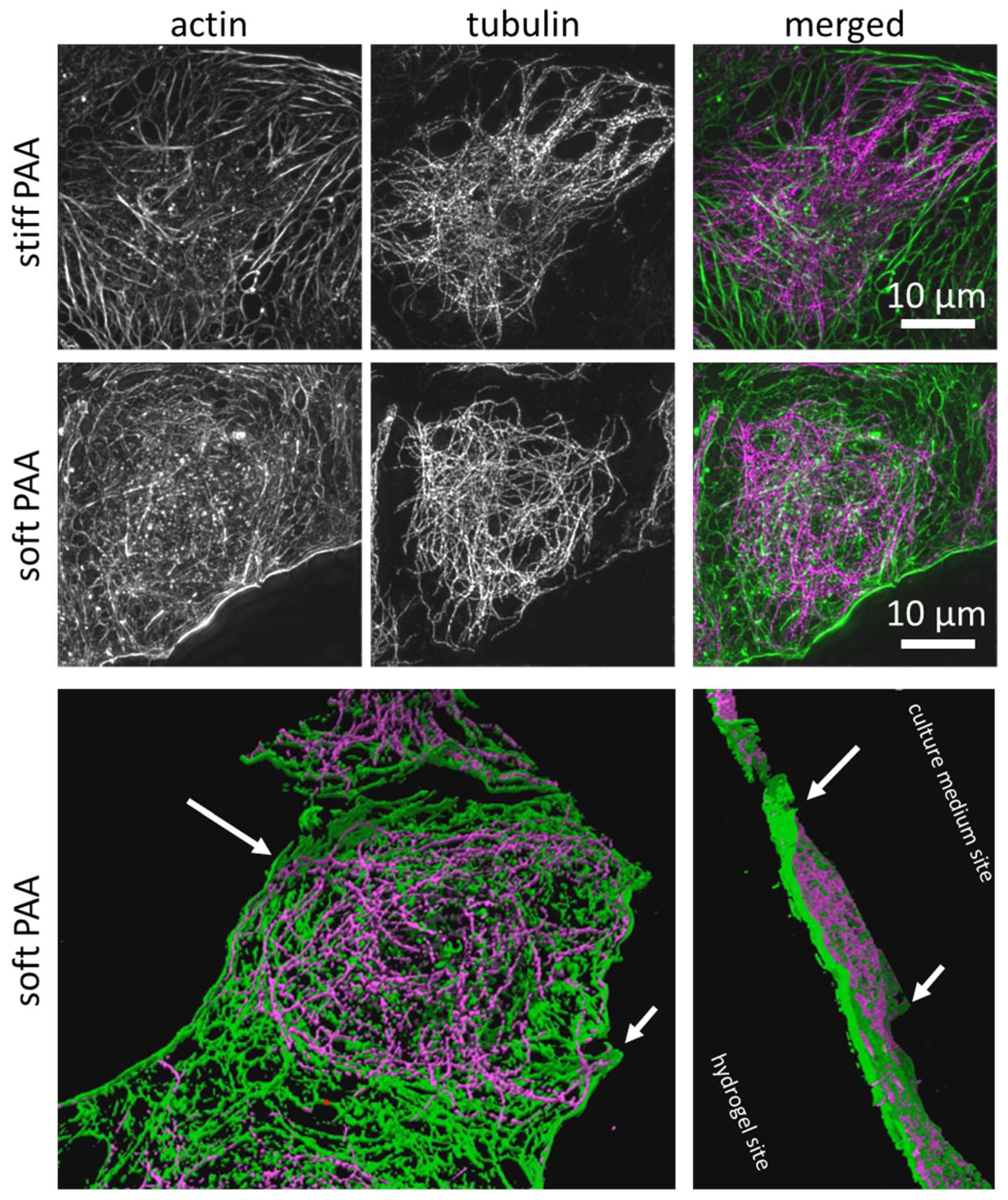
3.2. Characterization of LSECs on PAA Hydrogels
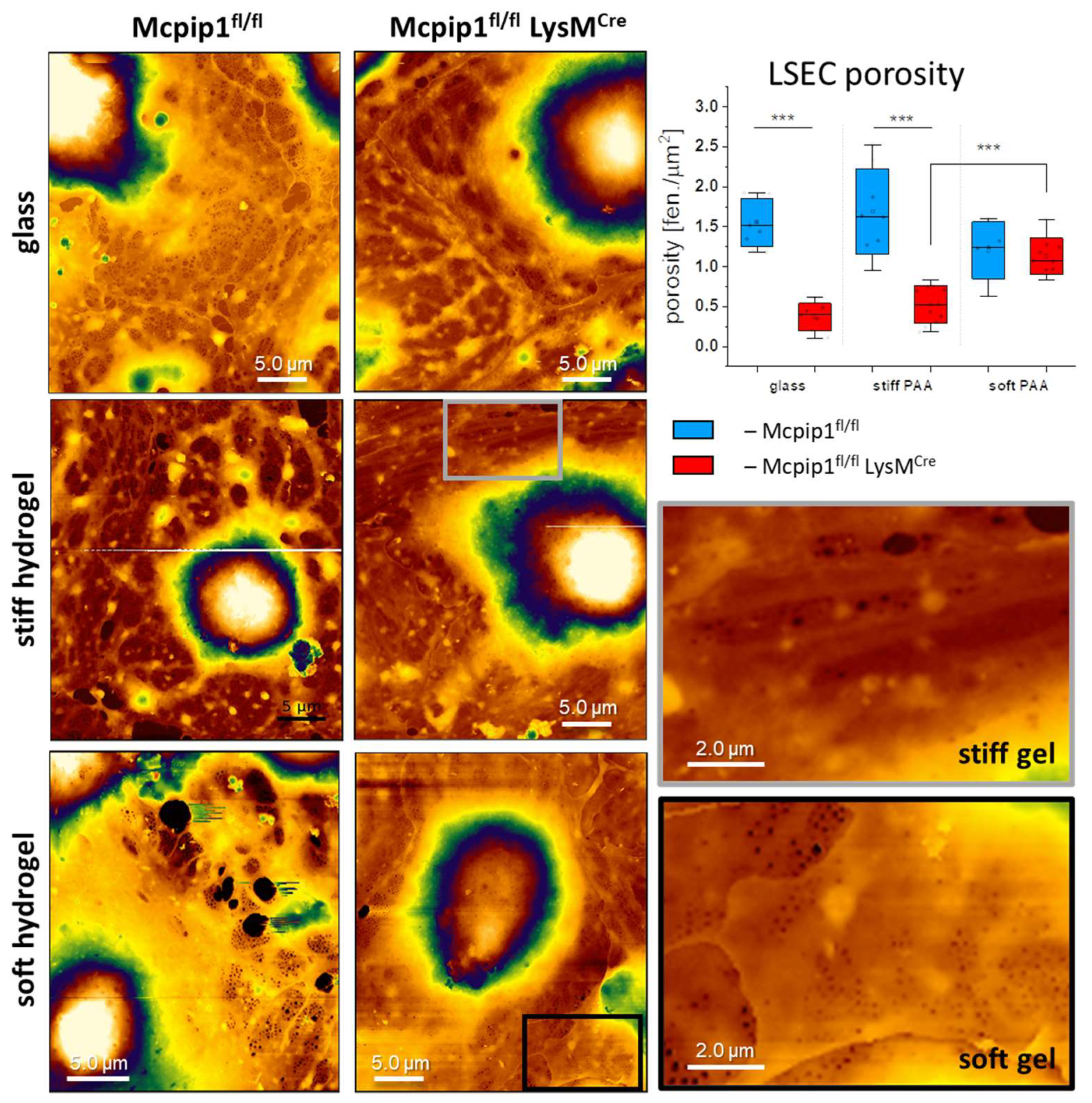
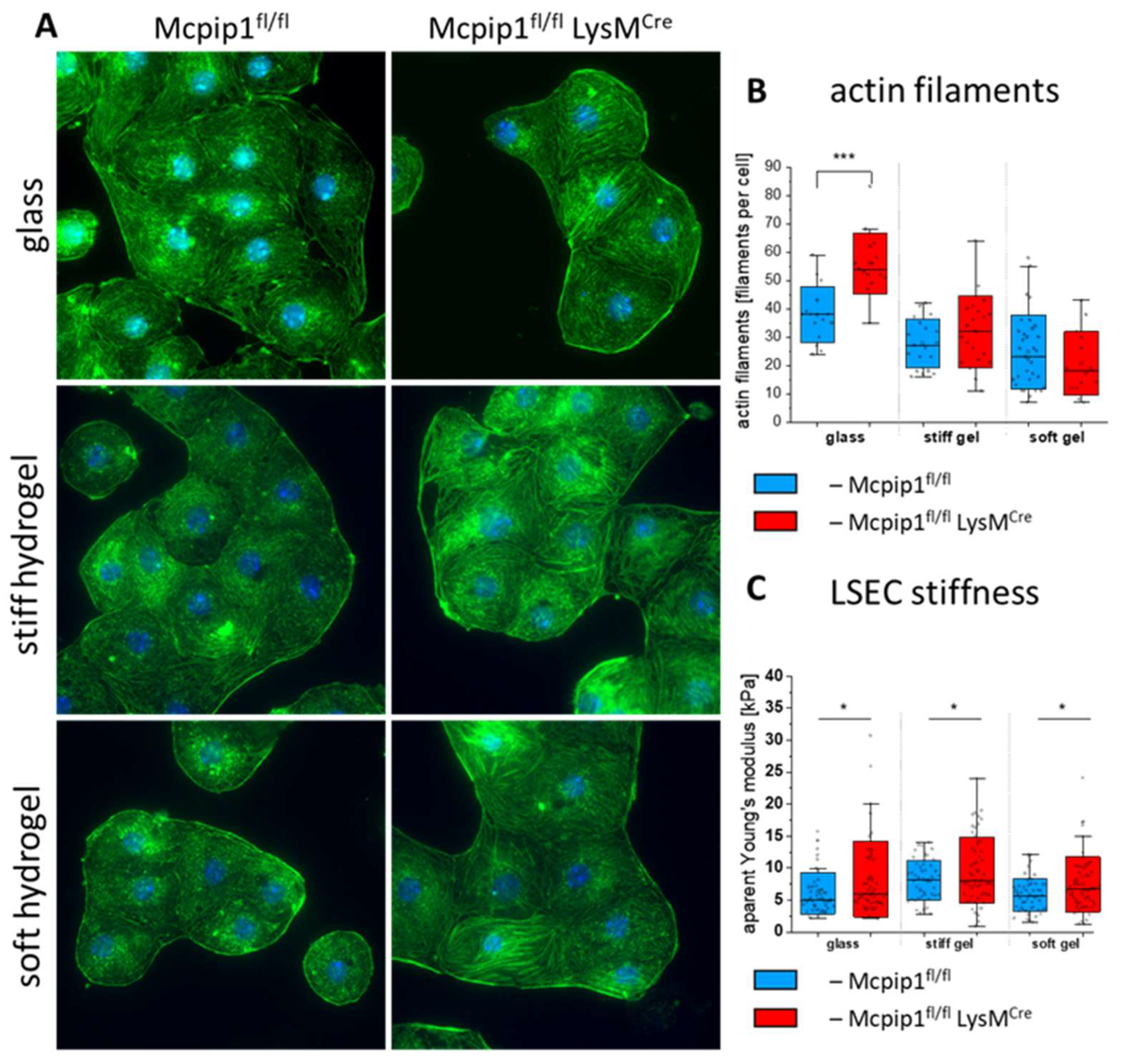
3.3. Characterization of LSECs from a Genetic Model of Systemic Inflammation Cultured on PAA Hydrogels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Zhang, X.; Zhou, J.; Li, W.; Shu, X.; Wu, Y.; Long, M. Multiscale Biomechanics and Mechanotransduction from Liver Fibrosis to Cancer. Adv. Drug Deliv. Rev. 2022, 188, 114448. [Google Scholar] [CrossRef] [PubMed]
- Kang, N. Mechanotransduction in Liver Diseases. Semin. Liver Dis. 2020, 40, 84–90. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Warren, A.; Cogger, V.C.; Smedsrød, B.; Sørensen, K.K.; De Cabo, R.; Fraser, R.; McCuskey, R.S. Old Age and the Hepatic Sinusoid. Anat. Rec. 2008, 291, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.; Cogger, V.C.; Dobbs, B.; Jamieson, H.; Warren, A.; Hilmer, S.N.; Le Couteur, D.G. The Liver Sieve and Atherosclerosis. Pathology 2012, 44, 181–186. [Google Scholar] [CrossRef]
- Wisse, E.; de Zanger, R.B.; Charels, K.; Van Der Smissen, P.; McCuskey, R.S. The Liver Sieve: Considerations Concerning the Structure and Function of Endothelial Fenestrae, the Sinusoidal Wall and the Space of Disse. Hepatology 1985, 5, 683–692. [Google Scholar] [CrossRef]
- Szafranska, K.; Holte, C.F.; Kruse, L.D.; McCourt, P.; Zapotoczny, B. The WHole Story about Fenestrations in Liver Sinusoidal Endothelial Cells. Front. Physiol. 2021, 12, 735573. [Google Scholar] [CrossRef] [PubMed]
- Horst, A.K.; Neumann, K.; Diehl, L.; Tiegs, G. Modulation of Liver Tolerance by Conventional and Nonconventional Antigen-Presenting Cells and Regulatory Immune Cells. Cell. Mol. Immunol. 2016, 13, 277–292. [Google Scholar] [CrossRef]
- Inverso, D.; Iannacone, M. Spatiotemporal Dynamics of Effector CD8+ T Cell Responses within the Liver. J. Leukoc. Biol. 2016, 99, 51–55. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Inverso, D.; Sironi, L.; Di Lucia, P.; Fioravanti, J.; Ganzer, L.; Fiocchi, A.; Vacca, M.; Aiolfi, R.; Sammicheli, S.; et al. Immunosurveillance of the Liver by Intravascular Effector CD8+ T Cells. Cell 2015, 161, 486–500. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Fraser, R.; Hilmer, S.; Rivory, L.P.; McLean, A.J. The Hepatic Sinusoid in Aging and Cirrhosis: Effects on Hepatic Substrate Disposition and Drug Clearance. Clin. Pharmacokinet. 2005, 44, 187–200. [Google Scholar] [CrossRef]
- Bhandari, S.; Larsen, A.K.; McCourt, P.; Smedsrød, B.; Sørensen, K.K. The Scavenger Function of Liver Sinusoidal Endothelial Cells in Health and Disease. Front. Physiol. 2021, 12, 757469. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.K.; McCourt, P.; Berg, T.; Crossley, C.; Le Couteur, D.; Wake, K.; Smedsrød, B. The Scavenger Endothelial Cell: A New Player in Homeostasis and Immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1217–R1230. [Google Scholar] [CrossRef]
- Simon-Santamaria, J.; Malovic, I.; Warren, A.; Oteiza, A.; Le Couteur, D.; Smedsrod, B.; McCourt, P.; Sorensen, K.K. Age-Related Changes in Scavenger Receptor-Mediated Endocytosis in Rat Liver Sinusoidal Endothelial Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Cogger, V.C.; Hilmer, S.N.; Sullivan, D.; Muller, M.; Fraser, R.; Le Couteur, D.G. Hyperlipidemia and Surfactants: The Liver Sieve Is a Link. Atherosclerosis 2006, 189, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wojnar-Lason, K.; Tyrankiewicz, U.; Kij, A.; Kurpinska, A.; Kaczara, P.; Kwiatkowski, G.; Wilkosz, N.; Giergiel, M.; Stojak, M.; Grosicki, M.; et al. Chronic Heart Failure Induces Early Defenestration of Liver Sinusoidal Endothelial Cells (LSECs) in Mice. Acta Physiol. 2024, 240, e14141. [Google Scholar] [CrossRef]
- Hunt, N.J.; Lockwood, G.P.; Warren, A.; Mao, H.; Mccourt, P.A.G.G.; Le Couteur, D.G.; Cogger, V.C.; Hunt, X.N.J.; Lockwood, G.P.; Warren, A.; et al. Manipulating Fenestrations in Young and Old Liver Sinusoidal Endothelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G144–G154. [Google Scholar] [CrossRef]
- Mueller, S.; Sandrin, L. Liver Stiffness: A Novel Parameter for the Diagnosis of Liver Disease. Hepatic Med. Evid. Res. 2010, 2, 49–67. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Kotlinowski, J.; Blacharczyk, O.; Giergiel, M.; Szymanowski, K.; Metwally, S.; Wojnar-Lason, K.; Dobosz, E.; Koziel, J.; Lekka, M.; et al. Early and Late Phases of Liver Sinusoidal Endothelial Cell (LSEC) Defenestration in Mouse Model of Systemic Inflammation. Cell. Mol. Biol. Lett. 2024, 29, 139. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Braet, F.; Wisse, E.; Lekka, M.; Szymonski, M. Biophysical Nanocharacterization of Liver Sinusoidal Endothelial Cells through Atomic Force Microscopy. Biophys. Rev. 2020, 12, 625–636. [Google Scholar] [CrossRef]
- Guixé-Muntet, S.; Ortega-Ribera, M.; Wang, C.; Selicean, S.; Andreu, I.; Kechagia, J.Z.; Fondevila, C.; Roca-Cusachs, P.; Dufour, J.F.; Bosch, J.; et al. Nuclear Deformation Mediates Liver Cell Mechanosensing in Cirrhosis. JHEP Rep. 2020, 2, 100145. [Google Scholar] [CrossRef]
- Shu, X.; Li, N.; Wu, Y.; Li, W.; Zhang, X.; Li, P.; Lü, D.; Lü, S.; Long, M. Mechanotransduction of Liver Sinusoidal Endothelial Cells under Varied Mechanical Stimuli. Acta Mech. Sin. 2021, 37, 201–217. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zhou, J.; Zhang, Z.; Wu, H.; Shu, X.; Li, W.; Wu, Y.; Du, Y.; Lü, D.; et al. FAK-P38 Signaling Serves as a Potential Target for Reverting Matrix Stiffness-Modulated Liver Sinusoidal Endothelial Cell Defenestration. Biomaterials 2024, 305, 122462. [Google Scholar] [CrossRef]
- Natarajan, V.; Moeller, M.; Casey, C.A.; Harris, E.N. Matrix Stiffness Regulates Liver Sinusoidal Endothelial Cell Function Mimicking Responses in Fatty Liver Disease. bioRxiv 2020. [Google Scholar] [CrossRef]
- Selicean, S.-E.; Felli, E.; Wang, C.; Nulan, Y.; Lozano, J.J.; Guixé-Muntet, S.; Ştefănescu, H.; Bosch, J.; Berzigotti, A.; Gracia-Sancho, J. Stiffness-Induced Modulation of ERG Transcription Factor in Chronic Liver Disease. npj Gut Liver 2024, 1, 7. [Google Scholar] [CrossRef]
- Pydyn, N.; Żurawek, D.; Kozieł, J.; Kus, E.; Wojnar-Lason, K.; Jasztal, A.; Fu, M.; Jura, J.; Kotlinowski, J. Role of Mcpip1 in Obesity-Induced Hepatic Steatosis as Determined by Myeloid and Liver-Specific Conditional Knockouts. FEBS J. 2021, 288, 6563–6580. [Google Scholar] [CrossRef]
- Syed, S.; Karadaghy, A.; Zustiak, S. Simple Polyacrylamide-Based Multiwell Stiffness Assay for the Study of Stiffness-Dependent Cell Responses. J. Vis. Exp. 2015, 2015, e52643. [Google Scholar] [CrossRef]
- Wouters, O.Y.; Ploeger, D.T.A.; van Putten, S.M.; Bank, R.A. 3,4-Dihydroxy-L-Phenylalanine as a Novel Covalent Linker of Extracellular Matrix Proteins to Polyacrylamide Hydrogels with a Tunable Stiffness. Tissue Eng. Part C Methods 2016, 22, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kus, E.; Kaczara, P.; Czyzynska-Cichon, I.; Szafrańska, K.; Zapotoczny, B.; Kij, A.; Sowińska, A.; Kotlinowski, J.; Mateuszuk, Ł.; Czarnowska, E.; et al. LSEC Fenestrae Are Preserved despite Pro-Inflammatory Phenotype of Liver Sinusoidal Endothelial Cells in Mice on High Fat Diet. Front. Physiol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Smedsrød, B.; Pertoft, H. Preparation of Pure Hepatocytes and Reticuloendothelial Cells in High Yield from a Single Rat Liver by Means of Percoll Centrifugation and Selective Adherence. J. Leukoc. Biol. 1985, 38, 213–230. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Braet, F.; Kus, E.; Ginda-Mäkelä, K.; Klejevskaja, B.; Campagna, R.; Chlopicki, S.; Szymonski, M. Actin-Spectrin Scaffold Supports Open Fenestrae in Liver Sinusoidal Endothelial Cells. Traffic 2019, 20, 932–942. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Owczarczyk, K.; Szafranska, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Morphology and Force Probing of Primary Murine Liver Sinusoidal Endothelial Cells. J. Mol. Recognit. 2017, 30, e2610. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczny, B.; Szafranska, K.; Owczarczyk, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Atomic Force Microscopy Reveals the Dynamic Morphology of Fenestrations in Live Liver Sinusoidal Endothelial Cells. Sci. Rep. 2017, 7, 7994. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Quantification of Fenestrations in Liver Sinusoidal Endothelial Cells by Atomic Force Microscopy. Micron 2017, 101, 48–53. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical Atomic Force Microscopy Procedure (SNAP) for Measuring Soft and Biological Samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.; Burel, J.-M.; Moore, J.; Blackburn, C.; Linkert, M.; Loynton, S.; MacDonald, D.; Moore, W.J.; Neves, C.; Patterson, A.; et al. OMERO: Flexible, Model-Driven Data Management for Experimental Biology. Nat. Methods 2012, 9, 245–253. [Google Scholar] [CrossRef]
- Zemła, J.; Iyer, P.S.; Pyka-Fościak, G.; Mermod, N.; Lekka, M. Rheological Properties of Skeletal Muscles in a Duchenne Muscular Dystrophy Murine Model before and after Autologous Cell Therapy. J. Biomech. 2021, 128, 110770. [Google Scholar] [CrossRef]
- Blomhoff, R.; Eskild, W.; Berg, T. Endocytosis of Formaldehyde-Treated Serum Albumin via Scavenger Pathway in Liver Endothelial Cells. Biochem. J. 1984, 218, 81–86. [Google Scholar] [CrossRef]
- Sneddon, I.N. The Relation between Load and Penetration in the Axisymmetric Boussinesq Problem for a Punch of Arbitrary Profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; Gonzalez-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. Materials Today Bio A Tough Act to Follow: Collagen Hydrogel Modi Fi Cations to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today 2021, 10, 100098. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of Cell Morphology and Differentiation by Substrates with Independently Tunable Elasticity and Viscous Dissipation. Nat. Commun. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.T.N.; Wen, Q.; Janmey, P.A.; Crocker, J.C.; Yodh, A.G. Rheology of Soft Materials. Annu. Rev. Condens. Matter Phys. 2010, 1, 301–322. [Google Scholar] [CrossRef]
- Szafranska, K.; Neuman, T.; Baster, Z.; Rajfur, Z.; Szelest, O.; Christopher, H.; Kubisiak, A.; Kus, E.; Wolfson, D.L.; Chlopicki, S.; et al. From Fixed-Dried to Wet-Fixed to Live-Comparative Super-Resolution Microscopy of Liver Sinusoidal Endothelial Cell Fenestrations. Nanophotonics 2022, 11, 2253–2270. [Google Scholar] [CrossRef] [PubMed]
- Mönkemöller, V.; Øie, C.; Hübner, W.; Huser, T.; McCourt, P. Multimodal Super-Resolution Optical Microscopy Visualizes the Close Connection between Membrane and the Cytoskeleton. Sci. Rep. 2015, 5, 16279. [Google Scholar] [CrossRef]
- Kruse, L.D.; Holte, C.; Struck, E.C.; Schürstedt, J.; Hübner, W.; Huser, T.; Szafranska, K. Hydrogen Peroxide Damage to Rat Liver Sinusoidal Endothelial Cells Is Prevented by n-Acetyl-Cysteine but Not GSH. Hepatol. Commun. 2025, 9, e0617. [Google Scholar] [CrossRef]
- Mao, H.; Kruse, L.D.; Li, R.; Oteiza, A.; Struck, E.C.; Schürstedt, J.; Hübner, W.; Cogger, V.C.; Couteur, D.L.; Wolfson, D.L.; et al. Impact of Oxidized Low-Density Lipoprotein on Rat Liver Sinusoidal Endothelial Cell Morphology and Function. NPJ Gut Liver 2024, 1, 9. [Google Scholar] [CrossRef]
- Cogger, V.C.; McNerney, G.P.; Nyunt, T.; DeLeve, L.D.; McCourt, P.; Smedsr??d, B.; Le Couteur, D.G.; Huser, T.R. Three-Dimensional Structured Illumination Microscopy of Liver Sinusoidal Endothelial Cell Fenestrations. J. Struct. Biol. 2010, 171, 382–388. [Google Scholar] [CrossRef]
- Braet, F.; Riches, J.; Geerts, W.; Jahn, K.A.; Wisse, E.; Frederik, P. Three-Dimensional Organization of Fenestrae Labyrinths in Liver Sinusoidal Endothelial Cells. Liver Int. 2009, 29, 603–613. [Google Scholar] [CrossRef]
- Hauke, L.; Primeßnig, A.; Eltzner, B.; Radwitz, J.; Huckemann, S.F.; Rehfeldt, F. FilamentSensor 2.0: An Open-Source Modular Toolbox for 2D/3D Cytoskeletal Filament Tracking. PLoS ONE 2023, 18, e0279336. [Google Scholar] [CrossRef]
- Hegmar, H.; Wester, A.; Aleman, S.; Backman, J.; Degerman, E.; Ekvall, H.; Lund, K.; Lundgren, Å.; Nasr, P.; Shahnavaz, A.; et al. Liver Stiffness Predicts Progression to Liver-Related Events in Patients with Chronic Liver Disease–A Cohort Study of 14 414 Patients. Liver Int. 2024, 44, 1689–1699. [Google Scholar] [CrossRef]
- Krivega, E.S.; Kotova, S.L.; Timashev, P.S.; Efremov, Y.M. Mechanical Characterization of Soft Biomaterials: Which Time and Spatial Scale to Choose? bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Buffinton, C.M.; Tong, K.J.; Blaho, R.A.; Buffinton, E.M.; Ebenstein, D.M. Comparison of Mechanical Testing Methods for Biomaterials: Pipette Aspiration, Nanoindentation, and Macroscale Testing. J. Mech. Behav. Biomed. Mater. 2015, 51, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Zemła, J.; Bobrowska, J.; Kubiak, A.; Zieliński, T.; Pabijan, J.; Pogoda, K.; Bobrowski, P.; Lekka, M. Indenting Soft Samples (Hydrogels and Cells) with Cantilevers Possessing Various Shapes of Probing Tip. Eur. Biophys. J. 2020, 49, 485–495. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Rausch, M.K.; Peppas, N.A. Cross-Evaluation of Stiffness Measurement Methods for Hydrogels. Polymer 2022, 258, 125316. [Google Scholar] [CrossRef]
- Shakado, S.; Sakisaka, S.; Noguchi, K.; Harada, M.; Maekawa, R.; Sata, M.; Tanikawa, K. Effects of Vascular Endothelial Growth Factor (VEGF) on Cultured Rat Sinusoidal Endothelial Cells. In Cells of the Hepatic Sinusoid; Kupfer Cell Foundation: Leiden, The Netherlands, 1996; Volume 6, pp. 79–81. [Google Scholar]
- Li, P.; Zhou, J.; Li, W.; Wu, H.; Hu, J.; Ding, Q.; Lü, S.; Pan, J.; Zhang, C.; Li, N.; et al. Characterizing Liver Sinusoidal Endothelial Cell Fenestrae on Soft Substrates upon AFM Imaging and Deep Learning. BBA Gen. Subj. 2020, 1864, 129702. [Google Scholar] [CrossRef]
- Zurawek, D.; Pydyn, N.; Major, P.; Szade, K.; Trzos, K.; Radkowiak, D.; Jura, J.; Kotlinowski, J. Diosmetin Alleviates TNF α -Induced Liver Inflammation by Improving Liver Sinusoidal Endothelial Cell Dysfunction. Biomed. Pharmacother. 2025, 183, 117843. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Giergiel, M.; Kwiatkowski, G.; Kurpinska, A.; Wojnar-Lason, K.; Kaczara, P.; Szymonski, M.; Lekka, M.; Kalvins, I.; Zapotoczny, B.; et al. Protein Disulfide Isomerase A1 Regulates Fenestration Dynamics in Primary Mouse Liver Sinusoidal Endothelial Cells (LSECs). Redox Biol. 2024, 72, 103162. [Google Scholar] [CrossRef]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-Related Intimal Stiffening Enhances Endothelial Permeability and Leukocyte Transmigration. Sci. Transl. Med. 2011, 3, 112ra122. [Google Scholar] [CrossRef]
- Yokomori, H.; Yoshimura, K.; Funakoshi, S.; Nagai, T.; Fujimaki, K.; Nomura, M.; Ishii, H.; Oda, M. Rho Modulates Hepatic Sinusoidal Endothelial Fenestrae via Regulation of the Actin Cytoskeleton in Rat Endothelial Cells. Lab. Investig. 2004, 84, 857–864. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Lekka, M.; Ahluwalia, B.S.; McCourt, P. Tuning of Liver Sieve: The Interplay between Actin and Myosin Regulatory Light Chain Regulates Fenestration Size and Number in Murine Liver Sinusoidal Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 9850. [Google Scholar] [CrossRef]

| Acrylamide Concentration [%] | Bisacrylamide Concentration [%] | Ingredient Quantity for 1 mL of Hydrogel [µL] | |||
|---|---|---|---|---|---|
| Acrylamide (40%) | Bisacrylamide (2%) | Deionized Water | |||
| Stiff hydrogel | 12 | 0.25 | 300 | 125 | 575 |
| Soft hydrogel | 3 | 0.6 | 75 | 300 | 625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borah, D.; Blacharczyk, O.; Szafranska, K.; Czyzynska-Cichon, I.; Metwally, S.; Szymanowski, K.; Hübner, W.; Kotlinowski, J.; Dobosz, E.; McCourt, P.; et al. Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation. Cells 2025, 14, 621. https://doi.org/10.3390/cells14080621
Borah D, Blacharczyk O, Szafranska K, Czyzynska-Cichon I, Metwally S, Szymanowski K, Hübner W, Kotlinowski J, Dobosz E, McCourt P, et al. Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation. Cells. 2025; 14(8):621. https://doi.org/10.3390/cells14080621
Chicago/Turabian StyleBorah, Dibakar, Oliwia Blacharczyk, Karolina Szafranska, Izabela Czyzynska-Cichon, Sara Metwally, Konrad Szymanowski, Wolfgang Hübner, Jerzy Kotlinowski, Ewelina Dobosz, Peter McCourt, and et al. 2025. "Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation" Cells 14, no. 8: 621. https://doi.org/10.3390/cells14080621
APA StyleBorah, D., Blacharczyk, O., Szafranska, K., Czyzynska-Cichon, I., Metwally, S., Szymanowski, K., Hübner, W., Kotlinowski, J., Dobosz, E., McCourt, P., Huser, T., Lekka, M., & Zapotoczny, B. (2025). Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation. Cells, 14(8), 621. https://doi.org/10.3390/cells14080621






