Ion Channel Regulation in Caveolae and Its Pathological Implications
Abstract
1. Introduction
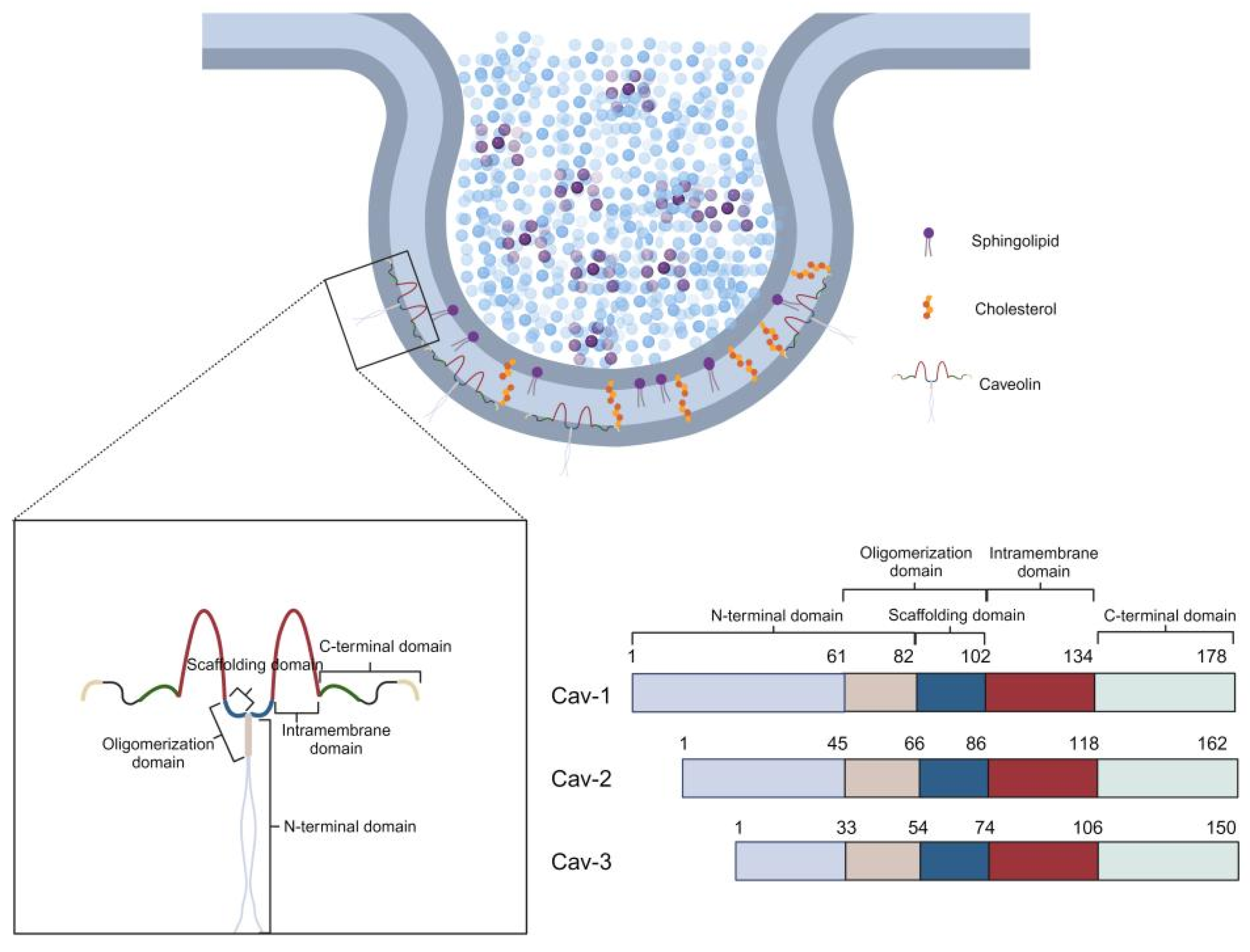
1.1. Discovery of Caveolae and Characterization of Caveolin Proteins
1.2. Physiological and Pathological Significance of Ion Channels and Their Association with Caveolae
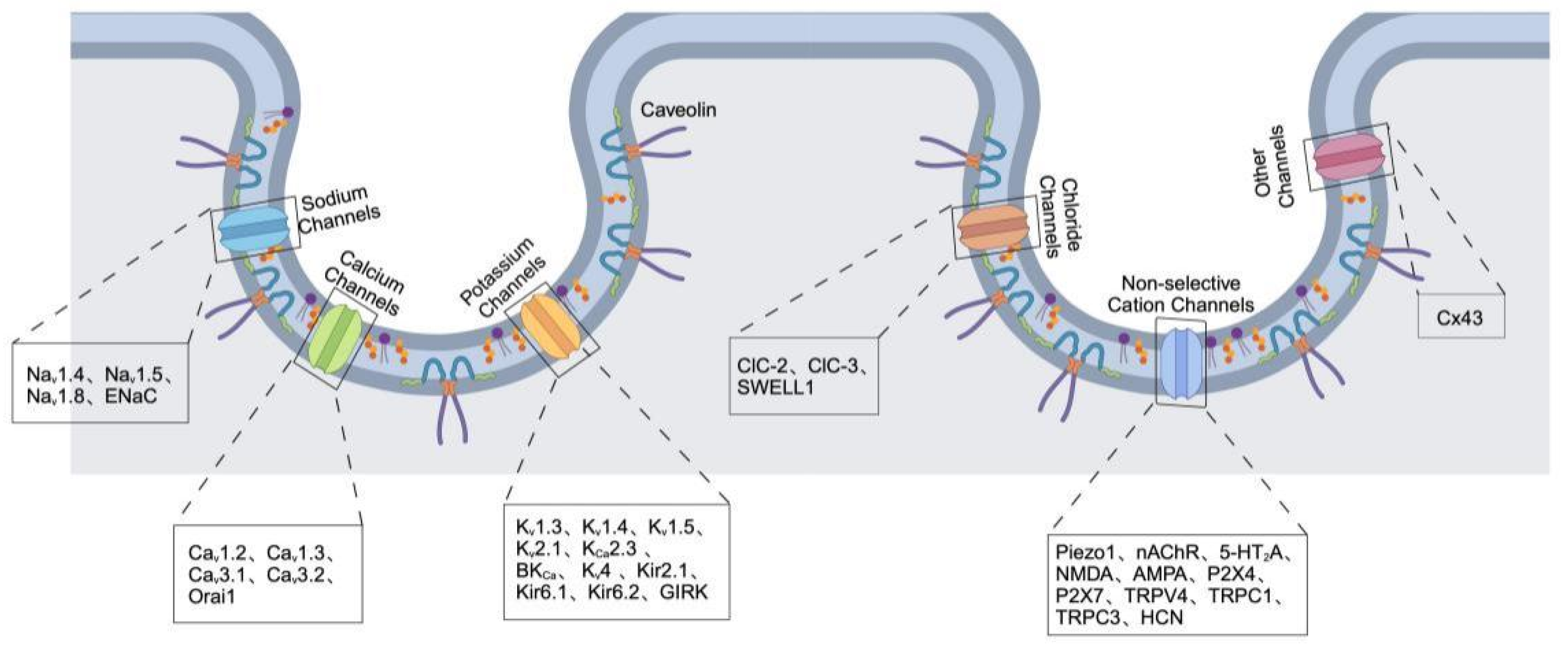
2. Ion Channels Localized in Caveolae
2.1. Sodium Channels
2.1.1. Voltage-Gated Sodium Channels (Nav)
2.1.2. Epithelial Sodium Channels (ENaC)
2.2. Calcium Channels
2.2.1. Voltage-Gated Calcium Channels (Cav)
2.2.2. Store-Operated Calcium Channels
2.3. Potassium Channels
2.3.1. Voltage-Gated Potassium Channels (Kv)
2.3.2. Inwardly Rectifying Potassium Channels
2.3.3. Two-Pore Domain Potassium Channels
2.4. Chloride Channels
2.5. Non-Selective Cation Channels
2.5.1. Mechanosensitive Ion Channels and Ligand-Gated Ion Channels
2.5.2. Transient Receptor Potential Channels
2.5.3. Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels
2.6. Other Channels
Gap Junction Channels
| Channel Type | Localization in Caveolae/Interaction with Caveolins | Caveolin Isoforms Involved | References |
|---|---|---|---|
| Sodium Channels | |||
| Nav1.4 | Localized within muscle caveolae, direct interaction | Cav-3 | [32,33] |
| Nav1.5 | Caveolae-localized; forms signaling complex affecting cardiac excitability | Cav-1, Cav-3 | [34,35,36] |
| Nav1.8 | Stabilized within caveolae by direct interaction | Cav-1 | [37] |
| ENaC | Caveolae-localized in endothelial cells; mechanosensitive sodium transport | Cav-1 | [39,40,41] |
| Calcium Channels | |||
| Cav1.2 | Caveolae-localized; regulate cardiac calcium signaling and pacemaker activity | Cav-3 | [29,42,43,44,45] |
| Cav1.3 | |||
| Cav3.1, Cav3.2 | Localized in caveolae; regulate cardiac pacing and calcium signaling | Cav-1, Cav-3 | [45,46,47] |
| Orai1 | Caveolae localization; modulates calcium influx | Cav-1 | [48] |
| Potassium Channels | |||
| Kv1.3 | Localized in caveolar lipid rafts; insulin signaling | Cav-1 | [49,98] |
| Kv1.4 | Tissue-specific localization in caveolar/non-caveolar rafts | Cav-2, Cav-3 | [50,51,52,53,54] |
| Kv1.5 | |||
| Kv2.1 | |||
| Kv4 | |||
| KCa2.3 (SK3) | Localized in caveolae | Cav-1 | [59,60,61] |
| BK Channels (hSlo1c, BKCa) | Localized in caveolae; regulate permeability and hypoxic response | Cav-1 | [57,58] |
| Kir2.1 | Localized in caveolae; regulate membrane potential and metabolism | Cav-1, Cav-3 | [62,63,64,65,66]. |
| Kir6.1 | |||
| Kir6.2 | |||
| GIRK | Localized in caveolae | Cav-3 | [67] |
| TASK-1 and TWIK-1 | Colocalized with Cav-1/3 | Cav-1, Cav-3 | [68,69] |
| Chloride Channels | |||
| ClC-2 | Localized in caveolae; regulate cell volume and mechanosensitivity | Cav-1, Cav-3 | [70,71,72] |
| ClC-3 | |||
| SWELL1 | |||
| Non-selective Cation Channels | |||
| Piezo1 | Colocalized in cardiomyocytes with Cav-3 | Cav-3 | [73] |
| nAChR, 5-HT2A, NMDA, AMPA, P2X4, P2X7 | Localized in caveolae; modulate neurotransmitter responses and excitability | Cav-1, Cav-3 | [75,76,77,78,99] |
| TRPV4, TRPC1, TRPC3 | Localized in caveolae; regulate thermal and mechanical responses | Cav-1, Cav-3 | [60,61,79,80,81,82,83,84] |
| HCN | Caveolae-localized; regulate cardiac pacing and rhythmicity | Cav-3 | [45,85] |
| Other Channels | |||
| Cx43 | Caveolae-localized; regulate intercellular communication and stability | Cav-1, Cav-2 | [94,95,96,97] |
3. Regulation of Ion Channels by Caveolae
3.1. Direct Interaction Between Caveolin Scaffolding Domains and Ion Channels
3.2. Lipid-Dependent Modulation of Ion Channel Function
| Caveolin/Caveolae Component | Interaction Partner | Key Interaction Domain or Mechanism | Effect on Ion Channel Localization, Trafficking, or Function | Reference |
|---|---|---|---|---|
| Caveolin-3 | Kir2.x (Kir channels) | Caveolin scaffolding domain (CSD); Caveolin-binding motif (CBM) | Regulates proper localization in caveolae; disruption alters channel distribution and functionand function | [100,101,102] |
| Caveolin-3 | Kir6.2/SUR2A (KATP channels) | Caveolin scaffolding domain peptide | Negative regulation; inhibits channel function | [103] |
| Caveolin-1 | TRPC5 | Amino acids 295–322 | Direct binding modulates channel localization and function | [104] |
| Caveolin-1 | TRPC1 | C-terminal domain | Regulates store-operated calcium entry; affects cardiac cell survival and function | [105,106] |
| Caveolin-1 | BK (MaxiK) channel (Slo1 <-subunit) | Slo1 sequence: YNMLCFGIY; Caveolin scaffolding domain residues 82–101 | Dual function: Limits surface expression; anchors channels in caveolin-rich domains; negatively regulates channel activity | [58,107,108] |
| Caveolin-1 | Connexin 43 (Cx43) | Scaffolding and C-terminal domains | Complex regulation of intercellular communication | [94] |
| Cholesterol (lipid) | TRPV4 | Cholesterol-rich caveolar domains | Cholesterol depletion enhances TRPV4-mediated Ca2+ influx | [110,111] |
| Cholesterol | KV7.1/KV7.4 | Cholesterol-dependent caveolin interactions | Cholesterol depletion disrupts interaction, alters channel localization | [112] |
| Cholesterol | KV1.5 | Cholesterol-rich lipid raft localization | Cholesterol depletion increases channel current capacity | [52] |
| Cholesterol/Sphingolipids | KV2.1 | Membrane lipid modification by sphingomyelinase D | Induces activation at more negative potentials | [113] |
| Cholesterol | CaV2.1 | Cholesterol depletion | Enhances channel current | [114] |
| Cholesterol | Orai1 | Cholesterol-rich domains | Depletion causes internalization and reduced Ca2+ influx; caveolin-1 reverses effects | [48] |
| PUFAs | Cardiac Na+ and L-type Ca2+ channels | Lipid microdomain modulation | Reduces currents; shortens action potential duration | [115,116] |
| PIP2 | Kir channels; Na+/Ca2+ exchangers; KATP channels | Membrane lipid modulation | Increased PIP2 activates Kir channels; depletion inhibits activity | [117] |
3.3. Compartmentalization of Signaling Cascades
3.4. Mechanosensitive Regulation of Ion Channels by Caveolae
3.5. Caveolae-Mediated Regulation of Ion Channels by Other Channels and Proteins
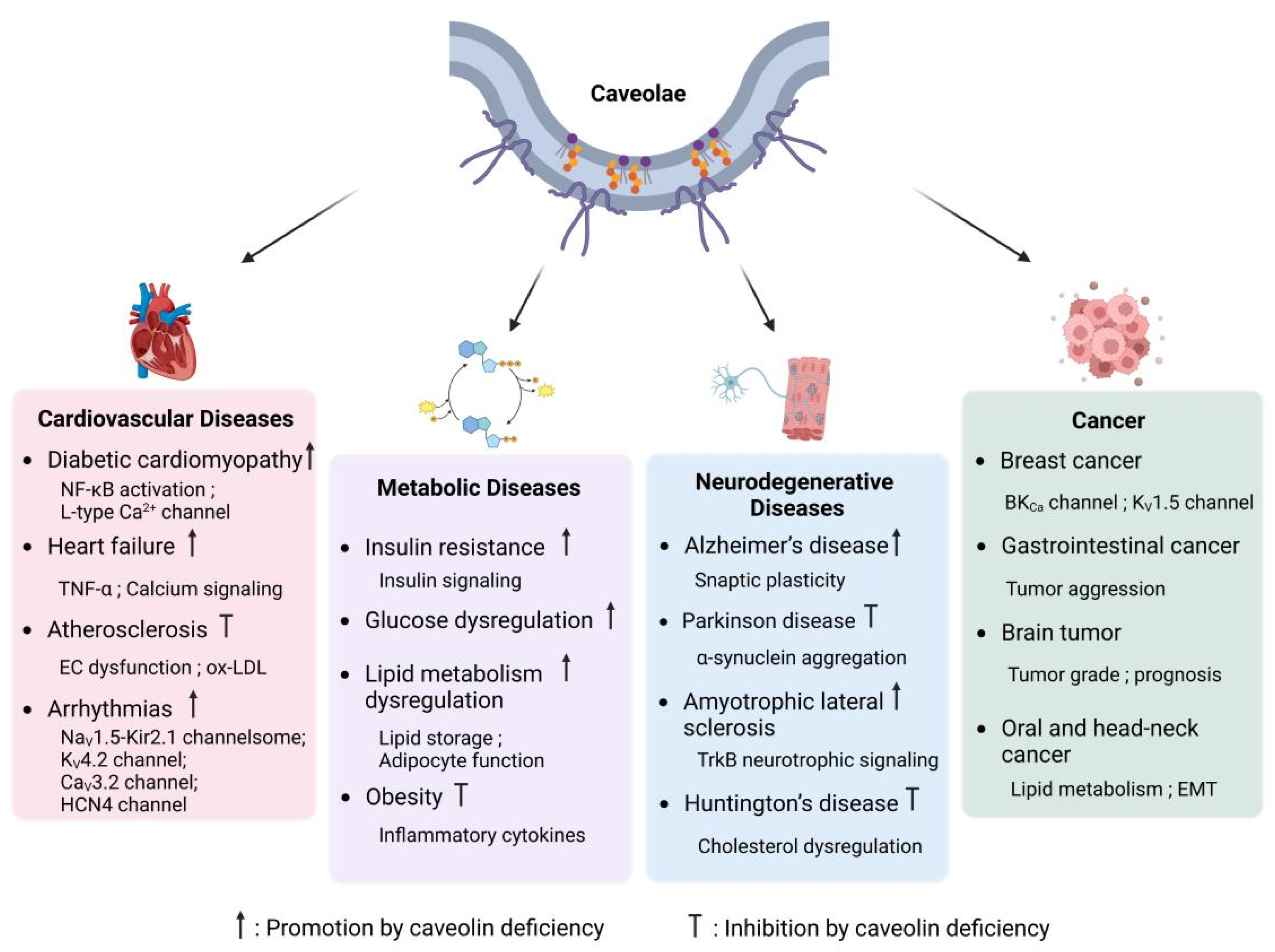
4. Caveolae and Diseases
4.1. Cardiovascular Diseases
4.1.1. Atherosclerosis
4.1.2. Diabetic Cardiomyopathy
4.1.3. Heart Failure
4.1.4. Arrhythmias
4.2. Metabolic Diseases
4.2.1. Insulin Resistance and Glucose Dysregulation
4.2.2. Obesity and Lipid Metabolism Dysregulation
4.3. Neurodegenerative Diseases
4.3.1. Alzheimer’s Disease and Cognitive Decline
4.3.2. Parkinson Disease and α-Synuclein Aggregation
4.3.3. Amyotrophic Lateral Sclerosis (ALS) and Neuroprotection
4.3.4. Huntington’s Disease and Cholesterol Dysregulation
4.4. Cancer
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palade, G.E. An Electron Microscope Study of the Mitochondrial Structure. J. Histochem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.S.; Glenney, J.R.; Anderson, R.G. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Murata, M.; Peranen, J.; Schreiner, R.; Wieland, F.; Kurzchalia, T.V.; Simons, K. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA 1995, 92, 10339–10343. [Google Scholar] [CrossRef]
- Scherer, P.E.; Okamoto, T.; Chun, M.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 131–135. [Google Scholar] [CrossRef]
- Tang, Z.; Scherer, P.E.; Okamoto, T.; Song, K.; Chu, C.; Kohtz, D.S.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996, 271, 2255–2261. [Google Scholar] [CrossRef]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214. [Google Scholar] [CrossRef]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef]
- Razani, B.; Engelman, J.A.; Wang, X.B.; Schubert, W.; Zhang, X.L.; Marks, C.B.; Macaluso, F.; Russell, R.G.; Li, M.; Pestell, R.G.; et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001, 276, 38121–38138. [Google Scholar] [CrossRef] [PubMed]
- Razani, B.; Lisanti, M.P. Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am. J. Physiol. Cell Physiol. 2001, 281, C1241–C1250. [Google Scholar] [CrossRef] [PubMed]
- Gazzerro, E.; Sotgia, F.; Bruno, C.; Lisanti, M.P.; Minetti, C. Caveolinopathies: From the biology of caveolin-3 to human diseases. Eur. J. Hum. Genet. 2010, 18, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Way, M.; Parton, R.G. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1995, 376, 108–112. [Google Scholar] [CrossRef]
- Scherer, P.E.; Lewis, R.Y.; Volonte, D.; Engelman, J.A.; Galbiati, F.; Couet, J.; Kohtz, D.S.; van Donselaar, E.; Peters, P.; Lisanti, M.P. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 1997, 272, 29337–29346. [Google Scholar] [CrossRef]
- Jung, W.; Sierecki, E.; Bastiani, M.; O’Carroll, A.; Alexandrov, K.; Rae, J.; Johnston, W.; Hunter, D.J.B.; Ferguson, C.; Gambin, Y.; et al. Cell-free formation and interactome analysis of caveolae. J. Cell Biol. 2018, 217, 2141–2165. [Google Scholar] [CrossRef]
- Parton, R.G.; Hanzal-Bayer, M.; Hancock, J.F. Biogenesis of caveolae: A structural model for caveolin-induced domain formation. J. Cell Sci. 2006, 119, 787–796. [Google Scholar] [CrossRef]
- Fiala, G.J.; Minguet, S. Caveolin-1: The Unnoticed Player in TCR and BCR Signaling. Adv. Immunol. 2018, 137, 83–133. [Google Scholar] [CrossRef]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Root, K.T.; Plucinsky, S.M.; Glover, K.J. Recent progress in the topology, structure, and oligomerization of caveolin: A building block of caveolae. Curr. Top. Membr. 2015, 75, 305–336. [Google Scholar] [CrossRef]
- Head, B.P.; Insel, P.A. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007, 17, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zhang, X.L.; Lisanti, M.P. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998, 436, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zhang, X.L.; Lisanti, M.P. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999, 448, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.M.; Head, B.P.; Patel, H.H. Caveolins as Regulators of Stress Adaptation. Mol. Pharmacol. 2018, 93, 277–285. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer: Sunderland, MA, USA, 2001; p. xviii. 814p. [Google Scholar]
- Ashcroft, F.M. Ion Channels and Disease: Channelopathies; Academic Press: San Diego, CA, USA, 2000; p. xxi. 481p. [Google Scholar]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Patel, H.H.; Murray, F.; Insel, P.A. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 359–391. [Google Scholar] [CrossRef]
- Balijepalli, R.C.; Foell, J.D.; Hall, D.D.; Hell, J.W.; Kamp, T.J. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 7500–7505. [Google Scholar] [CrossRef]
- Martens, J.R.; Navarro-Polanco, R.; Coppock, E.A.; Nishiyama, A.; Parshley, L.; Grobaski, T.D.; Tamkun, M.M. Differential targeting of Shaker-like potassium channels to lipid rafts. J. Biol. Chem. 2000, 275, 7443–7446. [Google Scholar] [CrossRef]
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002, 277, 8635–8647. [Google Scholar] [CrossRef]
- Yarbrough, T.L.; Lu, T.; Lee, H.C.; Shibata, E.F. Localization of cardiac sodium channels in caveolin-rich membrane domains: Regulation of sodium current amplitude. Circ. Res. 2002, 90, 443–449. [Google Scholar] [CrossRef]
- Murphy, R.M.; Mollica, J.P.; Lamb, G.D. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp. Cell Res. 2009, 315, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Vatta, M.; Ackerman, M.J.; Ye, B.; Makielski, J.C.; Ughanze, E.E.; Taylor, E.W.; Tester, D.J.; Balijepalli, R.C.; Foell, J.D.; Li, Z.; et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006, 114, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Rubino, R.; Besson, P.; Labbal, F.; Chevalier, S.; et al. NaV1.5 Na(+) channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Brisson, L.; Gillet, L.; Calaghan, S.; Besson, P.; Le Guennec, J.Y.; Roger, S.; Gore, J. Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene 2011, 30, 2070–2076. [Google Scholar] [CrossRef]
- Ohman, E.; Nilsson, A.; Madeira, A.; Sjogren, B.; Andren, P.E.; Svenningsson, P. Use of surface plasmon resonance coupled with mass spectrometry reveals an interaction between the voltage-gated sodium channel type X alpha-subunit and caveolin-1. J. Proteome Res. 2008, 7, 5333–5338. [Google Scholar] [CrossRef]
- Palygin, O.A.; Pettus, J.M.; Shibata, E.F. Regulation of caveolar cardiac sodium current by a single Gsalpha histidine residue. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1693–H1699. [Google Scholar] [CrossRef]
- Guo, D.; Liang, S.; Wang, S.; Tang, C.; Yao, B.; Wan, W.; Zhang, H.; Jiang, H.; Ahmed, A.; Zhang, Z.; et al. Role of epithelial Na+ channels in endothelial function. J. Cell Sci. 2016, 129, 290–297. [Google Scholar] [CrossRef]
- Hill, W.G.; Butterworth, M.B.; Wang, H.; Edinger, R.S.; Lebowitz, J.; Peters, K.W.; Frizzell, R.A.; Johnson, J.P. The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J. Biol. Chem. 2007, 282, 37402–37411. [Google Scholar] [CrossRef]
- Lee, I.H.; Campbell, C.R.; Song, S.H.; Day, M.L.; Kumar, S.; Cook, D.I.; Dinudom, A. The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J. Biol. Chem. 2009, 284, 12663–12669. [Google Scholar] [CrossRef]
- Tonegawa, K.; Otsuka, W.; Kumagai, S.; Matsunami, S.; Hayamizu, N.; Tanaka, S.; Moriwaki, K.; Obana, M.; Maeda, M.; Asahi, M.; et al. Caveolae-specific activation loop between CaMKII and L-type Ca2+ channel aggravates cardiac hypertrophy in alpha(1)-adrenergic stimulation. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H501–H514. [Google Scholar] [CrossRef]
- Best, J.M.; Kamp, T.J. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J. Mol. Cell Cardiol. 2012, 52, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Vivas, O.; Baudot, M.; Moreno, C.M. Aging Alters the Formation and Functionality of Signaling Microdomains Between L-type Calcium Channels and beta2-Adrenergic Receptors in Cardiac Pacemaker Cells. Front. Physiol. 2022, 13, 805909. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Ni, H.; Medvedev, R.Y.; Liu, F.; Alvarez-Baron, C.P.; Tyan, L.; Turner, D.G.P.; Warden, A.; Morotti, S.; Schrauth, T.A.; et al. Caveolar Compartmentalization of Pacemaker Signaling is Required for Stable Rhythmicity of Sinus Nodal Cells and is Disrupted in Heart Failure. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hashad, A.M.; Harraz, O.F.; Brett, S.E.; Romero, M.; Kassmann, M.; Puglisi, J.L.; Wilson, S.M.; Gollasch, M.; Welsh, D.G. Caveolae Link Ca(V)3.2 Channels to BK(Ca)-Mediated Feedback in Vascular Smooth Muscle. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2371–2381. [Google Scholar] [CrossRef]
- Markandeya, Y.S.; Fahey, J.M.; Pluteanu, F.; Cribbs, L.L.; Balijepalli, R.C. Caveolin-3 regulates protein kinase A modulation of the Ca(V)3.2 (alpha1H) T-type Ca2+ channels. J. Biol. Chem. 2011, 286, 2433–2444. [Google Scholar] [CrossRef]
- Bohorquez-Hernandez, A.; Gratton, E.; Pacheco, J.; Asanov, A.; Vaca, L. Cholesterol modulates the cellular localization of Orai1 channels and its disposition among membrane domains. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1481–1490. [Google Scholar] [CrossRef]
- Perez-Verdaguer, M.; Capera, J.; Ortego-Dominguez, M.; Bielanska, J.; Comes, N.; Montoro, R.J.; Camps, M.; Felipe, A. Caveolar targeting links Kv1.3 with the insulin-dependent adipocyte physiology. Cell Mol. Life Sci. 2018, 75, 4059–4075. [Google Scholar] [CrossRef]
- Eldstrom, J.; Van Wagoner, D.R.; Moore, E.D.; Fedida, D. Localization of Kv1.5 channels in rat and canine myocyte sarcolemma. FEBS Lett. 2006, 580, 6039–6046. [Google Scholar] [CrossRef]
- Shibata, E.F.; Brown, T.L.; Washburn, Z.W.; Bai, J.; Revak, T.J.; Butters, C.A. Autonomic regulation of voltage-gated cardiac ion channels. J. Cardiovasc. Electrophysiol. 2006, 17 (Suppl. S1), S34–S42. [Google Scholar] [CrossRef]
- Abi-Char, J.; Maguy, A.; Coulombe, A.; Balse, E.; Ratajczak, P.; Samuel, J.L.; Nattel, S.; Hatem, S.N. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J. Physiol. 2007, 582, 1205–1217. [Google Scholar] [CrossRef]
- Xia, F.; Leung, Y.M.; Gaisano, G.; Gao, X.; Chen, Y.; Fox, J.E.; Bhattacharjee, A.; Wheeler, M.B.; Gaisano, H.Y.; Tsushima, R.G. Targeting of voltage-gated K+ and Ca2+ channels and soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins to cholesterol-rich lipid rafts in pancreatic alpha-cells: Effects on glucagon stimulus-secretion coupling. Endocrinology 2007, 148, 2157–2167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallego, M.; Alday, A.; Alonso, H.; Casis, O. Adrenergic regulation of cardiac ionic channels: Role of membrane microdomains in the regulation of kv4 channels. Biochim. Biophys. Acta 2014, 1838, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Maguy, A.; Hebert, T.E.; Nattel, S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc. Res. 2006, 69, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.R.; Sakamoto, N.; Sullivan, S.A.; Grobaski, T.D.; Tamkun, M.M. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J. Biol. Chem. 2001, 276, 8409–8414. [Google Scholar] [CrossRef]
- Song, Y.; Wang, P.; Ma, J.; Xue, Y. C-terminus of human BKca channel alpha subunit enhances the permeability of the brain endothelial cells by interacting with caveolin-1 and triggering caveolin-1 intracellular trafficking. Neuromolecular Med. 2014, 16, 499–509. [Google Scholar] [CrossRef]
- Riddle, M.A.; Hughes, J.M.; Walker, B.R. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am. J. Physiol. Cell Physiol. 2011, 301, C1404–C1414. [Google Scholar] [CrossRef]
- Gao, Y.; Bertuccio, C.A.; Balut, C.M.; Watkins, S.C.; Devor, D.C. Dynamin- and Rab5-dependent endocytosis of a Ca2+-activated K+ channel, KCa2.3. PLoS ONE 2012, 7, e44150. [Google Scholar] [CrossRef]
- Goedicke-Fritz, S.; Kaistha, A.; Kacik, M.; Markert, S.; Hofmeister, A.; Busch, C.; Banfer, S.; Jacob, R.; Grgic, I.; Hoyer, J. Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur. J. Cell Biol. 2015, 94, 391–400. [Google Scholar] [CrossRef]
- Lu, T.; Wang, X.L.; Chai, Q.; Sun, X.; Sieck, G.C.; Katusic, Z.S.; Lee, H.C. Role of the endothelial caveolae microdomain in shear stress-mediated coronary vasorelaxation. J. Biol. Chem. 2017, 292, 19013–19023. [Google Scholar] [CrossRef]
- Garg, V.; Jiao, J.; Hu, K. Regulation of ATP-sensitive K+ channels by caveolin-enriched microdomains in cardiac myocytes. Cardiovasc. Res. 2009, 82, 51–58. [Google Scholar] [CrossRef]
- Puddu, A.; Salani, B.; Cordera, R.; Viviani, G.L.; Maggi, D. Caveolin-1 is essential for glimepiride-induced insulin secretion in the pancreatic betaTC-6 cell line. Biochem. Biophys. Res. Commun. 2008, 375, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Q.; Jana, K.; Rindler, M.J.; Coetzee, W.A. The trafficking protein, EHD2, positively regulates cardiac sarcolemmal K(ATP) channel surface expression: Role in cardioprotection. FASEB J. 2018, 32, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.M.; Purves, G.I.; Barrett-Jolley, R.; Dart, C. Interaction with caveolin-1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J. Physiol. 2010, 588, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Rosenhouse-Dantsker, A.; Gnanasambandam, R.; Epshtein, Y.; Chen, Z.; Sachs, F.; Minshall, R.D.; Levitan, I. Silencing of Kir2 channels by caveolin-1: Cross-talk with cholesterol. J. Physiol. 2014, 592, 4025–4038. [Google Scholar] [CrossRef]
- Cui, S.; Ho, W.K.; Kim, S.T.; Cho, H. Agonist-induced localization of Gq-coupled receptors and G protein-gated inwardly rectifying K+ (GIRK) channels to caveolae determines receptor specificity of phosphatidylinositol 4,5-bisphosphate signaling. J. Biol. Chem. 2010, 285, 41732–41739. [Google Scholar] [CrossRef]
- Kang, C.; Hernandez, V.A.; Hu, K. Functional interaction of the two-pore domain potassium channel TASK-1 and caveolin-3. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1537–1544. [Google Scholar] [CrossRef]
- Zuniga, R.; Mancilla, D.; Rojas, T.; Vergara, F.; Gonzalez, W.; Catalan, M.A.; Zuniga, L. A Direct Interaction between Cyclodextrins and TASK Channels Decreases the Leak Current in Cerebellar Granule Neurons. Biology 2022, 11, 1097. [Google Scholar] [CrossRef]
- Nighot, P.K.; Blikslager, A.T. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am. J. Physiol. Cell Physiol. 2012, 302, C178–C187. [Google Scholar] [CrossRef]
- Turner, D.G.P.; Tyan, L.; DeGuire, F.C.; Medvedev, R.Y.; Stroebel, S.J.; Lang, D.; Glukhov, A.V. Caveolin-3 prevents swelling-induced membrane damage via regulation of I(Cl,swell) activity. Biophys. J. 2022, 121, 1643–1659. [Google Scholar] [CrossRef]
- Egorov, Y.V.; Lang, D.; Tyan, L.; Turner, D.; Lim, E.; Piro, Z.D.; Hernandez, J.J.; Lodin, R.; Wang, R.; Schmuck, E.G.; et al. Caveolae-Mediated Activation of Mechanosensitive Chloride Channels in Pulmonary Veins Triggers Atrial Arrhythmogenesis. J. Am. Heart Assoc. 2019, 8, e012748. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wu, F.; Yu, Z.; Yang, L. Disruption of the interaction between caveolae and Piezo1 promotes pressure overload-induced cardiac remodeling. Biochem. Biophys. Res. Commun. 2024, 735, 150456. [Google Scholar] [CrossRef] [PubMed]
- Hezel, M.; de Groat, W.C.; Galbiati, F. Caveolin-3 promotes nicotinic acetylcholine receptor clustering and regulates neuromuscular junction activity. Mol. Biol. Cell 2010, 21, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mialet-Perez, J.; D’Angelo, R.; Villeneuve, C.; Ordener, C.; Negre-Salvayre, A.; Parini, A.; Vindis, C. Serotonin 5-HT2A receptor-mediated hypertrophy is negatively regulated by caveolin-3 in cardiomyoblasts and neonatal cardiomyocytes. J. Mol. Cell Cardiol. 2012, 52, 502–510. [Google Scholar] [CrossRef]
- Nakashima, M.; Suga, N.; Fukumoto, A.; Yoshikawa, S.; Matsuda, S. Caveolae with serotonin and NMDA receptors as promising targets for the treatment of Alzheimer’s disease. Int. J. Physiol. Pathophysiol. Pharmacol. 2024, 16, 96–110. [Google Scholar] [CrossRef]
- Nakashima, M.; Suga, N.; Yoshikawa, S.; Matsuda, S. Caveolae with GLP-1 and NMDA Receptors as Crossfire Points for the Innovative Treatment of Cognitive Dysfunction Associated with Neurodegenerative Diseases. Molecules 2024, 29, 3922. [Google Scholar] [CrossRef]
- Barth, K.; Weinhold, K.; Guenther, A.; Linge, A.; Gereke, M.; Kasper, M. Characterization of the molecular interaction between caveolin-1 and the P2X receptors 4 and 7 in E10 mouse lung alveolar epithelial cells. Int. J. Biochem. Cell Biol. 2008, 40, 2230–2239. [Google Scholar] [CrossRef]
- Das, R.; Goswami, C. TRPV4 expresses in bone cell lineages and TRPV4-R616Q mutant causing Brachyolmia in human reveals “loss-of-interaction” with cholesterol. Biochem. Biophys. Res. Commun. 2019, 517, 566–574. [Google Scholar] [CrossRef]
- Lakk, M.; Hoffmann, G.F.; Gorusupudi, A.; Enyong, E.; Lin, A.; Bernstein, P.S.; Toft-Bertelsen, T.; MacAulay, N.; Elliott, M.H.; Krizaj, D. Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. J. Lipid Res. 2021, 62, 100145. [Google Scholar] [CrossRef]
- Naik, J.S.; Walker, B.R. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflug. Arch. 2018, 470, 633–648. [Google Scholar] [CrossRef]
- Gervasio, O.L.; Whitehead, N.P.; Yeung, E.W.; Phillips, W.D.; Allen, D.G. TRPC1 binds to caveolin-3 and is regulated by Src kinase—Role in Duchenne muscular dystrophy. J. Cell Sci. 2008, 121, 2246–2255. [Google Scholar] [CrossRef]
- Bergdahl, A.; Gomez, M.F.; Dreja, K.; Xu, S.Z.; Adner, M.; Beech, D.J.; Broman, J.; Hellstrand, P.; Sward, K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ. Res. 2003, 93, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, A.; Narayanan, D.; Jaggar, J.H. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J. Biol. Chem. 2011, 286, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Song, Q.; Zhu, J.; Zhao, J.; Liu, Q.; Zhang, T.; Long, Z.; Li, J.; Wu, C.; Wang, Q.; et al. Interaction of Caveolin-3 and HCN is involved in the pathogenesis of diabetic cystopathy. Sci. Rep. 2016, 6, 24844. [Google Scholar] [CrossRef]
- Das Sarma, J.; Kaplan, B.E.; Willemsen, D.; Koval, M. Identification of rab20 as a potential regulator of connexin 43 trafficking. Cell Commun. Adhes. 2008, 15, 65–74. [Google Scholar] [CrossRef]
- Epifantseva, I.; Shaw, R.M. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim. Biophys. Acta Biomembr. 2018, 1860, 40–47. [Google Scholar] [CrossRef]
- Evans, W.H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459. [Google Scholar] [CrossRef]
- Salameh, A. Life cycle of connexins: Regulation of connexin synthesis and degradation. Adv. Cardiol. 2006, 42, 57–70. [Google Scholar] [CrossRef]
- Smyth, J.W.; Hong, T.T.; Gao, D.; Vogan, J.M.; Jensen, B.C.; Fong, T.S.; Simpson, P.C.; Stainier, D.Y.; Chi, N.C.; Shaw, R.M. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J. Clin. Investig. 2010, 120, 266–279. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 2018, 1860, 83–90. [Google Scholar] [CrossRef]
- Su, V.; Lau, A.F. Connexins: Mechanisms regulating protein levels and intercellular communication. FEBS Lett. 2014, 588, 1212–1220. [Google Scholar] [CrossRef]
- Andelova, K.; Egan Benova, T.; Szeiffova Bacova, B.; Sykora, M.; Prado, N.J.; Diez, E.R.; Hlivak, P.; Tribulova, N. Cardiac Connexin-43 Hemichannels and Pannexin1 Channels: Provocative Antiarrhythmic Targets. Int. J. Mol. Sci. 2020, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.L.; Schubert, W.; Spray, D.C.; Lisanti, M.P. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 2002, 41, 5754–5764. [Google Scholar] [CrossRef] [PubMed]
- Langlois, S.; Cowan, K.N.; Shao, Q.; Cowan, B.J.; Laird, D.W. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell 2008, 19, 912–928. [Google Scholar] [CrossRef]
- Ampey, B.C.; Morschauser, T.J.; Ramadoss, J.; Magness, R.R. Domain-Specific Partitioning of Uterine Artery Endothelial Connexin43 and Caveolin-1. Hypertension 2016, 68, 982–988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mattera, M.D. Changing practice. RN 1998, 61, 7. [Google Scholar]
- Perez-Verdaguer, M.; Capera, J.; Martinez-Marmol, R.; Camps, M.; Comes, N.; Tamkun, M.M.; Felipe, A. Caveolin interaction governs Kv1.3 lipid raft targeting. Sci. Rep. 2016, 6, 22453. [Google Scholar] [CrossRef]
- Lucas, K.C.; Hart, D.A.; Becker, R.W. Porcine proximal tubular cells (LLC-PK1) are able to tolerate high levels of lithium chloride in vitro: Assessment of the influence of 1-20 mM LiCl on cell death and alterations in cell biology and biochemistry. Cell Biol. Int. 2010, 34, 225–233. [Google Scholar] [CrossRef]
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997, 272, 6525–6533. [Google Scholar] [CrossRef]
- Vaidyanathan, R.; Reilly, L.; Eckhardt, L.L. Caveolin-3 Microdomain: Arrhythmia Implications for Potassium Inward Rectifier and Cardiac Sodium Channel. Front. Physiol. 2018, 9, 1548. [Google Scholar] [CrossRef]
- Vaidyanathan, R.; Van Ert, H.; Haq, K.T.; Morotti, S.; Esch, S.; McCune, E.C.; Grandi, E.; Eckhardt, L.L. Inward Rectifier Potassium Channels (Kir2.x) and Caveolin-3 Domain-Specific Interaction: Implications for Purkinje Cell-Dependent Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2018, 11, e005800. [Google Scholar] [CrossRef]
- Garg, V.; Sun, W.; Hu, K. Caveolin-3 negatively regulates recombinant cardiac K(ATP) channels. Biochem. Biophys. Res. Commun. 2009, 385, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.; Takahashi, N.; Yoshida, T.; Ogawa, N.; Ueda, Y.; Hamano, S.; Yamaguchi, K.; Sawamura, S.; Yamamoto, S.; Hara, Y.; et al. Dynamic remodeling of TRPC5 channel-caveolin-1-eNOS protein assembly potentiates the positive feedback interaction between Ca2+ and NO signals. J. Biol. Chem. 2024, 300, 107705. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, A.M.; Minshall, R.D.; Cool, D.R.; Skidgel, R.A.; Malik, A.B.; Tiruppathi, C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol. Pharmacol. 2006, 70, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Liu, S.Y.; Zhang, M.; Meng, J.R.; Tang, N.; Feng, Y.D.; Sun, Y.; Gao, Y.Y.; Zhou, L.; Cao, W.; et al. TRPC1 contributes to endotoxemia-induced myocardial dysfunction via mediating myocardial apoptosis and autophagy. Pharmacol. Res. 2022, 181, 106262. [Google Scholar] [CrossRef]
- Alioua, A.; Lu, R.; Kumar, Y.; Eghbali, M.; Kundu, P.; Toro, L.; Stefani, E. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J. Biol. Chem. 2008, 283, 4808–4817. [Google Scholar] [CrossRef]
- Wang, X.L.; Ye, D.; Peterson, T.E.; Cao, S.; Shah, V.H.; Katusic, Z.S.; Sieck, G.C.; Lee, H.C. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J. Biol. Chem. 2005, 280, 11656–11664. [Google Scholar] [CrossRef]
- Liu, J.; Oh, P.; Horner, T.; Rogers, R.A.; Schnitzer, J.E. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J. Biol. Chem. 1997, 272, 7211–7222. [Google Scholar] [CrossRef]
- Morales-Lazaro, S.L.; Rosenbaum, T. Multiple Mechanisms of Regulation of Transient Receptor Potential Ion Channels by Cholesterol. Curr. Top. Membr. 2017, 80, 139–161. [Google Scholar] [CrossRef]
- Kuwashima, Y.; Yanagawa, M.; Maekawa, M.; Abe, M.; Sako, Y.; Arita, M. TRPV4-dependent Ca2+ influx determines cholesterol dynamics at the plasma membrane. Biophys. J. 2024, 123, 867–884. [Google Scholar] [CrossRef]
- van der Horst, J.; Rognant, S.; Abbott, G.W.; Ozhathil, L.C.; Hagglund, P.; Barrese, V.; Chuang, C.Y.; Jespersen, T.; Davies, M.J.; Greenwood, I.A.; et al. Dynein regulates Kv7.4 channel trafficking from the cell membrane. J. Gen. Physiol. 2021, 153, e202012760. [Google Scholar] [CrossRef]
- Ramu, Y.; Xu, Y.; Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 2006, 442, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Singh, B.B. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009, 45, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Barhoumi, R.; Johnson, A.E.; Lupton, J.R.; Chapkin, R.S. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006, 20, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.F.; Gomez, A.M.; Morgan, J.P.; Lederer, W.J.; Leaf, A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. USA 1997, 94, 4182–4187. [Google Scholar] [CrossRef]
- Suh, B.C.; Hille, B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005, 15, 370–378. [Google Scholar] [CrossRef]
- Balijepalli, R.C.; Kamp, T.J. Caveolae, ion channels and cardiac arrhythmias. Prog. Biophys. Mol. Biol. 2008, 98, 149–160. [Google Scholar] [CrossRef]
- Cheng, J.; Valdivia, C.R.; Vaidyanathan, R.; Balijepalli, R.C.; Ackerman, M.J.; Makielski, J.C. Caveolin-3 suppresses late sodium current by inhibiting nNOS-dependent S-nitrosylation of SCN5A. J. Mol. Cell Cardiol. 2013, 61, 102–110. [Google Scholar] [CrossRef]
- Sampson, L.J.; Hayabuchi, Y.; Standen, N.B.; Dart, C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ. Res. 2004, 95, 1012–1018. [Google Scholar] [CrossRef]
- Alday, A.; Urrutia, J.; Gallego, M.; Casis, O. alpha1-adrenoceptors regulate only the caveolae-located subpopulation of cardiac K(V)4 channels. Channels 2010, 4, 168–178. [Google Scholar] [CrossRef][Green Version]
- Rybin, V.O.; Xu, X.; Lisanti, M.P.; Steinberg, S.F. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000, 275, 41447–41457. [Google Scholar] [CrossRef]
- Sampson, L.J.; Davies, L.M.; Barrett-Jolley, R.; Standen, N.B.; Dart, C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc. Res. 2007, 76, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kassmann, M.; Fan, G.; Gollasch, M. Arterial elementary calcium signaling in aging. Aging 2020, 12, 24476–24478. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Garg, V.; Yang, B.; Elton, T.S.; Hu, K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension 2008, 52, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bae, C.; Sachs, F.; Suchyna, T.M. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS ONE 2013, 8, e72894. [Google Scholar] [CrossRef]
- Turner, D.; Kang, C.; Mesirca, P.; Hong, J.; Mangoni, M.E.; Glukhov, A.V.; Sah, R. Electrophysiological and Molecular Mechanisms of Sinoatrial Node Mechanosensitivity. Front. Cardiovasc. Med. 2021, 8, 662410. [Google Scholar] [CrossRef]
- Kozera, L.; White, E.; Calaghan, S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS ONE 2009, 4, e8312. [Google Scholar] [CrossRef]
- Kong, Q.; Quan, Y.; Tian, G.; Zhou, J.; Liu, X. Purinergic P2 Receptors: Novel Mediators of Mechanotransduction. Front. Pharmacol. 2021, 12, 671809. [Google Scholar] [CrossRef]
- Romanenko, V.G.; Roser, K.S.; Melvin, J.E.; Begenisich, T. The role of cell cholesterol and the cytoskeleton in the interaction between IK1 and maxi-K channels. Am. J. Physiol. Cell Physiol. 2009, 296, C878–C888. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamura, H.; Ohya, S.; Imaizumi, Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and Cav1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J. Biol. Chem. 2013, 288, 36750–36761. [Google Scholar] [CrossRef]
- Tian, J.; Popal, M.S.; Huang, R.; Zhang, M.; Zhao, X.; Zhang, M.; Song, X. Caveolin as a Novel Potential Therapeutic Target in Cardiac and Vascular Diseases: A Mini Review. Aging Dis. 2020, 11, 378–389. [Google Scholar] [CrossRef]
- Gerbod-Giannone, M.C.; Dallet, L.; Naudin, G.; Sahin, A.; Decossas, M.; Poussard, S.; Lambert, O. Involvement of caveolin-1 and CD36 in native LDL endocytosis by endothelial cells. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Gutierrez-Pajares, J.L.; Iturrieta, J.; Lisanti, M.P.; Frank, P.G. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014, 356, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, P.; Sharma, A.; Bauer, P.M.; Marin, E.; Sessa, W.C. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J. Clin. Investig. 2011, 121, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.W.; Zu, X.Y.; Tuo, Q.H.; Chen, L.X.; Lei, X.Y.; Li, K.; Tang, C.K.; Liao, D.F. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol. Sin. 2010, 31, 1336–1342. [Google Scholar] [CrossRef]
- Forrester, S.J.; Elliott, K.J.; Kawai, T.; Obama, T.; Boyer, M.J.; Preston, K.J.; Yan, Z.; Eguchi, S.; Rizzo, V. Caveolin-1 Deletion Prevents Hypertensive Vascular Remodeling Induced by Angiotensin II. Hypertension 2017, 69, 79–86. [Google Scholar] [CrossRef]
- Wang, D.X.; Pan, Y.Q.; Liu, B.; Dai, L. Cav-1 promotes atherosclerosis by activating JNK-associated signaling. Biochem. Biophys. Res. Commun. 2018, 503, 513–520. [Google Scholar] [CrossRef]
- Zhou, L.J.; Chen, X.Y.; Liu, S.P.; Zhang, L.L.; Xu, Y.N.; Mu, P.W.; Geng, D.F.; Tan, Z. Downregulation of Cavin-1 Expression via Increasing Caveolin-1 Degradation Prompts the Proliferation and Migration of Vascular Smooth Muscle Cells in Balloon Injury-Induced Neointimal Hyperplasia. J. Am. Heart Assoc. 2017, 6, e005754. [Google Scholar] [CrossRef]
- Gong, W.; Jiao, Q.; Yuan, J.; Luo, H.; Liu, Y.; Zhang, Y.; Chen, Z.; Xu, X.; Bai, L.; Zhang, X. Cardioprotective and anti-inflammatory effects of Caveolin 1 in experimental diabetic cardiomyopathy. Clin. Sci. 2023, 137, 511–525. [Google Scholar] [CrossRef]
- Bryant, S.M.; Kong, C.H.T.; Cannell, M.B.; Orchard, C.H.; James, A.F. Loss of caveolin-3-dependent regulation of I(Ca) in rat ventricular myocytes in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H521–H529. [Google Scholar] [CrossRef]
- Bryant, S.M.; Kong, C.H.T.; Watson, J.J.; Gadeberg, H.C.; James, A.F.; Cannell, M.B.; Orchard, C.H. Caveolin 3-dependent loss of t-tubular I(Ca) during hypertrophy and heart failure in mice. Exp. Physiol. 2018, 103, 652–665. [Google Scholar] [CrossRef]
- Bryant, S.M.; Kong, C.H.T.; Watson, J.J.; Gadeberg, H.C.; Roth, D.M.; Patel, H.H.; Cannell, M.B.; James, A.F.; Orchard, C.H. Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular I(Ca) density in mouse ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1101–H1111. [Google Scholar] [CrossRef] [PubMed]
- Woodman, S.E.; Park, D.S.; Cohen, A.W.; Cheung, M.W.; Chandra, M.; Shirani, J.; Tang, B.; Jelicks, L.A.; Kitsis, R.N.; Christ, G.J.; et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J. Biol. Chem. 2002, 277, 38988–38997. [Google Scholar] [CrossRef] [PubMed]
- Feiner, E.C.; Chung, P.; Jasmin, J.F.; Zhang, J.; Whitaker-Menezes, D.; Myers, V.; Song, J.; Feldman, E.W.; Funakoshi, H.; Degeorge, B.R., Jr.; et al. Left ventricular dysfunction in murine models of heart failure and in failing human heart is associated with a selective decrease in the expression of caveolin-3. J. Card. Fail. 2011, 17, 253–263. [Google Scholar] [CrossRef]
- Miake, J.; Marban, E.; Nuss, H.B. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J. Clin. Investig. 2003, 111, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Chemin, J.; Taiakina, V.; Monteil, A.; Piazza, M.; Guan, W.; Stephens, R.F.; Kitmitto, A.; Pang, Z.P.; Dolphin, A.C.; Perez-Reyes, E.; et al. Calmodulin regulates Ca(v)3 T-type channels at their gating brake. J. Biol. Chem. 2017, 292, 20010–20031. [Google Scholar] [CrossRef]
- Markandeya, Y.S.; Phelan, L.J.; Woon, M.T.; Keefe, A.M.; Reynolds, C.R.; August, B.K.; Hacker, T.A.; Roth, D.M.; Patel, H.H.; Balijepalli, R.C. Caveolin-3 Overexpression Attenuates Cardiac Hypertrophy via Inhibition of T-type Ca2+ Current Modulated by Protein Kinase Calpha in Cardiomyocytes. J. Biol. Chem. 2015, 290, 22085–22100. [Google Scholar] [CrossRef]
- Barbuti, A.; Terragni, B.; Brioschi, C.; DiFrancesco, D. Localization of f-channels to caveolae mediates specific beta2-adrenergic receptor modulation of rate in sinoatrial myocytes. J. Mol. Cell Cardiol. 2007, 42, 71–78. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Kuwahara, K.; Takano, M.; Kinoshita, H.; Arai, Y.; Yasuno, S.; Nakagawa, Y.; Igata, S.; Usami, S.; Minami, T.; et al. Increased expression of HCN channels in the ventricular myocardium contributes to enhanced arrhythmicity in mouse failing hearts. J. Am. Heart Assoc. 2013, 2, e000150. [Google Scholar] [CrossRef]
- Kabayama, K.; Sato, T.; Saito, K.; Loberto, N.; Prinetti, A.; Sonnino, S.; Kinjo, M.; Igarashi, Y.; Inokuchi, J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 13678–13683. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Z.; Zhao, J.; Liu, C.; Yao, P.; Zhang, L.; Xie, D.; Lau, W.B.; Tsukuda, J.; Christopher, T.A.; et al. Nitrative Modification of Caveolin-3: A Novel Mechanism of Cardiac Insulin Resistance and a Potential Therapeutic Target Against Ischemic Heart Failure in Prediabetic Animals. Circulation 2023, 147, 1162–1179. [Google Scholar] [CrossRef]
- Pojoga, L.H.; Underwood, P.C.; Goodarzi, M.O.; Williams, J.S.; Adler, G.K.; Jeunemaitre, X.; Hopkins, P.N.; Raby, B.A.; Lasky-Su, J.; Sun, B.; et al. Variants of the caveolin-1 gene: A translational investigation linking insulin resistance and hypertension. J. Clin. Endocrinol. Metab. 2011, 96, E1288–E1292. [Google Scholar] [CrossRef] [PubMed]
- Oshikawa, J.; Otsu, K.; Toya, Y.; Tsunematsu, T.; Hankins, R.; Kawabe, J.; Minamisawa, S.; Umemura, S.; Hagiwara, Y.; Ishikawa, Y. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc. Natl. Acad. Sci. USA 2004, 101, 12670–12675. [Google Scholar] [CrossRef] [PubMed]
- Al Madhoun, A.; Kochumon, S.; Haddad, D.; Thomas, R.; Nizam, R.; Miranda, L.; Sindhu, S.; Bitar, M.S.; Ahmad, R.; Al-Mulla, F. Adipose Tissue Caveolin-1 Upregulation in Obesity Involves TNF-alpha/NF-kappaB Mediated Signaling. Cells 2023, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Nizam, R.; Al-Ozairi, E.; Goodson, J.M.; Melhem, M.; Davidsson, L.; Alkhandari, H.; Al Madhoun, A.; Shamsah, S.; Qaddoumi, M.; Alghanim, G.; et al. Caveolin-1 Variant Is Associated With the Metabolic Syndrome in Kuwaiti Children. Front. Genet. 2018, 9, 689. [Google Scholar] [CrossRef]
- Head, B.P.; Hu, Y.; Finley, J.C.; Saldana, M.D.; Bonds, J.A.; Miyanohara, A.; Niesman, I.R.; Ali, S.S.; Murray, F.; Insel, P.A.; et al. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J. Biol. Chem. 2011, 286, 33310–33321. [Google Scholar] [CrossRef]
- Head, B.P.; Peart, J.N.; Panneerselvam, M.; Yokoyama, T.; Pearn, M.L.; Niesman, I.R.; Bonds, J.A.; Schilling, J.M.; Miyanohara, A.; Headrick, J.; et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS ONE 2010, 5, e15697. [Google Scholar] [CrossRef]
- Tang, W.; Li, Y.; Li, Y.; Wang, Q. Caveolin-1, a novel player in cognitive decline. Neurosci. Biobehav. Rev. 2021, 129, 95–106. [Google Scholar] [CrossRef]
- Ha, T.Y.; Choi, Y.R.; Noh, H.R.; Cha, S.H.; Kim, J.B.; Park, S.M. Age-related increase in caveolin-1 expression facilitates cell-to-cell transmission of alpha-synuclein in neurons. Mol. Brain 2021, 14, 122. [Google Scholar] [CrossRef]
- Sawada, A.; Wang, S.; Jian, M.; Leem, J.; Wackerbarth, J.; Egawa, J.; Schilling, J.M.; Platoshyn, O.; Zemljic-Harpf, A.; Roth, D.M.; et al. Neuron-targeted caveolin-1 improves neuromuscular function and extends survival in SOD1(G93A) mice. FASEB J. 2019, 33, 7545–7554. [Google Scholar] [CrossRef]
- Trushina, E.; Canaria, C.A.; Lee, D.Y.; McMurray, C.T. Loss of caveolin-1 expression in knock-in mouse model of Huntington’s disease suppresses pathophysiology in vivo. Hum. Mol. Genet. 2014, 23, 129–144. [Google Scholar] [CrossRef]
- Trushina, E.; Singh, R.D.; Dyer, R.B.; Cao, S.; Shah, V.H.; Parton, R.G.; Pagano, R.E.; McMurray, C.T. Mutant huntingtin inhibits clathrin-independent endocytosis and causes accumulation of cholesterol in vitro and in vivo. Hum. Mol. Genet. 2006, 15, 3578–3591. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, J.; Klein, D. Caveolin-1, cancer and therapy resistance. Int. J. Cancer 2018, 143, 2092–2104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Senyuk, V.; Ma, K.; Chen, H.; Qin, X.; Li, S.; Liu, Y.; Gentile, S.; Minshall, R.D. Pharmacological Activation of Potassium Channel Kv11.1 with NS1643 Attenuates Triple Negative Breast Cancer Cell Migration by Promoting the Dephosphorylation of Caveolin-1. Cells 2022, 11, 2461. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Chen, L.; Zhang, H.; Wang, Z.; Liu, W.; Xie, X.; Xie, M. Caveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasion. Int. J. Mol. Sci. 2014, 15, 20706–20722. [Google Scholar] [CrossRef]
- Qu, C.; Sun, J.; Liu, Y.; Wang, X.; Wang, L.; Han, C.; Chen, Q.; Guan, T.; Li, H.; Zhang, Y.; et al. Caveolin-1 facilitated KCNA5 expression, promoting breast cancer viability. Oncol. Lett. 2018, 16, 4829–4838. [Google Scholar] [CrossRef]
- Eser Ocak, P.; Ocak, U.; Tang, J.; Zhang, J.H. The role of caveolin-1 in tumors of the brain—Functional and clinical implications. Cell Oncol. 2019, 42, 423–447. [Google Scholar] [CrossRef]
- Kamposioras, K.; Dinas, P.C.; Barriuoso, J.; Trachana, V.; Dimas, K. Caveolin-1 protein expression as a prognostic biomarker of gastrointestinal tumours: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e14065. [Google Scholar] [CrossRef]
- Wiechen, K.; Sers, C.; Agoulnik, A.; Arlt, K.; Dietel, M.; Schlag, P.M.; Schneider, U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am. J. Pathol. 2001, 158, 833–839. [Google Scholar] [CrossRef]
- Chen, X.J.; Bai, Y.T.; Xie, J.R.; Zhou, G. Lipid droplets’ functional protein caveolin-2 is associated with lipid metabolism-related molecule FABP5 and EMT marker E-cadherin in oral epithelial dysplasia. J. Clin. Pathol. 2024, 77, 330–337. [Google Scholar] [CrossRef]
- Bourseau-Guilmain, E.; Menard, J.A.; Lindqvist, E.; Indira Chandran, V.; Christianson, H.C.; Cerezo Magana, M.; Lidfeldt, J.; Marko-Varga, G.; Welinder, C.; Belting, M. Hypoxia regulates global membrane protein endocytosis through caveolin-1 in cancer cells. Nat. Commun. 2016, 7, 11371. [Google Scholar] [CrossRef]
- Diaz, M.I.; Diaz, P.; Bennett, J.C.; Urra, H.; Ortiz, R.; Orellana, P.C.; Hetz, C.; Quest, A.F.G. Caveolin-1 suppresses tumor formation through the inhibition of the unfolded protein response. Cell Death Dis. 2020, 11, 648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, J.; Mo, L.; Lv, X.; Du, Y.; Yang, H. Ion Channel Regulation in Caveolae and Its Pathological Implications. Cells 2025, 14, 631. https://doi.org/10.3390/cells14090631
Huo J, Mo L, Lv X, Du Y, Yang H. Ion Channel Regulation in Caveolae and Its Pathological Implications. Cells. 2025; 14(9):631. https://doi.org/10.3390/cells14090631
Chicago/Turabian StyleHuo, Jianyi, Liangzhu Mo, Xiaojing Lv, Yun Du, and Huaqian Yang. 2025. "Ion Channel Regulation in Caveolae and Its Pathological Implications" Cells 14, no. 9: 631. https://doi.org/10.3390/cells14090631
APA StyleHuo, J., Mo, L., Lv, X., Du, Y., & Yang, H. (2025). Ion Channel Regulation in Caveolae and Its Pathological Implications. Cells, 14(9), 631. https://doi.org/10.3390/cells14090631





