Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis
Abstract
:1. Overview
2. Neuronal Polarity Imposes Structural Constraints on the ER
3. ER-Resident Ca2+ Channels Are Required for the Regulation of Axodendritic Growth and Morphology
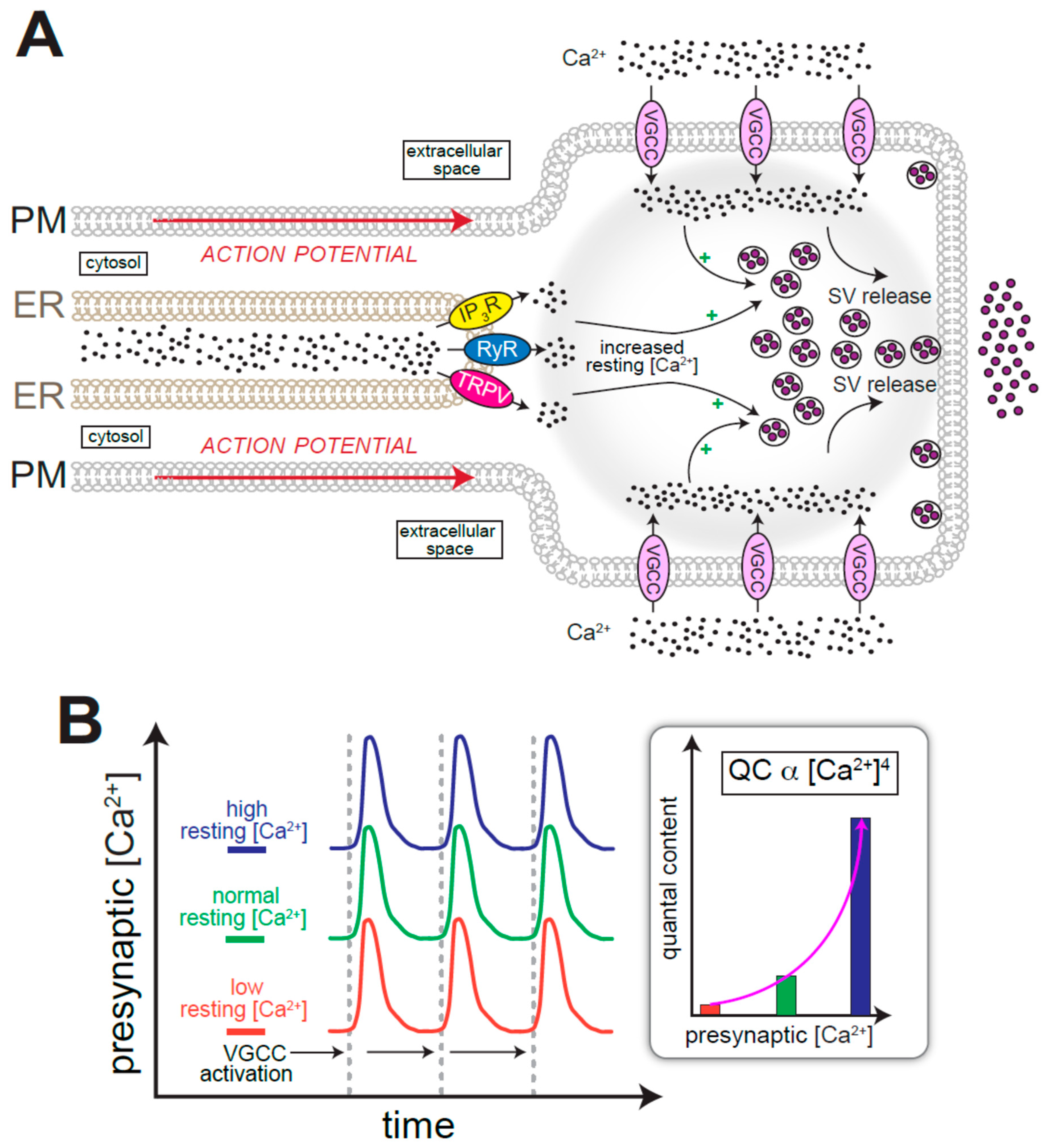
4. ER Ca2+ and the Regulation of Neurotransmission
5. ER Ca2+ Release Regulates Neuronal Gene Expression during Development, Synaptic Plasticity, and Cell Death
6. Relationship of ER Ca2+ Release with Neuronal Bioenergetics and Excitability
7. Mechanisms Involved in the Activation of ER Ca2+ Release in Neurons
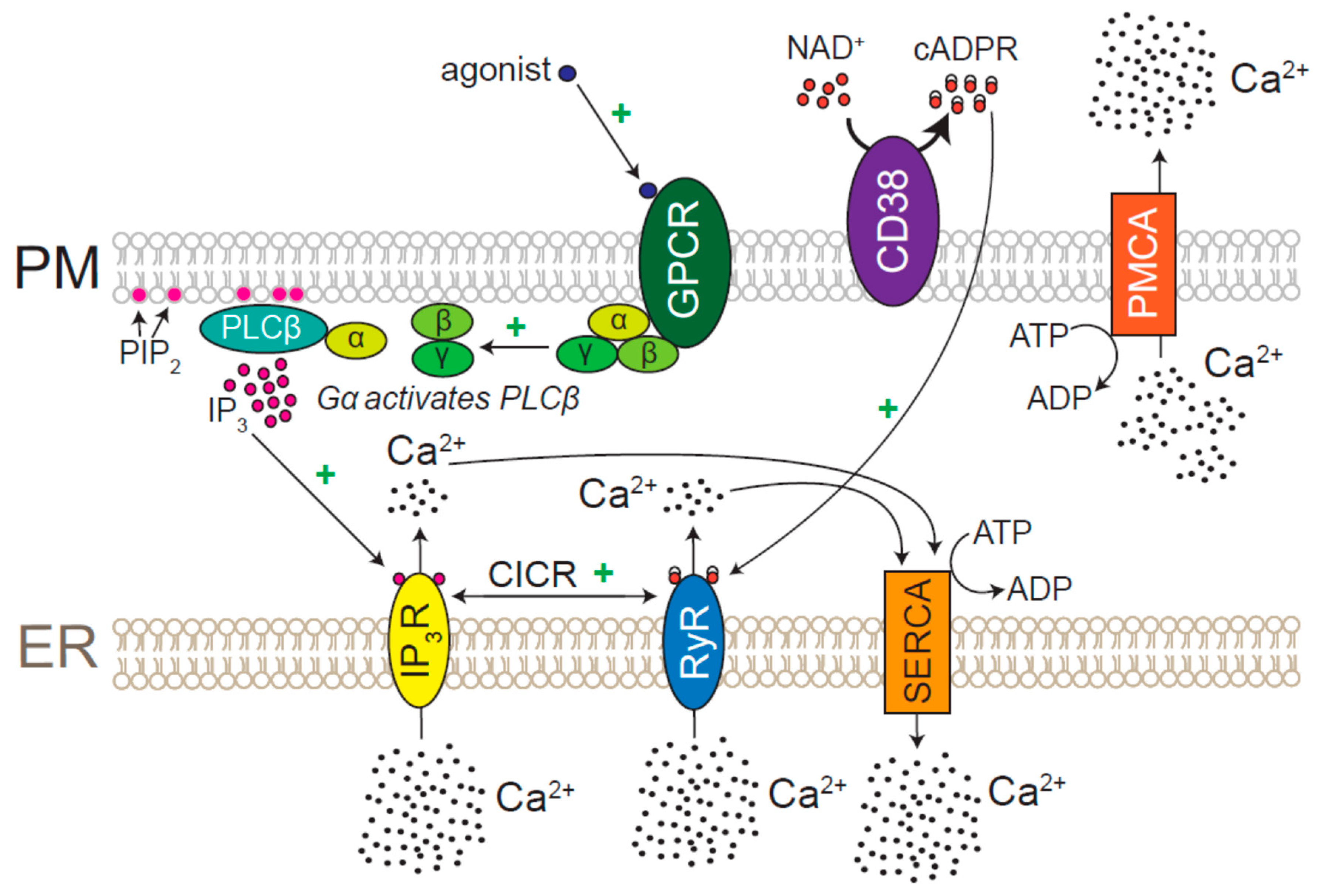
7.1. Role for CICR and Ca2+ Pumps in ER Ca2+ Homeostasis
7.2. Physiological Agonists of IP3Rs and RyRs
7.3. Mechanisms Involved in the Activation of ER-Resident TRP Channels
8. Pathologies Associated with Neuronal ER Ca2+ Dyshomeostasis
8.1. Autism Spectrum Disorder (ASD)
8.2. Lysosomal Storage Diseases (LSDs)
8.3. Neuropsychiatric Diseases
8.4. Peripheral Neuropathies
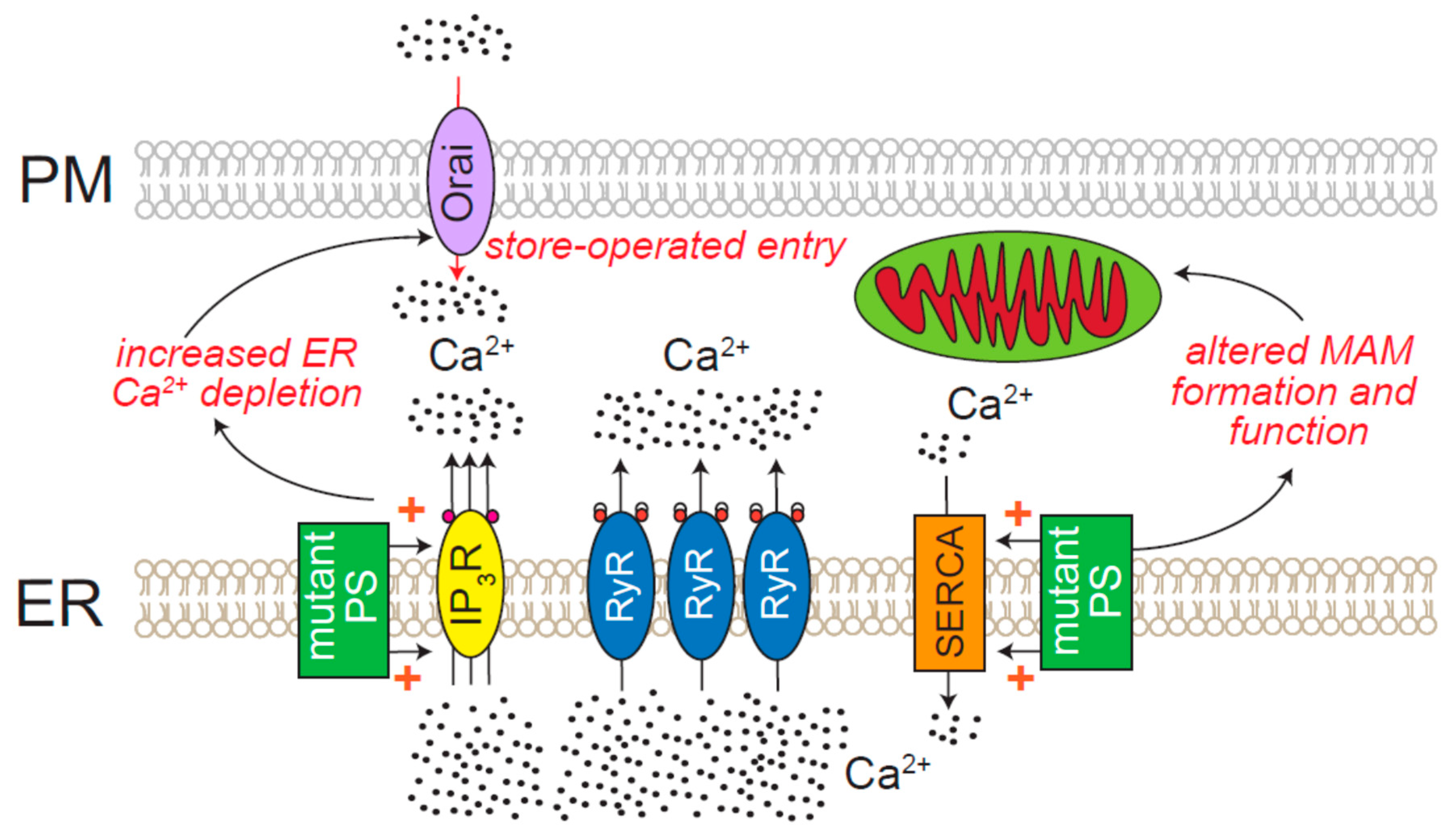
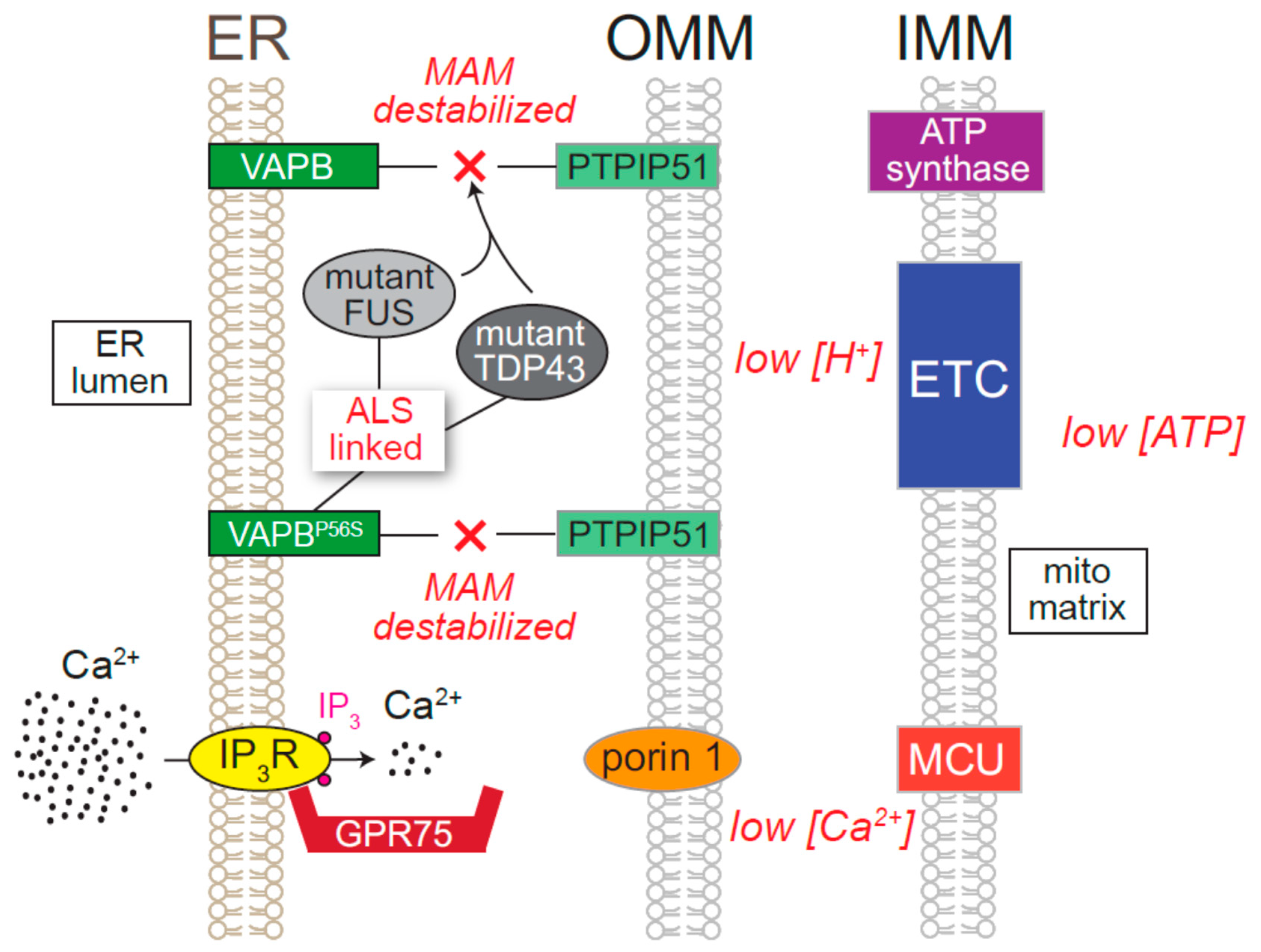
8.5. Neurodegenerative Diseases Directly Attributed to ER Ca2+ Release Channels
8.6. Age-Related Neurodegenerative Diseases
9. Closing Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Sotelo, C. Viewing the brain through the master hand of Ramon y Cajal. Nat. Rev. Neurosci. 2003, 4, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Toescu, E.C.; Verkhratsky, A.; Landfield, P.W. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004, 27, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M. Axonal endoplasmic reticulum is very narrow. J. Cell Sci. 2018, 131, jcs210450. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Shemesh, T.; Kasthuri, N.; Klemm, R.W.; Schalek, R.; Hayworth, K.J.; Hand, A.R.; Yankova, M.; Huber, G.; Lichtman, J.W.; et al. Stacked Endoplasmic Reticulum Sheets Are Connected by Helicoidal Membrane Motifs. Cell 2013, 154, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, M.; Bartol, T.; Sejnowski, T.; Rangamani, P. Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium. J. Gen. Physiol. 2019. [Google Scholar] [CrossRef]
- GRAY, E.G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J. Anat. 1959, 93, 420–433. [Google Scholar]
- Yalçın, B.; Zhao, L.; Stofanko, M.; O’Sullivan, N.C.; Kang, Z.H.; Roost, A.; Thomas, M.R.; Zaessinger, S.; Blard, O.; Patto, A.L.; et al. Modeling of axonal endoplasmic reticulum network by spastic paraplegia proteins. Elife 2017, 6. [Google Scholar] [CrossRef]
- Jedlicka, P.; Vlachos, A.; Schwarzacher, S.W.; Deller, T. A role for the spine apparatus in LTP and spatial learning. Behav. Brain Res. 2008, 192, 12–19. [Google Scholar] [CrossRef]
- Deller, T.; Korte, M.; Chabanis, S.; Drakew, A.; Schwegler, H.; Stefani, G.G.; Zuniga, A.; Schwarz, K.; Bonhoeffer, T.; Zeller, R.; et al. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc. Natl. Acad. Sci. 2003, 100, 10494–10499. [Google Scholar] [CrossRef] [Green Version]
- Spacek, J.; Harris, K.M. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 1997, 17, 190–203. [Google Scholar] [CrossRef]
- Cui-Wang, T.; Hanus, C.; Cui, T.; Helton, T.; Bourne, J.; Watson, D.; Harris, K.M.; Ehlers, M.D. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell 2012, 148, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Hanus, C.; Kochen, L.; tom Dieck, S.; Racine, V.; Sibarita, J.-B.; Schuman, E.M.; Ehlers, M.D. Synaptic Control of Secretory Trafficking in Dendrites. Cell Rep. 2014, 7, 1771–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.M.; Kim, S.H.; Chung, S.; Uhm, D.Y.; Park, M.K. Regional Interaction of Endoplasmic Reticulum Ca2+ Signals between Soma and Dendrites through Rapid Luminal Ca2+ Diffusion. J. Neurosci. 2006, 26, 12127–12136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toresson, H.; Grant, S.G.N. Dynamic distribution of endoplasmic reticulum in hippocampal neuron dendritic spines. Eur. J. Neurosci. 2005, 22, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Hanus, C.; Ehlers, M.D. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr. Opin. Neurobiol. 2016, 39, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Ishikawa, H. Three-dimensional distribution of smooth endoplasmic reticulum in myelinated axons. J. Electron Microsc. (Tokyo) 1976, 25, 141–149. [Google Scholar]
- O’Sullivan, N.C.; Jahn, T.R.; Reid, E.; O’Kane, C.J. Reticulon-like-1, the Drosophila orthologue of the Hereditary Spastic Paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet. 2012, 21, 3356–3365. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, C.; Delgado, R.; Ibacache, A.; Sierralta, J.; Couve, A. Drosophila Atlastin in motor neurons is required for locomotion and presynaptic function. J. Cell Sci. 2017, 130, 3507–3516. [Google Scholar] [CrossRef]
- Zhu, P.-P.; Soderblom, C.; Tao-Cheng, J.-H.; Stadler, J.; Blackstone, C. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum. Mol. Genet. 2006, 15, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Summerville, J.B.; Faust, J.F.; Fan, E.; Pendin, D.; Daga, A.; Formella, J.; Stern, M.; McNew, J.A. The effects of ER morphology on synaptic structure and function in Drosophila melanogaster. J. Cell Sci. 2016, 129, 1635–1648. [Google Scholar] [CrossRef]
- Lim, Y.; Cho, I.-T.; Schoel, L.J.; Cho, G.; Golden, J.A. Hereditary spastic paraplegia-linked REEP1 modulates endoplasmic reticulum/mitochondria contacts. Ann. Neurol. 2015, 78, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Farías, G.G.; Fréal, A.; Tortosa, E.; Stucchi, R.; Pan, X.; Portegies, S.; Will, L.; Altelaar, M.; Hoogenraad, C.C. Feedback-Driven Mechanisms between Microtubules and the Endoplasmic Reticulum Instruct Neuronal Polarity. Neuron 2019, 102, 184–201.e8. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.-O.; Chen, K.; Lin, Y.Q.; Chao, Y.; Duraine, L.; Lu, Z.; Yoon, W.H.; Sullivan, J.M.; Broadhead, G.T.; Sumner, C.J.; et al. A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission. Neuron 2014, 84, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Wada, F.; Nakata, A.; Tatsu, Y.; Ooashi, N.; Fukuda, T.; Nabetani, T.; Kamiguchi, H. Myosin Va and Endoplasmic Reticulum Calcium Channel Complex Regulates Membrane Export during Axon Guidance. Cell Rep. 2016, 15, 1329–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.V.; Mohan, P.S.; Kumar, A.; Yuan, A.; Montagna, L.; Campbell, J.; Veeranna; Espreafico, E.M.; Julien, J.P.; Nixon, R.A. The Myosin Va Head Domain Binds to the Neurofilament-L Rod and Modulates Endoplasmic Reticulum (ER) Content and Distribution within Axons. PLoS ONE 2011, 6, e17087. [Google Scholar] [CrossRef] [PubMed]
- Kittel, R.J.; Wichmann, C.; Rasse, T.M.; Fouquet, W.; Schmidt, M.; Schmid, A.; Wagh, D.A.; Pawlu, C.; Kellner, R.R.; Willig, K.I.; et al. Bruchpilot Promotes Active Zone Assembly, Ca2+ Channel Clustering, and Vesicle Release. Science 2006, 312, 1051–1054. [Google Scholar] [CrossRef]
- Kawasaki, F.; Zou, B.; Xu, X.; Ordway, R.W. Active Zone Localization of Presynaptic Calcium Channels Encoded by the cacophony Locus of Drosophila. J. Neurosci. 2004, 24, 282–285. [Google Scholar] [CrossRef]
- Koenig, J.H.; Ikeda, K. Contribution of Active Zone Subpopulation of Vesicles to Evoked and Spontaneous Release. J. Neurophysiol. 1999, 81, 1495–1505. [Google Scholar] [CrossRef]
- Pennetta, G.; Hiesinger, P.R.; Fabian-Fine, R.; Meinertzhagen, I.A.; Bellen, H.J. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron 2002, 35, 291–306. [Google Scholar] [CrossRef]
- Franco, B.; Bogdanik, L.; Bobinnec, Y.; Debec, A.; Bockaert, J.; Parmentier, M.-L.; Grau, Y. Shaggy, the Homolog of Glycogen Synthase Kinase 3, Controls Neuromuscular Junction Growth in Drosophila. J. Neurosci. 2004, 24, 6573–6577. [Google Scholar] [CrossRef]
- Roos, J.; Hummel, T.; Ng, N.; Klämbt, C.; Davis, G.W. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 2000, 26, 371–382. [Google Scholar] [CrossRef]
- Gögel, S.; Wakefield, S.; Tear, G.; Klämbt, C.; Gordon-Weeks, P.R. The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3β phosphorylation site in MAP1B. Mol. Cell. Neurosci. 2006, 33, 188–199. [Google Scholar] [CrossRef]
- Gong, Z. Two Interdependent TRPV Channel Subunits, Inactive and Nanchung, Mediate Hearing in Drosophila. J. Neurosci. 2004, 24, 9059–9066. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [Green Version]
- Nordman, J.C.; Kabbani, N. Microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation of a Gαq and IP 3 receptor pathway. FASEB J. 2014, 28, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Pavez, M.; Thompson, A.C.; Arnott, H.J.; Mitchell, C.B.; D’Atri, I.; Don, E.K.; Chilton, J.K.; Scott, E.K.; Lin, J.Y.; Young, K.M.; et al. STIM1 Is Required for Remodeling of the Endoplasmic Reticulum and Microtubule Cytoskeleton in Steering Growth Cones. J. Neurosci. 2019, 39, 5095–5114. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Nishiyama, M.; Henley, J.; Tessier-Lavigne, M.; Poo, M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 2000, 403, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H.; Berninger, B.; Inagaki, N.; Tessier-Lavigne, M.; Poo, M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 1999, 23, 139–148. [Google Scholar] [CrossRef]
- Grigoriev, I.; Gouveia, S.M.; van der Vaart, B.; Demmers, J.; Smyth, J.T.; Honnappa, S.; Splinter, D.; Steinmetz, M.O.; Putney, J.W.; Hoogenraad, C.C.; et al. STIM1 Is a MT-Plus-End-Tracking Protein Involved in Remodeling of the ER. Curr. Biol. 2008, 18, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Asanov, A.; Sherry, R.; Sampieri, A.; Vaca, L. A relay mechanism between EB1 and APC facilitate STIM1 puncta assembly at endoplasmic reticulum–plasma membrane junctions. Cell Calcium 2013, 54, 246–256. [Google Scholar] [CrossRef]
- Shim, S.; Zheng, J.Q.; Ming, G. A critical role for STIM1 in filopodial calcium entry and axon guidance. Mol. Brain 2013, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.B.; Gasperini, R.J.; Small, D.H.; Foa, L. STIM1 is necessary for store-operated calcium entry in turning growth cones. J. Neurochem. 2012, 122, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, P.J.; Wild, A.R.; Dell’Acqua, M.L.; Sather, W.A. STIM1 Ca2+ Sensor Control of L-type Ca2+-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. Cell Rep. 2017, 19, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Tshuva, R.Y.; Korkotian, E.; Segal, M. ORAI1-dependent synaptic plasticity in rat hippocampal neurons. Neurobiol. Learn. Mem. 2017, 140, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pchitskaya, E.; Kraskovskaya, N.; Chernyuk, D.; Popugaeva, E.; Zhang, H.; Vlasova, O.; Bezprozvanny, I. Stim2-Eb3 Association and Morphology of Dendritic Spines in Hippocampal Neurons. Sci. Rep. 2017, 7, 17625. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Brenowitz, S.D.; Hammer, J.A. Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat. Cell Biol. 2011, 13, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Langford, G.M. ER transport on actin filaments in squid giant axon: implications for signal transduction at synapse. FASEB J. 1999, 13, S248–S250. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and Regulation of Voltage-Gated Ca2+ Channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Dittman, J.S.; Ryan, T.A. The control of release probability at nerve terminals. Nat. Rev. Neurosci. 2019, 20, 177–186. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [Green Version]
- Awatramani, G.B.; Price, G.D.; Trussell, L.O. Modulation of Transmitter Release by Presynaptic Resting Potential and Background Calcium Levels. Neuron 2005, 48, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Peskin, C.S. Improved signaling as a result of randomness in synaptic vesicle release. Proc. Natl. Acad. Sci. USA 2015, 112, 14954–14959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, F.A.; Rahamimoff, R.; Rahamimoff, R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 1967, 193, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.; Beglopoulos, V.; Wines-Samuelson, M.; Zhang, D.; Dragatsis, I.; Südhof, T.C.; Shen, J. Presenilins are essential for regulating neurotransmitter release. Nature 2009, 460, 632–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakroborty, S.; Kim, J.; Schneider, C.; Jacobson, C.; Molgo, J.; Stutzmann, G.E. Early Presynaptic and Postsynaptic Calcium Signaling Abnormalities Mask Underlying Synaptic Depression in Presymptomatic Alzheimer’s Disease Mice. J. Neurosci. 2012, 32, 8341–8353. [Google Scholar] [CrossRef] [PubMed]
- Bardo, S.; Robertson, B.; Stephens, G.J. Presynaptic internal Ca2+ stores contribute to inhibitory neurotransmitter release onto mouse cerebellar Purkinje cells. Br. J. Pharmacol. 2002, 137, 529–537. [Google Scholar] [CrossRef]
- Galante, M.; Marty, A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J. Neurosci. 2003, 23, 11229–11234. [Google Scholar] [CrossRef]
- Unni, V.K. Calcium Release from Presynaptic Ryanodine-Sensitive Stores Is Required for Long-Term Depression at Hippocampal CA3-CA3 Pyramidal Neuron Synapses. J. Neurosci. 2004, 24, 9612–9622. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Yamaguchi, H.; Lai, F.A.; Shen, J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 15091–15096. [Google Scholar] [CrossRef] [Green Version]
- De Juan-Sanz, J.; Holt, G.T.; Schreiter, E.R.; de Juan, F.; Kim, D.S.; Ryan, T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 2017, 93, 867–881.e6. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Van Hook, M.J.; Thoreson, W.B. Ca2+ Diffusion through Endoplasmic Reticulum Supports Elevated Intraterminal Ca2+ Levels Needed to Sustain Synaptic Release from Rods in Darkness. J. Neurosci. 2015, 35, 11364–11373. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Križaj, D.; Thoreson, W.B. Intracellular calcium stores drive slow non-ribbon vesicle release from rod photoreceptors. Front. Cell. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emptage, N.J.; Reid, C.A.; Fine, A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 2001, 29, 197–208. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.H.; Choi, I.S.; Park, H.M.; Lee, M.G.; Choi, B.J.; Jang, I.S. Pregnenolone sulfate enhances spontaneous glutamate release by inducing presynaptic Ca2+-induced Ca2+ release. Neuroscience 2010, 171, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.-H.; Mei, L.; Mak, D.-O.D.; Hayashi, I.; Iwatsubo, T.; Kang, D.E.; Foskett, J.K. Gain-of-Function Enhancement of IP3 Receptor Modal Gating by Familial Alzheimer’s Disease-Linked Presenilin Mutants in Human Cells and Mouse Neurons. Sci. Signal. 2010, 3, ra22. [Google Scholar] [CrossRef] [PubMed]
- Ringsevjen, H.; Umbach Hansen, H.M.; Hussain, S.; Hvalby, Ø.; Jensen, V.; Walaas, S.I.; Davanger, S. Presynaptic increase in IP3 receptor type 1 concentration in the early phase of hippocampal synaptic plasticity. Brain Res. 2019, 1706, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.G.; Vogt, K.E.; Foster, K.A.; Regehr, W.G. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J. Neurosci. 2002, 22, 21–28. [Google Scholar] [CrossRef]
- Kikuma, K.; Li, X.; Kim, D.; Sutter, D.; Dickman, D.K. Extended Synaptotagmin Localizes to Presynaptic ER and Promotes Neurotransmission and Synaptic Growth in Drosophila. Genetics 2017, 207, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.F. Presynaptic Calcium Channels and the Depletion of Synaptic Cleft Calcium Ions. J. Neurophysiol. 2000, 83, 477–482. [Google Scholar] [CrossRef]
- Rusakov, D.A.; Fine, A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 2003, 37, 287–297. [Google Scholar] [CrossRef]
- Rabl, K.; Thoreson, W.B. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur. J. Neurosci. 2002, 16, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.G.; Sakmann, B. Depletion of calcium in the synaptic cleft of a calyx-type synapse in the rat brainstem. J. Physiol. 1999, 521 Pt 1, 123–133. [Google Scholar] [CrossRef]
- Gissel, C.; Doutheil, J.; Paschen, W. Temporal Analysis of Changes in Neuronal c-fos mRNA Levels Induced by Depletion of Endoplasmic Reticulum Calcium Stores: Effect of Clamping Cytoplasmic Calcium Activity at Resting Levels. J. Neurochem. 2002, 69, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Paschen, W.; Mengesdorf, T. Conditions associated with ER dysfunction activate homer 1a expression. J. Neurochem. 2003, 86, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.; Hardy, A.B.; Wheeler, M.B.; Belsham, D.D. Serotonin (5-HT) activation of immortalized hypothalamic neuronal cells through the 5-HT1B serotonin receptor. Endocrinology 2012, 153, 4862–4873. [Google Scholar] [CrossRef]
- Zhang, W.; Tingare, A.; Ng, D.C.-H.; Johnson, H.W.; Schell, M.J.; Lord, R.L.; Chawla, S. IP3-dependent intracellular Ca2+ release is required for cAMP-induced c-fos expression in hippocampal neurons. Biochem. Biophys. Res. Commun. 2012, 425, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, D.; Casadio, A.; Karl, K.A.; Serodio, P.; Kandel, E.R. CREB1 Encodes a Nuclear Activator, a Repressor, and a Cytoplasmic Modulator that Form a Regulatory Unit Critical for Long-Term Facilitation. Cell 1998, 95, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Kornhauser, J.M.; Cowan, C.W.; Shaywitz, A.J.; Dolmetsch, R.E.; Griffith, E.C.; Hu, L.S.; Haddad, C.; Xia, Z.; Greenberg, M.E. CREB Transcriptional Activity in Neurons Is Regulated by Multiple, Calcium-Specific Phosphorylation Events. Neuron 2002, 34, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Bito, H.; Deisseroth, K.; Tsien, R.W. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 1996, 87, 1203–1214. [Google Scholar] [CrossRef]
- Sheng, M.; McFadden, G.; Greenberg, M.E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 1990, 4, 571–582. [Google Scholar] [CrossRef]
- Nakamura, T.; Barbara, J.-G.; Nakamura, K.; Ross, W.N. Synergistic Release of Ca2+ from IP3-Sensitive Stores Evoked by Synaptic Activation of mGluRs Paired with Backpropagating Action Potentials. Neuron 1999, 24, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Jie, W.; Huang, L.; Wei, P.; Li, S.; Luo, Z.; Friedman, A.K.; Meredith, A.L.; Han, M.-H.; Zhu, X.-H.; et al. Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat. Neurosci. 2014, 17, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardingham, G.E.; Arnold, F.J.L.; Bading, H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 2001, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Chawla, S.; Johnson, C.M.; Bading, H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 1997, 385, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, C.P.; Bading, H. Nuclear Calcium Signaling. In Advances in Experimental Medicine and Biology; Springer: Vienna, Austria, 2012; Volume 970, pp. 377–405. [Google Scholar]
- Power, J.M.; Sah, P. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J. Neurosci. 2002, 22, 3454–3462. [Google Scholar] [CrossRef] [PubMed]
- Chamero, P.; Manjarres, I.M.; García-Verdugo, J.M.; Villalobos, C.; Alonso, M.T.; García-Sancho, J. Nuclear calcium signaling by inositol trisphosphate in GH3 pituitary cells. Cell Calcium 2008, 43, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.F.; Thrower, E.C.; Echevarria, W.; Koulen, P.; Hirata, K.; Bennett, A.M.; Ehrlich, B.E.; Nathanson, M.H. Nuclear and cytosolic calcium are regulated independently. Proc. Natl. Acad. Sci. USA 2003, 100, 2975–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, J.-P.; Matter, N.; Artault, J.-C.; Köppler, P.; Malviya, A.N. Inositol 1,4,5-Trisphosphate Receptor Is Located to the Inner Nuclear Membrane Vindicating Regulation of Nuclear Calcium Signaling by Inositol 1,4,5-Trisphosphate. J. Biol. Chem. 1996, 271, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Enslen, H.; Myung, P.S.; Maurer, R.A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994, 8, 2527–2539. [Google Scholar] [CrossRef]
- Xing, J.; Ginty, D.D.; Greenberg, M.E.; Bading, H. Coupling of the RAS-MAPK Pathway to Gene Activation by RSK2, a Growth Factor-Regulated CREB Kinase. Science 1996, 273, 959–963. [Google Scholar] [CrossRef]
- Carrasco, M.A.; Jaimovich, E.; Kemmerling, U.; Hidalgo, C. Signal transduction and gene expression regulated by calcium release from internal stores in excitable cells. Biol. Res. 2004, 37, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.-H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Giltnane, J.; Dolmetsch, R.; Staudt, L.M.; Rao, A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.; Shaw, K.T.-Y.; Carew, J.; Viola, J.P.B.; Luo, C.; Perrino, B.A.; Rao, A. Calcineurin Binds the Transcription Factor NFAT1 and Reversibly Regulates Its Activity. J. Biol. Chem. 1996, 271, 10884–10891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, J.; McCafffrey, P.G.; Miner, Z.; Kerppola, T.K.; Lambert, J.N.; Verdine, G.L.; Curran, T.; Rao, A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 1993, 365, 352–355. [Google Scholar] [CrossRef]
- Groth, R.D.; Mermelstein, P.G. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J. Neurosci. 2003, 23, 8125–8134. [Google Scholar] [CrossRef]
- Hernández-Ochoa, E.O.; Contreras, M.; Cseresnyés, Z.; Schneider, M.F. Ca2+ signal summation and NFATc1 nuclear translocation in sympathetic ganglion neurons during repetitive action potentials. Cell Calcium 2007, 41, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Usachev, Y.M. Mitochondrial Ca2+ Cycling Facilitates Activation of the Transcription Factor NFAT in Sensory Neurons. J. Neurosci. 2009, 29, 12101–12114. [Google Scholar] [CrossRef]
- Nguyen, T.; Lindner, R.; Tedeschi, A.; Forsberg, K.; Green, A.; Wuttke, A.; Gaub, P.; Di Giovanni, S. NFAT-3 Is a Transcriptional Repressor of the Growth-associated Protein 43 during Neuronal Maturation. J. Biol. Chem. 2009, 284, 18816–18823. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.; Franciscovich, A.; Bowers, M.; Sandstrom, D.J.; Sanyal, S. NFAT regulates pre-synaptic development and activity-dependent plasticity in Drosophila. Mol. Cell. Neurosci. 2011, 46, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Sintes, R.; Lucas, J.J. NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. J. Clin. Invest. 2010, 120, 2432–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanthi, S.; Deng, X.; Ladenheim, B.; McCoy, M.T.; Cluster, A.; Cai, N.-S.; Cadet, J.L. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 868–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blalock, E.M.; Chen, K.-C.; Sharrow, K.; Herman, J.P.; Porter, N.M.; Foster, T.C.; Landfield, P.W. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 2003, 23, 3807–3819. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Choi, J.K.; Shin, H.Y.; Jeon, Y.C.; Jeong, B.H.; Lee, H.G.; Kim, J.I.; Choi, E.K.; Carp, R.I.; Kim, Y.S. Altered expression of type 1 inositol 1,4,5-trisphosphate receptor in the Ngsk Prnp deficient mice. Neuroscience 2010, 167, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.A.; Carafoli, E.; Guerini, D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 5797–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, N.; deTombe, P.P.; Mignery, G.A. Calcineurin-NFATc Regulates Type 2 Inositol 1,4,5-Trisphosphate Receptor (InsP3R2) Expression during Cardiac Remodeling. J. Biol. Chem. 2014, 289, 6188. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, H.; Pathan, A.R.; Daalis, A.; Hubrack, S.; Abou-Jassoum, H.; Al-Naeimi, H.; Rusch, N.J.; Machaca, K. Inositol 1,4,5-Trisphosphate (IP3) Receptor Up-regulation in Hypertension Is Associated with Sensitization of Ca2+ Release and Vascular Smooth Muscle Contractility. J. Biol. Chem. 2013, 288, 32941–32951. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Breuer, M.E.; Koopman, W.J.; Koene, S.; Nooteboom, M.; Rodenburg, R.J.; Willems, P.H.; Smeitink, J.A.M. The role of mitochondrial OXPHOS dysfunction in the development of neurologic diseases. Neurobiol. Dis. 2013, 51, 27–34. [Google Scholar] [CrossRef]
- McCormack, J.G.; Denton, R.M. The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. Biochem. Soc. Trans. 1993, 21, 793–799. [Google Scholar] [CrossRef]
- Duchen, M.R. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 1992, 283 Pt 1, 41–50. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta - Bioenerg. 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchi, S.; Pinton, P. The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. J. Physiol. 2014, 592, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Lnenicka, G.A.; Grizzaffi, J.; Lee, B.; Rumpal, N. Ca2+ Dynamics along Identified Synaptic Terminals in Drosophila Larvae. J. Neurosci. 2006, 26, 12283–12293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, A.K.; Ivannikov, M.V.; Lu, Z.; Sugimori, M.; Llinas, R.R.; Macleod, G.T. Cytosolic Calcium Coordinates Mitochondrial Energy Metabolism with Presynaptic Activity. J. Neurosci. 2012, 32, 1233–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S. The ER–mitochondria interface: The social network of cell death. Biochim. Biophys. Acta - Mol. Cell Res. 2012, 1823, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decuypere, J.-P.; Monaco, G.; Bultynck, G.; Missiaen, L.; De Smedt, H.; Parys, J.B. The IP3 receptor–mitochondria connection in apoptosis and autophagy. Biochim. Biophys. Acta - Mol. Cell Res. 2011, 1813, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.-H.; Yang, J.; Parker, I.; et al. Essential Regulation of Cell Bioenergetics by Constitutive InsP3 Receptor Ca2+ Transfer to Mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiel, C.; Lallet-Daher, H.; Gitenay, D.; Gras, B.; Le Calvé, B.; Augert, A.; Ferrand, M.; Prevarskaya, N.; Simonnet, H.; Vindrieux, D.; et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, Q.; Wang, Q.; Ding, Y.; Ma, Z.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.-H. Binding of FUN14 Domain Containing 1 With Inositol 1,4,5-Trisphosphate Receptor in Mitochondria-Associated Endoplasmic Reticulum Membranes Maintains Mitochondrial Dynamics and Function in Hearts in Vivo. Circulation 2017, 136, 2248–2266. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Simpson, A.W.M. Elevation of Mitochondrial Calcium by Ryanodine-sensitive Calcium-induced Calcium Release. J. Biol. Chem. 2000, 275, 23661–23665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, A.; Matute, C.; Alberdi, E. Endoplasmic reticulum Ca2+ release through ryanodine and IP3 receptors contributes to neuronal excitotoxicity. Cell Calcium 2009, 46, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Liou, B.; Peng, Y.; Li, R.; Inskeep, V.; Zhang, W.; Quinn, B.; Dasgupta, N.; Blackwood, R.; Setchell, K.D.R.; Fleming, S.; et al. Modulating ryanodine receptors with dantrolene attenuates neuronopathic phenotype in Gaucher disease mice. Hum. Mol. Genet. 2016, ddw322. [Google Scholar] [CrossRef] [PubMed]
- Fergestad, T.; Bostwick, B.; Ganetzky, B. Metabolic disruption in drosophila bang-sensitive seizure mutants. Genetics 2006, 173, 1357–1364. [Google Scholar] [CrossRef]
- Le Masson, G.; Przedborski, S.; Abbott, L.F. A Computational Model of Motor Neuron Degeneration. Neuron 2014, 83, 975–988. [Google Scholar] [CrossRef] [Green Version]
- Stutzmann, G.E.; LaFerla, F.M.; Parker, I. Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate. J. Neurosci. 2003, 23, 758–765. [Google Scholar] [CrossRef]
- Ryu, C.; Jang, D.C.; Jung, D.; Kim, Y.G.; Shim, H.G.; Ryu, H.-H.; Lee, Y.-S.; Linden, D.J.; Worley, P.F.; Kim, S.J. STIM1 Regulates Somatic Ca2+ Signals and Intrinsic Firing Properties of Cerebellar Purkinje Neurons. J. Neurosci. 2017, 37, 8876–8894. [Google Scholar] [CrossRef]
- Stutzmann, G.E.; Mattson, M.P. Endoplasmic reticulum Ca 2+ handling in excitable cells in health and disease. Pharmacol. Rev. 2011, 63, 700–727. [Google Scholar] [CrossRef] [PubMed]
- Kato, B.M.; Rubel, E.W. Glutamate Regulates IP3-Type and CICR Stores in the Avian Cochlear Nucleus. J. Neurophysiol. 1999, 81, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Neymotin, S.A.; McDougal, R.A.; Sherif, M.A.; Fall, C.P.; Hines, M.L.; Lytton, W.W. Neuronal Calcium Wave Propagation Varies with Changes in Endoplasmic Reticulum Parameters: A Computer Model. Neural Comput. 2015, 27, 898–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesch, R.E.; Weinreich, D.; Kao, J.P.Y. Localized IP3-Evoked Ca2+ Release Activates a K+ Current in Primary Vagal Sensory Neurons. J. Neurophysiol. 2004, 91, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Keizer, J.; Levine, L. Ryanodine receptor adaptation and Ca2+(-)induced Ca2+ release-dependent Ca2+ oscillations. Biophys. J. 1996, 71, 3477–3487. [Google Scholar] [CrossRef] [Green Version]
- Zahradník, I.; Györke, S.; Zahradníková, A. Calcium Activation of Ryanodine Receptor Channels—Reconciling RyR Gating Models with Tetrameric Channel Structure. J. Gen. Physiol. 2005, 126, 515. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.; Keizer, J.; Sanderson, M.J. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J. 1995, 9, 1463–1472. [Google Scholar] [CrossRef]
- Politi, A.; Gaspers, L.D.; Thomas, A.P.; Höfer, T. Models of IP3 and Ca2+ oscillations: frequency encoding and identification of underlying feedbacks. Biophys. J. 2006, 90, 3120–3133. [Google Scholar] [CrossRef]
- Tang, Y.; Othmer, H.G. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys. J. 1994, 67, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Tu, L.; Chen, D.; Tan, T.; Wang, Y.; Wang, S. TRPV4 channels stimulate Ca2+-induced Ca2+ release in mouse neurons and trigger endoplasmic reticulum stress after intracerebral hemorrhage. Brain Res. Bull. 2019, 146, 143–152. [Google Scholar] [CrossRef]
- Richter, T.A.; Kolaj, M.; Renaud, L.P. Low Voltage-Activated Ca2+ Channels Are Coupled to Ca2+-Induced Ca2+ Release in Rat Thalamic Midline Neurons. J. Neurosci. 2005, 25, 8267–8271. [Google Scholar] [CrossRef] [PubMed]
- Usachev, Y.M.; Thayer, S.A. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J. Neurosci. 1997, 17, 7404–7414. [Google Scholar] [CrossRef] [PubMed]
- Groten, C.J.; Rebane, J.T.; Hodgson, H.M.; Chauhan, A.K.; Blohm, G.; Magoski, N.S. Ca2+ removal by the plasma membrane Ca2+-ATPase influences the contribution of mitochondria to activity-dependent Ca2+ dynamics in Aplysia neuroendocrine cells. J. Neurophysiol. 2016, 115, 2615–2634. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Nishizawa, M.; Onodera, O. Roles of inositol 1,4,5-trisphosphate receptors in spinocerebellar ataxias. Neurochem. Int. 2016, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Hosoi, E.; Bando, M.; Sakata-Haga, H.; Lee, N.-S.; Jeong, Y.-G.; Fukui, Y. Differential alterations in expressions of ryanodine receptor subtypes in cerebellar cortical neurons of an ataxic mutant, rolling mouse Nagoya. Neuroscience 2008, 152, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H.; Salmina, A.B.; Olovyannikova, R.Y.; Hashii, M.; Yokoyama, S.; Koizumi, K.; Jin, D.; Liu, H.-X.; Lopatina, O.; Amina, S.; et al. Cyclic ADP-ribose as a universal calcium signal molecule in the nervous system. Neurochem. Int. 2007, 51, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Litosch, I. Decoding Gαq signaling. Life Sci. 2016, 152, 99–106. [Google Scholar] [CrossRef]
- Kang, D.-S.; Yang, Y.R.; Lee, C.; Kim, S.; Ryu, S.H.; Suh, P.-G. Roles of phosphoinositide-specific phospholipase Cγ1 in brain development. Adv. Biol. Regul. 2016, 60, 167–173. [Google Scholar] [CrossRef]
- Sandal, M.; Paltrinieri, D.; Carloni, P.; Musiani, F.; Giorgetti, A. Structure/function relationships of phospholipases C Beta. Curr. Protein Pept. Sci. 2013, 14, 650–657. [Google Scholar] [CrossRef]
- Pawelczyk, T.; Matecki, A. Localization of phospholipase C delta3 in the cell and regulation of its activity by phospholipids and calcium. Eur. J. Biochem. 1998, 257, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, T.-J.; Lee, Y.H.; Baek, K.J.; Suh, P.-G.; Ryu, S.H.; Kim, K.-T. Phospholipase C-δ1 Is Activated by Capacitative Calcium Entry That Follows Phospholipase C-β Activation upon Bradykinin Stimulation. J. Biol. Chem. 1999, 274, 26127–26134. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, Y.; Zhang, Z.; Cui, K.; Wang, S.; Yuan, X.; Wu, C.; Poo, M.; Duan, S. Nerve growth cone guidance mediated by G protein–coupled receptors. Nat. Neurosci. 2002, 5, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ding, Y.-Q.; Hong, Y.; Feng, Z.; Navarre, S.; Xi, C.-X.; Zhu, X.-J.; Wang, C.-L.; Ackerman, S.L.; Kozlowski, D.; et al. Phosphatidylinositol transfer protein-α in netrin-1-induced PLC signalling and neurite outgrowth. Nat. Cell Biol. 2005, 7, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lopez, S.; Tkatch, T.; Perez-Garci, E.; Galarraga, E.; Bargas, J.; Hamm, H.; Surmeier, D.J. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J. Neurosci. 2000, 20, 8987–8995. [Google Scholar] [CrossRef] [PubMed]
- Cruzblanca, H.; Koh, D.-S.; Hille, B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 7151–7156. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.C.; Nichols, D.E. Serotonin 5-HT 2A receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J. Neurochem. 2006, 99, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, V.; Girardot, F.; Audin, W.; Tricoire, H. Control of oxidative stress resistance by IP3 kinase in Drosophila melanogaster. Free Radic. Biol. Med. 2002, 33, 1250–1259. [Google Scholar] [CrossRef]
- Galione, A.; McDougall, A.; Busa, W.; Willmott, N.; Gillot, I.; Whitaker, M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science 1993, 261, 348–352. [Google Scholar] [CrossRef]
- Mészáros, L.G.; Bak, J.; Chu, A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature 1993, 364, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Sitsapesan, R.; McGarry, S.J.; Williams, A.J. Cyclic ADP-ribose, the ryanodine receptor and Ca2+ release. Trends Pharmacol. Sci. 1995, 16, 386–391. [Google Scholar] [CrossRef]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, J.; Wei, W.; Lam, C.M.C.; Zhao, Y.-J.; Dong, M.; Zhang, L.-R.; Zhang, L.-H.; Lee, H.-C. CD38/cADPR/Ca2+ pathway promotes cell proliferation and delays nerve growth factor-induced differentiation in PC12 cells. J. Biol. Chem. 2009, 284, 29335–29342. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Aarhus, R.; Graeff, R.; Gurnack, M.E.; Walseth, T.F. Cyclic ADP ribose activation of the ryanodine receptor is mediated by calmodulin. Nature 1994, 370, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tashjian, A.H. Calmodulin is a selective mediator of Ca(2+)-induced Ca2+ release via the ryanodine receptor-like Ca2+ channel triggered by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1995, 92, 3244–3248. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Summerhill, R.J.; Fruen, B.R.; Churchill, G.C.; Galione, A. Calmodulin dissociation mediates desensitization of the cADPR-induced Ca2+ release mechanism. Curr. Biol. 2002, 12, 2018–2022. [Google Scholar] [CrossRef]
- Pollock, J.; Crawford, J.H.; Wootton, J.F.; Seabrook, G.R.; Scott, R.H. Metabotropic glutamate receptor activation and intracellular cyclic ADP-ribose release Ca2+from the same store in cultured DRG neurones. Cell Calcium 1999, 26, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.H.; Wootton, J.F.; Seabrook, G.R.; Scott, R.H. Activation of Ca2+-Dependent Currents in Dorsal Root Ganglion Neurons by Metabotropic Glutamate Receptors and Cyclic ADP-Ribose Precursors. J. Neurophysiol. 1997, 77, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Budde, T.; Sieg, F.; Braunewell, K.H.; Gundelfinger, E.D.; Pape, H.C. Ca2+-induced Ca2+ release supports the relay mode of activity in thalamocortical cells. Neuron 2000, 26, 483–492. [Google Scholar] [CrossRef]
- Reyes-Harde, M.; Potter, B.V.L.; Galione, A.; Stanton, P.K. Induction of Hippocampal LTD Requires Nitric-Oxide-Stimulated PKG Activity and Ca 2+ Release from Cyclic ADP-Ribose-Sensitive Stores. J. Neurophysiol. 1999, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Harde, M.; Empson, R.; Potter, B.V.L.; Galione, A.; Stanton, P.K. Evidence of a role for cyclic ADP-ribose in long-term synaptic depression in hippocampus. Proc. Natl. Acad. Sci. USA 1999, 96, 4061–4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mothet, J.-P.; Fossier, P.; Meunier, F.-M.; Stinnakre, J.; Tauc, L.; Baux, G. Cyclic ADP-ribose and calcium-induced calcium release regulate neurotransmitter release at a cholinergic synapse of Aplysia. J. Physiol. 1998, 507, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, M.; Benzing, T.; Simmen, T.; Tauber, R.; Buchholz, B.; Feliciangeli, S.; Huber, T.B.; Schermer, B.; Kramer-Zucker, A.; Höpker, K.; et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005, 24, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidaux, G.; Gordienko, D.; Shapovalov, G.; Farfariello, V.; Borowiec, A.; Iamshanova, O.; Lemonnier, L.; Gueguinou, M.; Guibon, R.; Fromont, G.; et al. 4TM-TRPM8 channels are new gatekeepers of the ER-mitochondria Ca2+ transfer. Biochim. Biophys. Acta - Mol. Cell Res. 2018, 1865, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Koulen, P.; Cai, Y.; Geng, L.; Maeda, Y.; Nishimura, S.; Witzgall, R.; Ehrlich, B.E.; Somlo, S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002, 4, 191–197. [Google Scholar] [CrossRef]
- Wegierski, T.; Steffl, D.; Kopp, C.; Tauber, R.; Buchholz, B.; Nitschke, R.; Kuehn, E.W.; Walz, G.; Köttgen, M. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009, 28, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Agosto, M.A.; Anastassov, I.A.; Robichaux, M.A.; Wensel, T.G. A Large Endoplasmic Reticulum-Resident Pool of TRPM1 in Retinal ON-Bipolar Cells. eNeuro 2018, 5, ENEURO.0143-18.2018. [Google Scholar] [CrossRef]
- Imler, E.; Zinsmaier, K.E. TRPV1 Channels: Not So Inactive on the ER. Neuron 2014, 84, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Wisnoskey, B.J.; Sinkins, W.G.; Schilling, W.P. Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem. J. 2003, 372, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Lotteau, S.; Ducreux, S.; Romestaing, C.; Legrand, C.; Van Coppenolle, F. Characterization of Functional TRPV1 Channels in the Sarcoplasmic Reticulum of Mouse Skeletal Muscle. PLoS ONE 2013, 8, e58673. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Sandín, S.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The Endoplasmic Reticulum of Dorsal Root Ganglion Neurons Contains Functional TRPV1 Channels. J. Biol. Chem. 2009, 284, 32591–32601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Tanaka, H.; Yamaguchi, M.; Takemori, S.; Nakamura, A.; Kohama, K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kárai, L.J.; Russell, J.T.; Iadarola, M.J.; Oláh, Z. Vanilloid Receptor 1 Regulates Multiple Calcium Compartments and Contributes to Ca2+-induced Ca2+ Release in Sensory Neurons. J. Biol. Chem. 2004, 279, 16377–16387. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.; Fleig, A.; Stokes, A.; Kinet, J.-P.; Penner, R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem. J. 2003, 371, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Olah, Z.; Szabo, T.; Karai, L.; Hough, C.; Fields, R.D.; Caudle, R.M.; Blumberg, P.M.; Iadarola, M.J. Ligand-induced Dynamic Membrane Changes and Cell Deletion Conferred by Vanilloid Receptor 1. J. Biol. Chem. 2001, 276, 11021–11030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Liu, M.-C.; Magoulas, C.; Priestley, J.V.; Willmott, N.J. Versatile Regulation of Cytosolic Ca2+ by Vanilloid Receptor I in Rat Dorsal Root Ganglion Neurons. J. Biol. Chem. 2003, 278, 5462–5472. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.D.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000, 129, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numazaki, M.; Tominaga, T.; Takeuchi, K.; Murayama, N.; Toyooka, H.; Tominaga, M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. USA 2003, 100, 8002–8006. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/Calmodulin Modulates TRPV1 Activation by Capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.L.; Medvedeva, Y.V.; Ayyagari, T.E.; Schmunk, G.; Gargus, J.J. Intracellular calcium dysregulation in autism spectrum disorder: An analysis of converging organelle signaling pathways. Biochim. Biophys. Acta - Mol. Cell Res. 2018, 1865, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Schmunk, G.; Boubion, B.J.; Smith, I.F.; Parker, I.; Gargus, J.J. Shared functional defect in IP3R-mediated calcium signaling in diverse monogenic autism syndromes. Transl. Psychiatry 2015, 5, e643-10. [Google Scholar] [CrossRef] [PubMed]
- Schmunk, G.; Nguyen, R.L.; Ferguson, D.L.; Kumar, K.; Parker, I.; Gargus, J.J. High-Throughput screen detects calcium signaling dysfunction in typical sporadic autism spectrum disorder. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keil, K.P.; Sethi, S.; Wilson, M.D.; Silverman, J.L.; Pessah, I.N.; Lein, P.J. Genetic mutations in Ca2+ signaling alter dendrite morphology and social approach in juvenile mice. Genes, Brain Behav. 2019, 18, e12526. [Google Scholar] [CrossRef]
- Matsuo, N.; Tanda, K.; Nakanishi, K.; Yamasaki, N.; Toyama, K.; Takao, K.; Takeshima, H.; Miyakawa, T. Comprehensive behavioral phenotyping of ryanodine receptor type3 (RyR3) knockout mice: Decreased social contact duration in two social interaction tests. Front. Behav. Neurosci. 2009, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soueid, J.; Kourtian, S.; Makhoul, N.J.; Makoukji, J.; Haddad, S.; Ghanem, S.S.; Kobeissy, F.; Boustany, R.-M. RYR2, PTDSS1 and AREG genes are implicated in a Lebanese population-based study of copy number variation in autism. Sci. Rep. 2016, 6, 19088. [Google Scholar] [CrossRef]
- Deng, P.-Y.; Rotman, Z.; Blundon, J.A.; Cho, Y.; Cui, J.; Cavalli, V.; Zakharenko, S.S.; Klyachko, V.A. FMRP Regulates Neurotransmitter Release and Synaptic Information Transmission by Modulating Action Potential Duration via BK Channels. Neuron 2013, 77, 696–711. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; di Ronza, A.; Lotfi, P.; Sardiello, M. Lysosomes and Brain Health. Annu. Rev. Neurosci. 2018, 41, 255–276. [Google Scholar] [CrossRef]
- Pelled, D.; Trajkovic-Bodennec, S.; Lloyd-Evans, E.; Sidransky, E.; Schiffmann, R.; Futerman, A.H. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol. Dis. 2005, 18, 83–88. [Google Scholar] [CrossRef]
- Korkotian, E.; Segal, M.; Schwarz, A.; Pelled, D.; Futerman, A.H.; Schwarzmann, G. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J. Biol. Chem. 1999, 274, 21673–21678. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.T.; Mu, T.-W.; Palmer, A.E.; Kelly, J.W. Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis. Nat. Chem. Biol. 2010, 6, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessitore, A.; del P. Martin, M.; Sano, R.; Ma, Y.; Mann, L.; Ingrassia, A.; Laywell, E.D.; Steindler, D.A.; Hendershot, L.M.; d’Azzo, A. GM1-Ganglioside-Mediated Activation of the Unfolded Protein Response Causes Neuronal Death in a Neurodegenerative Gangliosidosis. Mol. Cell 2004, 15, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chung, C.; Shen, D.; Xu, H.; Lieberman, A.P. Ryanodine receptor antagonists adapt NPC1 proteostasis to ameliorate lipid storage in Niemann–Pick type C disease fibroblasts. Hum. Mol. Genet. 2012, 21, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Annunziata, I.; Patterson, A.; Moshiach, S.; Gomero, E.; Opferman, J.; Forte, M.; d’Azzo, A. GM1-Ganglioside Accumulation at the Mitochondria-Associated ER Membranes Links ER Stress to Ca2+-Dependent Mitochondrial Apoptosis. Mol. Cell 2009, 36, 500–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelled, D.; Lloyd-Evans, E.; Riebeling, C.; Jeyakumar, M.; Platt, F.M.; Futerman, A.H. Inhibition of Calcium Uptake via the Sarco/Endoplasmic Reticulum Ca2+-ATPase in a Mouse Model of Sandhoff Disease and Prevention by Treatment with N -Butyldeoxynojirimycin. J. Biol. Chem. 2003, 278, 29496–29501. [Google Scholar] [CrossRef] [PubMed]
- Atakpa, P.; Thillaiappan, N.B.; Mataragka, S.; Prole, D.L.; Taylor, C.W. IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes. Cell Rep. 2018, 25, 3180–3193.e7. [Google Scholar] [CrossRef] [Green Version]
- López-Sanjurjo, C.I.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013, 126, 289–300. [Google Scholar] [CrossRef]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Zhang, Z.; Toro, L.; Dong, X.-P. BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev. Cell 2015, 33, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Patel, S. Getting close. Lysosome-ER contact sites tailor Ca2+ signals. Cell Calcium 2019, 80, 194–196. [Google Scholar] [CrossRef]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, B.S.; Eden, E.R.; Hockey, L.N.; Yates, E.; Futter, C.E.; Patel, S. An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 2017, 18, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain. Cell. Mol. Neurobiol. 2018, 38, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Smith, K.; Jones, L.A.; Burge, S.M.; Munro, C.S.; Tavadia, S.; Craddock, N. The neuropsychiatric phenotype in Darier disease. Br. J. Dermatol. 2010, 163, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Craddock, N.; Owen, M.; Burge, S.; Kurian, B.; Thomas, P.; McGuffin, P. Familial Cosegregation of Major Affective Disorder and Darier’s Disease (Keratosis Follicularis). Br. J. Psychiatry 1994, 164, 355–358. [Google Scholar] [CrossRef]
- Jones, I.; Jacobsen, N.; Green, E.K.; Elvidge, G.P.; Owen, M.J.; Craddock, N. Evidence for familial cosegregation of major affective disorder and genetic markers flanking the gene for Darier’s disease. Mol. Psychiatry 2002, 7, 424–427. [Google Scholar] [CrossRef]
- Wang, S.-L.; Yang, S.-F.; Chen, C.-C.; Tsai, P.-T.; Chai, C.-Y. Darier’s disease associated with bipolar affective disorder: a case report. Kaohsiung J. Med. Sci. 2002, 18, 622–626. [Google Scholar]
- Takeichi, T.; Sugiura, K.; Nakamura, Y.; Fujio, Y.; Konohana, I.; Akiyama, M. Darier’s Disease Complicated by Schizophrenia Caused by a Novel ATP2A2 Mutation. Acta Derm. Venereol. 2016, 96, 993–994. [Google Scholar] [CrossRef]
- Gold, P.W.; Licinio, J.; Pavlatou, M.G. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-γ systems. Mol. Psychiatry 2013, 18, 154–165. [Google Scholar] [CrossRef]
- Galeotti, N.; Vivoli, E.; Bartolini, A.; Ghelardini, C. A gene-specific cerebral types 1, 2, and 3 RyR protein knockdown induces an antidepressant-like effect in mice. J. Neurochem. 2008, 106, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Alda, M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol. Psychiatry 2015, 20, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sade, Y.; Toker, L.; Kara, N.Z.; Einat, H.; Rapoport, S.; Moechars, D.; Berry, G.T.; Bersudsky, Y.; Agam, G. IP3 accumulation and/or inositol depletion: two downstream lithium’s effects that may mediate its behavioral and cellular changes. Transl. Psychiatry 2016, 6, e968. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- De Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Barneo-Muñoz, M.; Juárez, P.; Civera-Tregón, A.; Yndriago, L.; Pla-Martin, D.; Zenker, J.; Cuevas-Martín, C.; Estela, A.; Sánchez-Aragó, M.; Forteza-Vila, J.; et al. Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy. PLoS Genet. 2015, 11, e1005115. [Google Scholar] [CrossRef] [PubMed]
- Pla-Martín, D.; Rueda, C.B.; Estela, A.; Sánchez-Piris, M.; González-Sánchez, P.; Traba, J.; de la Fuente, S.; Scorrano, L.; Renau-Piqueras, J.; Alvarez, J.; et al. Silencing of the Charcot–Marie–Tooth disease-associated gene GDAP1 induces abnormal mitochondrial distribution and affects Ca2+ homeostasis by reducing store-operated Ca2+ entry. Neurobiol. Dis. 2013, 55, 140–151. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, P.; Pla-Martín, D.; Martínez-Valero, P.; Rueda, C.B.; Calpena, E.; del Arco, A.; Palau, F.; Satrústegui, J. CMT-linked loss-of-function mutations in GDAP1 impair store-operated Ca2+ entry-stimulated respiration. Sci. Rep. 2017, 7, 42993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanoye, C.G.; Sakakura, M.; Follis, R.M.; Trevisan, A.J.; Narayan, M.; Li, J.; Sanders, C.R.; Carter, B.D. Peripheral myelin protein 22 modulates store-operated calcium channel activity, providing insights into Charcot-Marie-Tooth disease etiology. J. Biol. Chem. 2019, 294, 12054–12065. [Google Scholar] [CrossRef] [PubMed]
- Filadi, R.; Greotti, E.; Turacchio, G.; Luini, A.; Pozzan, T.; Pizzo, P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl. Acad. Sci. USA 2015, 112, E2174-81. [Google Scholar] [CrossRef]
- Singaravelu, K.; Nelson, C.; Bakowski, D.; de Brito, O.M.; Ng, S.-W.; Di Capite, J.; Powell, T.; Scorrano, L.; Parekh, A.B. Mitofusin 2 Regulates STIM1 Migration from the Ca2+ Store to the Plasma Membrane in Cells with Depolarized Mitochondria. J. Biol. Chem. 2011, 286, 12189–12201. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.A.; Kennerson, M.L.; Anney, R.J.; Matsuura, T.; Nicholson, G.A.; Salimi-Tari, P.; Gardner, R.J.M.; Storey, E.; Forrest, S.M. Spinocerebellar ataxia type 15 (sca15) maps to 3p24.2-3pter:: exclusion of the ITPR1 gene, the human orthologue of an ataxic mouse mutant. Neurobiol. Dis. 2003, 13, 147–157. [Google Scholar] [CrossRef]
- Van de Leemput, J.; Chandran, J.; Knight, M.A.; Holtzclaw, L.A.; Scholz, S.; Cookson, M.R.; Houlden, H.; Gwinn-Hardy, K.; Fung, H.-C.; Lin, X.; et al. Deletion at ITPR1 Underlies Ataxia in Mice and Spinocerebellar Ataxia 15 in Humans. PLoS Genet. 2007, 3, e108. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Prontera, P.; Di Gregorio, E.; Rossi, V.; Parnetti, L.; Rossi, A.; Donti, E.; Brusco, A.; Calabresi, P.; Tambasco, N. A novel spinocerebellar ataxia type 15 family with involuntary movements and cognitive decline. Eur. J. Neurol. 2011, 18, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Marelli, C.; van de Leemput, J.; Johnson, J.O.; Tison, F.; Thauvin-Robinet, C.; Picard, F.; Tranchant, C.; Hernandez, D.G.; Huttin, B.; Boulliat, J.; et al. SCA15 Due to Large ITPR1 Deletions in a Cohort of 333 White Families with Dominant Ataxia. Arch. Neurol. 2011, 68, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Helbig, K.L.; Harmuth, F.; Deconinck, T.; Tanpaiboon, P.; Sun, B.; Guo, W.; Wang, R.; Palmaer, E.; Tang, S.; et al. De novo ITPR1 variants are a recurrent cause of early-onset ataxia, acting via loss of channel function. Eur. J. Hum. Genet. 2018, 26, 1623–1634. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Ohba, C.; Iai, M.; Hirabayashi, S.; Osaka, H.; Hiraide, T.; Saitsu, H.; Matsumoto, N. Sporadic infantile-onset spinocerebellar ataxia caused by missense mutations of the inositol 1,4,5-triphosphate receptor type 1 gene. J. Neurol. 2015, 262, 1278–1284. [Google Scholar] [CrossRef]
- Wittig, E.O.; Moreira, C.A.; Freire-Maia, N.; Vianna-Morgante, A.M.; Opitz, J.M.; Reynolds, J.F. Partial aniridia, cerebellar ataxia, and mental deficiency (gillespie syndrome) in two brothers. Am. J. Med. Genet. 1988, 30, 703–708. [Google Scholar] [CrossRef]
- Paganini, L.; Pesenti, C.; Milani, D.; Fontana, L.; Motta, S.; Sirchia, S.M.; Scuvera, G.; Marchisio, P.; Esposito, S.; Cinnante, C.M.; et al. A novel splice site variant in ITPR1 gene underlying recessive Gillespie syndrome. Am. J. Med. Genet. Part A 2018, 176, 1427–1431. [Google Scholar] [CrossRef]
- Gerber, S.; Alzayady, K.J.; Burglen, L.; Brémond-Gignac, D.; Marchesin, V.; Roche, O.; Rio, M.; Funalot, B.; Calmon, R.; Durr, A.; et al. Recessive and Dominant De Novo ITPR1 Mutations Cause Gillespie Syndrome. Am. J. Hum. Genet. 2016, 98, 971–980. [Google Scholar] [CrossRef] [Green Version]
- McEntagart, M.; Williamson, K.A.; Rainger, J.K.; Wheeler, A.; Seawright, A.; De Baere, E.; Verdin, H.; Bergendahl, L.T.; Quigley, A.; Rainger, J.; et al. A Restricted Repertoire of De Novo Mutations in ITPR1 Cause Gillespie Syndrome with Evidence for Dominant-Negative Effect. Am. J. Hum. Genet. 2016, 98, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Nakagawa, T.; Inoue, T.; Nagata, E.; Tanaka, K.; Takano, H.; Kuno, J.; Sakakibara, S.; Yamada, M.; Yoneshima, H.; et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature 1996, 379, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.P.; Hirouchi, T.; Hisatsune, C.; Lynch, B.; Murphy, R.; Dunne, A.M.; Miyamoto, A.; Ennis, S.; van der Spek, N.; O’Hici, B.; et al. A novel gain-of-function mutation in the ITPR1 suppressor domain causes spinocerebellar ataxia with altered Ca2+ signal patterns. J. Neurol. 2017, 264, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.N.; Williamson, K.A.; FitzPatrick, D.R. The genetic architecture of aniridia and Gillespie syndrome. Hum. Genet. 2019, 138, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium hypothesis of Alzheimer’s disease. Pflügers Arch. - Eur. J. Physiol. 2010, 459, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Lyckman, A.; Oddo, S.; LaFerla, F.M.; Querfurth, H.W.; Shtifman, A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J. Neurochem. 2008, 105, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Stutzmann, G.E.; Caccamo, A.; LaFerla, F.M.; Parker, I. Dysregulated IP3 Signaling in Cortical Neurons of Knock-In Mice Expressing an Alzheimer’s-Linked Mutation in Presenilin1 Results in Exaggerated Ca2+ Signals and Altered Membrane Excitability. J. Neurosci. 2004, 24, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.-O.D.; Cheung, K.-H.; Toglia, P.; Foskett, J.K.; Ullah, G. Analyzing and Quantifying the Gain-of-Function Enhancement of IP3 Receptor Gating by Familial Alzheimer’s Disease-Causing Mutants in Presenilins. PLoS Comput. Biol. 2015, 11, e1004529. [Google Scholar] [CrossRef]
- Cheung, K.H.; Shineman, D.; Müller, M.; Cárdenas, C.; Mei, L.; Yang, J.; Tomita, T.; Iwatsubo, T.; Lee, V.M.Y.; Foskett, J.K. Mechanism of Ca2+ Disruption in Alzheimer’s Disease by Presenilin Regulation of InsP3 Receptor Channel Gating. Neuron 2008, 58, 871–883. [Google Scholar] [CrossRef]
- Chan, S.L.; Mayne, M.; Holden, C.P.; Geiger, J.D.; Mattson, M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000, 275, 18195–18200. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Wei, H. Dantrolene: From Malignant Hyperthermia to Alzheimer’s Disease. CNS Neurol. Disord. - Drug Targets 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Demuro, A.; Akbari, Y.; Hitt, B.D.; Smith, I.F.; Parker, I.; LaFerla, F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid β production. J. Cell Biol. 2008, 181, 1107–1116. [Google Scholar] [PubMed]
- Zampese, E.; Fasolato, C.; Kipanyula, M.J.; Bortolozzi, M.; Pozzan, T.; Pizzo, P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA 2011, 108, 2777–2782. [Google Scholar] [PubMed]
- Area-Gomez, E.; del Carmen Lara Castillo, M.; Tambini, M.D.; Guardia-Laguarta, C.; de Groof, A.J.C.; Madra, M.; Ikenouchi, J.; Umeda, M.; Bird, T.D.; Sturley, S.L.; et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012, 31, 4106–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Rodriguez, M.; Hernando-Perez, E.; Nuñez, L.; Villalobos, C. Amyloid β Oligomers Increase ER-Mitochondria Ca2+ Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca2+ Remodeling. Front. Cell. Neurosci. 2019, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Wu, H.-Y.; Arbel-Ornath, M.; Hashimoto, T.; Matsouaka, R.; Fan, Z.; Spires-Jones, T.L.; Betensky, R.A.; Bacskai, B.J.; Hyman, B.T. Inhibition of the NFAT Pathway Alleviates Amyloid Beta Neurotoxicity in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2012, 32, 3176–3192. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Ferreiro, E.; Cardoso, S.M.; Oliveira, C.R.; Pereira, C.M.F. ER Stress-Mediated Apoptotic Pathway Induced by Aβ Peptide Requires the Presence of Functional Mitochondria. J. Alzheimer’s Dis. 2010, 20, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.; Resende, R.; Costa, R.; Oliveira, C.R.; Pereira, C.M.F. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol. Dis. 2006, 23, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.O.; Lacor, P.N.; Ferreira, I.L.; Resende, R.; Auberson, Y.P.; Klein, W.L.; Oliveira, C.R.; Rego, A.C.; Pereira, C.M.F. Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N -methyl-D-aspartate receptor in mature hippocampal cultures treated with amyloid-β oligomers. Aging Cell 2012, 11, 823–833. [Google Scholar] [CrossRef]
- Giacomello, M.; Barbiero, L.; Zatti, G.; Squitti, R.; Binetti, G.; Pozzan, T.; Fasolato, C.; Ghidoni, R.; Pizzo, P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol. Dis. 2005, 18, 638–648. [Google Scholar] [CrossRef]
- Zatti, G.; Ghidoni, R.; Barbiero, L.; Binetti, G.; Pozzan, T.; Fasolato, C.; Pizzo, P. The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular stores. Neurobiol. Dis. 2004, 15, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ryazantseva, M.; Skobeleva, K.; Kaznacheyeva, E. Familial Alzheimer’s disease-linked presenilin-1 mutation M146V affects store-operated calcium entry: Does gain look like loss? Biochimie 2013, 95, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Zatti, G.; Burgo, A.; Giacomello, M.; Barbiero, L.; Ghidoni, R.; Sinigaglia, G.; Florean, C.; Bagnoli, S.; Binetti, G.; Sorbi, S.; et al. Presenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium 2006, 39, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Suaga, P.; Bravo-San Pedro, J.M.; González-Polo, R.A.; Fuentes, J.M.; Niso-Santano, M. ER-mitochondria signaling in Parkinson’s disease review-article. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein binds to the ER–mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Caraveo, G.; Auluck, P.K.; Whitesell, L.; Chung, C.Y.; Baru, V.; Mosharov, E.V.; Yan, X.; Ben-Johny, M.; Soste, M.; Picotti, P.; et al. Calcineurin determines toxic versus beneficial responses to α-synuclein. Proc. Natl. Acad. Sci. USA 2014, 111, E3544-52. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, L.; Lin, X.; Liu, G.; Yu, J.; Parisiadou, L.; Xie, C.; Ding, J.; Cai, H. A calcineurin- and NFAT-dependent pathway is involved in -synuclein-induced degeneration of midbrain dopaminergic neurons. Hum. Mol. Genet. 2014, 23, 6567–6574. [Google Scholar] [CrossRef] [Green Version]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: a clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Higo, T.; Hamada, K.; Hisatsune, C.; Nukina, N.; Hashikawa, T.; Hattori, M.; Nakamura, T.; Mikoshiba, K. Mechanism of ER Stress-Induced Brain Damage by IP3 Receptor. Neuron 2010, 68, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.S.; Tu, H.; Chan, E.Y.W.; Maximov, A.; Wang, Z.; Wellington, C.L.; Hayden, M.R.; Bezprozvanny, I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 2003, 39, 227–239. [Google Scholar] [CrossRef]
- Kaltenbach, L.S.; Romero, E.; Becklin, R.R.; Chettier, R.; Bell, R.; Phansalkar, A.; Strand, A.; Torcassi, C.; Savage, J.; Hurlburt, A.; et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007, 3, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ryskamp, D.A.; Liang, X.; Egorova, P.; Zakharova, O.; Hung, G.; Bezprozvanny, I. Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model. J. Neurosci. 2016, 36, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-S.; Guo, C.; Wang, H.; Chen, X.; Bezprozvanny, I. Neuroprotective Effects of Inositol 1,4,5-Trisphosphate Receptor C-Terminal Fragment in a Huntington’s Disease Mouse Model. J. Neurosci. 2009, 29, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.L.; Mitne-Neto, M.; Silva, H.C.A.; Richieri-Costa, A.; Middleton, S.; Cascio, D.; Kok, F.; Oliveira, J.R.M.; Gillingwater, T.; Webb, J.; et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004, 75, 822–831. [Google Scholar] [CrossRef] [PubMed]
- De Vos, K.J.; Mórotz, G.M.; Stoica, R.; Tudor, E.L.; Lau, K.-F.; Ackerley, S.; Warley, A.; Shaw, C.E.; Miller, C.C.J. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 2012, 21, 1299–1311. [Google Scholar] [CrossRef]
- Mitne-Neto, M.; Machado-Costa, M.; Marchetto, M.C.N.; Bengtson, M.H.; Joazeiro, C.A.; Tsuda, H.; Bellen, H.J.; Silva, H.C.A.; Oliveira, A.S.B.; Lazar, M.; et al. Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum. Mol. Genet. 2011, 20, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Stoica, R.; Paillusson, S.; Gomez-Suaga, P.; Mitchell, J.C.; Lau, D.H.; Gray, E.H.; Sancho, R.M.; Vizcay-Barrena, G.; De Vos, K.J.; Shaw, C.E.; et al. ALS / FTD -associated FUS activates GSK -3β to disrupt the VAPB – PTPIP 51 interaction and ER –mitochondria associations. EMBO Rep. 2016, 17, 1326–1342. [Google Scholar] [CrossRef]
- Stoica, R.; De Vos, K.J.; Paillusson, S.; Mueller, S.; Sancho, R.M.; Lau, K.F.; Vizcay-Barrena, G.; Lin, W.L.; Xu, Y.F.; Lewis, J.; et al. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Aggad, D.; Vérièpe, J.; Tauffenberger, A.; Parker, J.A. TDP-43 Toxicity Proceeds via Calcium Dysregulation and Necrosis in Aging Caenorhabditis elegans Motor Neurons. J. Neurosci. 2014, 34, 12093. [Google Scholar] [CrossRef]
- Nguyen, L.; Lucke-Wolds, B.P.; Mookerjee, S.; Kaushal, N.; Matsumoto, R.R. Sigma-1 Receptors and Neurodegenerative Diseases: Towards a Hypothesis of Sigma-1 Receptors as Amplifiers of Neurodegeneration and Neuroprotection. Adv. Exp. Med. Biol. 2017, 964, 133. [Google Scholar] [PubMed]
- Dafinca, R.; Scaber, J.; Ababneh, N.; Lalic, T.; Weir, G.; Christian, H.; Vowles, J.; Douglas, A.G.L.; Fletcher-Jones, A.; Browne, C.; et al. C9orf72 Hexanucleotide Expansions Are Associated with Altered Endoplasmic Reticulum Calcium Homeostasis and Stress Granule Formation in Induced Pluripotent Stem Cell-Derived Neurons from Patients with Amyotrophic Lateral Sclerosis and Frontotemporal Demen. Stem Cells 2016, 34, 2063–2078. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. https://doi.org/10.3390/cells8101232
Karagas NE, Venkatachalam K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells. 2019; 8(10):1232. https://doi.org/10.3390/cells8101232
Chicago/Turabian StyleKaragas, Nicholas E., and Kartik Venkatachalam. 2019. "Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis" Cells 8, no. 10: 1232. https://doi.org/10.3390/cells8101232
APA StyleKaragas, N. E., & Venkatachalam, K. (2019). Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells, 8(10), 1232. https://doi.org/10.3390/cells8101232



