Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models
Abstract
1. Introduction
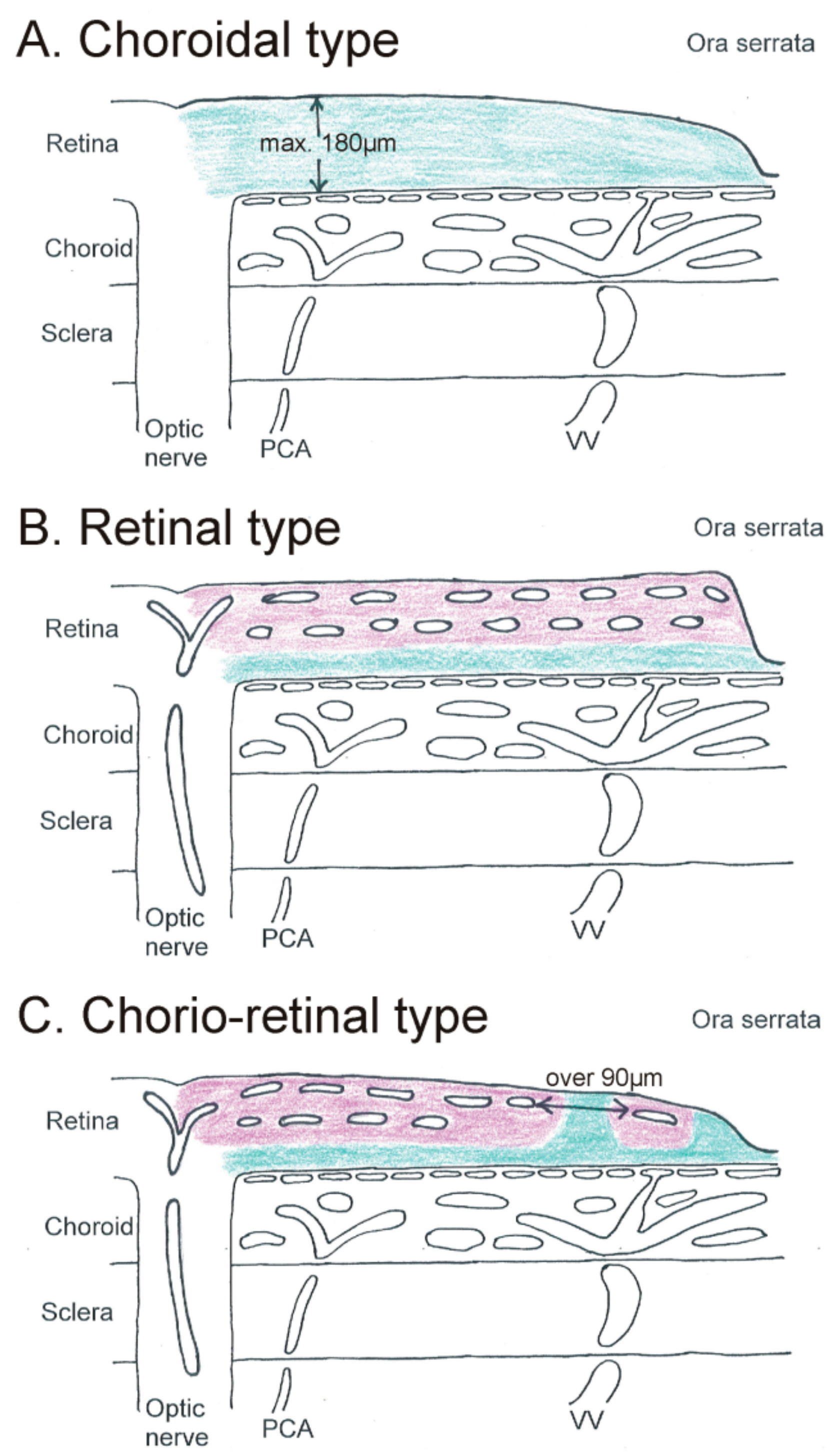
2. Diffusion Distance: The Impact of Vascularization on Retinal Ganglion Cell Nutrition
2.1. Choroidal Type of Retinal Ganglion Cell Nutrition
2.2. Retinal Type of Retinal Ganglion Cell Nutrition
2.3. Chorio-Retinal Nutrition of Retinal Ganglion Cells
3. Concentration Difference of Nutrients
3.1. Oxygen Tension
3.2. Glucose Metabolism
3.3. Amino Acids and Lipids
4. Diffusing and Host Materials: The Impact of Intraocular Pressure on Retinal Ganglion Cell Nutrition
5. Temperature
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2018, 63, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog. Retin. Eye Res. 2018, 62, 58–76. [Google Scholar] [CrossRef] [PubMed]
- De Schaepdrijver, L.; Simoens, P.; Lauwers, H.; De Geest, J.P. Retinal vascular patterns in domestic animals. Res. Vet. Sci. 1989, 47, 34–42. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J.; Alder, V.A.; Su, E.N.; Yu, P.K. Intraretinal oxygen distribution and choroidal regulation in the avascular retina of guinea pigs. Am. J. Physiol. 1996, 270, H965–H973. [Google Scholar] [CrossRef]
- Ninomiya, H.; Inomata, T.; Kanemaki, N. Microvascular architecture of the rabbit eye: A scanning electron microscopic study of vascular corrosion casts. J. Vet. Med. Sci. 2008, 70, 887–892. [Google Scholar] [CrossRef]
- Buttery, R.G.; Hinrichsen, C.F.; Weller, W.L.; Haight, J.R. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Res. 1991, 31, 169–187. [Google Scholar] [CrossRef]
- Cuthbertson, R.A.; Mandel, T.E. Anatomy of the mouse retina. Endothelial cell-pericyte ratio and capillary distribution. Invest. Ophthalmol. Vis. Sci. 1986, 27, 1659–1664. [Google Scholar]
- Kerr, B.A.; West, X.Z.; Kim, Y.W.; Zhao, Y.; Tischenko, M.; Cull, R.M.; Phares, T.W.; Peng, X.D.; Bernier-Latmani, J.; Petrova, T.V.; et al. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat. Commun. 2016, 7, 10960. [Google Scholar] [CrossRef]
- Rust, R.; Grönnert, L.; Dogançay, B.; Schwab, M.E. A Revised View on Growth and Remodeling in the Retinal Vasculature. Sci. Rep. 2019, 9, 3263. [Google Scholar] [CrossRef]
- Bhutto, I.A.; Amemiya, T. Corrosion cast demonstration of retinal vasculature of normal Wistar-Kyoto rats. Acta Anat. 1995, 153, 290–300. [Google Scholar] [CrossRef]
- Ninomiya, H.; Kuno, H. Microvasculature of the rat eye: Scanning electron microscopy of vascular corrosion casts. Vet. Ophthalmol. 2001, 4, 55–59. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Magno, A.L.; Ramos, D.; Catita, J.; McMenamin, P.G.; Chen, F.K.; Rakoczy, E.P.; Ruberte, J. Angiography reveals novel features of the retinal vasculature in healthy and diabetic mice. Exp. Eye Res. 2015, 138, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Bloodworth, J.M., Jr.; Gutgesell, H.P., Jr.; Engerman, R.L. Retinal vasculature of the pig. Light and electron microscope studies. Exp. Eye Res. 1965, 4, 174–178. [Google Scholar] [CrossRef]
- Simoens, P.; De Schaepdrijver, L.; Lauwers, H. Morphologic and clinical study of the retinal circulation in the miniature pig. A: Morphology of the retinal microvasculature. Exp. Eye Res. 1992, 54, 965–973. [Google Scholar] [CrossRef]
- Ninomiya, H.; Inomata, T. Microvascular anatomy of the pig eye: Scanning electron microscopy of vascular corrosion casts. J. Vet. Med. Sci. 2006, 68, 1149–1154. [Google Scholar] [CrossRef]
- Michaelson, I.C. Vascular morphogenesis in the retina of the cat. J. Anat. 1949, 83, 64. [Google Scholar]
- Jacot, J.L.; Laver, N.M.; Glover, J.P.; Lazarous, D.F.; Unger, E.F.; Robison, W.G., Jr. Histological evaluation of the canine retinal vasculature following chronic systemic administration of basic fibroblast growth factor. J. Anat. 1996, 188, 349–354. [Google Scholar]
- May, C.A. An unusual distribution of neuronal Nitric Oxide Synthase (nNOS) neurons in the porcine retina next to the Ora serrata. J. Vet. Sci. Technol. 2013, 4, e147. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inomata, H. Relation between Superficial Capillaries and foveal structure in the Human Retina. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1698–1705. [Google Scholar]
- Song, Y.; Mellott, J.G.; Winer, J.A. Microvascular organization of the cat inferior colliculus. Hear Res. 2011, 274, 5–12. [Google Scholar] [CrossRef]
- Wenner, Y.; Wismann, S.; Preising, M.N.; Jäger, M.; Pons-Kühnemann, J.; Lorenz, B. Normative values of peripheral retinal thickness measured with Spectralis OCT in healthy young adults. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Fanea, L. Reference 3 T MRI parameters of the normal human eye. Phys. Med. 2018, 47, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; May, C.A. Nutrition and vascular supply of retinal ganglion cells during human development. Front. Neurol. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Niemeyer, G. Glucose concentration and retinal function. Clin. Neurosci. 1997, 4, 327–335. [Google Scholar]
- Pérezleón, J.A.; Osorio-Paz, I.; Francois, L.; Salceda, R. Immunohistochemical localization of glycogen synthase and GSK3β: Control of glycogen content in retina. Neurochem. Res. 2013, 38, 1063–1069. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Low oxygen consumption in the inner retina of the visual streak of the rabbit. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, 419–423. [Google Scholar] [CrossRef]
- Cringle, S.J.; Yu, D.Y. Regulation of Oxygen Tension in the Mammalian Retina During Systemic Hyperoxia Is Species Dependent. Adv. Exp. Med. Biol. 2018, 1072, 241–244. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J.; Alder, V.; Su, E.N. Intraretinal oxygen distribution in the rat with graded systemic hyperoxia and hypercapnia. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2082–2087. [Google Scholar]
- Landers, M.B., 3rd. Retinal oxygenation via the choroidal circulation. Trans. Am. Ophthalmol. Soc. 1978, 76, 528–556. [Google Scholar]
- Dreher, Z.; Robinson, S.R.; Distler, C. Müller cells in vascular and avascular retinae: A survey of seven mammals. J. Comp. Neurol. 1992, 323, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Sato, K. Reassessment of histochemistry of retinal glycogen. Exp. Eye Res. 1975, 21, 489–497. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Grosche, J.; Reichenbach, A.; Hamprecht, B. Immunocytochemical demonstration of glycogen phosphorylase in Müller (glial) cells of the mammalian retina. Glia 1994, 12, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer-Guglielmi, B.; Francke, M.; Reichenbach, A.; Fleckenstein, B.; Jung, G.; Hamprecht, B. Glycogen phosphorylase isozyme pattern in mammalian retinal Müller (glial) cells and in astrocytes of retina and optic nerve. Glia 2005, 49, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Rungger-Brändle, E.; Kolb, H.; Niemeyer, G. Histochemical demonstration of glycogen in neurons of the cat retina. Investig. Ophthalmol. Vis. Sci. 1996, 37, 702–715. [Google Scholar]
- Magalhàes, M.M.; Coimbra, A. Electron microscope radioautographic study of glycogen synthesis in the rabbit retina. J. Cell Biol. 1970, 47, 263–275. [Google Scholar] [CrossRef]
- Wasilewa, P.; Hockwin, O.; Korte, I. Glycogen concentration changes in retina, vitreous body and other eye tissues caused by disturbances of blood circulation. Albrecht von Graefes Arch. Klin. Exp. Ophthalmol. 1976, 199, 115–120. [Google Scholar] [CrossRef]
- Toft-Kehler, A.K.; Skytt, D.M.; Kolko, M. A Perspective on the Müller Cell-Neuron Metabolic Partnership in the Inner Retina. Mol. Neurobiol. 2018, 55, 5353–5361. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef]
- Trost, A.; Bruckner, D.; Rivera, F.J.; Reitsamer, H.A. Pericytes in the Retina. Adv. Exp. Med. Biol. 2019, 1122, 1–26. [Google Scholar] [CrossRef]
- Phipps, J.A.; Dixon, M.A.; Jobling, A.I.; Wang, A.Y.; Greferath, U.; Vessey, K.A.; Fletcher, E.L. The renin-angiotensin system and the retinal neurovascular unit: A role in vascular regulation and disease. Exp. Eye Res. 2019, 187, 107753. [Google Scholar] [CrossRef] [PubMed]
- Coffe, V.; Carbajal, R.C.; Salceda, R. Glycogen metabolism in the rat retina. J. Neurochem. 2004, 88, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Törnquist, P.; Alm, A. Retinal and choroidal contribution to retinal metabolism in vivo. A study in pigs. Acta Physiol. Scand. 1979, 106, 351–357. [Google Scholar] [CrossRef]
- Padnick-Silver, L.; Linsenmeier, R.A. Quantification of in vivo anaerobic metabolism in the normal cat retina through intraretinal pH measurements. Vis. Neurosci. 2002, 19, 793–806. [Google Scholar] [CrossRef]
- Léveillard, T.; Sahel, J.A. Metabolic and redox signaling in the retina. Cell. Mol. Life Sci. 2017, 74, 3649–3665. [Google Scholar] [CrossRef]
- Ola, M.S.; LaNoue, K.F. Molecular basis for increased lactate formation in the Müller glial cells of retina. Brain Res. Bull. 2019, 144, 158–163. [Google Scholar] [CrossRef]
- Militante, J.D.; Lombardini, J.B. Taurine: Evidence of physiological function in the retina. Nutr. Neurosci. 2002, 5, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, Q.; Wang, J.; Liu, F.; Mi, M.; Xu, H.; Chen, F.; Zeng, K. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res. 2009, 1279, 131–138. [Google Scholar] [CrossRef]
- Zeng, K.; Xu, H.; Chen, K.; Zhu, J.; Zhou, Y.; Zhang, Q.; Mantian, M. Effects of taurine on glutamate uptake and degradation in Müller cells under diabetic conditions via antioxidant mechanism. Mol. Cell. Neurosci. 2010, 45, 192–199. [Google Scholar] [CrossRef]
- Schnebelen, C.; Pasquis, B.; Salinas-Navarro, M.; Joffre, C.; Creuzot-Garcher, C.P.; Vidal-Sanz, M.; Bron, A.M.; Bretillon, L.; Acar, N. A dietary combination of omega-3 and omega-6 polyunsaturated fatty acids is more efficient than single supplementations in the prevention of retinal damage induced by elevation of intraocular pressure in rats. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Fourgeux, C.; Martine, L.; Pasquis, B.; Maire, M.A.; Acar, N.; Creuzot-Garcher, C.; Bron, A.; Bretillon, L. Steady-state levels of retinal 24S-hydroxycholesterol are maintained by glial cells intervention after elevation of intraocular pressure in the rat. Acta Ophthalmol. 2012, 90, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Fourgeux, C.; Martine, L.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C.P.; Bretillon, L. In vivo consequences of cholesterol-24S-hydroxylase (CYP46A1) inhibition by voriconazole on cholesterol homeostasis and function in the rat retina. Biochem. Biophys. Res. Commun. 2014, 446, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, S.M.; Asadi, F.; Rajabian, M.R.; Ostadhassan, H.; Crasta, M. Effect of body position, eyelid manipulation, and manual jugular compression on intraocular pressure in clinically normal cats. Vet. Ophthalmol. 2018, 21, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Mood, M.; Mehdi-Rajaei, S.; Sadjadi, R.; Selk-Ghaffari, M.; Williams, D.L. Twenty-four-Hour Measurement of Intraocular Pressure in Guinea Pigs (Cavia porcellus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 95–97. [Google Scholar]
- Qiu, Y.; Yang, H.; Lei, B. Effects of three commonly used anesthetics on intraocular pressure in mouse. Curr. Eye Res. 2014, 39, 365–369. [Google Scholar] [CrossRef]
- Di, Y.; Luo, X.M.; Qiao, T.; Lu, N. Intraocular pressure with rebound tonometry and effects of topical intraocular pressure reducing medications in guinea pigs. Int. J. Ophthalmol. 2017, 10, 186–190. [Google Scholar] [CrossRef]
- Katz, R.S.; Henkind, P.; Weitzman, E.D. The circadian rhythm of the intraocular pressure in the New Zealand White rabbit. Investig. Ophthalmol. 1975, 14, 775–780. [Google Scholar]
- Wang, X.; Dong, J.; Wu, Q. Twenty-four-hour measurement of IOP in rabbits using rebound tonometer. Vet. Ophthalmol. 2013, 16, 423–428. [Google Scholar] [CrossRef]
- Aihara, M.; Lindsey, J.D.; Weinreb, R.N. Twenty-four-hour pattern of mouse intraocular pressure. Exp. Eye Res. 2003, 77, 681–686. [Google Scholar] [CrossRef]
- Sugimoto, E.; Aihara, M.; Ota, T.; Araie, M. Effect of light cycle on 24-hour pattern of mouse intraocular pressure. J. Glaucoma 2006, 15, 505–511. [Google Scholar] [CrossRef]
- Nissirios, N.; Goldblum, D.; Rohrer, K.; Mittag, T.; Danias, J. Noninvasive determination of intraocular pressure (IOP) in nonsedated mice of 5 different inbred strains. J. Glaucoma 2007, 16, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J.; Georg, B.; Hannibal, J.; Jørgensen, H.L. Role of light and the circadian clock in the rhythmic oscillation of intraocular pressure: Studies in VPAC2 receptor and PACAP deficient mice. Exp. Eye Res. 2018, 169, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Sugiyama, K.; Van Gelder, R.N. Adrenal and Glucocorticoid Effects on the Circadian Rhythm of Murine Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5641–5647. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.C.; Hartwick, A.T.; Twa, M.D. Circadian rhythm of intraocular pressure in the adult rat. Chronobiol. Int. 2015, 32, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, M.J.; Sande, P.H.; Bernades, J.M.; Aba, M.A.; Rosenstein, R.E. Circadian rhythm of intraocular pressure in cats. Vet. Ophthalmol. 2007, 10, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Telle, M.R.; Chen, N.; Shinsako, D.; Kiland, J.A.; Oikawa, K.; Møller Trane, R.; McLellan, G.J. Relationship between corneal sensitivity, corneal thickness, corneal diameter, and intraocular pressure in normal cats and cats with congenital glaucoma. Vet. Ophthalmol. 2019, 22, 4–12. [Google Scholar] [CrossRef]
- Giannetto, C.; Piccione, G.; Giudice, E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet. Ophthalmol. 2009, 12, 302–305. [Google Scholar] [CrossRef]
- Martín-Suárez, E.; Molleda, C.; Tardón, R.; Galán, A.; Gallardo, J.; Molleda, J. Diurnal variations of central corneal thickness and intraocular pressure in dogs from 8:00 am to 8:00 pm. Can. Vet. J. 2014, 55, 361–365. [Google Scholar]
- Liu, G.; Cull, G.; Wang, L.; Bui, B.V. Hypercapnia Impairs Vasoreactivity to Changes in Blood Pressure and Intraocular Pressure in Rat Retina. Optom. Vis. Sci. 2019, 96, 470–476. [Google Scholar] [CrossRef]
- Rutkowski, P.; May, C.A. The peripheral and central Humphrey visual field. Morphological changes during aging. BMC Ophthalmol. 2017, 17, 127. [Google Scholar] [CrossRef]
- Ali, M.A. Temperature and vision. Rev. Can. Biol. 1975, 34, 131–177. [Google Scholar] [PubMed]
- Salido, E.M.; Dorfman, D.; Bordone, M.; Chianelli, M.; González Fleitas, M.F.; Rosenstein, R.E. Global and ocular hypothermic preconditioning protect the rat retina from ischemic damage. PLoS ONE 2013, 8, e61656. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, M.; Schnichels, S.; Hermann, T.; Hurst, J.; Feldkaemper, M.; Arango-Gonzalez, B.; Ueffing, M.; Bartz-Schmidt, K.U.; Zeck, G.; Spitzer, M.S. Hypothermia Protects and Prolongs the Tolerance Time of Retinal Ganglion Cells against Ischemia. PLoS ONE 2016, 11, e0148616. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.; Mutter, M.; Gustafsson, E.; Gustafsson, L.; Vaegler, M.; Schultheiss, M.; Müller, S.; Yoeruek, E.; Schrader, M.; Münch, T.A. Hypothermia Promotes Survival of Ischemic Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Larrayoz, I.M.; Rey-Funes, M.; Contartese, D.S.; Rolón, F.; Sarotto, A.; Dorfman, V.B.; Loidl, C.F.; Martínez, A. Cold Shock Proteins Are Expressed in the Retina Following Exposure to Low Temperatures. PLoS ONE 2016, 11, e0161458. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Hurst, J.; Dias Blak, M.; Herrmann, T.; Melchinger, M.; Bartz-Schmidt, K.U.; Zeck, G.; Schultheiss, M.; Spitzer, M.S.; Schnichels, S. Hypothermia protects retinal ganglion cells against hypoxia-induced cell death in a retina organ culture model. Clin. Exp. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Kwong, J.M.; Lam, T.T.; Caprioli, J. Hyperthermic pre-conditioning protects retinal neurons from N-methyl-D-aspartate (NMDA)-induced apoptosis in rat. Brain Res. 2003, 970, 119–130. [Google Scholar] [CrossRef]
- Birge, R.R.; Vought, B.W. Energetics of rhodopsin photobleaching: Photocalorimetric studies of energy storage in early and late intermediates. In Vertebrate Phototransduction and the Visual Cycle; Palczewski, K., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 143–163. [Google Scholar]
- Kefalov, V.J. Phototransduction: Phototransduction in cones. Encycl. Eye 2010, 3, 389–396. [Google Scholar]
- Piyathaisere, D.V.; Margalit, E.; Chen, S.J.; Shyu, J.S.; D’Anna, S.A.; Weiland, J.D.; Grebe, R.R.; Grebe, L.; Fujii, G.; Kim, S.Y.; et al. Heat effects on the retina. Ophthalmic Surg. Lasers Imaging Retin. 2003, 34, 114–120. [Google Scholar] [CrossRef]
- Glickman, R.D. Phototoxicity to the retina: Mechanisms of damage. Int. J. Toxicol. 2002, 21, 473–490. [Google Scholar] [CrossRef]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye 2016, 30, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A.; Gordon, L.K. Retinal ganglion cell disorders: Types and treatments. Prog. Retin. Eye Res. 2002, 21, 465–484. [Google Scholar] [CrossRef]
- Hankeln, T.; Wystub, S.; Laufs, T.; Schmidt, M.; Gerlach, F.; Saaler-Reinhardt, S.; Reuss, S.; Burmester, T. The cellular and subcellular localization of neuroglobin and cytoglobin—A clue to their function? IUBMB Life 2004, 56, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D. Oxygen Saturation and Retinopathy of Prematurity. Clin. Perinatol. 2019, 46, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Barac, I.R.; Pop, M.D.; Gheorghe, A.I.; Taban, C. Neovascular secondary glaucoma, etiology and pathogenesis. Rom. J. Ophthalmol. 2015, 59, 24–28. [Google Scholar]
- Havens, S.J.; Gulati, V. Neovascular Glaucoma. Dev. Ophthalmol. 2016, 55, 196–204. [Google Scholar]
- Ishikawa, M. Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Tsai, T.; Reinehr, S.; Maliha, A.M.; Joachim, S.C. Immune Mediated Degeneration and Possible Protection in Glaucoma. Front. Neurosci. 2019, 13, 931. [Google Scholar] [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef]
- Rutkowski, P.; May, C.A. Long-term follow-up of ocular hypertension: A plea for peripheral visual field analysis. Int. J. Case Stud. 2017, 6, 11–14. [Google Scholar]
- Podell, B.K.; Ackart, D.F.; Richardson, M.A.; DiLisio, J.E.; Pulford, B.; Basaraba, R.J. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis. Model. Mech. 2017, 10, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Faiq, M.A.; Sofi, R.A. A glimpse into the mysteries of glaucoma: From theories to clinics. Oman J. Ophthalmol. 2019, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, H.; Hauser, M.A.; Liu, Y. Update on the genetics of primary open-angle glaucoma. Exp. Eye Res. 2019, 188, 107795. [Google Scholar] [CrossRef]
| Species | Vascular Supply of the Inner Retina | Normal Intraocular Pressure Range | ||
|---|---|---|---|---|
| Choroidal | Chorioretinal | Retinal | ||
| Guinea pig | X | 10–15 mmHg [4] | ||
| Rabbit | (Visual streak) | 8–16 mmHg | ||
| Pig | X | no in vivo data | ||
| Cat | X | 16–21 mmHg | ||
| Dog | X | 15–21 mmHg | ||
| Primate/Human | X | Majority 14–16 mmHg | ||
| Mouse | X | 15–20 mmHg | ||
| Rat | X | 15–25 mmHg | ||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, C.A. Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models. Cells 2019, 8, 1254. https://doi.org/10.3390/cells8101254
May CA. Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models. Cells. 2019; 8(10):1254. https://doi.org/10.3390/cells8101254
Chicago/Turabian StyleMay, Christian Albrecht. 2019. "Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models" Cells 8, no. 10: 1254. https://doi.org/10.3390/cells8101254
APA StyleMay, C. A. (2019). Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models. Cells, 8(10), 1254. https://doi.org/10.3390/cells8101254




