PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development
Abstract
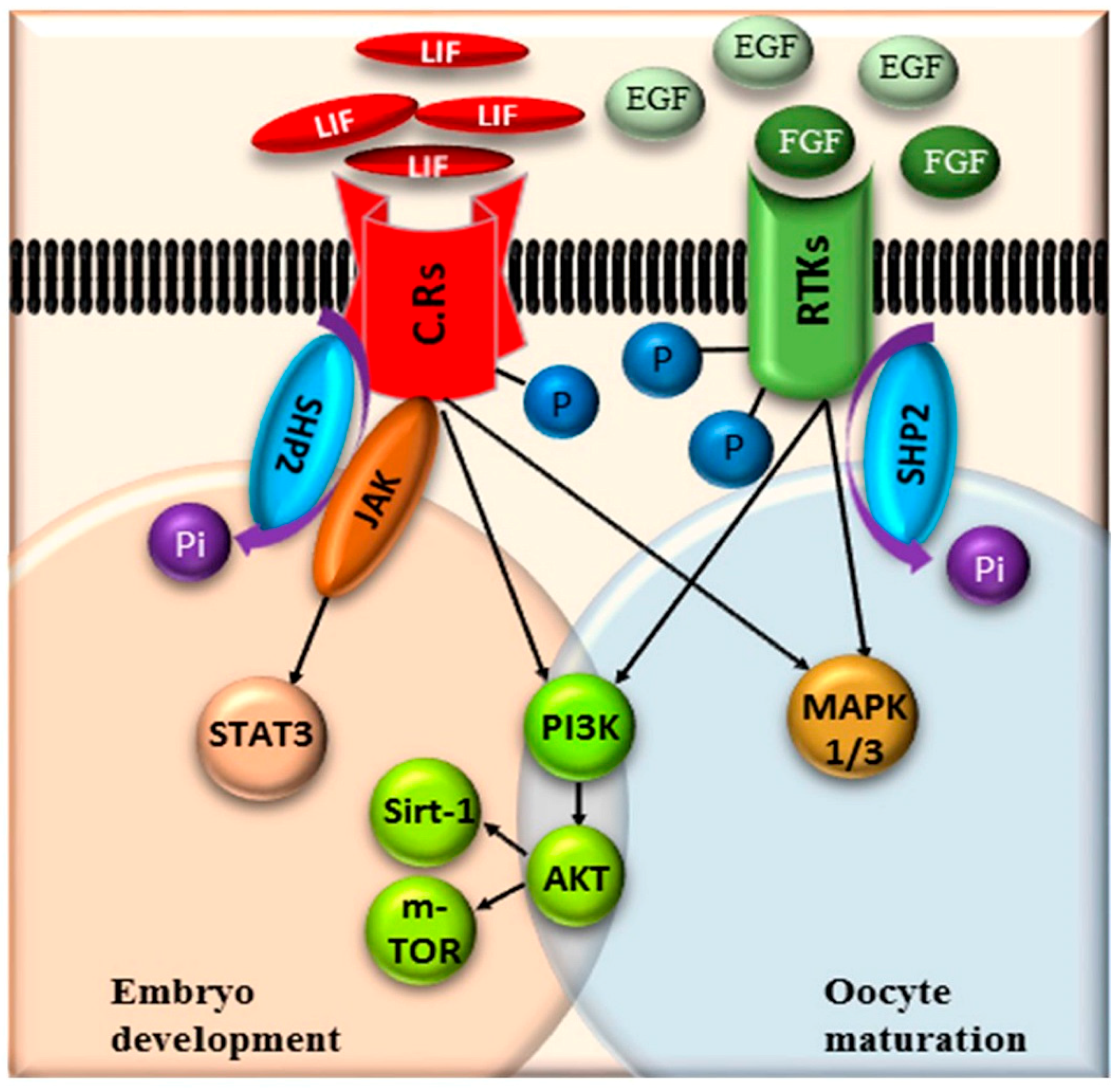
:1. Introduction
2. Material and Methods
2.1. Experimental Design
- 1
- SOF-BE1: SOF;
- 2
- SOF + EGF + CIS: COMBO;
- 3
- SOF + CIS: CISPLATIN;
- 4
- SOF + EGF: CONTROL, as this is our standard control media.
2.2. Oocyte Collection and In Vitro Maturation
2.3. In Vitro Fertilization and In Vitro Culture
2.4. Histological Analysis
2.5. Immunofluorescence
2.6. TUNEL Assay
2.7. H2DCFDA Assay (Reactive Oxygen Species (ROS) Detection)
2.8. Extraction of mRNA and cDNA Synthesis
2.9. Real-Time Polymerase Chain Reaction
2.10. Invasion Assay
2.11. Protein Extraction
2.12. Western Blot Analysis
2.13. Antibodies
2.14. Statistical Analysis
3. Results
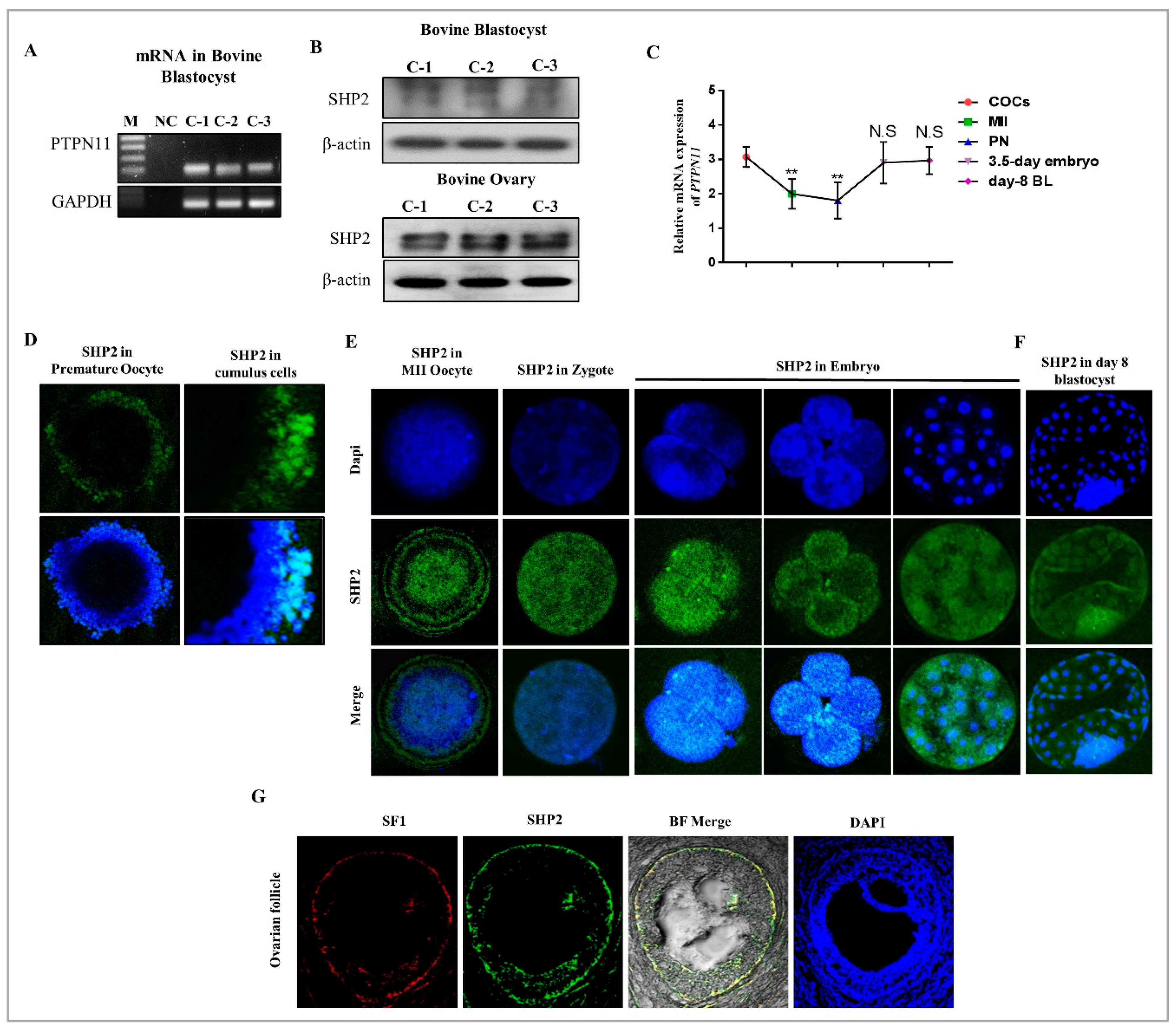
3.1. SHP2 Expression in Bovine Ovary, Oocytes and Blastocysts
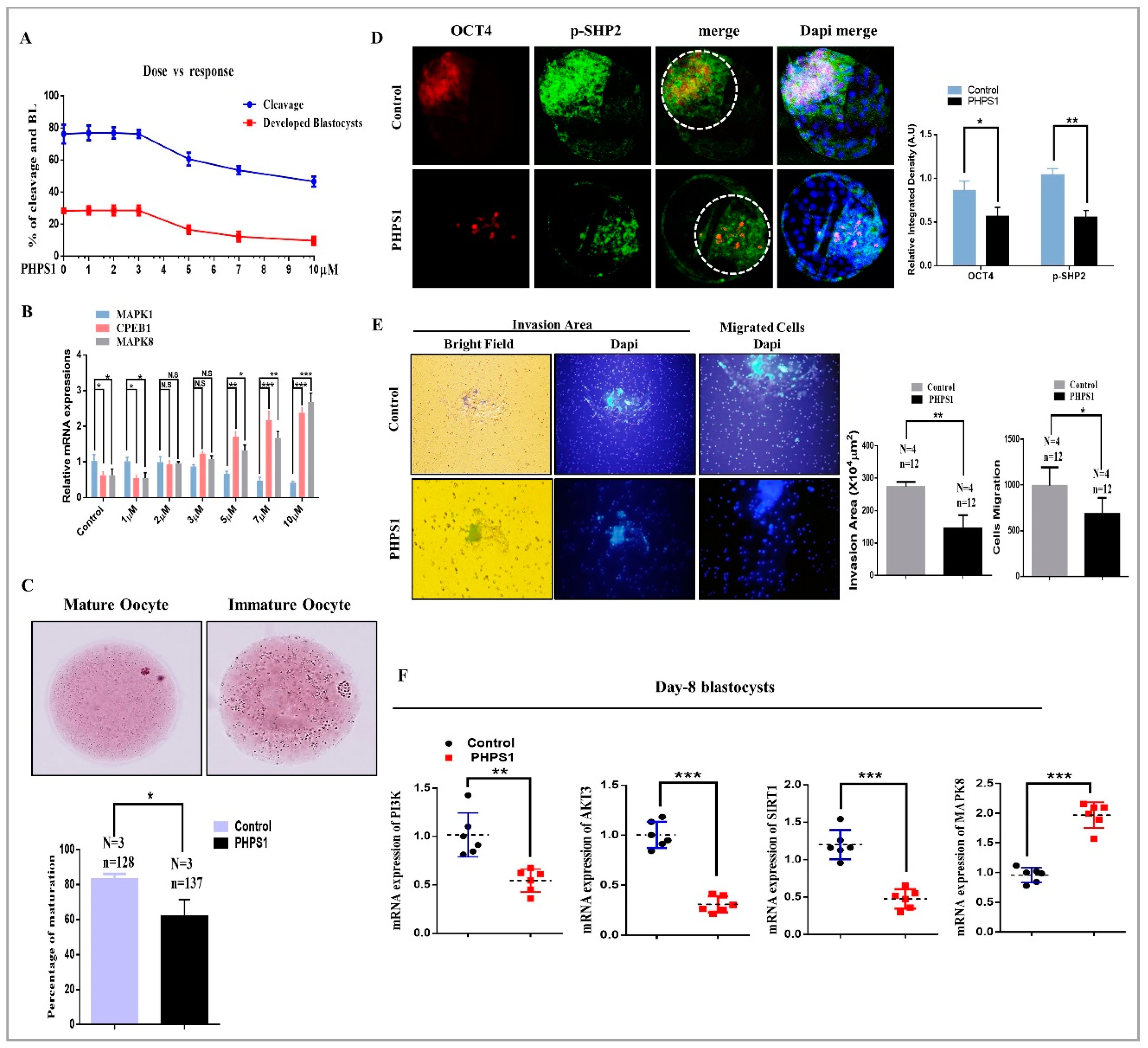
3.2. SHP2 Inhibition Compromise Oocyte Maturation and Embryo Development
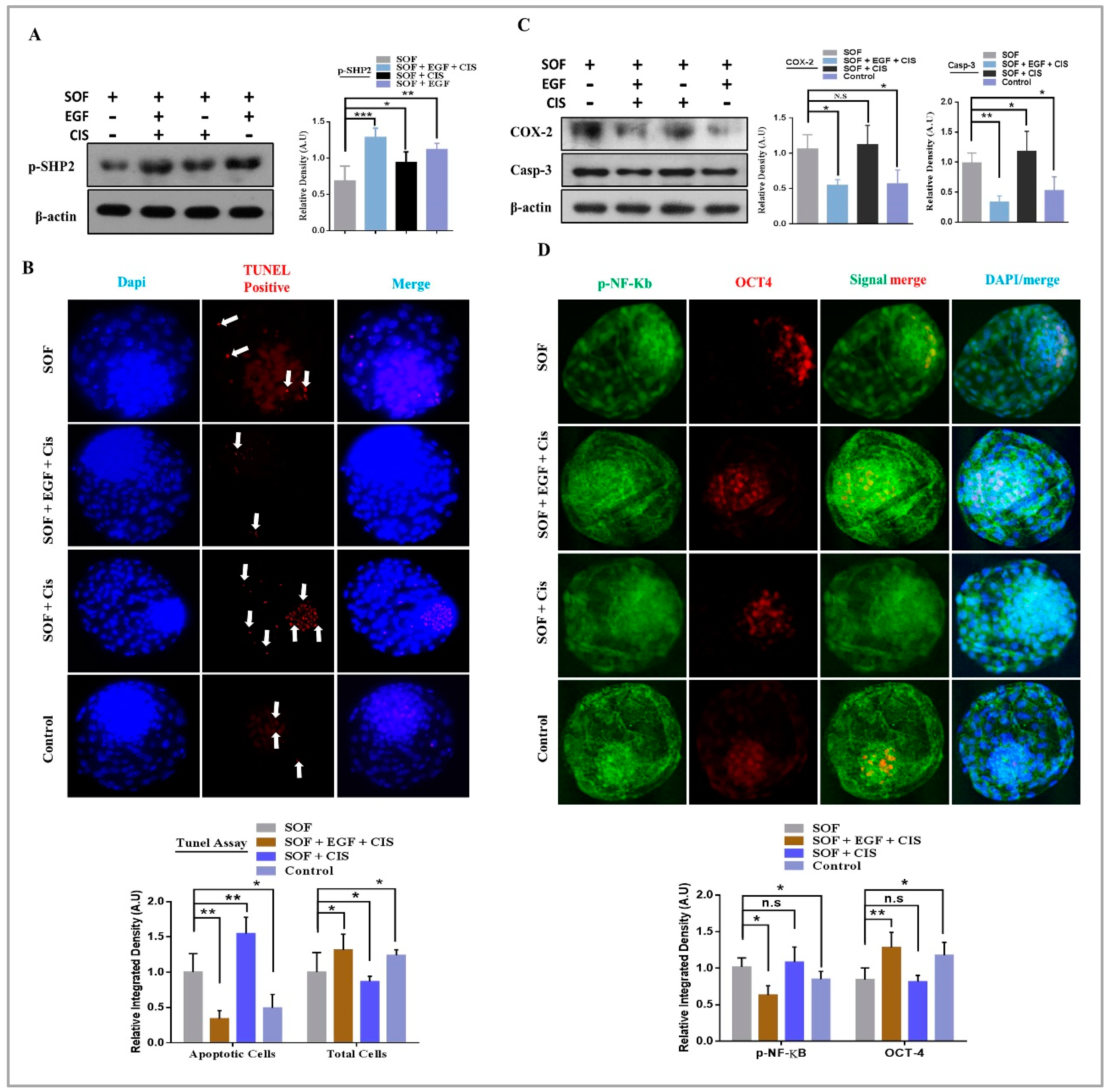
3.3. EGF Neutralize Cisplatin Induced Apoptosis and Enhance Rate of Blastocysts Development
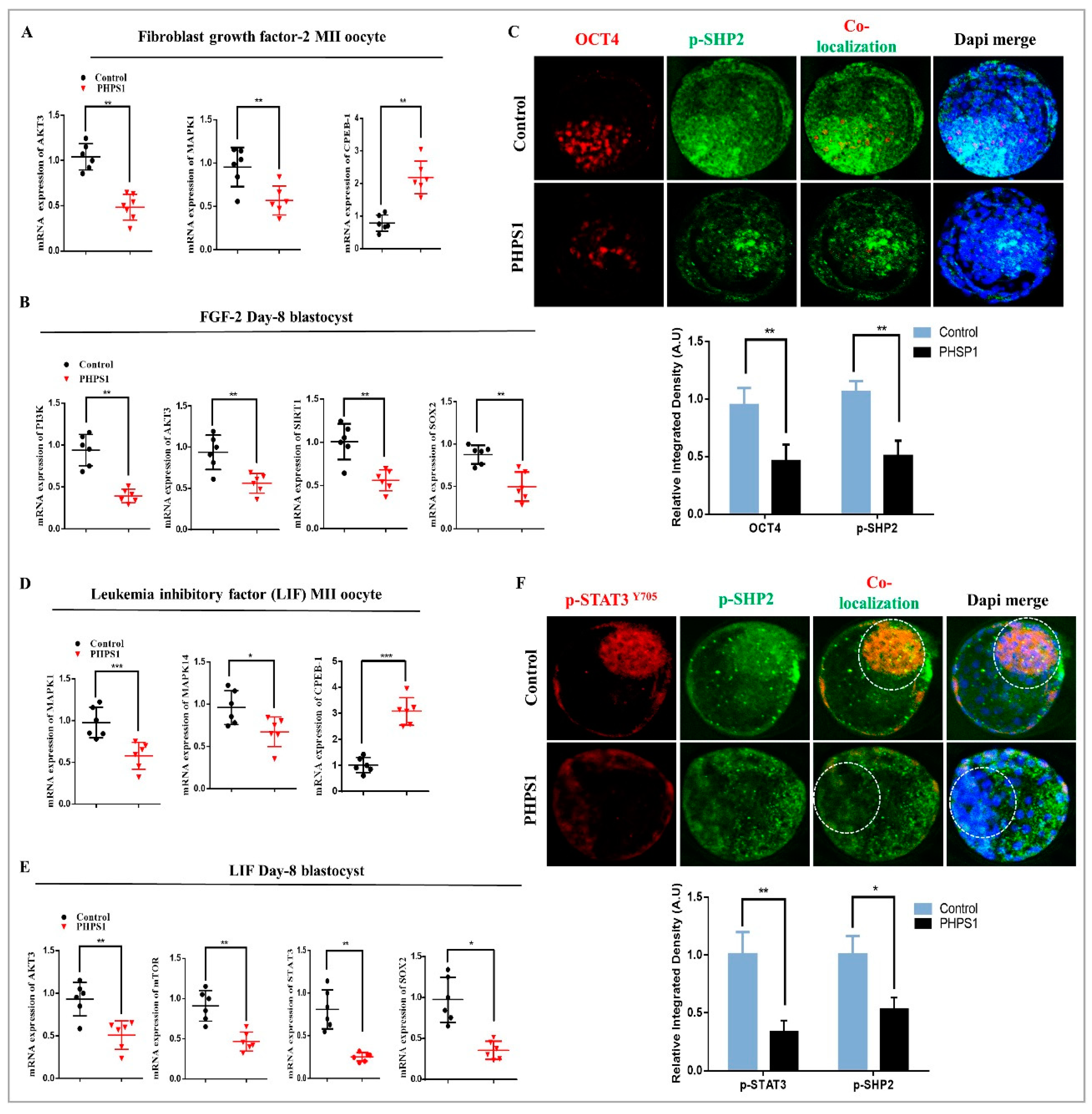
3.4. Consistent SHP2 Expression in the Presence of Ligand, Enhance RTK Downstream Pathway Proteins Without Effecting Chromatin Stability
3.5. SHP2 is Essential for RTK and Cytokine Receptor Signal Transduction During Early Developmental Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watson, A.J.; De Sousa, P.; Caveney, A.; Barcroft, L.C.; Natale, D.; Urquhart, J.; Westhusin, M.E. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol. Reprod. 2000, 62, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, M.N.; Guimaraes, A.L.S.; Leme, L.O.; Mauricio, M.F.; Dode, M.A.N. Effect of prematuration and maturation with fibroblast growth factor 10 (FGF10) on in vitro development of bovine oocytes. Theriogenology 2017, 102, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Carolan, C.; Van Langendonckt, A.; Donnay, I.; Khatir, H.; Mermillod, P. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol. Reprod. 1996, 54, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Sheng, X.; Cui, X.; Gu, M.; Liu, Y.; Qi, X.; Xing, S.; Guo, Y. Epidermal growth factor-mediated mitogen-activated protein kinase3/1 pathway is conducive to in vitro maturation of sheep oocytes. PLoS ONE 2015, 10, e0120418. [Google Scholar] [CrossRef]
- Neira, J.A.; Tainturier, D.; Pena, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef]
- Sha, Q.Q.; Dai, X.X.; Dang, Y.; Tang, F.; Liu, J.; Zhang, Y.L.; Fan, H.Y. A MAPK cascade couples maternal mRNA translation and degradation to meiotic cell cycle progression in mouse oocytes. Development 2017, 144, 452–463. [Google Scholar] [CrossRef]
- Uzbekova, S.; Arlot-Bonnemains, Y.; Dupont, J.; Dalbies-Tran, R.; Papillier, P.; Pennetier, S.; Thelie, A.; Perreau, C.; Mermillod, P.; Prigent, C.; et al. Spatio-temporal expression patterns of aurora kinases a, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol. Reprod. 2008, 78, 218–233. [Google Scholar] [CrossRef]
- Chen, J.; Torcia, S.; Xie, F.; Lin, C.J.; Cakmak, H.; Franciosi, F.; Horner, K.; Onodera, C.; Song, J.S.; Cedars, M.I.; et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat. Cell Biol. 2013, 15, 1415–1423. [Google Scholar] [CrossRef]
- Park, J.Y.; Su, Y.Q.; Ariga, M.; Law, E.; Jin, S.L.; Conti, M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef]
- Wang, L.M.; Feng, H.L.; Ma, Y.; Cang, M.; Li, H.J.; Yan, Z.; Zhou, P.; Wen, J.X.; Bou, S.; Liu, D.J. Expression of IGF receptors and its ligands in bovine oocytes and preimplantation embryos. Anim. Reprod. Sci. 2009, 114, 99–108. [Google Scholar] [CrossRef]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonks, N.K.; Neel, B.G. From form to function: Signaling by protein tyrosine phosphatases. Cell 1996, 87, 365–368. [Google Scholar] [CrossRef]
- Arregui, C.O.; Balsamo, J.; Lilien, J. Regulation of signaling by protein-tyrosine phosphatases: Potential roles in the nervous system. Neurochem. Res. 2000, 25, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Morales, L.D.; Jang, I.S.; Cho, Y.Y.; Kim, D.J. Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling. Int. J. Mol. Sci. 2018, 19, 2708. [Google Scholar] [CrossRef]
- Kheilova, K.; Petr, J.; Zalmanova, T.; Kucerova-Chrpova, V.; Rehak, D. Src family kinases are involved in the meiotic maturation of porcine oocytes. Reprod. Fertility Dev. 2015, 27, 1097–1105. [Google Scholar] [CrossRef]
- Dance, M.; Montagner, A.; Salles, J.P.; Yart, A.; Raynal, P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell. Signal. 2008, 20, 453–459. [Google Scholar] [CrossRef]
- Neel, B.G.; Gu, H.; Pao, L. The Shping news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Kontaridis, M.I.; Liu, X.; Zhang, L.; Bennett, A.M. Role of SHP-2 in fibroblast growth factor receptor-mediated suppression of myogenesis in C2C12 myoblasts. Mol. Cell. Biol. 2002, 22, 3875–3891. [Google Scholar] [CrossRef]
- Agazie, Y.M.; Hayman, M.J. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell. Biol. 2003, 23, 7875–7886. [Google Scholar] [CrossRef]
- Araki, T.; Nawa, H.; Neel, B.G. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J. Biol. Chem. 2003, 278, 41677–41684. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Tezuka, M.; Yoshida, Y.; Fukada, T.; Ohtani, T.; Yamanaka, Y.; Nishida, K.; Nakajima, K.; Hibi, M.; Hirano, T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol. 1998, 18, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Agazie, Y.M.; Hayman, M.J. Development of an efficient substrate-trapping mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J. Biol. Chem. 2003, 278, 13952–13958. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Matsumoto, K.; Nishida, E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 2004, 279, 22992–22995. [Google Scholar] [CrossRef] [PubMed]
- Hanke, S.; Mann, M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol. Cell. Proteom. 2009, 8, 519–534. [Google Scholar] [CrossRef]
- Mo, X.; Wu, G.; Yuan, D.; Jia, B.; Liu, C.; Zhu, S.; Hou, Y. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2014, 81, 608–618. [Google Scholar] [CrossRef]
- Grossmann, K.S.; Rosario, M.; Birchmeier, C.; Birchmeier, W. The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 2010, 106, 53–89. [Google Scholar] [CrossRef]
- Cunnick, J.M.; Meng, S.; Ren, Y.; Desponts, C.; Wang, H.G.; Djeu, J.Y.; Wu, J. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 2002, 277, 9498–9504. [Google Scholar] [CrossRef]
- Fan, H.Y.; Sun, Q.Y. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol. Reprod. 2004, 70, 535–547. [Google Scholar] [CrossRef]
- Torner, H.; Kubelka, M.; Heleil, B.; Tomek, W.; Aim, H.; Kuzmina, T.; Guiard, V. Dynamics of meiosis and protein kinase activities in bovine oocytes correlated to prolactin treatment and follicle size. Theriogenology 2001, 55, 885–899. [Google Scholar] [CrossRef]
- Wang, L.; Iorio, C.; Yan, K.; Yang, H.; Takeshita, S.; Kang, S.; Neel, B.G.; Yang, W. A ERK/RSK-mediated negative feedback loop regulates M-CSF-evoked PI3K/AKT activation in macrophages. FASEB J. Off. Publ. Am. Soc. Exp. Biol. 2018, 32, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, C.; Perreau, C.; Dupont, J.; Uzbekova, S.; Prigent, C.; Mermillod, P. Several signaling pathways are involved in the control of cattle oocyte maturation. Mol. Reprod. Dev. 2004, 69, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Yu, Z.H.; Zeng, L.; Zhang, S.; Bai, Y.; Miao, J.; Chen, L.; Xie, J.; Zhang, Z.Y. SHP2 phosphatase as a novel therapeutic target for melanoma treatment. Oncotarget 2016, 7, 73817–73829. [Google Scholar] [CrossRef] [PubMed]
- Tomek, W.; Smiljakovic, T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction 2005, 130, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, K.; Grosskopf, S.; Lum, C.T.; Wurtele, M.; Roder, N.; von Kries, J.P.; Rosario, M.; Rademann, J.; Birchmeier, W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc. Natl. Acad. Sci. USA 2008, 105, 7275–7280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.C.; Chu, C.Y.; Lin, J.J.; Lo, L.C. Selective activation of SHP2 activity by cisplatin revealed by a novel chemical probe-based assay. Biochem. Biophys. Res. Commun. 2010, 391, 230–234. [Google Scholar] [CrossRef]
- Mesalam, A.; Kong, R.; Khan, I.; Chowdhury, M.; Choi, B.H.; Kim, S.W.; Cho, K.W.; Jin, J.I.; Kong, I.K. Effect of charcoal:dextran stripped fetal bovine serum on in vitro development of bovine embryos. Reprod. Biol. 2017, 17, 312–319. [Google Scholar] [CrossRef]
- Chowdhury, M.M.R.; Mesalam, A.; Khan, I.; Joo, M.D.; Lee, K.L.; Xu, L.; Afrin, F.; Kong, I.K. Improved developmental competence in embryos treated with lycopene during in vitro culture system. Mol. Reprod. Dev. 2018, 85, 46–61. [Google Scholar] [CrossRef]
- Gonzalez-Marrero, I.; Hernandez-Abad, L.G.; Carmona-Calero, E.M.; Castaneyra-Ruiz, L.; Abreu-Reyes, J.A.; Castaneyra-Perdomo, A. Systemic Hypertension Effects on the Ciliary Body and Iris. An Immunofluorescence Study with Aquaporin 1, Aquaporin 4, and Na (+), K (+) ATPase in Hypertensive Rats. Cells 2018, 7, 210. [Google Scholar] [CrossRef]
- Deb, G.K.; Dey, S.R.; Bang, J.I.; Cho, S.J.; Park, H.C.; Lee, J.G.; Kong, I.K. 9-cis retinoic acid improves developmental competence and embryo quality during in vitro maturation of bovine oocytes through the inhibition of oocyte tumor necrosis factor-alpha gene expression. J. Anim. Sci. 2011, 89, 2759–2767. [Google Scholar] [CrossRef]
- Khan, I.; Lee, K.L.; Xu, L.; Mesalam, A.; Chowdhury, M.M.; Joo, M.D.; Ihsan Ul, H.; Mirza, B.; Kong, I.K. Improvement of in vitro-produced bovine embryo treated with coagulansin-A under heat-stressed condition. Reproduction 2017, 153, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavropoulou, M.P.; Poulios, C.; Michalopoulos, N.; Gatzou, A.; Chrisafi, S.; Mantalovas, S.; Papavramidis, T.; Daskalaki, E.; Sofou, E.; Kotsa, K.; et al. A Role for Circular Non-Coding RNAs in the Pathogenesis of Sporadic Parathyroid Adenomas and the Impact of Gender-Specific Epigenetic Regulation. Cells 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Katafuchi, R.; Yanase, T.; Ikeda, K.; Tanaka, H.; Fujimi, S. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.N.M.; Campbell, A.; Devito, L.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef]
- Naidansuren, P.; Park, C.W.; Kim, S.H.; Nanjidsuren, T.; Park, J.J.; Yun, S.J.; Sim, B.W.; Hwang, S.; Kang, M.H.; Ryu, B.Y.; et al. Molecular characterization of bovine placental and ovarian 20alpha-hydroxysteroid dehydrogenase. Reproduction 2011, 142, 723–731. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Haraguchi, H.; Hirota, Y.; Saito-Fujita, T.; Tanaka, T.; Shimizu-Hirota, R.; Harada, M.; Akaeda, S.; Hiraoka, T.; Matsuo, M.; Matsumoto, L.; et al. Mdm2-p53-SF1 pathway in ovarian granulosa cells directs ovulation and fertilization by conditioning oocyte quality. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 2610–2620. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, H.C.; Chou, C.C.; Hur, S.S.; Osterday, K.; del Alamo, J.C.; Lasheras, J.C.; Chien, S. Shp2 plays a crucial role in cell structural orientation and force polarity in response to matrix rigidity. Proc. Natl. Acad. Sci. USA 2013, 110, 2840–2845. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, J.; Wang, Z.; Ji, W.; Tian, R.; Zhang, F.; Niu, R. Shp2 Plays a Critical Role in IL-6-Induced EMT in Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 395. [Google Scholar] [CrossRef]
- Yang, W.; Klaman, L.D.; Chen, B.; Araki, T.; Harada, H.; Thomas, S.M.; George, E.L.; Neel, B.G. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 2006, 10, 317–327. [Google Scholar] [CrossRef]
- Caraglia, M.; Tagliaferri, P.; Marra, M.; Giuberti, G.; Budillon, A.; Gennaro, E.D.; Pepe, S.; Vitale, G.; Improta, S.; Tassone, P.; et al. EGF activates an inducible survival response via the RAS-> Erk-1/2 pathway to counteract interferon-alpha-mediated apoptosis in epidermoid cancer cells. Cell Death Differ. 2003, 10, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Zhou, Y.; Chui, C.H.; Lam, K.H.; Law, S.; Chan, A.S.; Li, X.; Lam, A.K.; Tang, J.C.O. Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma. Cells 2018, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, F.; Uppangala, S.; Asampille, G.; Salian, S.R.; Kalthur, G.; Talevi, R.; Atreya, H.S.; Adiga, S.K. Spent embryo culture medium metabolites are related to the in vitro attachment ability of blastocysts. Sci. Rep. 2018, 8, 17025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005, 1, 2005.0010. [Google Scholar] [CrossRef]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef]
- Yuan, J.; Pu, M.; Zhang, Z.; Lou, Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle 2009, 8, 1747–1753. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.G.; Xu, L.; Zhang, S.; Mesalam, A.; Lee, K.L.; Liu, H.; Joo, M.D.; Idrees, M.; Kong, I.K. Polydatin and I-CBP112 protects early bovine embryo against nicotinamide-induced mitochondrial dysfunction. Theriogenology 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Fields, S.D.; Hansen, P.J.; Ealy, A.D. Fibroblast growth factor requirements for in vitro development of bovine embryos. Theriogenology 2011, 75, 1466–1475. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Ripamonte, P.; Buratini, J.; Portela, V.M.; Price, C.A. Fibroblast growth factor-2 regulation of Sprouty and NR4A genes in bovine ovarian granulosa cells. J. Cell. Physiol. 2011, 226, 1820–1827. [Google Scholar] [CrossRef]
- Sirotkin, A.V. The Role and Application of Sirtuins and mTOR Signaling in the Control of Ovarian Functions. Cells 2016, 5, 42. [Google Scholar] [CrossRef]
- Meng, F.; Forrester-Gauntlett, B.; Turner, P.; Henderson, H.; Oback, B. Signal Inhibition Reveals JAK/STAT3 Pathway as Critical for Bovine Inner Cell Mass Development. Biol. Reprod. 2015, 93, 132. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Cao, F.; Jiang, H.; Li, A.; Li, J.; Qiu, L.; Shen, H.; Chang, W.; Zhou, C.; et al. SHP2 associates with nuclear localization of STAT3: Significance in progression and prognosis of colorectal cancer. Sci. Rep. 2017, 7, 17597. [Google Scholar] [CrossRef] [PubMed]
- Deb, T.B.; Wong, L.; Salomon, D.S.; Zhou, G.; Dixon, J.E.; Gutkind, J.S.; Thompson, S.A.; Johnson, G.R. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J. Biol. Chem. 1998, 273, 16643–16646. [Google Scholar] [CrossRef] [PubMed]
- Saxton, T.M.; Ciruna, B.G.; Holmyard, D.; Kulkarni, S.; Harpal, K.; Rossant, J.; Pawson, T. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat. Genet. 2000, 24, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Saxton, T.M.; Henkemeyer, M.; Gasca, S.; Shen, R.; Rossi, D.J.; Shalaby, F.; Feng, G.S.; Pawson, T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997, 16, 2352–2364. [Google Scholar] [CrossRef]
- Qian, H.; Xu, J.; Lalioti, M.D.; Gulle, K.; Sakkas, D. Oocyte numbers in the mouse increase after treatment with 5-aminoisoquinolinone: A potent inhibitor of poly (ADP-ribosyl) ation. Biol. Reprod. 2010, 82, 1000–1007. [Google Scholar] [CrossRef]
- Ran, H.; Kong, S.; Zhang, S.; Cheng, J.; Zhou, C.; He, B.; Xin, Q.; Lydon, J.P.; DeMayo, F.J.; Feng, G.S.; et al. Nuclear Shp2 directs normal embryo implantation via facilitating the ERalpha tyrosine phosphorylation by the Src kinase. Proc. Natl. Acad. Sci. USA 2017, 114, 4816–4821. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Garcia-Herreros, M.; O'Shea, L.C.; Hensey, C.; Lonergan, P.; Fair, T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef] [Green Version]
- Chediek Dall’Acqua, P.; Barros Nunes, G.; Rodrigues da Silva, C.; Fontes, P.K.; Fabio Gouveia Nogueira, M.; Lombardi Lopes, F.; Marinho, M.; Zoccal Mingoti, G. Differences in embryonic gene expression and quality indicate the benefit of epidermal growth factor receptor inhibitor during prematuration to improve competence in bovine oocytes. Reprod. Domest. Anim. = Zuchthyg. 2019, 54, 666–677. [Google Scholar] [CrossRef]
- Liang, C.G.; Su, Y.Q.; Fan, H.Y.; Schatten, H.; Sun, Q.Y. Mechanisms regulating oocyte meiotic resumption: Roles of mitogen-activated protein kinase. Mol. Endocrinol. 2007, 21, 2037–2055. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, E.W.; van Tol, L.T.; Van de Velde, H.; Wubbolts, R.; Welling, M.; Geijsen, N.; Roelen, B.A. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 2012, 139, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, Y.; Wei, L.; Liu, W.; Tian, Y.; Chen, B.; Lin, X.; Li, Y.; Feng, G.S.; Lu, Z. Tyrosine phosphatase Shp2 mediates the estrogen biological action in breast cancer via interaction with the estrogen extranuclear receptor. PLoS ONE 2014, 9, e102847. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, C.J.; Salvador, I.; Cebrian-Serrano, A.; Lopera, R.; Silvestre, M.A. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality. Anim. Int. J. Anim. Biosci. 2013, 7, 455–462. [Google Scholar] [CrossRef]
- Wang, J.J.; Ge, W.; Liu, J.C.; Klinger, F.G.; Dyce, P.W.; De Felici, M.; Shen, W. Complete in vitro oogenesis: Retrospects and prospects. Cell Death Differ. 2017, 24, 1845–1852. [Google Scholar] [CrossRef]
- De Matos, D.G.; Miller, K.; Scott, R.; Tran, C.A.; Kagan, D.; Nataraja, S.G.; Clark, A.; Palmer, S. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation. Fertil. Steril. 2008, 90, 2367–2375. [Google Scholar] [CrossRef]
- Furcht, C.M.; Buonato, J.M.; Skuli, N.; Mathew, L.K.; Munoz Rojas, A.R.; Simon, M.C.; Lazzara, M.J. Multivariate signaling regulation by SHP2 differentially controls proliferation and therapeutic response in glioma cells. J. Cell Sci. 2014, 127, 3555–3567. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, Y.; Miyoshi, S.; Fukuda, K.; Nishiyama, N.; Ikegami, Y.; Tanimoto, K.; Murata, M.; Takahashi, E.; Shimoda, K.; Hirano, T.; et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J. Mol. Cell. Cardiol. 2007, 43, 710–716. [Google Scholar] [CrossRef]
- Li, M.; Gao, J.; Li, D.; Yin, Y. CEP55 Promotes Cell Motility via JAK2–STAT3–MMPs Cascade in Hepatocellular Carcinoma. Cells 2018, 7, 99. [Google Scholar] [CrossRef]
- Wu, D.; Pang, Y.; Ke, Y.; Yu, J.; He, Z.; Tautz, L.; Mustelin, T.; Ding, S.; Huang, Z.; Feng, G.S. A conserved mechanism for control of human and mouse embryonic stem cell pluripotency and differentiation by shp2 tyrosine phosphatase. PLoS ONE 2009, 4, e4914. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, Z.; Li, Y.; Liu, W.; Zhang, S.; Wang, B.; Tian, Y.; Zhao, Y.; Ran, H.; Liu, W.; et al. Deletion of the tyrosine phosphatase Shp2 in Sertoli cells causes infertility in mice. Sci. Rep. 2015, 5, 12982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, A.; Hiniker, S.M.; Ramanand, S.G.; Nyati, S.; Hegde, A.; Helman, A.; Menawat, R.; Bhojani, M.S.; Lawrence, T.S.; Nyati, M.K. Role of epidermal growth factor receptor degradation in cisplatin-induced cytotoxicity in head and neck cancer. Cancer Res. 2010, 70, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]

| Name | Accession No. | Order Name | Sequence (5′-3′) | ProductSize (bp) |
|---|---|---|---|---|
| PTPN11 | XM_002694590.6 | F | GGCACAGTACTACAACTCAA | 100 |
| R | TGGTCTCAGCTAATTTGCTT | |||
| MAPK1 | NM_175793 | F | CCGTGTTGCAGATCCAGAC | 130 |
| R | GACGGACCAGATGTCGATG | |||
| MAPK14 | NM_001102 | F | GCTGTCGACCTGCTGGAGAAGATG | 110 |
| R | TCGTCGTCAGGATCGTGGTACTGG | |||
| CPEB1 | XM_864691 | F | GTGTGGAGTGGCCTGGTAAG | 114 |
| R | GAGAGCAAGCCTGAAGCAAG | |||
| MAPK8 | NM_001192974.1 | F | GACGCTTGATTGCATGTAAA | 153 |
| R | TACCTCAAAGGGCTTCATTC | |||
| mTOR | XM_002694043.6 | F | TTAACAGGGTTCGAGACAAG | 114 |
| R | AGAGGTTTTCATGGGATGTC | |||
| PI3K | NM_174574.1 234 | F | TCAACCATGACTGTGTGCCA | 234 |
| R | CCATCAGCATCAAATTGGGCA | |||
| AKT3 | NM_001191309.1 | F | AGCTGTTTTTCCATTTGTCG | 94 |
| R | TGTAGATAGTCCAAGGCAGA | |||
| SIRT1 | NM_001192980.2 | F | CAACGGTTTCCATTCGTGTG | 138 |
| R | GTTCGAGGATCTGTGCCAAT | |||
| BAX | NM_173894 | F | CACCAAGAAGCTGAGCGAGTGT | 118 |
| R | TCGGAAAAAGACCTCTCGGGGA | |||
| BCL-2 | NM_001166486.1 | F | TGGATGACCGAGTACCTGAA | 123 |
| R | GAGACAGCCAGGAGAAATCAAA | |||
| SOX2 | NM_001105463 | F | CTATGACCAGCT CGCAGA | 152 |
| R | GGAAGAAGAGGTAACCACG | |||
| STAT3 | NM_001012671 | F | CTCTCCCCACTTCTGCCAAG | 118 |
| R | AGGGGTCACAACTGCTGCTC | |||
| GAPDH | NM_001034034 | F | CCCAGAATATCATCCCTGCT | 185 |
| R | CTGCTTCACCACCTTCTTGA |
| Groups | No. of Presumed Zygotes | No. of Cleavage Embryo (% ± SEM) | No. of Blastocysts (% ± SEM) |
|---|---|---|---|
| Control | 339 | 254 (75.71 ± 2.76) a | 84 (31.86 ± 1.16) a |
| PHPS1 | 316 | 154 (50.00 ± 6.44) b | 47 (16.14 ± 2.53) b |
| Groups | No. of Presumed Zygotes | No. of Cleavage Embryos (% ± SEM) | No. of Blastocysts (% ± SEM) |
|---|---|---|---|
| SOF | 789 | 475 (68.25 ± 2.14) a | 175 (20.25 ± 0.86) a |
| COMBO | 748 | 633 (84.00 ± 1.45) b | 294 (41.00 ± 0.94) c |
| CISPLATIN | 770 | 480 (66.25 ± 1.33) a | 149 (18.00 ± 1.00) a |
| CONTROL | 769 | 599 (79.75 ± 1.63) b | 240 (31.25 ± 0.56) b |
| Groups | No. of Presumed Zygotes | No. of Cleavage Embryos (% ± SEM) | No. of Blastocysts (% ± SEM) |
|---|---|---|---|
| SOF + FGF-2 | 456 | 335 (74.88 ± 3.34) a | 255 (32.22 ± 1.66) a |
| SOF + FGF-2 + PHPS1 | 432 | 211 (48.58 ± 3.64) c | 154 (15.00 ± 1.03) b |
| SOF + EGF + LIF | 452 | 369 (81.78 ± 1.54) a | 259 (33.33 ± 2.88) a |
| SOF + EGF + LIF + PHPS1 | 448 | 257 (57.89 ± 1.89) b | 171 (13.67 ± 1.59) b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.; Xu, L.; Song, S.-H.; Joo, M.-D.; Lee, K.-L.; Muhammad, T.; El Sheikh, M.; Sidrat, T.; Kong, I.-K. PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development. Cells 2019, 8, 1272. https://doi.org/10.3390/cells8101272
Idrees M, Xu L, Song S-H, Joo M-D, Lee K-L, Muhammad T, El Sheikh M, Sidrat T, Kong I-K. PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development. Cells. 2019; 8(10):1272. https://doi.org/10.3390/cells8101272
Chicago/Turabian StyleIdrees, Muhammad, Lianguang Xu, Seok-Hwan Song, Myeong-Don Joo, Kyeong-Lim Lee, Tahir Muhammad, Marwa El Sheikh, Tabinda Sidrat, and Il-Keun Kong. 2019. "PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development" Cells 8, no. 10: 1272. https://doi.org/10.3390/cells8101272
APA StyleIdrees, M., Xu, L., Song, S.-H., Joo, M.-D., Lee, K.-L., Muhammad, T., El Sheikh, M., Sidrat, T., & Kong, I.-K. (2019). PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development. Cells, 8(10), 1272. https://doi.org/10.3390/cells8101272








