Co-Translational Insertion of Aquaporins into Liposome for Functional Analysis via an E. coli Based Cell-Free Protein Synthesis System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid and DNA Manipulation
2.2. CFPS
2.3. Liposome Preparation
2.4. Fluorescence Quantification
2.5. Sample Preparation for Water Permeability Measurements
2.6. Preparation of Giant Unilamellar Vesicles (GUVs) from Large Unilamellar Vesicles (LUVs)
2.7. Water Permeability Measurements
2.8. Confocal Fluorescence Microscopy
3. Results
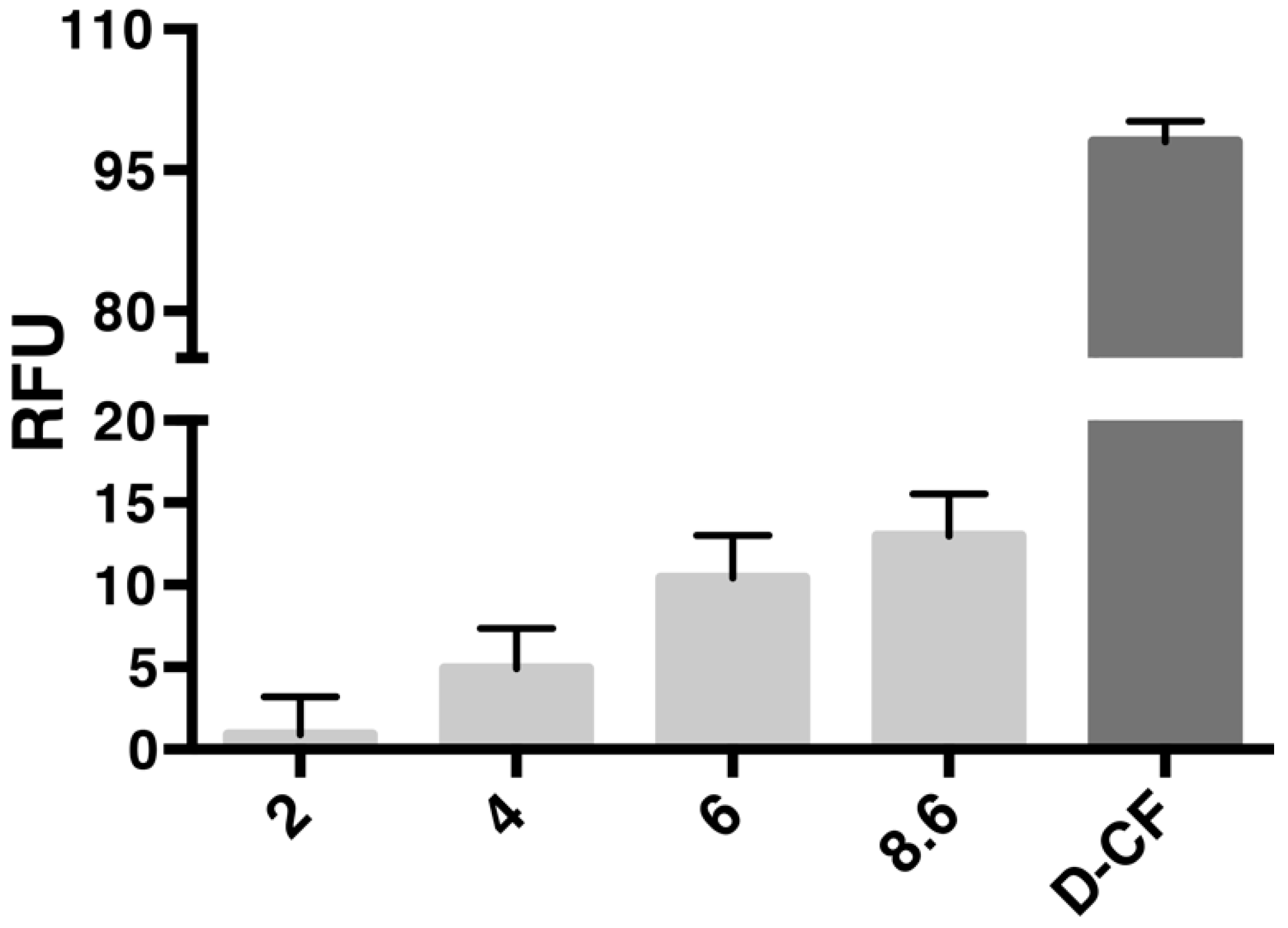
3.1. Co-Translational Incorporation of AqpZ-sGFP into Preformed Liposomes
3.2. Improving Homogeneity of l-CFPS Produced AqpZ-sGFP Proteo-Liposomes for Functional Analysis of Water Permeability
3.3. Visualization of Aquaporin Insertion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin Z incorporation. Coll. Surf. B Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Coutable, A.; Thibault, C.; Chalmeau, J.; Francois, J.M.; Vieu, C.; Noireaux, V.; Trevisiol, E. Preparation of tethered-lipid bilayers on gold surfaces for the incorporation of integral membrane proteins synthesized by cell-free expression. Langmuir 2014, 30, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, F.; Tozawa, Y. Cell-free expression—Making a mark. Curr. Opin. Struct. Biol. 2013, 23, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Dotsch, V.; Kaldenhoff, R.; Bernhard, F. Artificial environments for the co-translational stabilization of cell-free expressed proteins. PLoS ONE 2013, 8, e56637. [Google Scholar] [CrossRef]
- Kai, L.; Orban, E.; Henrich, E.; Proverbio, D.; Dotsch, V.; Bernhard, F. Insoluble Proteins; Humana Press: New York, NY, USA, 2015; Volume 1258, Chapter 7; pp. 125–143. [Google Scholar]
- Schneider, B.; Junge, F.; Shirokov, V.A.; Durst, F.; Schwarz, D.; Dotsch, V.; Bernhard, F. Heterologous Expression of Membrane Proteins; Humana Press: New York, NY, USA, 2010; Volume 601, Chapter 11; pp. 165–186. [Google Scholar]
- Schwarz, D.; Daley, D.; Beckhaus, T.; Dotsch, V.; Bernhard, F. Cell-free expression profiling of E. coli inner membrane proteins. Proteomics 2010, 10, 1762–1779. [Google Scholar] [CrossRef]
- Hovijitra, N.T.; Wuu, J.J.; Peaker, B.; Swartz, J.R. Cell-free synthesis of functional aquaporin Z in synthetic liposomes. Biotechnol. Bioeng. 2009, 104, 40–49. [Google Scholar] [CrossRef]
- Kai, L.; Kaldenhoff, R.; Lian, J.; Zhu, X.; Dotsch, V.; Bernhard, F.; Cen, P.; Xu, Z. Preparative scale production of functional mouse aquaporin 4 using different cell-free expression modes. PLoS ONE 2010, 5, e12972. [Google Scholar] [CrossRef]
- Muller-Lucks, A.; Gena, P.; Frascaria, D.; Altamura, N.; Svelto, M.; Beitz, E.; Calamita, G. Preparative scale production and functional reconstitution of a human aquaglyceroporin (AQP3) using a cell free expression system. New Biotechnol. 2013, 30, 545–551. [Google Scholar] [CrossRef]
- Von Bulow, J.; Muller-Lucks, A.; Kai, L.; Bernhard, F.; Beitz, E. Functional characterization of a novel aquaporin from Dictyostelium discoideum amoebae implies a unique gating mechanism. J. Biol. Chem. 2012, 287, 7487–7494. [Google Scholar] [CrossRef]
- Kai, L.; Kaldenhoff, R. A refined model of water and CO(2) membrane diffusion: Effects and contribution of sterols and proteins. Sci. Rep. 2014, 4, 6665. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Kai, L.; Uehlein, N. Aquaporins and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta 2014, 1840, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Roos, C.; Kai, L.; Proverbio, D.; Ghoshdastider, U.; Filipek, S.; Dotsch, V.; Bernhard, F. Co-translational association of cell-free expressed membrane proteins with supplied lipid bilayers. Mol. Membr. Biol. 2013, 30, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Uehlein, N.; Sdorra, S.; Fischer, M.; Ayaz, M.; Belastegui-Macadam, X.; Heckwolf, M.; Lachnit, M.; Pede, N.; Priem, N.; et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 2010, 285, 31253–31260. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Junge, F.; Durst, F.; Frolich, N.; Schneider, B.; Reckel, S.; Sobhanifar, S.; Dotsch, V.; Bernhard, F. Preparative scale expression of membrane proteins in Escherichia coli-based continuous exchange cell-free systems. Nat. Protoc. 2007, 2, 2945–2957. [Google Scholar] [CrossRef]
- Kai, L.; Roos, C.; Haberstock, S.; Proverbio, D.; Ma, Y.; Junge, F.; Karbyshev, M.; Dotsch, V.; Bernhard, F. Systems for the cell-free synthesis of proteins. Methods Mol. Biol. 2012, 800, 201–225. [Google Scholar] [CrossRef]
- Tsumoto, K.; Matsuo, H.; Tomita, M.; Yoshimura, T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Coll. Surf. B Biointerfaces 2009, 68, 98–105. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Agre, P. Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc. Natl. Acad. Sci. USA 2001, 98, 2888–2893. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Sugishita, K.; Yoneyama, S.; Akada, K.; Ueha, M.; Nakamura, A.; Kobayashi, S. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim. Biophys. Acta 2000, 1467, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef]
- Fischer, G.; Kosinska-Eriksson, U.; Aponte-Santamaria, C.; Palmgren, M.; Geijer, C.; Hedfalk, K.; Hohmann, S.; de Groot, B.L.; Neutze, R.; Lindkvist-Petersson, K. Crystal structure of a yeast aquaporin at 1.15 angstrom reveals a novel gating mechanism. PLoS Biol. 2009, 7, e1000130. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, W.; Schenk, A.D.; Johanson, U.; Braun, T.; de Groot, B.L.; Fotiadis, D.; Kjellbom, P.; Engel, A. The 5A structure of heterologously expressed plant aquaporin SoPIP2;1. J. Mol. Biol. 2005, 350, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Hiroaki, Y.; Tani, K.; Kamegawa, A.; Gyobu, N.; Nishikawa, K.; Suzuki, H.; Walz, T.; Sasaki, S.; Mitsuoka, K.; Kimura, K.; et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006, 355, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, L.; Enberg, J.; Neutze, R.; Goran Karlsson, B.; Pedersen, A. Expression screening of membrane proteins with cell-free protein synthesis. Protein Expr. Purif. 2012, 82, 218–225. [Google Scholar] [CrossRef]
- Rigaud, J.L.; Pitard, B.; Levy, D. Reconstitution of membrane proteins into liposomes: Application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1995, 1231, 223–246. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- Lentz, B.R.; Lee, J.K. Poly (ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: A mechanism in common with viral fusion and secretory vesicle release? (Review). Mol. Membr. Biol. 1999, 16, 279–296. [Google Scholar] [CrossRef]
- Duzgunes, N.; Wilschut, J.; Fraley, R.; Papahadjopoulos, D. Studies on the Mechanism of Membrane-Fusion—Role of Headgroup Composition in Calcium-Induced and Magnesium-Induced Fusion of Mixed Phospholipid-Vesicles. Biochim. Biophys. Acta 1981, 642, 182–195. [Google Scholar] [CrossRef]
- Proverbio, D.; Roos, C.; Beyermann, M.; Orban, E.; Dotsch, V.; Bernhard, F. Functional properties of cell-free expressed human endothelin A and endothelin B receptors in artificial membrane environments. Biochim. Biophys. Acta 2013, 1828, 2182–2192. [Google Scholar] [CrossRef] [Green Version]
- Klammt, C.; Schwarz, D.; Fendler, K.; Haase, W.; Dotsch, V.; Bernhard, F. Evaluation of detergents for the soluble expression of α-helical and β-barrel-type integral membrane proteins by a preparative scale individual cell-free expression system. FEBS J. 2005, 272, 6024–6038. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; le Maire, M.; Moller, J.V. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys. J. 1998, 75, 2932–2946. [Google Scholar] [CrossRef]
- Geertsma, E.R.; Nik Mahmood, N.A.; Schuurman-Wolters, G.K.; Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 2008, 3, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knol, J.; Sjollema, K.; Poolman, B. Detergent-mediated reconstitution of membrane proteins. Biochemistry 1998, 37, 16410–16415. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.M.; Rivers, R.L.; Zeidel, M.L.; Roberts, D.M. Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry 1999, 38, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Habel, J.E.; Shen, Y.X.; Meier, W.P.; Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 2012, 134, 18631–18637. [Google Scholar] [CrossRef]
- Sun, G.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. Stabilization and immobilization of aquaporin reconstituted lipid vesicles for water purification. Coll. Surf. B Biointerfaces 2013, 102, 466–471. [Google Scholar] [CrossRef]
- Wang, H.; Chung, T.S.; Tong, Y.W.; Jeyaseelan, K.; Armugam, A.; Chen, Z.; Hong, M.; Meier, W. Highly permeable and selective pore-spanning biomimetic membrane embedded with aquaporin Z. Small 2012, 8, 1185–1190. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. AQP4 antibodies in neuromyelitis optica: Diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 2010, 6, 383–392. [Google Scholar] [CrossRef]



| Sample | Diameter (nm) | k | Pf (μm/s) | Normalization (%) |
|---|---|---|---|---|
| Control | 180 | 5.38 ± 0.16 | 44.88 ± 1.33 | 100 |
| AqpZ-sGFP-Dialysis | 210 | 15.49 ± 0.96 | 150.60 ± 9.33 | 335.6 |
| AqpZ-sGFP-Biobeads | 210 | 15.08 ± 0.62 | 146.61 ± 6.03 | 326.7 |
| NtPIP2;1-Dialysis | 195 | 54.23 ± 0.99 | 489.58 ± 8.94 | 1090.9 |
| NtPIP2;1-Biobeads | 195 | 39.49 ± 0.59 | 356.51 ± 5.33 | 794.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, K.; Trung, T.N.; Zhu, Y.; Kaldenhoff, R.; Kai, L. Co-Translational Insertion of Aquaporins into Liposome for Functional Analysis via an E. coli Based Cell-Free Protein Synthesis System. Cells 2019, 8, 1325. https://doi.org/10.3390/cells8111325
Yue K, Trung TN, Zhu Y, Kaldenhoff R, Kai L. Co-Translational Insertion of Aquaporins into Liposome for Functional Analysis via an E. coli Based Cell-Free Protein Synthesis System. Cells. 2019; 8(11):1325. https://doi.org/10.3390/cells8111325
Chicago/Turabian StyleYue, Ke, Tran Nam Trung, Yiyong Zhu, Ralf Kaldenhoff, and Lei Kai. 2019. "Co-Translational Insertion of Aquaporins into Liposome for Functional Analysis via an E. coli Based Cell-Free Protein Synthesis System" Cells 8, no. 11: 1325. https://doi.org/10.3390/cells8111325
APA StyleYue, K., Trung, T. N., Zhu, Y., Kaldenhoff, R., & Kai, L. (2019). Co-Translational Insertion of Aquaporins into Liposome for Functional Analysis via an E. coli Based Cell-Free Protein Synthesis System. Cells, 8(11), 1325. https://doi.org/10.3390/cells8111325





