Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular–Subventricular Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and In Vivo Treatments
2.2. Immunohistochemistry
2.3. Cell Counting
2.4. Flow Cytometry
2.5. Laser Capture Microdissection (LCM)
2.6. Neurosphere Cultures
2.7. RNA Extraction and RT-PCR
2.8. Combined HPLC and Western Blot (WB)
2.9. Statistical Analysis
3. Results
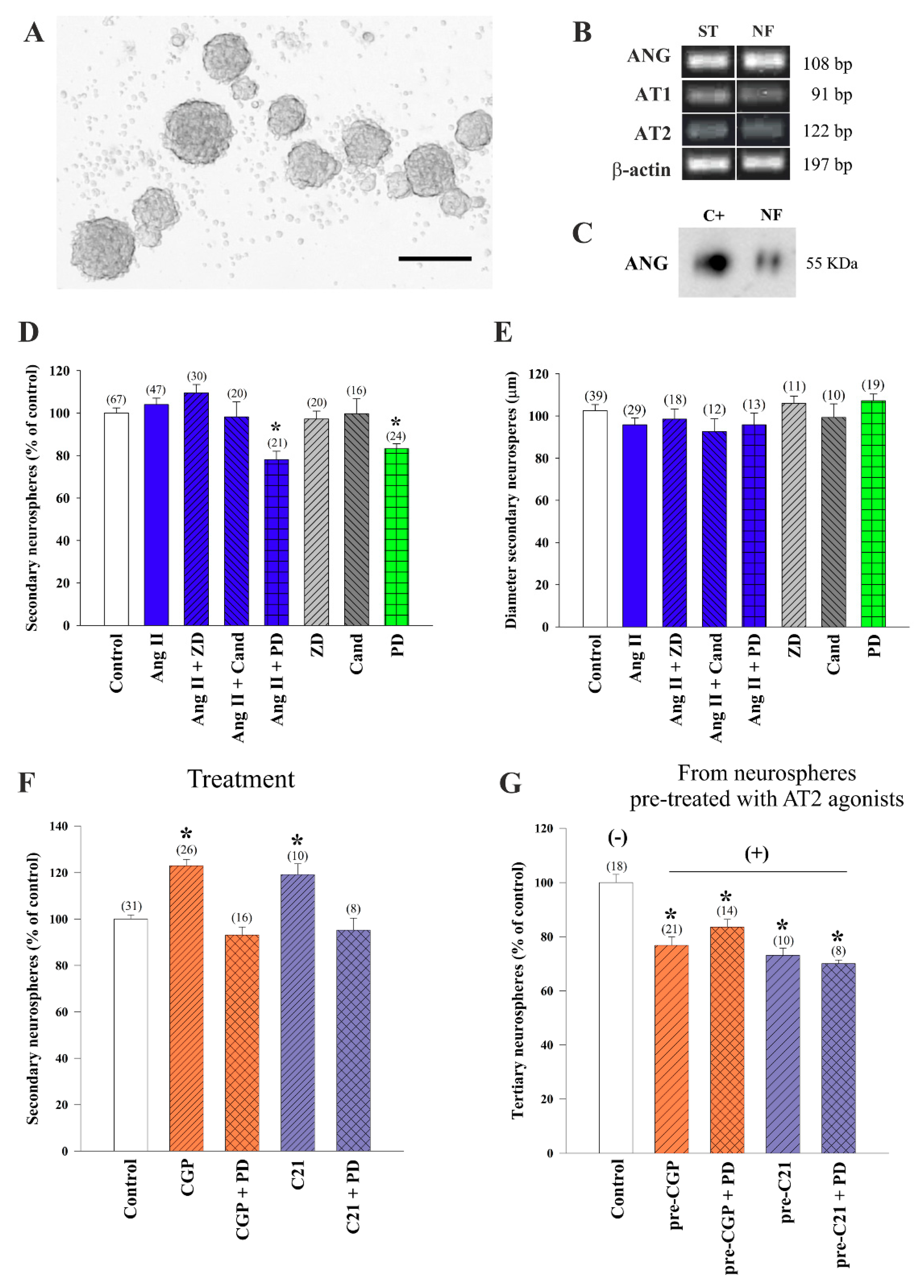
3.1. AT2 Receptors Mediated Promoting Effects of Ang II on Neurosphere Formation
3.2. AT2 Receptor Agonist C21 Stimulated Activity in the V-SVZ Niche
3.3. AT1 Receptor-Dependent Restraining Action on Adult Neurogenesis Was Counteracted by Stimulatory AT2 Receptor Activity
3.4. Cross-Regulation of AT1 and AT2 Receptors Underlies Ang II Effects on Neurosphere-Forming Cells
3.5. Stimulatory Effects of AT2 Receptor Activation on Young and Aged NSC Involved MasR
3.6. AT1 Receptor Involvement in the RAS Actions on Adult Neurogenesis in Rat
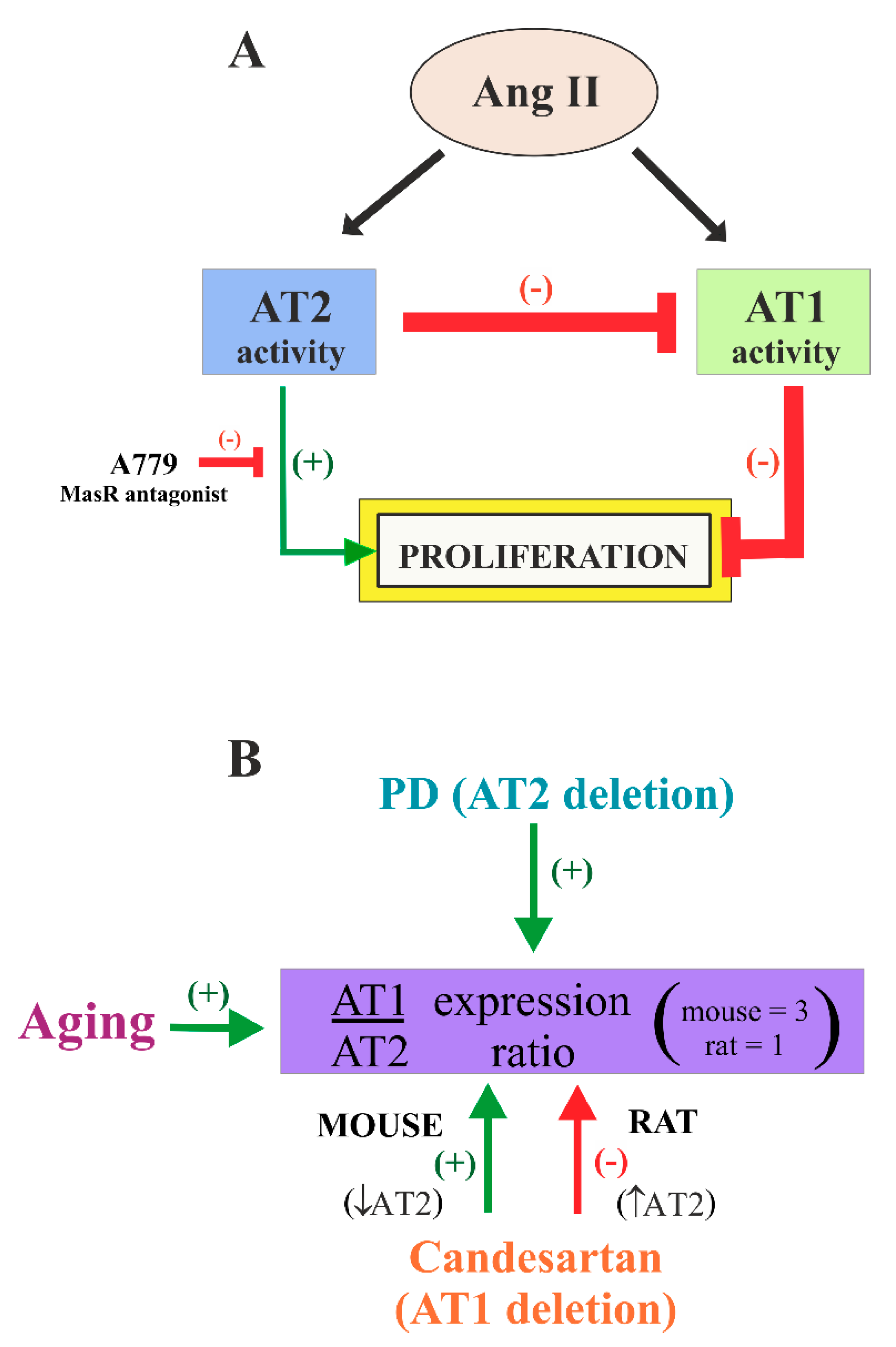
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Pathania, M.; Yan, L.D.; Bordey, A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology 2010, 58, 865–876. [Google Scholar] [CrossRef]
- Christie, K.J.; Turnley, A.M. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front. Cell. Neurosci. 2013, 6, 70. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Conover, J.C.; Shook, B.A. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011, 2, 49–63. [Google Scholar] [PubMed]
- Soriano-Canton, R.; Perez-Villalba, A.; Morante-Redolat, J.M.; Marques-Torrejon, M.A.; Pallas, M.; Perez-Sanchez, F.; Farinas, I. Regulation of the p19(Arf)/p53 pathway by histone acetylation underlies neural stem cell behavior in senescence-prone SAMP8 mice. Aging Cell 2015, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Apple, D.M.; Solano-Fonseca, R.; Kokovay, E. Neurogenesis in the aging brain. Biochem. Pharmacol. 2017, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Conover, J.C.; Todd, K.L. Development and aging of a brain neural stem cell niche. Exp. Gerontol. 2017, 94, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Chung, O.; Csikos, T.; Culman, J.; Gallinat, S.; Gohlke, P.; Hohle, S.; Meffert, S.; Stoll, M.; Stroth, U.; et al. Angiotensin receptors. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1996, 14, 95. [Google Scholar]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, T.N.; Jones, S.M.; Ahmadi, F.A.; Hoover, B.R.; Snell, L.D.; Skoch, J.; Jhaveri, V.V.; Poczobutt, A.M.; Weyhenmeyer, J.A.; Zawada, W.M. Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol. Neurodegener. 2007, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: Relevance to progression of the disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pallares, J.; Rey, P.; Parga, J.A.; Munoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008, 31, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Kaschina, E.; Unger, T. The AT2 receptor—A matter of love and hate. Peptides 2005, 26, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, C.; Ren, H.; Han, Y.; He, D.; Zhou, L.; Hopfer, U.; Jose, P.A.; Zeng, C. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J. Hypertens. 2012, 30, 1176–1184. [Google Scholar] [CrossRef]
- Becker, L.K.; Etelvino, G.M.; Walther, T.; Santos, R.A.; Campagnole-Santos, M.J. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1416. [Google Scholar] [CrossRef]
- Freund, M.; Walther, T.; von Bohlen und Halbach, O. Immunohistochemical localization of the angiotensin-(1–7) receptor Mas in the murine forebrain. Cell Tissue Res. 2012, 348, 29–35. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Quiroz, C.R.; Parga, J.A.; Guerra, M.J.; Labandeira-Garcia, J.L. Angiotensin II increases differentiation of dopaminergic neurons from mesencephalic precursors via angiotensin type 2 receptors. Eur. J. Neurosci. 2004, 20, 1489–1498. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O.; Albrecht, D. The CNS renin-angiotensin system. Cell Tissue Res. 2006, 326, 599–616. [Google Scholar] [CrossRef]
- Cook, V.I.; Grove, K.L.; McMenamin, K.M.; Carter, M.R.; Harding, J.W.; Speth, R.C. The AT2 angiotensin receptor subtype predominates in the 18 day gestation fetal rat brain. Brain Res. 1991, 560, 334–336. [Google Scholar] [CrossRef]
- Millan, M.A.; Jacobowitz, D.M.; Aguilera, G.; Catt, K.J. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci. USA 1991, 88, 11440–11444. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cheda, B.; Costa-Besada, M.A.; Valenzuela, R.; Perez-Costas, E.; Melendez-Ferro, M.; Labandeira-Garcia, J.L. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 2017, 8, e3044. [Google Scholar] [CrossRef] [PubMed]
- Sakata, A.; Mogi, M.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Fujita, T.; Iwai, M.; Ito, M.; Horiuchi, M. Sex-different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009, 1300, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Goel, R.; Shukla, S.; Shukla, R.; Hanif, K. Angiotensin Receptor Blockade by Inhibiting Glial Activation Promotes Hippocampal Neurogenesis Via Activation of Wnt/beta-Catenin Signaling in Hypertension. Mol. Neurobiol. 2018, 55, 5282–5298. [Google Scholar] [CrossRef] [PubMed]
- Drews, H.J.; Yenkoyan, K.; Lourhmati, A.; Buadze, M.; Kabisch, D.; Verleysdonk, S.; Petschak, S.; Beer-Hammer, S.; Davtyan, T.; Frey, W.H.; et al. Intranasal Losartan Decreases Perivascular Beta Amyloid, Inflammation, and the Decline of Neurogenesis in Hypertensive Rats. Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Klempin, F.; Mosienko, V.; Matthes, S.; Villela, D.C.; Todiras, M.; Penninger, J.M.; Bader, M.; Santos, R.A.S.; Alenina, N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. Cmls 2018, 75, 3625–3634. [Google Scholar] [CrossRef]
- Koyama, Y.; Mukuda, T.; Hamasaki, S.; Nakane, H.; Kaidoh, T. Short-term Heat Exposure Promotes Hippocampal Neurogenesis via Activation of Angiotensin II Type 1 Receptor in Adult Rats. Neuroscience 2018, 385, 121–132. [Google Scholar] [CrossRef]
- Krasniqi, S.; Daci, A. Role of the Angiotensin Pathway and its Target Therapy in Epilepsy Management. Int. J. Mol. Sci. 2019, 20, 726. [Google Scholar] [CrossRef]
- Ito, M.; Oliverio, M.I.; Mannon, P.J.; Best, C.F.; Maeda, N.; Smithies, O.; Coffman, T.M. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc. Natl. Acad. Sci. USA 1995, 92, 3521–3525. [Google Scholar] [CrossRef]
- Ichiki, T.; Labosky, P.A.; Shiota, C.; Okuyama, S.; Imagawa, Y.; Fogo, A.; Niimura, F.; Ichikawa, I.; Hogan, B.L.; Inagami, T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 1995, 377, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Borrajo, A.; Valenzuela, R.; Lanciego, J.L.; Labandeira-Garcia, J.L. Critical period for dopaminergic neuroprotection by hormonal replacement in menopausal rats. Neurobiol. Aging 2015, 36, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Namsolleck, P.; Boato, F.; Schwengel, K.; Paulis, L.; Matho, K.S.; Geurts, N.; Thone-Reineke, C.; Lucht, K.; Seidel, K.; Hallberg, A.; et al. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol. Dis. 2013, 51, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Rompe, F.; Kaschina, E.; Namsolleck, P.; Grzesiak, A.; Funke-Kaiser, H.; Bader, M.; Unger, T. The past, present and future of angiotensin II type 2 receptor stimulation. J. Renin-Angiotensin-Aldosterone Syst. Jraas. 2010, 11, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2015, 128, 95–109. [Google Scholar] [CrossRef]
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, A.K.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlen, A.; et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004, 47, 5995–6008. [Google Scholar] [CrossRef]
- Delgado, A.C.; Ferron, S.R.; Vicente, D.; Porlan, E.; Perez-Villalba, A.; Trujillo, C.M.; D’Ocon, P.; Farinas, I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 2014, 83, 572–585. [Google Scholar] [CrossRef] [Green Version]
- Giles, M.E.; Fernley, R.T.; Nakamura, Y.; Moeller, I.; Aldred, G.P.; Ferraro, T.; Penschow, J.D.; McKinley, M.J.; Oldfield, B.J. Characterization of a specific antibody to the rat angiotensin II AT1 receptor. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1999, 47, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Obermuller, N.; Gentili, M.; Gauer, S.; Gretz, N.; Weigel, M.; Geiger, H.; Gassler, N. Immunohistochemical and mRNA localization of the angiotensin II receptor subtype 2 (AT2) in follicular granulosa cells of the rat ovary. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2004, 52, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ortega, M.; Esteban, V.; Suzuki, Y.; Ruperez, M.; Mezzano, S.; Ardiles, L.; Justo, P.; Ortiz, A.; Egido, J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int. Suppl. 2003, 86, 21. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, R.; Costa-Besada, M.A.; Iglesias-Gonzalez, J.; Perez-Costas, E.; Villar-Cheda, B.; Garrido-Gil, P.; Melendez-Ferro, M.; Soto-Otero, R.; Lanciego, J.L.; Henrion, D.; et al. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016, 7, e2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Bendtsen, T.F.; Korbo, L.; Marcussen, N.; Moller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; Sorensen, F.B.; Vesterby, A. Some new simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis Acta Pathol. Microbiol. Et Immunol. Scand. 1988, 96, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Perez-Villalba, A.; Sirerol-Piquer, M.S.; Belenguer, G.; Soriano-Canton, R.; Munoz-Manchado, A.B.; Villadiego, J.; Alarcon-Aris, D.; Soria, F.N.; Dehay, B.; Bezard, E.; et al. Synaptic Regulator alpha-Synuclein in Dopaminergic Fibers Is Essentially Required for the Maintenance of Subependymal Neural Stem Cells. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 814–825. [Google Scholar] [CrossRef] [Green Version]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Gil, P.; Fernandez-Rodriguez, P.; Rodriguez-Pallares, J.; Labandeira-Garcia, J.L. Laser capture microdissection protocol for gene expression analysis in the brain. Histochem. Cell Biol. 2017, 148, 299–311. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Rodriguez-Perez, A.I.; Fernandez-Rodriguez, P.; Lanciego, J.L.; Labandeira-Garcia, J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the rat and monkey striatal neurons and glial cells. Brain Struct. Funct. 2017, 222, 2559–2571. [Google Scholar] [CrossRef]
- Belenguer, G.; Domingo-Muelas, A.; Ferron, S.R.; Morante-Redolat, J.M.; Farinas, I. Isolation, culture and analysis of adult subependymal neural stem cells. Differ. Res. Biol. Divers. 2016, 91, 28–41. [Google Scholar] [CrossRef]
- Ferron, S.R.; Andreu-Agullo, C.; Mira, H.; Sanchez, P.; Marques-Torrejon, M.A.; Farinas, I. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat. Protoc. 2007, 2, 849–859. [Google Scholar] [CrossRef]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Ruiz, C.; Villar-Cheda, B.; Dominguez-Meijide, A.; Garrido-Gil, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-Related Overactivity of the Angiotensin/AT1 Axis Decreases Sirtuin 3 Levels in the Substantia Nigra, which Induces Vulnerability to Oxidative Stress and Neurodegeneration. J. Gerontol. Serg. Abiol. Sci. Med. Sci. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.Y.; Pillai, B.; Dhandapani, K.M.; Ergul, A.; Fagan, S.C. Role of interleukin-10 in the neuroprotective effect of the Angiotensin Type 2 Receptor agonist, compound 21, after ischemia/reperfusion injury. Eur. J. Pharmacol. 2017, 799, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaschina, E.; Grzesiak, A.; Li, J.; Foryst-Ludwig, A.; Timm, M.; Rompe, F.; Sommerfeld, M.; Kemnitz, U.R.; Curato, C.; Namsolleck, P.; et al. Angiotensin II type 2 receptor stimulation: A novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 2008, 118, 2523–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Besada, M.A.; Valenzuela, R.; Garrido-Gil, P.; Villar-Cheda, B.; Parga, J.A.; Lanciego, J.L.; Labandeira-Garcia, J.L. Paracrine and Intracrine Angiotensin 1-7/Mas Receptor Axis in the Substantia Nigra of Rodents, Monkeys, and Humans. Mol. Neurobiol. 2018, 55, 5847–5867. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pallares, J.; Parga, J.A.; Munoz, A.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007, 103, 145–156. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brainbehaviorand Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; Deleo, A.M.; Pastrana, E.; Doetsch, F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Chaker, Z.; Codega, P.; Doetsch, F. A mosaic world: Puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 640–658. [Google Scholar] [CrossRef]
- Mich, J.K.; Signer, R.A.; Nakada, D.; Pineda, A.; Burgess, R.J.; Vue, T.Y.; Johnson, J.E.; Morrison, S.J. Prospective identification of functionally distinct stem cells and neurosphere-initiating cells in adult mouse forebrain. eLife 2014, 3, e02669. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Rodriguez-Pallares, J.; Valenzuela, R.; Munoz, A.; Guerra, M.J.; Baltatu, O.C.; Labandeira-Garcia, J.L. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: Implications for progression of Parkinson’s disease. Eur. J. Neurosci. 2010, 32, 1695–1706. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging 2012, 33, 204-e1. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Dominguez-Meijide, A.; Lanciego, J.L.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopaminergic degeneration is enhanced by chronic brain hypoperfusion and inhibited by angiotensin receptor blockage. Age 2013, 35, 1675–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvan, V.; Jin, K. Neurogenesis in the aging brain. Clin. Interv. Aging 2007, 2, 605–610. [Google Scholar] [PubMed] [Green Version]
- Rodriguez-Perez, A.I.; Valenzuela, R.; Villar-Cheda, B.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: Role of the brain angiotensin system. Brain A J. Neurol. 2012, 135, 124–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar-Cheda, B.; Dominguez-Meijide, A.; Valenzuela, R.; Granado, N.; Moratalla, R.; Labandeira-Garcia, J.L. Aging-related dysregulation of dopamine and angiotensin receptor interaction. Neurobiol. Aging 2014, 35, 1726–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, T. Inhibiting angiotensin receptors in the brain: Possible therapeutic implications. Curr. Med. Res. Opin. 2003, 19, 449–451. [Google Scholar] [CrossRef]
- Chao, J.; Yang, L.; Buch, S.; Gao, L. Angiotensin II increased neuronal stem cell proliferation: Role of AT2R. PLoS ONE 2013, 8, e63488. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, G.H.; Kim, Y.M.; Lee, B.W.; Nam, H.Y.; Sim, U.C.; Choo, S.J.; Yu, S.W.; Kim, J.J.; Kim Kwon, Y.; et al. Angiotensin II Causes Apoptosis of Adult Hippocampal Neural Stem Cells and Memory Impairment Through the Action on AMPK-PGC1alpha Signaling in Heart Failure. Stem Cells Transl. Med. 2017, 6, 1491–1503. [Google Scholar] [CrossRef] [Green Version]
- Platel, J.C.; Bordey, A. The multifaceted subventricular zone astrocyte: From a metabolic and pro-neurogenic role to acting as a neural stem cell. Neuroscience 2016, 323, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Gil-Perotin, S.; Duran-Moreno, M.; Cebrian-Silla, A.; Ramirez, M.; Garcia-Belda, P.; Garcia-Verdugo, J.M. Adult neural stem cells from the subventricular zone: A review of the neurosphere assay. Anat. Rec. 2013, 296, 1435–1452. [Google Scholar] [CrossRef]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 1999, 96, 11619–11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, O.; Quinones-Hinojosa, A. Astrocytes as neural stem cells in the adult brain. J. Stem Cells 2012, 7, 181–188. [Google Scholar]
- Nuyt, A.M.; Lenkei, Z.; Corvol, P.; Palkovits, M.; Llorens-Cortes, C. Ontogeny of angiotensin II type 1 receptor mRNAs in fetal and neonatal rat brain. J. Comp. Neurol. 2001, 440, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Valenzuela, R.; Borrajo, A.; Rodriguez-Perez, A.I. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 67. [Google Scholar] [PubMed]
- Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Topchiy, E.; Panzhinskiy, E.; Griffin, W.S.; Barger, S.W.; Das, M.; Zawada, W.M. Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation. Dev. Neurosci. 2013, 35, 293–305. [Google Scholar] [CrossRef]
- Parga, J.A.; Rodriguez-Pallares, J.; Joglar, B.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Effect of inhibitors of NADPH oxidase complex and mitochondrial ATP-sensitive potassium channels on generation of dopaminergic neurons from neurospheres of mesencephalic precursors. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2010, 239, 3247–3259. [Google Scholar] [CrossRef]
- Rodgers, K.E.; Xiong, S.; Steer, R.; diZerega, G.S. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 2000, 18, 287–294. [Google Scholar] [CrossRef]
- Ping, G.; Qian, W.; Song, G.; Zhaochun, S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol. Biochem. Behav. 2014, 124, 5–12. [Google Scholar] [CrossRef]
- Jenrow, K.A.; Brown, S.L.; Liu, J.; Kolozsvary, A.; Lapanowski, K.; Kim, J.H. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat. Oncol. 2010, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenrow, K.A.; Liu, J.; Brown, S.L.; Kolozsvary, A.; Lapanowski, K.; Kim, J.H. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J. Neuro-Oncol. 2011, 101, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Mukuda, T.; Koyama, Y.; Hamasaki, S.; Kaidoh, T.; Furukawa, Y. Systemic angiotensin II and exercise-induced neurogenesis in adult rat hippocampus. Brain Res. 2014, 1588, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Forbes, M.E.; Lee, W.H.; Lee, Y.W.; Riddle, D.R. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat. Res. 2011, 176, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakai, T.; Yoshioka, H.; Yagi, T.; Kato, T.; Kinouchi, H. Effects of valsartan on neuroprotection and neurogenesis after ischemia. Neuroreport 2011, 22, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Umschweif, G.; Liraz-Zaltsman, S.; Shabashov, D.; Alexandrovich, A.; Trembovler, V.; Horowitz, M.; Shohami, E. Angiotensin receptor type 2 activation induces neuroprotection and neurogenesis after traumatic brain injury. Neurother. J. Am. Soc. Exp. Neurother. 2014, 11, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Umschweif, G.; Shabashov, D.; Alexandrovich, A.G.; Trembovler, V.; Horowitz, M.; Shohami, E. Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 1381–1390. [Google Scholar] [CrossRef]
- Meffert, S.; Stoll, M.; Steckelings, U.M.; Bottari, S.P.; Unger, T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol. Cell. Endocrinol. 1996, 122, 59–67. [Google Scholar] [CrossRef]
- Suzuki, J.; Iwai, M.; Nakagami, H.; Wu, L.; Chen, R.; Sugaya, T.; Hamada, M.; Hiwada, K.; Horiuchi, M. Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation 2002, 106, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Wolf, G.; Harendza, S.; Schroeder, R.; Wenzel, U.; Zahner, G.; Butzmann, U.; Freeman, R.S.; Stahl, R.A. Angiotensin II’s antiproliferative effects mediated through AT2-receptors depend on down-regulation of SM-20. Lab. Investig. A J. Tech. Methods Pathol. 2002, 82, 1305–1317. [Google Scholar] [CrossRef] [Green Version]
- Cassis, P.; Conti, S.; Remuzzi, G.; Benigni, A. Angiotensin receptors as determinants of life span. Pflug. Arch. Eur. J. Physiol. 2010, 459, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Daynac, M.; Morizur, L.; Chicheportiche, A.; Mouthon, M.A.; Boussin, F.D. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci. Rep. 2016, 6, 21505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalamakis, G.; Brune, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catala-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 140–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Q.; Dhande, I.; Samuel, P.; Hussain, T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin II type 2 receptor/angiotensin (1-7)/Mas receptor and exhibit greater high-fat diet-induced kidney injury. J. Renin-Angiotensin-Aldosterone Syst. Jraas. 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, J.; Villela, D.C.; Teichmann, A.; Munter, L.M.; Mayer, M.C.; Mardahl, M.; Kirsch, S.; Namsolleck, P.; Lucht, K.; Benz, V.; et al. Evidence for Heterodimerization and Functional Interaction of the Angiotensin Type 2 Receptor and the Receptor MAS. Hypertension 2017, 69, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Ali, Q.; Samuel, P.; Steckelings, U.M.; Hussain, T. Angiotensin II Type 2 Receptor and Receptor Mas Are Colocalized and Functionally Interdependent in Obese Zucker Rat Kidney. Hypertension 2017, 70, 831–838. [Google Scholar] [CrossRef]
- O’Keeffe, G.C.; Barker, R.A.; Caldwell, M.A. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle 2009, 8, 2888–2894. [Google Scholar]
- Khan, F.; Spicarova, Z.; Zelenin, S.; Holtback, U.; Scott, L.; Aperia, A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2008, 295, 1110. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Liu, Y.; Wang, Z.; He, D.; Huang, L.; Yu, P.; Zheng, S.; Jones, J.E.; Asico, L.D.; Hopfer, U.; et al. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ. Res. 2006, 99, 494–500. [Google Scholar] [CrossRef] [Green Version]






| Primer | Accession Number | Forward Sequence (5′–3′) | Reverse Sequence (3′–5′) | |
|---|---|---|---|---|
| Rat | AT1R | NM_030985.4 | GTTAAGGGCCATTTTGTTTTTCTGG | TTGTCTGGATAAATCACACAACCC |
| AT2R | NM_012494.3 | AACATCTGCTGAAGACCAATAG | AGAAGGTCAGAACATGGAAGG | |
| β-act | NM_031144.3 | TCGTGCGTGACATTAAAGAG | TGCCACAGGATTCCATACC | |
| Mouse | Angiotensinogen (ANG) | NM_007428.4 | CTGCTGGCTGAGGACAAG | CGAGGAGGATGCTATTGAGAA |
| AT1R | NM_177322.3 | CTCTGCTGCTCTCCCGGACTTAA | AGGGCCATTTTGCTTTTCTGGGT | |
| AT2R | NM_007429.5 | CTGGCAAGCATCTTATGTAGTTC | CAAGCATTCACACCTAAGTATTCA | |
| β-act | NM_007393.5 | TCGTGCGTGACATTAAAGAG | TGCCACAGGATTCCATACC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garrote, M.; Perez-Villalba, A.; Garrido-Gil, P.; Belenguer, G.; Parga, J.A.; Perez-Sanchez, F.; Labandeira-Garcia, J.L.; Fariñas, I.; Rodriguez-Pallares, J. Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular–Subventricular Zone. Cells 2019, 8, 1551. https://doi.org/10.3390/cells8121551
Garcia-Garrote M, Perez-Villalba A, Garrido-Gil P, Belenguer G, Parga JA, Perez-Sanchez F, Labandeira-Garcia JL, Fariñas I, Rodriguez-Pallares J. Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular–Subventricular Zone. Cells. 2019; 8(12):1551. https://doi.org/10.3390/cells8121551
Chicago/Turabian StyleGarcia-Garrote, Maria, Ana Perez-Villalba, Pablo Garrido-Gil, German Belenguer, Juan A. Parga, Francisco Perez-Sanchez, Jose Luis Labandeira-Garcia, Isabel Fariñas, and Jannette Rodriguez-Pallares. 2019. "Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular–Subventricular Zone" Cells 8, no. 12: 1551. https://doi.org/10.3390/cells8121551






