Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Reagents
2.2. HOG and MO3.13 Cell Culture and Differentiation
2.3. Co-Culture Experiments
2.4. Glucose and Serum Deprivation Experiments With HOG and MO3.13
2.5. RNA Isolation and cDNA Synthesis
2.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Immunocytochemistry
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
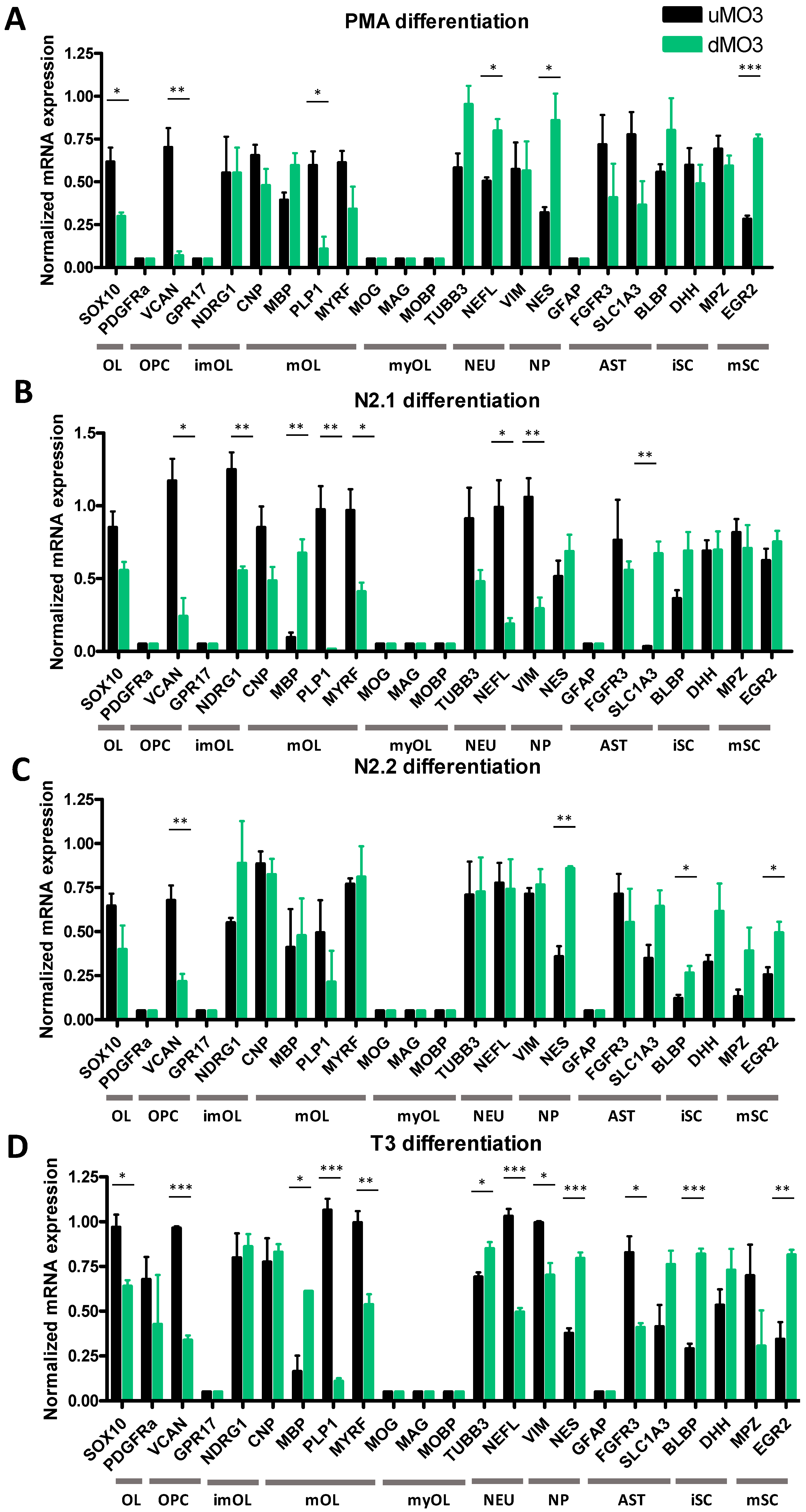
3.1. Differentiation of HOG Cells Does Not Induce a MyOL Expression Profile
3.2. Differentiation of MO3.13 Cells Does Not Induce a MyOL Expression Profile
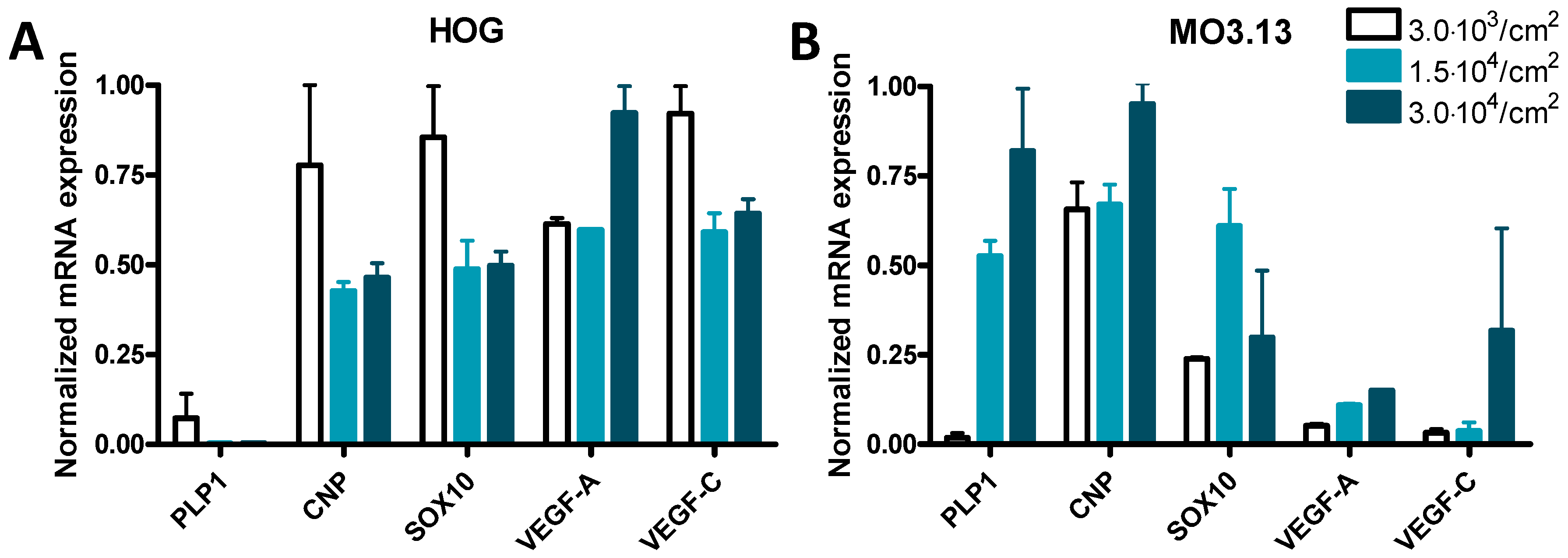
3.3. Cell Density Affects mRNA Expression in HOG and MO3.13 Cells
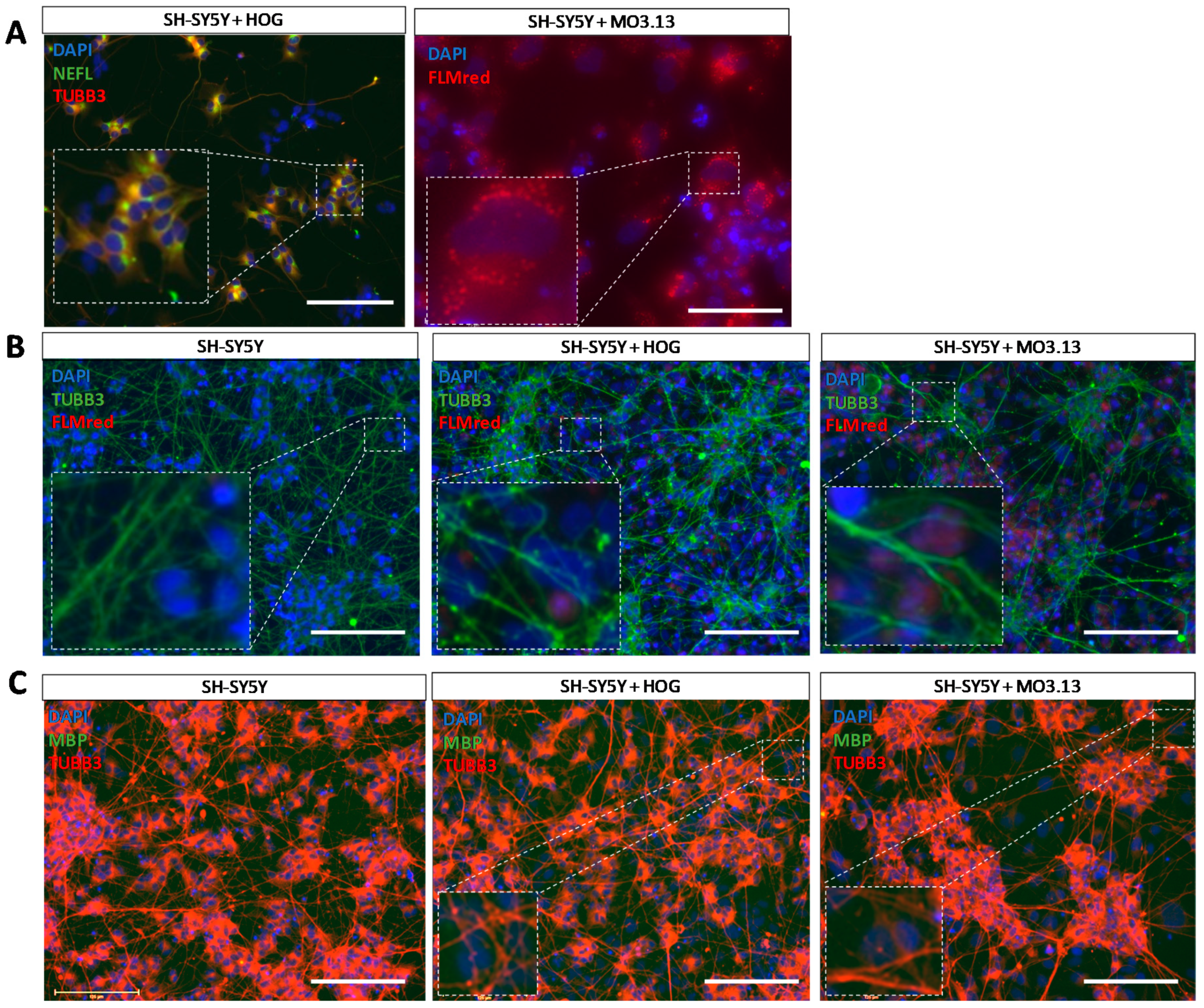
3.4. Co-Cultures of HOG or MO3.13 Cells with Neuronal SH-SY5Y Cells Do Not Display Myelin Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef]
- Santos, A.K.; Vieira, M.S.; Vasconcellos, R.; Goulart, V.A.M.; Kihara, A.H.; Resende, R.R. Decoding cell signalling and regulation of oligodendrocyte differentiation. Semin Cell Dev. Biol. 2018. [Google Scholar] [CrossRef]
- Van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef]
- Dugas, J.C.; Tai, Y.C.; Speed, T.P.; Ngai, J.; Barres, B.A. Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 2006, 26, 10967–10983. [Google Scholar] [CrossRef]
- Post, G.R.; Dawson, G. Characterization of a cell line derived from a human oligodendroglioma. Mol. Chem. Neuropathol. 1992, 16, 303–317. [Google Scholar] [CrossRef]
- McLaurin, J.; Trudel, G.C.; Shaw, I.T.; Antel, J.P.; Cashman, N.R. A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J. Neurobiol. 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Schoenfeld, R.; Wong, A.; Silva, J.; Li, M.; Itoh, A.; Horiuchi, M.; Itoh, T.; Pleasure, D.; Cortopassi, G. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 2010, 10, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Buntinx, M.; Moreels, M.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Steels, P.; Stinissen, P.; Ameloot, M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J. Neurosci. Res. 2004, 76, 834–845. [Google Scholar] [CrossRef]
- Issa, Y.; Watts, D.C.; Duxbury, A.J.; Brunton, P.A.; Watson, M.B.; Waters, C.M. Mercuric chloride: Toxicity and apoptosis in a human oligodendroglial cell line MO3.13. Biomaterials 2003, 24, 981–987. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Crespillo, A.J.; Fraile-Ramos, A.; Tabares, E.; Alcina, A.; Lopez-Guerrero, J.A. Role of the small GTPase Rab27a during herpes simplex virus infection of oligodendrocytic cells. BMC Microbiol. 2012, 12, 265. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ray, S.K. Diverse Biological Functions of Sphingolipids in the CNS: Ceramide and Sphingosine Regulate Myelination in Developing Brain but Stimulate Demyelination during Pathogenesis of Multiple Sclerosis. J. Neurol. Psychol. 2017, 5. [Google Scholar] [CrossRef]
- Won, J.S.; Kim, J.; Paintlia, M.K.; Singh, I.; Singh, A.K. Role of endogenous psychosine accumulation in oligodendrocyte differentiation and survival: Implication for Krabbe disease. Brain Res. 2013, 1508, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Guest, P.C.; Iwata, K.; Kato, T.A.; Steiner, J.; Schmitt, A.; Turck, C.W.; Martins-de-Souza, D. MK-801 treatment affects glycolysis in oligodendrocytes more than in astrocytes and neuronal cells: Insights for schizophrenia. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Buntinx, M.; Vanderlocht, J.; Hellings, N.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Ameloot, M.; Stinissen, P.; Steels, P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: Morphology and oligodendrocyte-specific gene expression. J. Neurocytol. 2003, 32, 25–38. [Google Scholar] [CrossRef]
- Slaets, H.; Dumont, D.; Vanderlocht, J.; Noben, J.P.; Leprince, P.; Robben, J.; Hendriks, J.; Stinissen, P.; Hellings, N. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up-regulation of 14-3-3. Proteomics 2008, 8, 1237–1247. [Google Scholar] [CrossRef]
- Smith, T.; Ghandour, M.S.; Wood, P.L. Detection of N-acetyl methionine in human and murine brain and neuronal and glial derived cell lines. J. Neurochem. 2011, 118, 187–194. [Google Scholar] [CrossRef]
- Hestvik, A.L.; Skorstad, G.; Vartdal, F.; Holmoy, T. Idiotope-specific CD4(+) T cells induce apoptosis of human oligodendrocytes. J. Autoimmun. 2009, 32, 125–132. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Crespillo, A.J.; Garcia, B.; Dorado, L.A.; Martin, B.; Tabares, E.; Krummenacher, C.; de Castro, F.; Lopez-Guerrero, J.A. The effect of cellular differentiation on HSV-1 infection of oligodendrocytic cells. PLoS ONE 2014, 9, e89141. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Crespillo, A.J.; Praena, B.; Tabares, E.; Revilla, Y.; Garcia, E.; Fraile-Ramos, A.; Baron, W.; Krummenacher, C.; Lopez-Guerrero, J.A. Role of Proteolipid Protein in HSV-1 Entry in Oligodendrocytic Cells. PLoS ONE 2016, 11, e0147885. [Google Scholar] [CrossRef]
- Bello-Morales, R.; de Marco, M.C.; Aranda, J.F.; Matesanz, F.; Alcina, A.; Lopez-Guerrero, J.A. Characterization of the MAL2-positive compartment in oligodendrocytes. Exp. Cell Res. 2009, 315, 3453–3465. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Perez-Hernandez, M.; Rejas, M.T.; Matesanz, F.; Alcina, A.; Lopez-Guerrero, J.A. Interaction of PLP with GFP-MAL2 in the human oligodendroglial cell line HOG. PLoS ONE 2011, 6, e19388. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Praena, B.; de la Nuez, C.; Rejas, M.T.; Guerra, M.; Galan-Ganga, M.; Izquierdo, M.; Calvo, V.; Krummenacher, C.; Lopez-Guerrero, J.A. Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Crespillo, A.J.; Praena, B.; Bello-Morales, R.; Lerma, L.; Vazquez-Calvo, A.; Martin-Acebes, M.A.; Tabares, E.; Sobrino, F.; Lopez-Guerrero, J.A. Inhibition of herpes virus infection in oligodendrocyte cultured cells by valproic acid. Virus Res. 2016, 214, 71–79. [Google Scholar] [CrossRef]
- De Arriba Zerpa, G.A.; Saleh, M.C.; Fernandez, P.M.; Guillou, F.; Espinosa de los Monteros, A.; de Vellis, J.; Zakin, M.M.; Baron, B. Alternative splicing prevents transferrin secretion during differentiation of a human oligodendrocyte cell line. J. Neurosci. Res. 2000, 61, 388–395. [Google Scholar] [CrossRef]
- Lily, O.; Palace, J.; Vincent, A. Serum autoantibodies to cell surface determinants in multiple sclerosis: A flow cytometric study. Brain 2004, 127, 269–279. [Google Scholar] [CrossRef]
- Podbielska, M.; Szulc, Z.M.; Kurowska, E.; Hogan, E.L.; Bielawski, J.; Bielawska, A.; Bhat, N.R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016, 57, 2028–2039. [Google Scholar] [CrossRef] [Green Version]
- Busto, R.; Serna, J.; Perianes-Cachero, A.; Quintana-Portillo, R.; Garcia-Seisdedos, D.; Canfran-Duque, A.; Paino, C.L.; Lerma, M.; Casado, M.E.; Martin-Hidalgo, A.; et al. Ellagic acid protects from myelin-associated sphingolipid loss in experimental autoimmune encephalomyelitis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 958–967. [Google Scholar] [CrossRef]
- Craighead, M.; Pole, J.; Waters, C. Caspases mediate C2-ceramide-induced apoptosis of the human oligodendroglial cell line, MO3.13. Neurosci. Lett. 2000, 278, 125–128. [Google Scholar] [CrossRef]
- Craighead, M.W.; Tiwari, P.; Keynes, R.G.; Waters, C.M. Human oligodendroglial cell line, MO3.13, can be protected from apoptosis using the general caspase inhibitor zVAD-FMK. J. Neurosci. Res. 1999, 57, 236–243. [Google Scholar] [CrossRef]
- Buntinx, M.; Gielen, E.; Van Hummelen, P.; Raus, J.; Ameloot, M.; Steels, P.; Stinissen, P. Cytokine-induced cell death in human oligodendroglial cell lines. II: Alterations in gene expression induced by interferon-gamma and tumor necrosis factor-alpha. J. Neurosci. Res. 2004, 76, 846–861. [Google Scholar] [CrossRef]
- Cantarella, G.; Risuglia, N.; Lombardo, G.; Lempereur, L.; Nicoletti, F.; Memo, M.; Bernardini, R. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligodendrocytic cell line: Evidence for multiple sites of interactions. Cell Death Differ. 2004, 11, 503–511. [Google Scholar] [CrossRef]
- Lim, C.K.; Smythe, G.A.; Stocker, R.; Brew, B.J.; Guillemin, G.J. Characterization of the kynurenine pathway in human oligodendrocytes. Int. Congr. Ser. 2007, 1304, 213–217. [Google Scholar] [CrossRef]
- Bourikas, D.; Mir, A.; Walmsley, A.R. LINGO-1-mediated inhibition of oligodendrocyte differentiation does not require the leucine-rich repeats and is reversed by p75(NTR) antagonists. Mol. Cell Neurosci. 2010, 45, 363–369. [Google Scholar] [CrossRef]
- Boscia, F.; D’Avanzo, C.; Pannaccione, A.; Secondo, A.; Casamassa, A.; Formisano, L.; Guida, N.; Sokolow, S.; Herchuelz, A.; Annunziato, L. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012, 19, 562–572. [Google Scholar] [CrossRef]
- Heffernan, C.; Sumer, H.; Guillemin, G.J.; Manuelpillai, U.; Verma, P.J. Design and screening of a glial cell-specific, cell penetrating peptide for therapeutic applications in multiple sclerosis. PLoS ONE 2012, 7, e45501. [Google Scholar] [CrossRef]
- Mastronardi, F.G.; Min, W.; Wang, H.; Winer, S.; Dosch, M.; Boggs, J.M.; Moscarello, M.A. Attenuation of experimental autoimmune encephalomyelitis and nonimmune demyelination by IFN-beta plus vitamin B12: Treatment to modify notch-1/sonic hedgehog balance. J. Immunol. 2004, 172, 6418–6426. [Google Scholar] [CrossRef]
- Ramesh, G.; Benge, S.; Pahar, B.; Philipp, M.T. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J. Neuroinflammation 2012, 9, 72. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Fevrier, H.B.; Philipp, M.T. Non-viable Borrelia burgdorferi induce inflammatory mediators and apoptosis in human oligodendrocytes. Neurosci Lett. 2013, 556, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Vidal, P.M.; Lemmens, E.; Avila, A.; Vangansewinkel, T.; Chalaris, A.; Rose-John, S.; Hendrix, S. ADAM17 is a survival factor for microglial cells in vitro and in vivo after spinal cord injury in mice. Cell Death Dis. 2013, 4, e954. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Philipp, M.T. The MEK/ERK pathway is the primary conduit for Borrelia burgdorferi-induced inflammation and P53-mediated apoptosis in oligodendrocytes. Apoptosis 2014, 19, 76–89. [Google Scholar] [CrossRef]
- Wuest, S.C.; Mexhitaj, I.; Chai, N.R.; Romm, E.; Scheffel, J.; Xu, B.; Lane, K.; Wu, T.; Bielekova, B. A complex role of herpes viruses in the disease process of multiple sclerosis. PLoS ONE 2014, 9, e105434. [Google Scholar] [CrossRef]
- Redpath, G.M.; Woolger, N.; Piper, A.K.; Lemckert, F.A.; Lek, A.; Greer, P.A.; North, K.N.; Cooper, S.T. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol. Biol. Cell 2014, 25, 3037–3048. [Google Scholar] [CrossRef]
- Accetta, R.; Damiano, S.; Morano, A.; Mondola, P.; Paterno, R.; Avvedimento, E.V.; Santillo, M. Reactive Oxygen Species Derived from NOX3 and NOX5 Drive Differentiation of Human Oligodendrocytes. Front. Cell Neurosci. 2016, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Dooley, D.; Lemmens, E.; Ponsaerts, P.; Hendrix, S. Interleukin-25 is detrimental for recovery after spinal cord injury in mice. J. Neuroinflammation 2016, 13, 101. [Google Scholar] [CrossRef]
- Ballerini, P.; Diomede, F.; Petragnani, N.; Cicchitti, S.; Merciaro, I.; Cavalcanti, M.; Trubiani, O. Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine 2017, 96, 261–272. [Google Scholar] [CrossRef]
- Boghozian, R.; McKenzie, B.A.; Saito, L.B.; Mehta, N.; Branton, W.G.; Lu, J.; Baker, G.B.; Noorbakhsh, F.; Power, C. Suppressed oligodendrocyte steroidogenesis in multiple sclerosis: Implications for regulation of neuroinflammation. Glia 2017, 65, 1590–1606. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Philipp, M.T. Receptor tyrosine kinases play a significant role in human oligodendrocyte inflammation and cell death associated with the Lyme disease bacterium Borrelia burgdorferi. J. Neuroinflammation 2017, 14, 110. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, G.; Martinez, A.N.; Martin, D.S.; Philipp, M.T. Effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in glial and neuronal cells of the central nervous system. J. Neuroinflammation 2017, 14, 28. [Google Scholar] [CrossRef]
- Chiou, B.; Lucassen, E.; Sather, M.; Kallianpur, A.; Connor, J. Semaphorin4A and H-ferritin utilize Tim-1 on human oligodendrocytes: A novel neuro-immune axis. Glia 2018, 66, 1317–1330. [Google Scholar] [CrossRef]
- Navarrete, C.; Carrillo-Salinas, F.; Palomares, B.; Mecha, M.; Jimenez-Jimenez, C.; Mestre, L.; Feliu, A.; Bellido, M.L.; Fiebich, B.L.; Appendino, G.; et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J. Neuroinflammation 2018, 15, 64. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Philipp, M.T. Intracellular TLR7 is activated in human oligodendrocytes in response to Borrelia burgdorferi exposure. Neurosci. Lett. 2018, 671, 38–42. [Google Scholar] [CrossRef]
- Singh, I.; Samuvel, D.J.; Choi, S.; Saxena, N.; Singh, A.K.; Won, J. Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model. Immunology 2018, 154, 434–451. [Google Scholar] [CrossRef] [Green Version]
- Haq, E.; Giri, S.; Singh, I.; Singh, A.K. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J. Neurochem. 2003, 86, 1428–1440. [Google Scholar] [CrossRef]
- Giri, S.; Khan, M.; Rattan, R.; Singh, I.; Singh, A.K. Krabbe disease: Psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J. Lipid Res. 2006, 47, 1478–1492. [Google Scholar] [CrossRef]
- Issa, Y.; Brunton, P.; Waters, C.M.; Watts, D.C. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity. Dent. Mater. 2008, 24, 281–287. [Google Scholar] [CrossRef]
- Bishop, A.; Hobbs, K.G.; Eguchi, A.; Jeffrey, S.; Smallwood, L.; Pennie, C.; Anderson, J.; Estevez, A.G. Differential sensitivity of oligodendrocytes and motor neurons to reactive nitrogen species: Implications for multiple sclerosis. J. Neurochem. 2009, 109, 93–104. [Google Scholar] [CrossRef]
- Nazarenus, M.; Abasolo, I.; García-Aranda, N.; Voccoli, V.; Rejman, J.; Cecchini, M.; Schwartz, S.; RiveraGil, P.; Parak, W.J. Polymer Capsules as a Theranostic Tool for a Universal In Vitro Screening Assay-The Case of Lysosomal Storage Diseases. Part. Part. Syst. Charact. 2015, 32, 991–998. [Google Scholar] [CrossRef]
- Del Grosso, A.; Antonini, S.; Angella, L.; Tonazzini, I.; Signore, G.; Cecchini, M. Lithium improves cell viability in psychosine-treated MO3.13 human oligodendrocyte cell line via autophagy activation. J. Neurosci. Res. 2016, 94, 1246–1260. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamashina, K.; Ishikawa, M.; Gotoh, M.; Yagishita, S.; Iwasa, K.; Maruyama, K.; Murakami-Murofushi, K.; Yoshikawa, K. Protective and therapeutic role of 2-carba-cyclic phosphatidic acid in demyelinating disease. J. Neuroinflammation 2017, 14, 142. [Google Scholar] [CrossRef]
- Chen, C.D.; Li, H.; Liang, J.; Hixson, K.; Zeldich, E.; Abraham, C.R. The anti-aging and tumor suppressor protein Klotho enhances differentiation of a human oligodendrocytic hybrid cell line. J. Mol. Neurosci. 2015, 55, 76–90. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, X.; Shen, J.; Xie, B.; Zhao, X.; Zhang, Z.; Cao, Q.; Shen, Y.; Qiu, M. A Novel Approach for Amplification and Purification of Mouse Oligodendrocyte Progenitor Cells. Front. Cell Neurosci. 2016, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, F.; Urra, O.; Alberdi, E.; Matute, C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012, 3, e268. [Google Scholar] [CrossRef]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar]
- Shipley, M.M.; Mangold, C.A.; Kuny, C.V.; Szpara, M.L. Differentiated Human SH-SY5Y Cells Provide a Reductionist Model of Herpes Simplex Virus 1 Neurotropism. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2004, 2, 51–62. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Hofmann, K.; Rodriguez-Rodriguez, R.; Gaebler, A.; Casals, N.; Scheller, A.; Kuerschner, L. Astrocytes and oligodendrocytes in grey and white matter regions of the brain metabolize fatty acids. Sci. Rep. 2017, 7, 10779. [Google Scholar] [CrossRef]
- Monsma, P.C.; Brown, A. FluoroMyelin Red is a bright, photostable and non-toxic fluorescent stain for live imaging of myelin. J. Neurosci. Methods 2012, 209, 344–350. [Google Scholar] [CrossRef]
- Pepper, R.E.; Pitman, K.A.; Cullen, C.L.; Young, K.M. How Do Cells of the Oligodendrocyte Lineage Affect Neuronal Circuits to Influence Motor Function, Memory and Mood? Front. Cell Neurosci. 2018, 12, 399. [Google Scholar] [CrossRef] [Green Version]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Roth, H.J.; Kronquist, K.E.; Kerlero de Rosbo, N.; Crandall, B.F.; Campagnoni, A.T. Evidence for the expression of four myelin basic protein variants in the developing human spinal cord through cDNA cloning. J. Neurosci. Res. 1987, 17, 321–328. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Sullivan, P.; Sharabi, Y.; Goldstein, D.S. DOPAL is transmissible to and oligomerizes alpha-synuclein in human glial cells. Auton Neurosci. 2016, 194, 46–51. [Google Scholar] [CrossRef]
- Najm, F.J.; Madhavan, M.; Zaremba, A.; Shick, E.; Karl, R.T.; Factor, D.C.; Miller, T.E.; Nevin, Z.S.; Kantor, C.; Sargent, A.; et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015, 522, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.C.; Giddens, M.M.; Schaefer, S.A.; Hall, R.A. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc. Natl. Acad. Sci. USA 2013, 110, 9529–9534. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, H.; Wang, S.; Koito, H.; Li, J.; Ye, F.; Hoang, J.; Escobar, S.S.; Gow, A.; Arnett, H.A.; et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 2009, 12, 1398–1406. [Google Scholar] [CrossRef]
- Steven, A.; Goldman, N.J.K. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef]
- Pukos, N.; Yoseph, R.; McTigue, D.M. To Be or Not to Be: Environmental Factors that Drive Myelin Formation during Development and after CNS Trauma. Neuroglia 2018, 1, 63–90. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, N.A.; Fuss, B. Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp. Neurol. 2016, 283, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Bardy, C.; van den Hurk, M.; Eames, T.; Marchand, C.; Hernandez, R.V.; Kellogg, M.; Gorris, M.; Galet, B.; Palomares, V.; Brown, J.; et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad Sci. USA 2015, 112, E2725–E2734. [Google Scholar] [CrossRef] [Green Version]
- Welsh, T.G.; Kucenas, S. Purinergic signaling in oligodendrocyte development and function. J. Neurochem. 2018, 145, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Barateiro, A.; Fernandes, A. Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochim. Biophys. Acta 2014, 1843, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Nixon, R.A. Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res. Bull. 2016, 126, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263. [Google Scholar] [CrossRef]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Tung, V.W.; Aghajanian, J.; Xu, Z. Antagonistic roles of neurofilament subunits NF-H and NF-M against NF-L in shaping dendritic arborization in spinal motor neurons. J. Cell Biol. 1998, 140, 1167–1176. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Gao, X.; Lin, S.H.; Ren, F.; Li, J.T.; Chen, J.J.; Yao, C.B.; Yang, H.B.; Jiang, S.X.; Yan, G.Q.; Wang, D.; et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 2016, 7, 11960. [Google Scholar] [CrossRef] [Green Version]
- Marin-Husstege, M.; Muggironi, M.; Liu, A.X.; Casaccia-Bonnefil, P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci. 2002, 22, 10333–10345. [Google Scholar] [CrossRef]
- Long, P.M.; Moffett, J.R.; Namboodiri, A.M.; Viapiano, M.S.; Lawler, S.E.; Jaworski, D.M. N-acetylaspartate (NAA) and N-acetylaspartylglutamate (NAAG) promote growth and inhibit differentiation of glioma stem-like cells. J. Biol. Chem. 2013, 288, 26188–26200. [Google Scholar] [CrossRef]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Ottaviani, L.; Andreoli, R.; Ciociola, T.; Lagrasta, C.A.M.; Tardito, S.; Bussolati, O. Oligodendroglioma Cells Lack Glutamine Synthetase and Are Auxotrophic for Glutamine, but Do not Depend on Glutamine Anaplerosis for Growth. Int. J. Mol. Sci. 2018, 19, 1099. [Google Scholar] [CrossRef]
- Dai, Z.M.; Sun, S.; Wang, C.; Huang, H.; Hu, X.; Zhang, Z.; Lu, Q.R.; Qiu, M. Stage-specific regulation of oligodendrocyte development by Wnt/beta-catenin signaling. J. Neurosci. 2014, 34, 8467–8473. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Han, X.; Yu, Y.; Ye, F.; Chen, Y.; Hoang, T.; Xu, X.; Mi, Q.S.; Xin, M.; et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 2010, 65, 612–626. [Google Scholar] [CrossRef]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef]
- De Faria, O., Jr.; Cui, Q.L.; Bin, J.M.; Bull, S.J.; Kennedy, T.E.; Bar-Or, A.; Antel, J.P.; Colman, D.R.; Dhaunchak, A.S. Regulation of miRNA 219 and miRNA Clusters 338 and 17-92 in Oligodendrocytes. Front. Genet. 2012, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Gasque, P.; Morgan, B.P. Complement regulatory protein expression by a human oligodendrocyte cell line: Cytokine regulation and comparison with astrocytes. Immunology 1996, 89, 338–347. [Google Scholar] [CrossRef]
- Yao, S.Y.; Natarajan, C.; Sriram, S. nNOS mediated mitochondrial injury in LPS stimulated oligodendrocytes. Mitochondrion 2012, 12, 336–344. [Google Scholar] [CrossRef]
- Jurewicz, A.; Matysiak, M.; Andrzejak, S.; Selmaj, K. TRAIL-induced death of human adult oligodendrocytes is mediated by JNK pathway. Glia 2006, 53, 158–166. [Google Scholar] [CrossRef]
- Kastriti, M.E.; Adameyko, I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr. Opin. Neurobiol. 2017, 47, 196–202. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef]
- Nishiyama, A.; Suzuki, R.; Zhu, X. NG2 cells (polydendrocytes) in brain physiology and repair. Front. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef]
- Varini, K.; Benzaria, A.; Taieb, N.; Di Scala, C.; Azmi, A.; Graoudi, S.; Maresca, M. Mislocalization of the exitatory amino-acid transporters (EAATs) in human astrocytoma and non-astrocytoma cancer cells: Effect of the cell confluence. J. Biomed. Sci. 2012, 19, 10. [Google Scholar] [CrossRef]
- Kashima, T.; Tiu, S.N.; Merrill, J.E.; Vinters, H.V.; Dawson, G.; Campagnoni, A.T. Expression of oligodendrocyte-associated genes in cell lines derived from human gliomas and neuroblastomas. Cancer Res. 1993, 53, 170–175. [Google Scholar]
- Gotz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef]
- Magistri, M.; Khoury, N.; Mazza, E.M.; Velmeshev, D.; Lee, J.K.; Bicciato, S.; Tsoulfas, P.; Faghihi, M.A. A comparative transcriptomic analysis of astrocytes differentiation from human neural progenitor cells. Eur. J. Neurosci. 2016, 44, 2858–2870. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Lang, J.; Sohn, J.; Hammond, E.; Chang, M.; Pleasure, D. Canonical Wnt signaling in the oligodendroglial lineage--puzzles remain. Glia 2015, 63, 1671–1693. [Google Scholar] [CrossRef]
- Gilthorpe, J.D.; Oozeer, F.; Nash, J.; Calvo, M.; Bennett, D.L.H.; Lumsden, A.; Pini, A. Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000Research 2013, 2, 148. [Google Scholar] [CrossRef]




| Differentiation Medium | Components |
|---|---|
| N2.1 medium | Eagle’s Minimum Essential Medium (EMEM) 1% antibiotic-antimycotic (AA), 50 μg/mL apo-transferrin, 16 μg/mL putrescine, 0.5 μg/mL human insulin, 30 nM sodium selenite, 30 nM triiodothyronine (T3), 500 uM 3-isobutyl-1-methylxanthine (IBMX), 500 uM dibutyryl cAMP (db-cAMP) |
| N2.2 medium | EMEM 1% AA, 50 μg/mL apo-transferrin, 16 μg/mL putrescine, 0.5 μg/mL human insulin, 30 nM sodium selenite, 30 nM T3, 10 ng/mL D-biotin, 50 nM hydrocortisone, 4 μM progesterone |
| T3 medium | EMEM 1% AA, 30 nM T3 |
| PMA medium | Gibco Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12(1:1)) 1% AA, 100 nM phorbol 12-myristate 13-acetate (PMA) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Kleijn, K.M.A.; Zuure, W.A.; Peijnenborg, J.; Heuvelmans, J.M.; Martens, G.J.M. Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning. Cells 2019, 8, 1096. https://doi.org/10.3390/cells8091096
De Kleijn KMA, Zuure WA, Peijnenborg J, Heuvelmans JM, Martens GJM. Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning. Cells. 2019; 8(9):1096. https://doi.org/10.3390/cells8091096
Chicago/Turabian StyleDe Kleijn, Kim M. A., Wieteke A. Zuure, Jolien Peijnenborg, Josje M. Heuvelmans, and Gerard J. M. Martens. 2019. "Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning" Cells 8, no. 9: 1096. https://doi.org/10.3390/cells8091096
APA StyleDe Kleijn, K. M. A., Zuure, W. A., Peijnenborg, J., Heuvelmans, J. M., & Martens, G. J. M. (2019). Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning. Cells, 8(9), 1096. https://doi.org/10.3390/cells8091096





