Disruption of Beta-Cell Mitochondrial Networks by the Orphan Nuclear Receptor Nor1/Nr4a3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Human Islets
2.4. Plasmid Transfection
2.5. Mitochondria Staining
2.6. Glucose Oxidation
2.7. Glucose Uptake
2.8. ATP Production
2.9. Mitochondrial Membrane Potential
2.10. Mitochondrial Ultrastructure
2.11. Quantification of Mitophagy
2.12. Western Blot
2.13. Citrate Synthase Activity
2.14. High-Resolution Respirometry
2.15. Statistical Analysis
3. Results
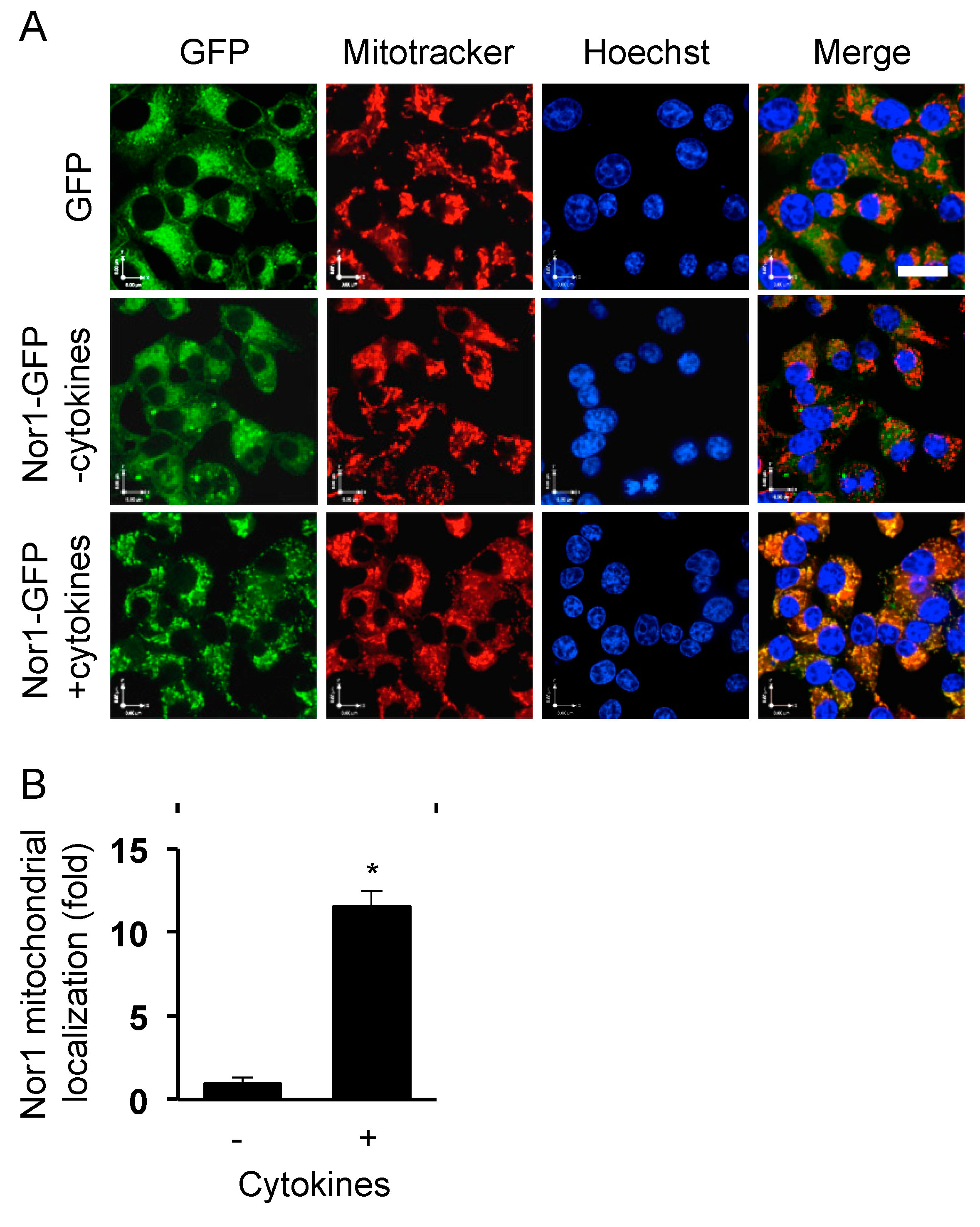
3.1. Nor1 Translocates to the Mitochondria in INS832/13 Cells Exposed to Proinflammatory Cytokines
3.2. Nor1 Affects Mitochondrial Function and Reduces Insulin Secretion in INS832/13 Cells
3.3. Nor1 Induces Mitochondrial Fractionation in Beta Cells
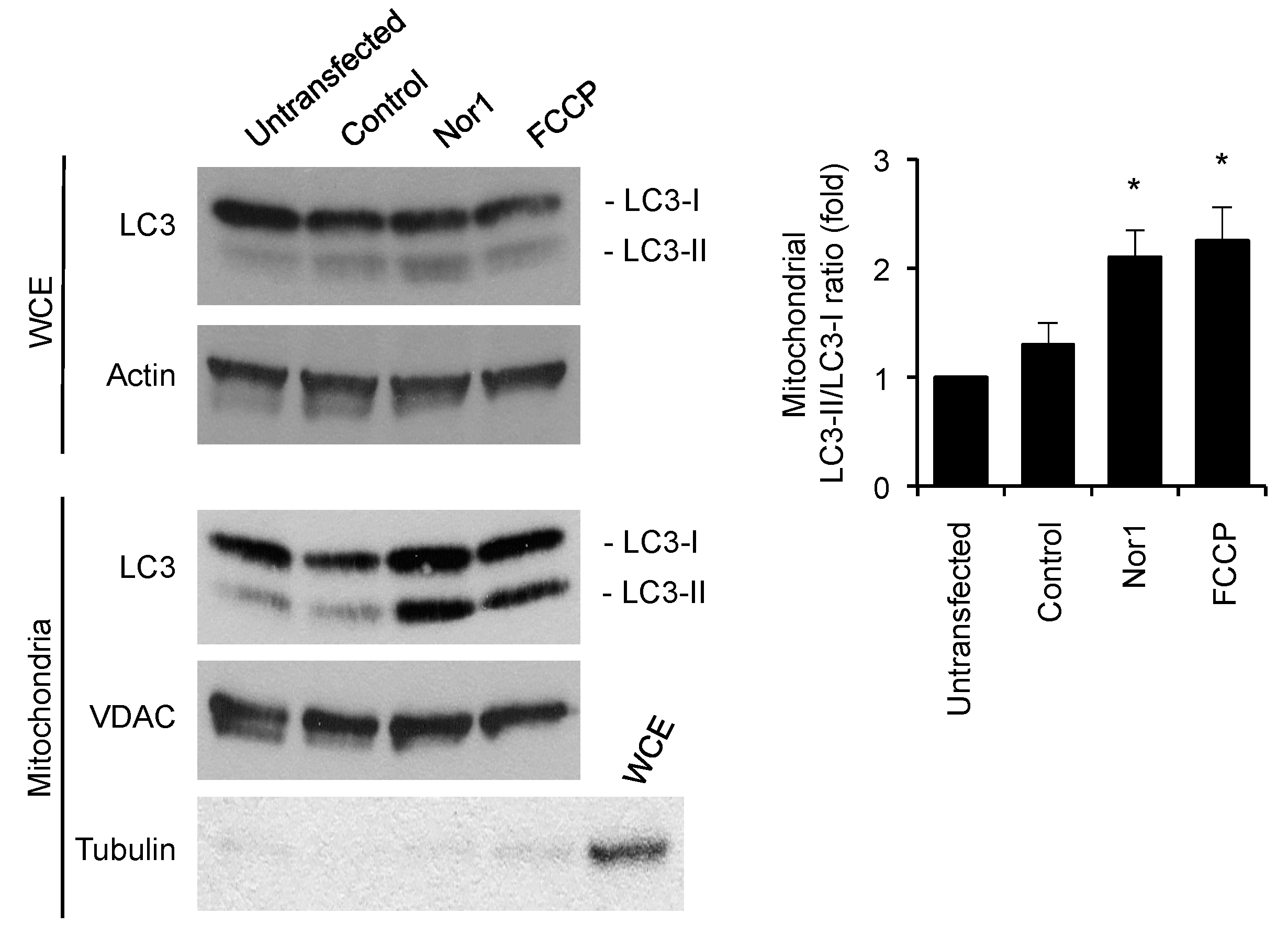
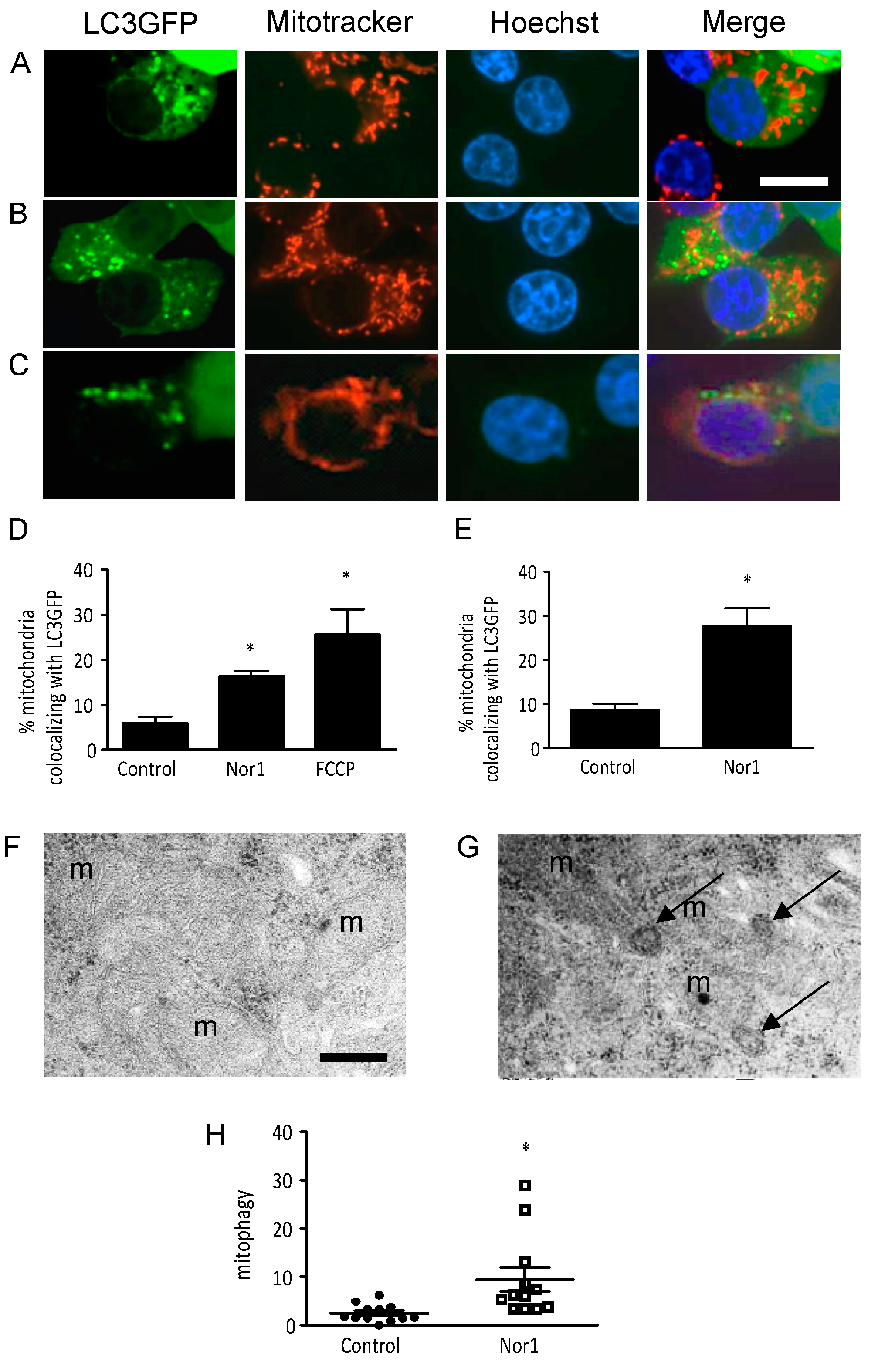
3.4. Nor1 Increases Mitophagy in Beta Cells
3.5. Nor1 and Mitochondrial Content in Beta Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saltiel, A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 2001, 104, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.J.; Bonadonna, R.C. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care 2013, 36, S113–S119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, J.J.; Breuer, T.G.; Bonadonna, R.C.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Schrader, H.; Menge, B.A. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia 2012, 55, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. Type 2 diabetes-a matter of beta-cell life and death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef]
- Close, A.F.; Dadheech, N.; Villela, B.S.; Rouillard, C.; Buteau, J. The orphan nuclear receptor nor1/nr4a3 is a negative regulator of beta-cell mass. J. Biol. Chem. 2019, 294, 4889–4897. [Google Scholar] [CrossRef] [Green Version]
- Ordelheide, A.M.; Gerst, F.; Rothfuss, O.; Heni, M.; Haas, C.; Thielker, I.; Herzberg-Schafer, S.; Bohm, A.; Machicao, F.; Ullrich, S.; et al. Nor-1, a novel incretin-responsive regulator of insulin genes and insulin secretion. Mol. Metab. 2013, 2, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, P.; Staiger, H.; Stancakova, A.; Schafer, S.A.; Kirchhoff, K.; Ullrich, S.; Ranta, F.; Gallwitz, B.; Stefan, N.; Machicao, F.; et al. Common polymorphisms within the nr4a3 locus, encoding the orphan nuclear receptor nor-1, are associated with enhanced beta-cell function in non-diabetic subjects. BMC Med. Genet. 2009, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Fu, Y.; Yu, C.; Wang, S.; Zhang, Y.; Zong, C.; Xu, T.; Liu, Y.; Li, X.; Wang, X. Elevation of nr4a3 expression and its possible role in modulating insulin expression in the pancreatic beta cell. PLoS ONE 2014, 9, e91462. [Google Scholar] [CrossRef]
- Close, A.F.; Rouillard, C.; Buteau, J. Nr4a orphan nuclear receptors in glucose homeostasis: A minireview. Diabetes Metab. 2013, 39, 478–484. [Google Scholar] [CrossRef]
- Zhang, X.K. Targeting nur77 translocation. Expert Opin. Targets 2007, 11, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Toth, K.; Sarang, Z.; Garabuczi, E.; Szondy, Z. Retinoids induce nur77-dependent apoptosis in mouse thymocytes. Biochim. Biophys. Acta 2015, 1853, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.Z.; Zhao, B.X.; Zhao, W.X.; Li, L.; Zhang, B.; Wu, Q. Akt phosphorylates the tr3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis 2008, 29, 2078–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapak, A.; Stasik, I.; Ziolo, E.; Strzadala, L. Apoptosis of lymphoma cells is abolished due to blockade of cytochrome c release despite nur77 mitochondrial targeting. Apoptosis 2007, 12, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Rud, J.; Olson, C.M., Jr.; Anguita, J.; Osborne, B.A. Phosphorylation of nur77 by the mek-erk-rsk cascade induces mitochondrial translocation and apoptosis in t cells. J. Immunol. 2009, 183, 3268–3277. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Bushue, N.; Bu, P.; Wan, Y.J. Induction and intracellular localization of nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem. Pharm. 2010, 79, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; Burger, M.L.; Whang, H.; Winoto, A. Protein kinase c regulates mitochondrial targeting of nur77 and its family member nor-1 in thymocytes undergoing apoptosis. Eur. J. Immunol. 2010, 40, 2041–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmeier, H.E.; Mulder, H.; Chen, G.; Henkel-Rieger, R.; Prentki, M.; Newgard, C.B. Isolation of ins-1-derived cell lines with robust atp-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000, 49, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buteau, J.; Shlien, A.; Foisy, S.; Accili, D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein foxo1. J. Biol. Chem. 2007, 282, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, W.J.; Chow, A.K.; Schulz, R.; Daniel, E.E. Matrix metalloproteinase-2, caveolins, focal adhesion kinase and c-kit in cells of the mouse myocardium. J. Cell Mol. Med. 2007, 11, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Katragadda, D.; Batchu, S.N.; Cho, W.J.; Chaudhary, K.R.; Falck, J.R.; Seubert, J.M. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J. Mol. Cell. Cardiol. 2009, 46, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L. Fine structure of the autophagosome. Methods Mol. Biol. 2008, 445, 11–28. [Google Scholar] [PubMed]
- Gnaiger, E.; Steinlechner-Maran, R.; Mendez, G.; Eberl, T.; Margreiter, R. Control of mitochondrial and cellular respiration by oxygen. J. Bioenerg. Biomembr. 1995, 27, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lemieux, H.; Hoppel, C.L.; Hanson, R.W.; Hakimi, P.; Croniger, C.M.; Puchowicz, M.; Anderson, V.E.; Fujioka, H.; Stavnezer, E. Peroxisome proliferator-activated receptor gamma (ppargamma) mediates a ski oncogene-induced shift from glycolysis to oxidative energy metabolism. J. Biol. Chem. 2011, 286, 40013–40024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnaiger, E.; Kuznetsov, A.V.; Schneeberger, S.; Seiler, R.; Brandacher, G.; Steurer, W.; Margreiter, R. Mitochondria in the cold. In Life in the Cold; Heldmaier, G., Klingenspor, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 431–442. [Google Scholar]
- Gnaiger, E.; Kuznetsov, A.V.; Lassnig, B.; Fuchs, A.; Reck, M.; Renner, K.; Stadlmann, S.; Rieger, G.; Margreiter, R. High-resolution respirometry. Optimum permeabilization of the cell membrane by digitonin. In Biothermokinetics in the Post Genomic Era; Larsson, C., Påhlman, I.-L., Gustafsson, L., Eds.; Chalmers Reproservice: Göteborg, Sweden, 1998; pp. 89–95. [Google Scholar]
- Giovannini, C.; Matarrese, P.; Scazzocchio, B.; Sanchez, M.; Masella, R.; Malorni, W. Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in caco-2 intestinal cells. FEBS Lett. 2002, 523, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Leal, A.M.; de Queiroz, J.D.; de Medeiros, S.R.; Lima, T.K.; Agnez-Lima, L.F. Violacein induces cell death by triggering mitochondrial membrane hyperpolarization in vitro. BMC Microbiol. 2015, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Chandel, N.S.; Li, X.X.; Schumacker, P.T.; Colombini, M.; Thompson, C.B. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA 2000, 97, 4666–4671. [Google Scholar] [CrossRef] [Green Version]
- Pearen, M.A.; Muscat, G.E. Minireview: Nuclear hormone receptor 4a signaling: Implications for metabolic disease. Mol. Endocrinol. 2010, 24, 1891–1903. [Google Scholar] [CrossRef] [Green Version]
- Briand, O.; Helleboid-Chapman, A.; Ploton, M.; Hennuyer, N.; Carpentier, R.; Pattou, F.; Vandewalle, B.; Moerman, E.; Gmyr, V.; Kerr-Conte, J.; et al. The nuclear orphan receptor nur77 is a lipotoxicity sensor regulating glucose-induced insulin secretion in pancreatic beta-cells. Mol. Endocrinol. 2012, 26, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Cui, S.; Zong, C.; Gao, W.; Xu, T.; Gao, P.; Chen, J.; Qin, D.; Guan, Q.; Liu, Y.; et al. The orphan nuclear receptor nr4a1 protects pancreatic beta-cells from endoplasmic reticulum (er) stress-mediated apoptosis. J. Biol. Chem. 2015, 290, 20687–20699. [Google Scholar] [CrossRef] [Green Version]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.; Solomon, T.P.J.; Affourtit, C. Pro-inflammatory cytokines attenuate glucose-stimulated insulin secretion from ins-1e insulinoma cells by restricting mitochondrial pyruvate oxidation capacity-novel mechanistic insight from real-time analysis of oxidative phosphorylation. PLoS ONE 2018, 13, e0199505. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Pedriali, G.; Sbano, L.; Iannitti, T.; Giorgi, C.; Pinton, P. Intersection of mitochondrial fission and fusion machinery with apoptotic pathways: Role of mcl-1. Biol. Cell 2016, 108, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suen, D.F.; Norris, K.L.; Youle, R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef] [Green Version]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Masini, M.; Bugliani, M.; Lupi, R.; del Guerra, S.; Boggi, U.; Filipponi, F.; Marselli, L.; Masiello, P.; Marchetti, P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 2009, 52, 1083–1086. [Google Scholar] [CrossRef] [Green Version]
- Bouzas-Rodriguez, J.; Zarraga-Granados, G.; Sanchez-Carbente Mdel, R.; Rodriguez-Valentin, R.; Gracida, X.; Anell-Rendon, D.; Covarrubias, L.; Castro-Obregon, S. The nuclear receptor nr4a1 induces a form of cell death dependent on autophagy in mammalian cells. PLoS ONE 2012, 7, e46422. [Google Scholar] [CrossRef] [Green Version]




| Sex | Age | BMI | |
|---|---|---|---|
| 1 | Female | 50 | 44 |
| 2 | Female | 68 | 21.7 |
| 3 | Male | 62 | 23.8 |
| 4 | Male | 61 | 29.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Close, A.-F.; Dadheech, N.; Lemieux, H.; Wang, Q.; Buteau, J. Disruption of Beta-Cell Mitochondrial Networks by the Orphan Nuclear Receptor Nor1/Nr4a3. Cells 2020, 9, 168. https://doi.org/10.3390/cells9010168
Close A-F, Dadheech N, Lemieux H, Wang Q, Buteau J. Disruption of Beta-Cell Mitochondrial Networks by the Orphan Nuclear Receptor Nor1/Nr4a3. Cells. 2020; 9(1):168. https://doi.org/10.3390/cells9010168
Chicago/Turabian StyleClose, Anne-Françoise, Nidheesh Dadheech, Hélène Lemieux, Qian Wang, and Jean Buteau. 2020. "Disruption of Beta-Cell Mitochondrial Networks by the Orphan Nuclear Receptor Nor1/Nr4a3" Cells 9, no. 1: 168. https://doi.org/10.3390/cells9010168
APA StyleClose, A.-F., Dadheech, N., Lemieux, H., Wang, Q., & Buteau, J. (2020). Disruption of Beta-Cell Mitochondrial Networks by the Orphan Nuclear Receptor Nor1/Nr4a3. Cells, 9(1), 168. https://doi.org/10.3390/cells9010168





