Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Hematopoietic Cells
2.3. Enumeration of Hematopoietic Cells
2.4. Mobilization
2.5. Albumin Quantification
2.6. rhG-CSF Quantification
2.7. Statistics
3. Results
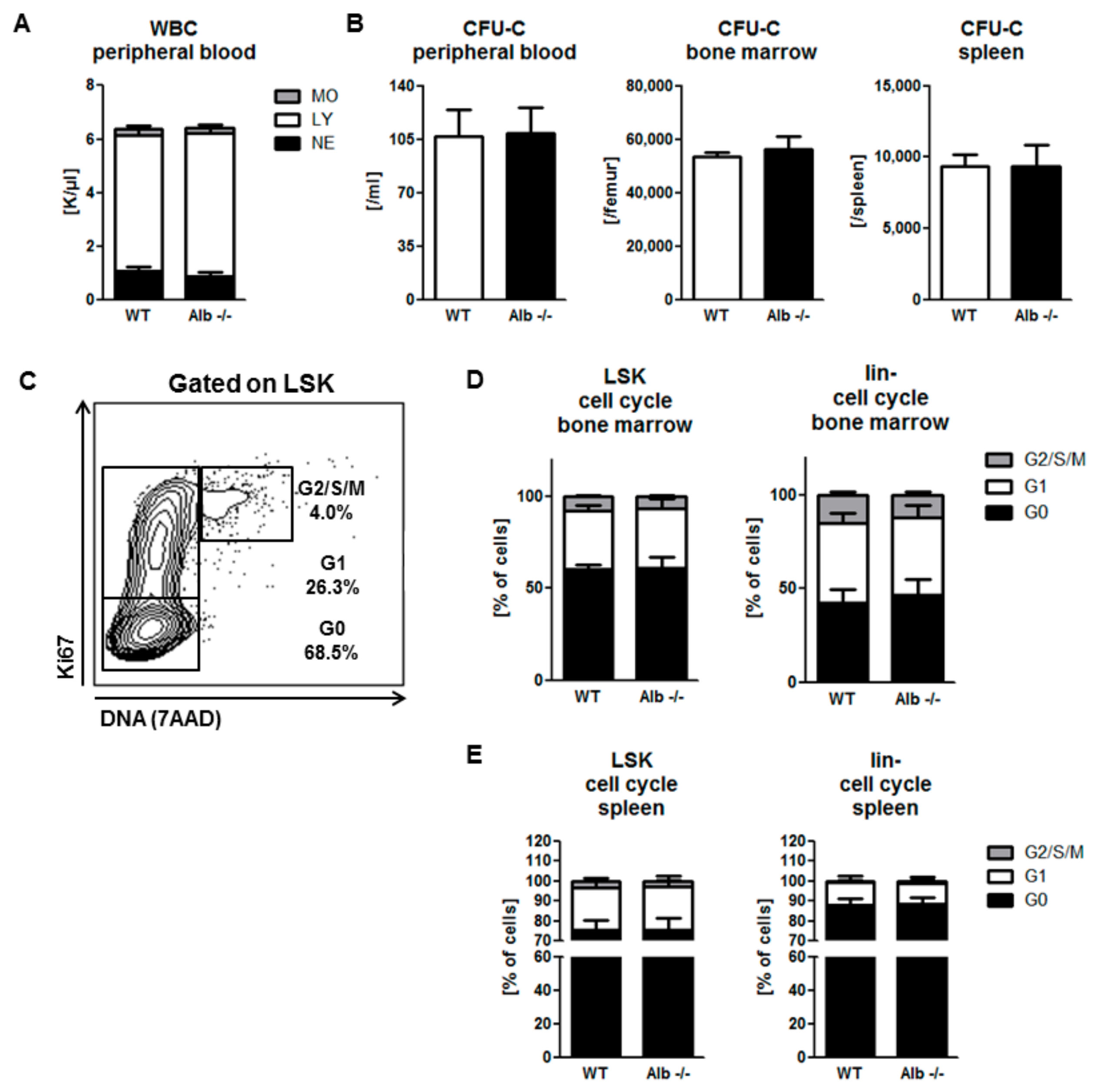
3.1. Homeostatic Hematopoiesis is Unaffected by Albumin Deficiency
3.2. Role of Albumin in the Pharmacodynamics of the Small-Molecule CXCR4 Antagonist AMD3100
3.3. Role of Albumin in G-CSF-Induced Mobilization
3.4. Human Albumin Substitution in the G-CSF Mobilization Setting
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.T. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic press: San Diego, CA, USA, 1995. [Google Scholar]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Meloun, B.; Moravek, L.; Kostka, V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975, 58, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Bern, M.; Sand, K.M.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release. 2015, 211, 144–162. [Google Scholar] [CrossRef]
- Zhivkova, Z.D. Studies on drug-human serum albumin binding: The current state of the matter. Curr Pharm. Des. 2015, 21, 1817–1830. [Google Scholar] [CrossRef] [Green Version]
- Roopenian, D.C.; Low, B.E.; Christianson, G.J.; Proetzel, G.; Sproule, T.J.; Wiles, M.V. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs 2015, 7, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Koot, B.G.; Houwen, R.; Pot, D.J.; Nauta, J. Congenital analbuminaemia: Biochemical and clinical implications. A case report and literature review. Eur. J. Pediatr. 2004, 163, 664–670. [Google Scholar] [CrossRef]
- Bonig, H.; Papayannopoulou, T. Mobilization of hematopoietic stem/progenitor cells: General principles and molecular mechanisms. Methods Mol. Biol. 2012, 904, 1–14. [Google Scholar]
- Bonig, H.; Watts, K.L.; Chang, K.H.; Kiem, H.P.; Papayannopoulou, T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells 2009, 27, 836–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpova, D.; Bonig, H. Concise Review: CXCR4/CXCL12 Signaling in Immature Hematopoiesis—Lessons From Pharmacological and Genetic Models. Stem Cells 2015, 33, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Holig, K. G-CSF in Healthy Allogeneic Stem Cell Donors. Transfus. Med. Hemother. 2013, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.S.; Cameron, D.A.; Pettengell, R.; Bohlius, J.; Crawford, J.; Ellis, M.; Kearney, N.; Lyman, G.H.; Tjan-Heijnen, V.C.; Walewski, J.; et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur. J. Cancer 2006, 42, 2433–2453. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Nicola, N.A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J. Cell Physiol. 1983, 116, 198–206. [Google Scholar] [CrossRef]
- Morrison, S.J.; Wright, D.E.; Weissman, I.L. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl. Acad. Sci. USA 1997, 94, 1908–1913. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.E.; Cheshier, S.H.; Wagers, A.J.; Randall, T.D.; Christensen, J.L.; Weissman, I.L. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood 2001, 97, 2278–2285. [Google Scholar] [CrossRef] [Green Version]
- Lapid, K.; Glait-Santar, C.; Gur-Cohen, S.; Canaani, J.; Kollet, O.; Lapidot, T. Egress and Mobilization of Hematopoietic Stem and Progenitor Cells: A Dynamic Multi-facet Process. Available online: https://www.stembook.org (accessed on 16 December 2019).
- Bonig, H.; Papayannopoulou, T. Hematopoietic stem cell mobilization: Updated conceptual renditions. Leukemia 2013, 27, 24–31. [Google Scholar] [CrossRef]
- Klein, G.; Schmal, O.; Aicher, W.K. Matrix metalloproteinases in stem cell mobilization. Matrix Biol. 2015, 44–46, 175–183. [Google Scholar] [CrossRef]
- Christopher, M.J.; Liu, F.; Hilton, M.J.; Long, F.; Link, D.C. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 2009, 114, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; David, T.S.; Avi, M.; Grigori, N.; Grigori, N.E.; et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Levesque, J.P. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails the cells that make blood and bone. Exp. Hematol. 2006, 34, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Poursine-Laurent, J.; Link, D.C. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not flt-3 ligand. Blood 1997, 90, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Orschell, C.M.; Clapp, D.W.; Hangoc, G.; Cooper, S.; Plett, P.A.; Liles, W.C.; Li, X.; Graham-Evans, B.; Timothy, B.C.; et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005, 201, 1307–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, C.W.; Flexner, C.; MacFarland, R.T.; Giandomenico, C.; Fuchs, E.J.; Redpath, E.; Bridger, G.; Henson, G.W. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Chemother. 2000, 44, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Stute, N.; Santana, V.M.; Rodman, J.H.; Schell, M.J.; Ihle, J.N.; Evans, W.E. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood 1992, 79, 2849–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturgill, M.G.; Huhn, R.D.; Drachtman, R.A.; Ettinger, A.G.; Ettinger, L.J. Pharmacokinetics of intravenous recombinant human granulocyte colony-stimulating factor (rhG-CSF) in children receiving myelosuppressive cancer chemotherapy: Clearance increases in relation to absolute neutrophil count with repeated dosing. Am. J. Hematol. 1997, 54, 124–130. [Google Scholar] [CrossRef]
- EMEA. CHMP Assessment Report for Mozobil 2009. Available online: https://www.ema.europa.eu/en/documents/assessment-report/mozobil-epar-public-assessment-report_en.pdf (accessed on 16 December 2019).
- Bonig, H.; Priestley, G.V.; Oehler, V.; Papayannopoulou, T. Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC. Exp. Hematol. 2007, 35, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Winkler, I.G.; Wiercinska, E.; Barbier, V.; Nowlan, B.; Bonig, H.; Levesque, J.P. Mobilization of hematopoietic stem cells with highest self-renewal by G-CSF precedes clonogenic cell mobilization peak. Exp. Hematol. 2016, 44, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Bonig, H.; Priestley, G.V.; Nilsson, L.M.; Jiang, Y.; Papayannopoulou, T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood 2004, 104, 2299–2306. [Google Scholar] [CrossRef]
- Rosenkilde, M.M.; Gerlach, L.O.; Jakobsen, J.S.; Skerlj, R.T.; Bridger, G.J.; Schwartz, T.W. Molecular mechanism of AMD3100 antagonism in the CXCR4 receptor: Transfer of binding site to the CXCR3 receptor. J. Biol. Chem. 2004, 279, 3033–3041. [Google Scholar] [CrossRef] [Green Version]
- Merlin, E.; Piguet, C.; Auvrignon, A.; Rubie, H.; Demeocq, F.; Kanold, J. The pros and cons of split-dose granulocyte colony-stimulating factor alone rather than a single high dose for hematopoietic progenitor cell mobilization in small children (<15 kg) with solid tumors. Haematologica 2006, 91, 1004–1005. [Google Scholar]
- Carrion, R.; Serrano, D.; Gomez-Pineda, A.; Diez-Martin, J.L. A randomised study of 10 microg/kg/day (single dose) vs 2 x 5 microg/kg/day (split dose) G-CSF as stem cell mobilisation regimen in high-risk breast cancer patients. Bone Marrow Transplant. 2003, 32, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Van Der Auwera, P.; Platzer, E.; Xu, Z.X.; Schulz, R.; Feugeas, O.; Capdeville, R.; David, J.E. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25-8315) in healthy volunteers: Comparison with single and multiple daily doses of filgrastim. Am. J. Hematol. 2001, 66, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kaneko, T. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor in the rat. Single and multiple dosing studies. Drug Metab. Dispos. 1991, 19, 200–204. [Google Scholar] [PubMed]
- Kroger, N.; Renges, H.; Kruger, W.; Gutensohn, K.; Loliger, C.; Carrero, I.; Lourdes, C.; Axel, R.Z. A randomized comparison of once versus twice daily recombinant human granulocyte colony-stimulating factor (filgrastim) for stem cell mobilization in healthy donors for allogeneic transplantation. Br. J. Haematol. 2000, 111, 761–765. [Google Scholar] [PubMed]
- Tanaka, H.; Satake-Ishikawa, R.; Ishikawa, M.; Matsuki, S.; Asano, K. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor conjugated to polyethylene glycol in rats. Cancer Res. 1991, 51, 3710–3714. [Google Scholar]
- Kim, M.G.; Han, N.; Lee, E.K.; Kim, T. Pegfilgrastim vs filgrastim in PBSC mobilization for autologous hematopoietic SCT: A systematic review and meta-analysis. Bone Marrow Transplant. 2015, 50, 523–530. [Google Scholar] [CrossRef]
- Do, B.H.; Kang, H.J.; Song, J.A.; Nguyen, M.T.; Park, S.; Yoo, J.; Do, B.H.; Kang, H.J.; Song, J.A.; Nguyen, M.T.; et al. Granulocyte colony-stimulating factor (GCSF) fused with Fc Domain produced from E. coli is less effective than Polyethylene Glycol-conjugated GCSF. Sci Rep. 2017, 7, 6480. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zhang, Y.; Tian, H.; Chen, X.; Cai, D.; Yao, W.; Gao, X. Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin. Biomed. Res. Int. 2013, 2013, 107238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Wen, X.F.; Yang, Z.Y.; Wu, Y.L.; Lu, Y.; Zhou, L.F. Development and characterization of a novel fusion protein of a mutated granulocyte colony-stimulating factor and human serum albumin in Pichia pastoris. PLoS ONE 2014, 9, e115840. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.C.; Guttman, D.E. The binding of drugs by plasma proteins. J. Pharm. Sci. 1968, 57, 895–918. [Google Scholar] [CrossRef] [PubMed]
- Vallner, J.J. Binding of drugs by albumin and plasma protein. J. Pharm. Sci. 1977, 66, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Gonzalez, D.; Derendorf, H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2010, 99, 1107–1122. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danner, E.; Bonig, H.; Wiercinska, E. Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice. Cells 2020, 9, 4. https://doi.org/10.3390/cells9010004
Danner E, Bonig H, Wiercinska E. Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice. Cells. 2020; 9(1):4. https://doi.org/10.3390/cells9010004
Chicago/Turabian StyleDanner, Eva, Halvard Bonig, and Eliza Wiercinska. 2020. "Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice" Cells 9, no. 1: 4. https://doi.org/10.3390/cells9010004




