Development and Validation of a Fully GMP-Compliant Process for Manufacturing Stromal Vascular Fraction: A Cost-Effective Alternative to Automated Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Donor Specifications
2.2. AT Harvesting
2.3. Development of the LG SVF Isolation Process
2.4. Inter-Donor Comparative Analysis between Manual and Reference Protocol
2.5. Celution Protocol for SVF Isolation
2.6. Intra-Donor Comparative Analysis between the LG and Reference Protocols
2.7.1. Concentration of Viable Nucleated Cells (VNCs) and Cell Viability
2.7.2. Colony-Forming Unit Fibroblast (CFU-F) Assay
2.7.3. Flow Cytometry Analysis of SVF Cell Subsets
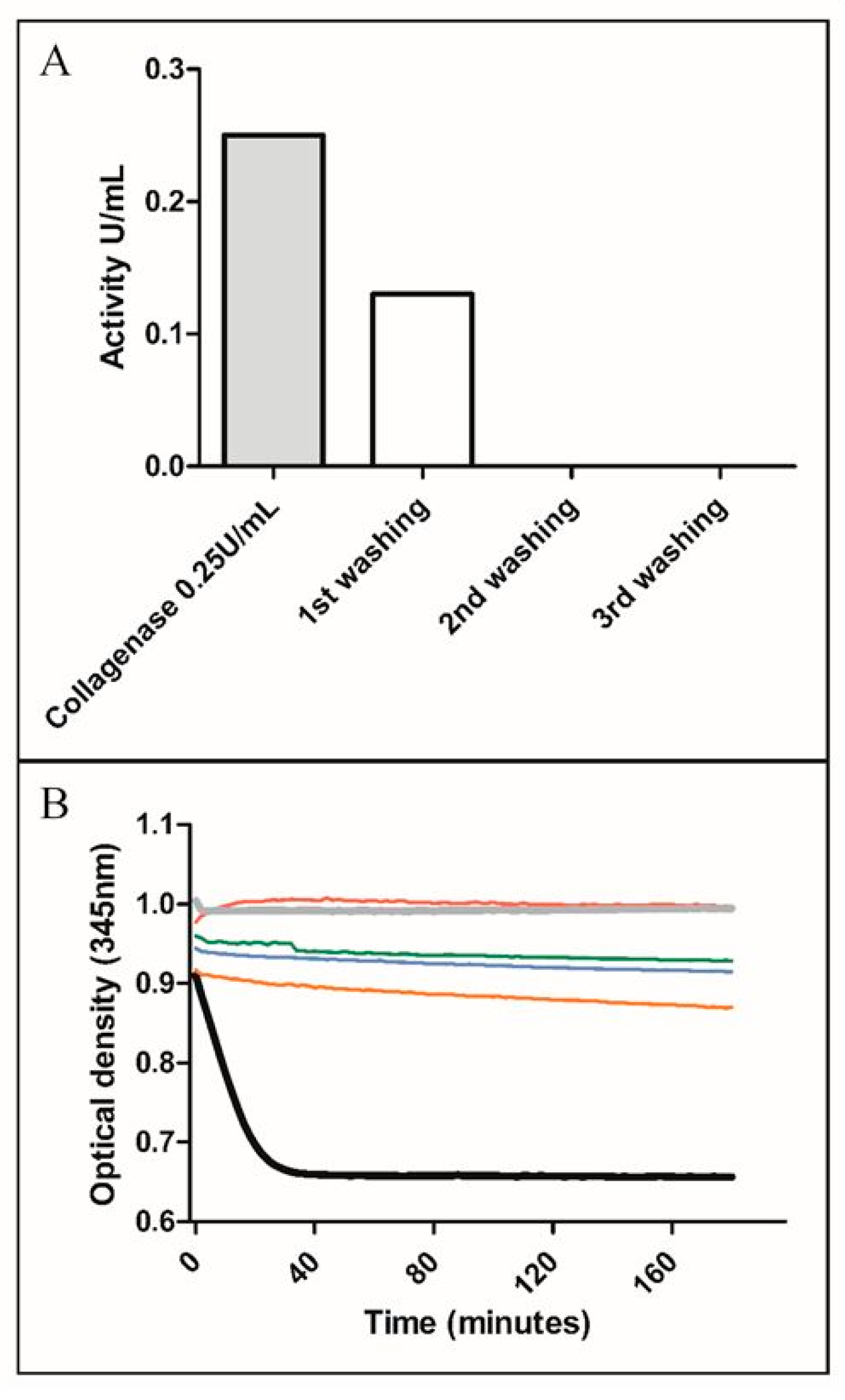
2.7.4. Collagenase Quantification
2.7.5. Tube Formation Assay
2.7.6. Spheroid-Based Sprouting Assay
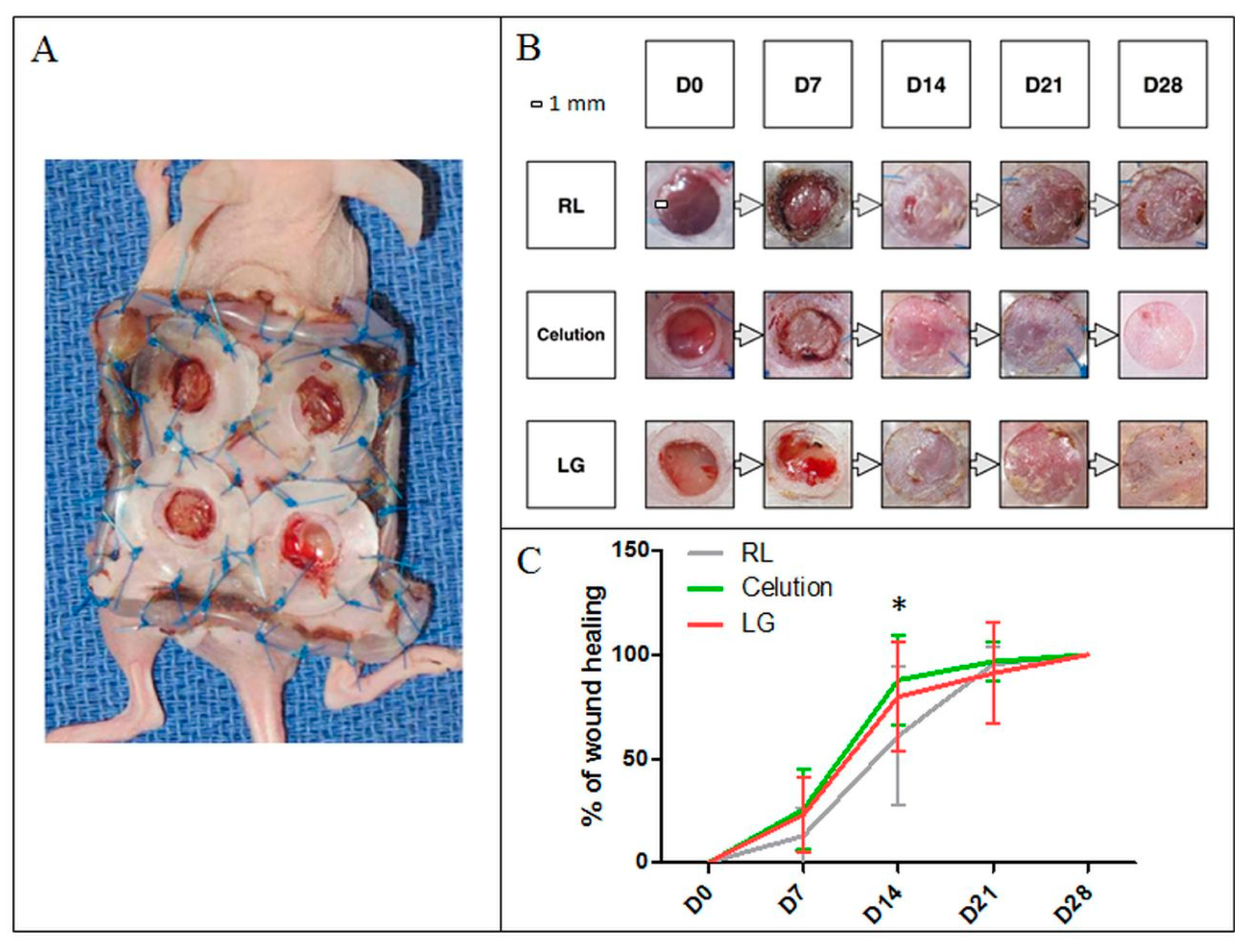
2.7.7. In Vivo Experiments
2.7.8. Statistical Analyses
3. Results
3.1. Development of the LG Protocol
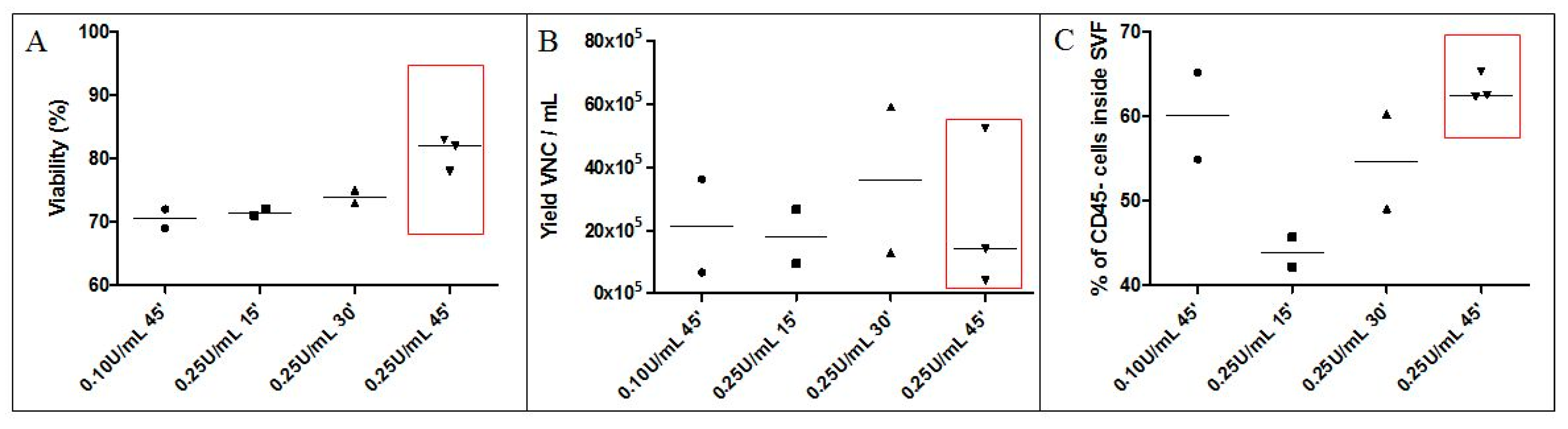
3.1.1. Collagenase Digestion Conditions (Time, Concentration)
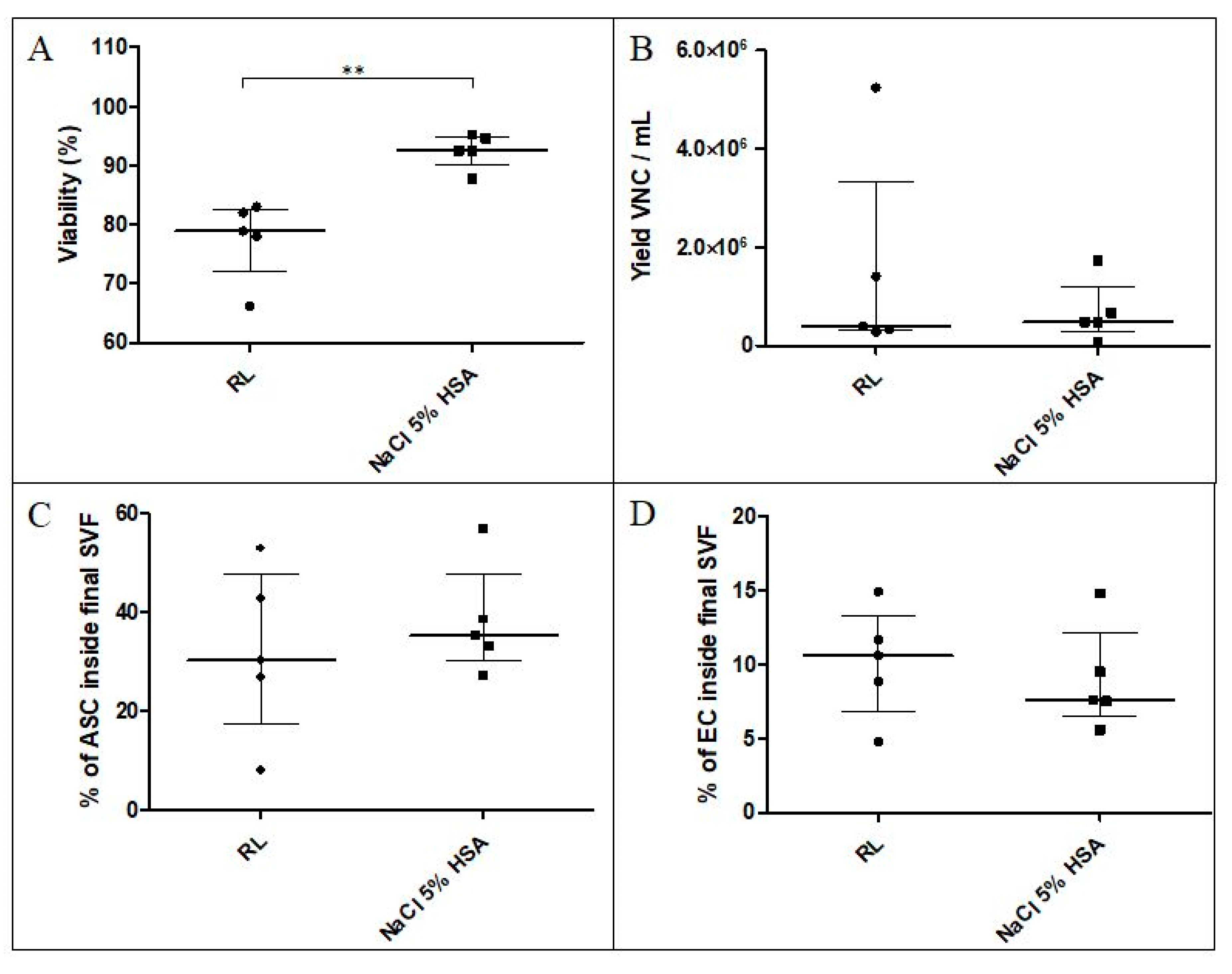
3.1.2. Washing Solution and Final Excipient for SVF Cells
3.1.3. Validation of the Necessary Number of Washings
3.2. Comparability of LG and Celution-Based Protocols for SVF Manufacturing
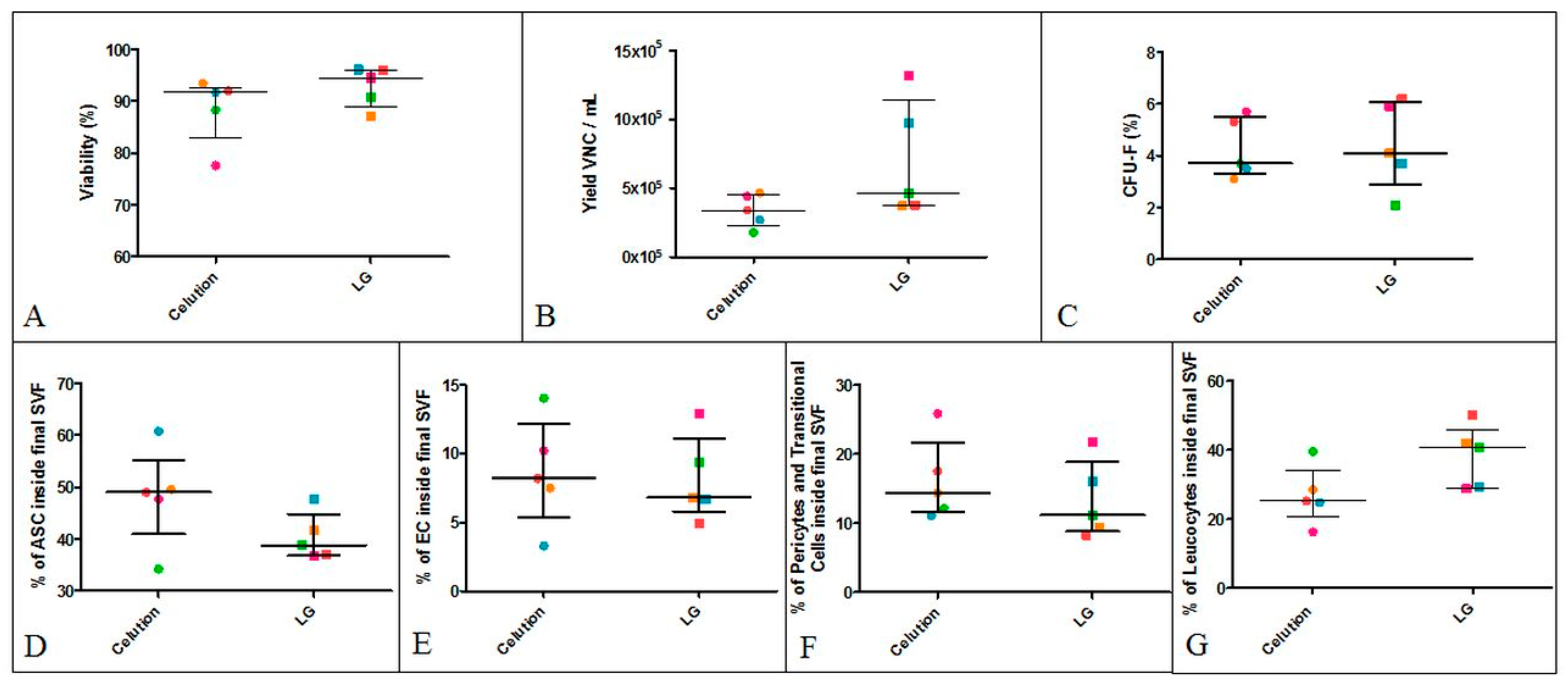
3.2.1. Inter-Donor Comparative Analysis of SVF Produced using the Celution Device or LG Standardized Protocol
3.2.2. Intra-Donor Comparative Analysis of SVF Produced Using the Celution Device or Final LG Protocol
3.2.3. Sterility Testing
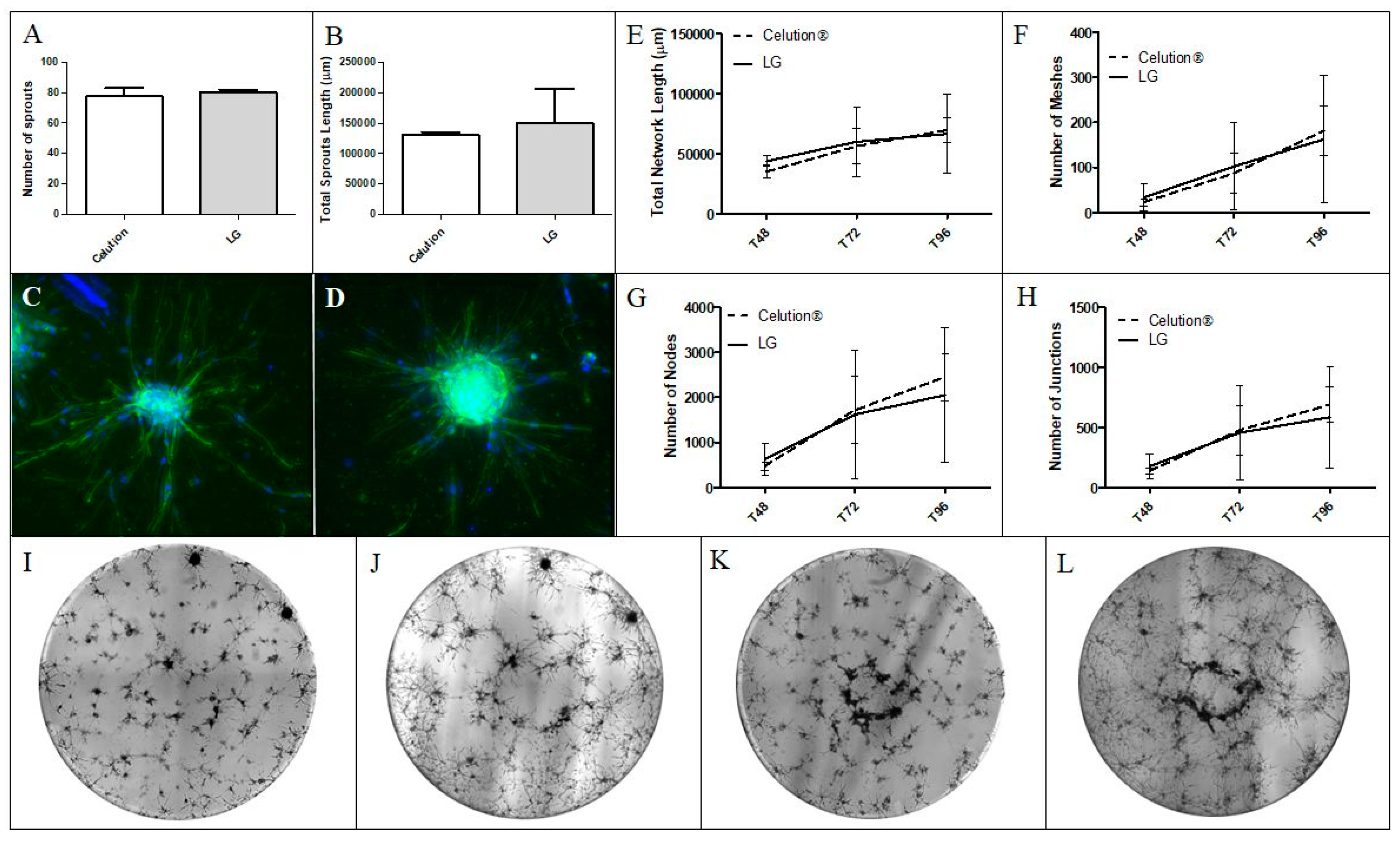
3.2.4. Analysis of the SVF Angiogenic Capacity In Vitro
3.2.5. Analysis of the Angiogenic Capacity In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mojallal, A.; Foyatier, J.-L. Historique de l’utilisation du tissu adipeux comme produit de comblement en chirurgie plastique. Ann. Chir. Plast. Esthét. 2004, 49, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.; Zavan, B.; Galiè, M.; Di Vittorio, L.; Gardin, C.; Bianchi, A.E. Autologous Fat Transfer for Facial Augmentation: Surgery and Regeneration. J. Craniofac. Surg. 2019, 30, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.V.; Alfonso, Z.; Zhang, R.; Leung, J.; Wu, B.; Ignarro, L.J. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12167–12172. [Google Scholar] [CrossRef] [Green Version]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and Therapeutic Perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Dubey, N.; Mishra, V.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.M.; Hur, S.-M.; Park, K.-Y.; Kim, C.-K.; Kim, Y.-M.; Kim, H.-S.; Shin, H.-C.; Won, M.-H.; Ha, K.-S.; Kwon, Y.-G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul. Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. In Mesenchymal Stem Cells; Gnecchi, M., Ed.; Springer: New York, NY, USA, 2016; Volume 1416, pp. 123–146. [Google Scholar]
- L., P.K.; Kandoi, S.; Misra, R.; S., V.; K., R.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrin, I.; Beloqui, I.; Zabaleta, L.; Salcedo, J.M.; Trigueros, C.; Martin, A.G. Isolation, Culture, and Expansion of Mesenchymal Stem Cells. In Stem Cell Banking; Crook, J.M., Ludwig, T.E., Eds.; Springer: New York, NY, USA, 2017; Volume 1590, pp. 177–190. [Google Scholar]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desando, G.; Bartolotti, I.; Martini, L.; Giavaresi, G.; Nicoli Aldini, N.; Fini, M.; Roffi, A.; Perdisa, F.; Filardo, G.; Kon, E.; et al. Regenerative Features of Adipose Tissue for Osteoarthritis Treatment in a Rabbit Model: Enzymatic Digestion Versus Mechanical Disruption. Int. J. Mol. Sci. 2019, 20, 2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Chae, D.-S.; Ryu, H.A.; Kim, S.-W. Transplantation of human adipose tissue derived-SVF enhance liver function through high anti-inflammatory property. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 2019, 1864, 158526. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.B.; Berman, M.H.; See, J.R. Personal cell therapy for interstitial cystitis with autologous stromal vascular fraction stem cells. Ther. Adv. Urol. 2019, 11, 175628721986859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef]
- Magalon, J.; Velier, M.; Simoncini, S.; François, P.; Bertrand, B.; Daumas, A.; Benyamine, A.; Boissier, R.; Arnaud, L.; Lyonnet, L.; et al. Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 391–398. [Google Scholar] [CrossRef]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.-R.; Nguyen, P.-S.; Chabannon, C.; Colavolpe, N.; Reynier, J.-C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehranfar, S.; Abdi Rad, I.; Mostafavi, E.; Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019, 47, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef]
- Comella, K.; Parcero, J.; Bansal, H.; Perez, J.; Lopez, J.; Agrawal, A.; Ichim, T. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eglinger, J.; Karsjens, H.; Lammert, E. Quantitative assessment of angiogenesis and pericyte coverage in human cell-derived vascular sprouts. Inflamm. Regen. 2017, 37, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiano, R.D.; Michaels, V.J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef] [Green Version]
- Maria, A.T.J.; Toupet, K.; Maumus, M.; Fonteneau, G.; Le Quellec, A.; Jorgensen, C.; Guilpain, P.; Noël, D. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J. Autoimmun. 2016, 70, 31–39. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/Pericyte Interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue–Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, C.R.; Kim, K.J.; Ryu, Y.H.; Kim, E.; Han, Y.N.; Moon, S.-H.; Rhie, J.-W. Optimal Condition of Isolation from an Adipose Tissue-Derived Stromal Vascular Fraction for the Development of Automated Systems. Tissue Eng. Regen. Med. 2020, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, J.; Pratta, A.-S.; Abbassi, N.; Fabre, H.; Rodriguez, F.; Debard, C.; Adobati, J.; Boucher, F.; Mallein-Gerin, F.; Auxenfans, C.; et al. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Breast Reconstruction with Enhanced Stromal Vascular Fraction Fat Grafting: What Is the Best Method? Plast. Reconstr. Surg. 2015, 3, e406. [Google Scholar] [CrossRef]
- Doi, K.; Tanaka, S.; Iida, H.; Eto, H.; Kato, H.; Aoi, N.; Kuno, S.; Hirohi, T.; Yoshimura, K. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis: SVF from lipoaspirates using automated processing. J. Tissue Eng. Regen. Med. 2013, 7, 864–870. [Google Scholar] [CrossRef]
- Güven, S.; Karagianni, M.; Schwalbe, M.; Schreiner, S.; Farhadi, J.; Bula, S.; Bieback, K.; Martin, I.; Scherberich, A. Validation of an Automated Procedure to Isolate Human Adipose Tissue–Derived Cells by Using the Sepax® Technology. Tissue Eng. Part C Methods 2012, 18, 575–582. [Google Scholar] [CrossRef]
- Kim, S.K. Adipose Stromal Vascular Fraction Isolation: A Head-to-Head Comparison of Four Commercial Cell Separation Systems. Plast. Reconstr. Surg. 2014, 133, 889e. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.C.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Halvorsen, Y.D.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell–Associated Markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Ellenhorn, J.D.I. Adipose Stromal Vascular Fraction Isolation: A Head-to-Head Comparison of Four Commercial Cell Separation Systems. Plast. Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Hicok, K.C.; Shanahan, R.; Zhu, M.; Miller, S.; Arm, D.M. The Celution ® System: Automated Processing of Adipose-Derived Regenerative Cells in a Functionally Closed System. Adv. Wound Care 2014, 3, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minonzio, G.; Corazza, M.; Mariotta, L.; Gola, M.; Zanzi, M.; Gandolfi, E.; De Fazio, D.; Soldati, G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology 2014, 69, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condé-Green, A.; Rodriguez, R.L.; Slezak, S.; Singh, D.P.; Goldberg, N.H.; McLenithan, J. Comparison between Stromal Vascular Cells’ Isolation with Enzymatic Digestion and Mechanical Processing of Aspirated Adipose Tissue. Plast. Reconstr. Surg. 2014, 134, 54. [Google Scholar] [CrossRef]
- Dos-Anjos Vilaboa, S.; Navarro-Palou, M.; Llull, R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy 2014, 16, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- SundarRaj, S.; Deshmukh, A.; Priya, N.; Krishnan, V.S.; Cherat, M.; Majumdar, A.S. Development of a System and Method for Automated Isolation of Stromal Vascular Fraction from Adipose Tissue Lipoaspirate. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; Da Ponte, A.; Tessitori, G.; et al. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. Ther. 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]

| Celution Protocol | LG Protocol | |||
|---|---|---|---|---|
| AT harvesting | Using Khouri cannula | Using Khouri cannula | ||
| AT repackaging | Packaging in 60-mL Luer Lock syringes | NA | ||
| Device preparation | Tensioning Celution device, settlement of consumables, and seal check | NA | ||
| AT transfer | AT transfer in Celution device | AT transfer in Puregraft device | ||
| Settling and discarding infiltration liquid | Automated | Visual check of discarding infiltration liquid | Discarding infiltration liquid performed using Puregraft device | |
| Weighing | Automated | At least 100 mL of AT needed | NA | |
| AT washing | Automated | From two to five washings with RL and visual check | Three washings using RL | |
| Weighing | Automated | No particular volume required | AT volume between 50 and 250 mL | |
| Dilution | NA | Two-fold dilution with RL | ||
| Incubation | NA | Incubation of the diluted AT for 15 min at 37 °C | ||
| Enzyme preparation | Celase reconstitution | Collagenase thawing (previously reconstituted at 20 U/mL) | ||
| Digestion | Automated | Injection of 5 mL of reconstituted Celase into Celution device | Injection of collagenase at 0.25 U/mL into Puregraft device for 45 min at 37 °C under agitation | |
| Concentration | Automated | Visual check of the concentration | Closed Circuit | Cellular concentration by centrifugation (400 g, 5 min) |
| Filtration | NA | Closed Circuit | Cell strainer 200-µm porosity | |
| Washings | Automated | With RL | Closed Circuit | Two washings with NaCl 5% HSA |
| Collection of SVF | SVF resuspended in RL | SVF resuspended in NaCl 5% HSA | ||
| Viability | Yield of VNCs (×105/mL AT) | CFU-F | ASC | EC | Pericytes and Transitional Cells | Leukocytes (Macrophages, Neutrophils, and Lymphocytes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LG Protocol | Celution Device | LG Protocol | Celution Device | LG Protocol | Celution Device | LG Protocol | Celution Device | LG Protocol | Celution Device | LG Protocol | Celution Device | LG Protocol | Celution Device | |
| Average | 93.05% | 87.00% | 6.30 | 2.50 | 4.05% | 4.80% | 39.50% | 43.99% | 8.40% | 7.66% | 12.70% | 10.29% | 39.50% | 38.06% |
| Standard deviation | 3.50% | 4.00% | 4.20 | 1.23 | 1.70% | 1.70% | 4.70% | 11.49% | 2.80% | 6.15% | 5.20% | 7.95% | 8.80% | 12.45% |
| Median | 94.15% | 87.30% | 4.20 | 2.52 | 3.92% | 5.00% | 37.90% | 44.00% | 8.07% | 6.00% | 10.64% | 8.00% | 41.25% | 37.00% |
| 25% Percentile | 89.83% | 84.60% | 3.35 | 1.50 | 2.24% | 3.42% | 36.25% | 36.50% | 6.25% | 5.00% | 8.67% | 3.00% | 29.15% | 31.50% |
| 75% Percentile | 96.03% | 90.00% | 10.58 | 3.18 | 5.97% | 5.90% | 43.31% | 49.50% | 10.43% | 8.50% | 17.45% | 14.50% | 47.50% | 49.00% |
| Minimum | 87.20% | 77.60% | 3.20 | 0.77 | 2.07% | 1.30% | 34.90% | 25.50% | 4.90% | 2.70% | 8.30% | 1.00% | 28.70% | 12.70% |
| Maximum | 96.10% | 93.40% | 13.20 | 5.76 | 6.20% | 8.00% | 47.70% | 81.40% | 12.90% | 37.10% | 21.80% | 32.20% | 49.90% | 63.60% |
| Studies | SVF Extraction Method | Number of Batches | Viability (%) | Yield of VNCs (×105/mL AT) |
|---|---|---|---|---|
| [45] | Non-automated | 18 | 93.90 | 4.00 |
| [46] | Non-automated | 44 | >50.00 | 3.08 |
| [42] | Non-automated | 11 | >90.00 | 1.60 |
| Sepax | >90.00 | 2.60 | ||
| [41] | Non-automated | 6 | 82.40 | 7.01 |
| Icellator | 80.70 | 7.02 | ||
| [47] | Celution | 5 | 93.00 | 2.40 |
| Lipokit | 72.00 | 0.35 | ||
| PNC Multistation | 57.00 | 1.70 | ||
| CHA-Station | 87.00 | 0.05 | ||
| [48] | Celution | 31 | 87.70 | 3.60 |
| [49] | Non-automated | 130 | 85.05 | 1.81 |
| [50] | Non-automated | 9 | 80–90 | 2.30 |
| [51] | GID SVF1 | 52 | 83.00 | 7.19 |
| [52] | Non-automated | 11 | 96.00 | 2.05 |
| [39] | Non-automated | 4 | 75.80 | 7.95 |
| GID SVF1 | 81.47 | 4.25 | ||
| Non-automated (Puregraft) | 77.45 | 2.50 | ||
| Stem.pras with Duografter | 69.30 | 5.35 | ||
| [53] | Non-automated | 19 | 82.20 | 1.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
François, P.; Giraudo, L.; Veran, J.; Bertrand, B.; Dumoulin, C.; Aboudou, H.; Grimaud, F.; Vogtensperger, M.; Velier, M.; Arnaud, L.; et al. Development and Validation of a Fully GMP-Compliant Process for Manufacturing Stromal Vascular Fraction: A Cost-Effective Alternative to Automated Methods. Cells 2020, 9, 2158. https://doi.org/10.3390/cells9102158
François P, Giraudo L, Veran J, Bertrand B, Dumoulin C, Aboudou H, Grimaud F, Vogtensperger M, Velier M, Arnaud L, et al. Development and Validation of a Fully GMP-Compliant Process for Manufacturing Stromal Vascular Fraction: A Cost-Effective Alternative to Automated Methods. Cells. 2020; 9(10):2158. https://doi.org/10.3390/cells9102158
Chicago/Turabian StyleFrançois, Pauline, Laurent Giraudo, Julie Veran, Baptiste Bertrand, Chloé Dumoulin, Houssein Aboudou, Fanny Grimaud, Marie Vogtensperger, Mélanie Velier, Laurent Arnaud, and et al. 2020. "Development and Validation of a Fully GMP-Compliant Process for Manufacturing Stromal Vascular Fraction: A Cost-Effective Alternative to Automated Methods" Cells 9, no. 10: 2158. https://doi.org/10.3390/cells9102158
APA StyleFrançois, P., Giraudo, L., Veran, J., Bertrand, B., Dumoulin, C., Aboudou, H., Grimaud, F., Vogtensperger, M., Velier, M., Arnaud, L., Lyonnet, L., Simoncini, S., Guillet, B., Dignat-George, F., Magalon, J., & Sabatier, F. (2020). Development and Validation of a Fully GMP-Compliant Process for Manufacturing Stromal Vascular Fraction: A Cost-Effective Alternative to Automated Methods. Cells, 9(10), 2158. https://doi.org/10.3390/cells9102158





