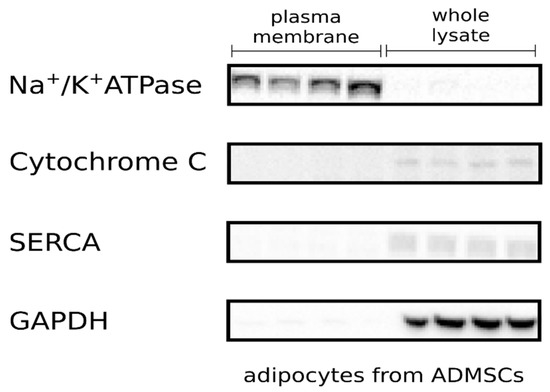
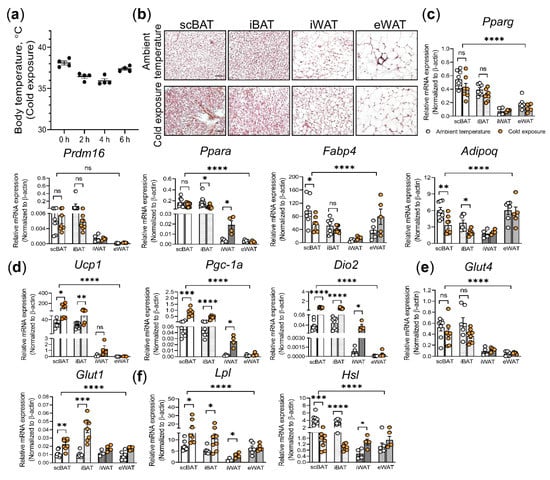
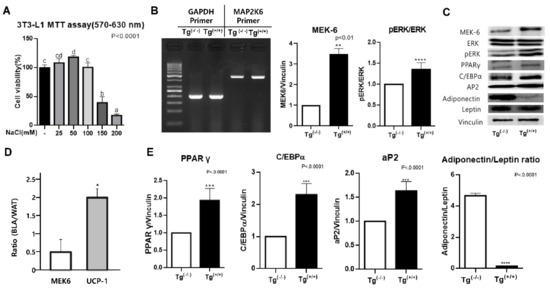
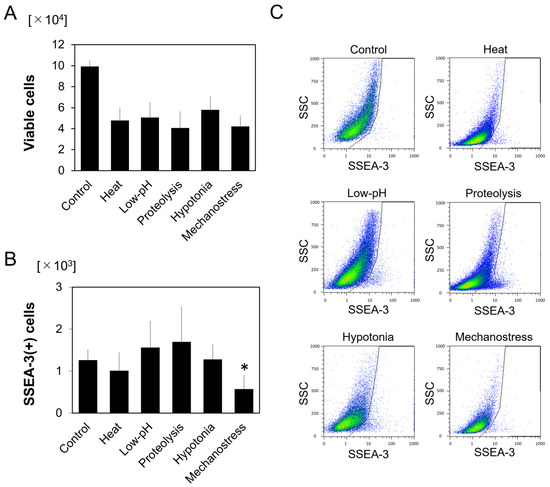
Research on Adipose Stem Cells
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 95500Editor
Interests: mesenchymal stem cells; adipose; regeneration; SVF; exosomes; therapy; tissue engineering
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
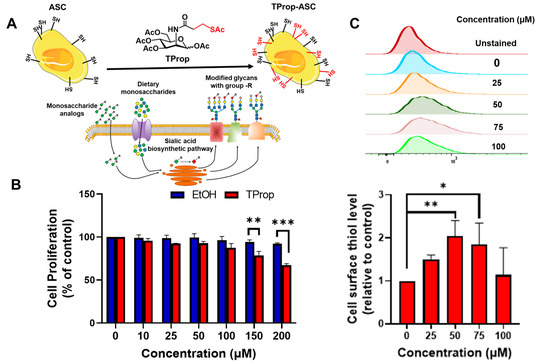
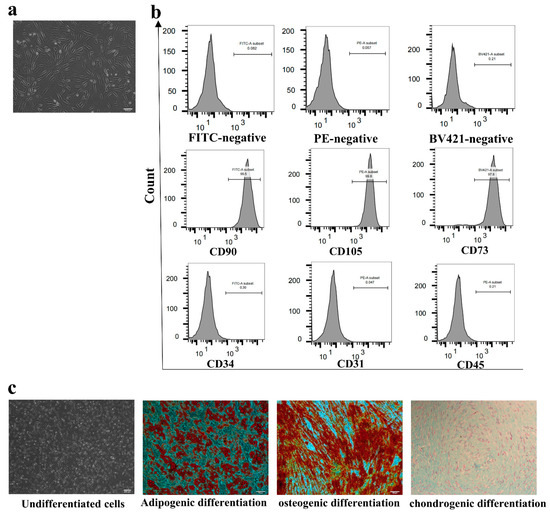
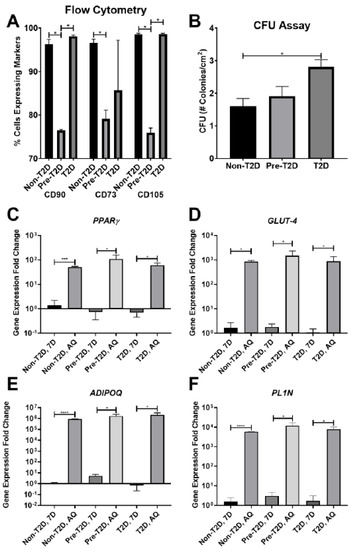
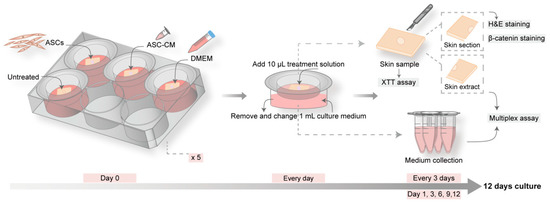
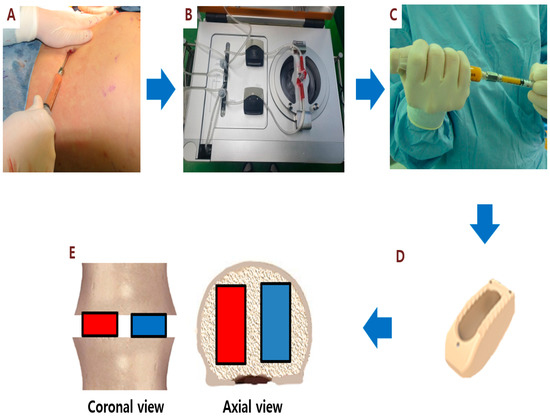
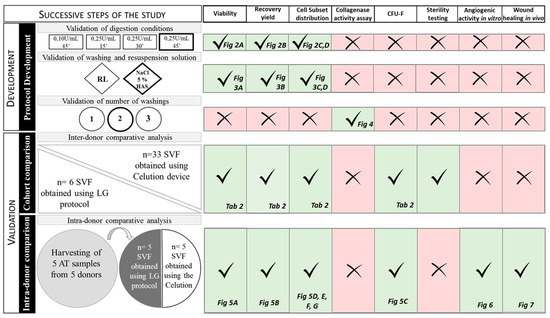
Stem cell therapies have shown potential benefits in a variety of human diseases. Adipose tissue is a rich source of cells with regenerative potential. Adipose-tissue‐derived cell products, including the stromal vascular fraction (SVF) and stromal/stem cells (ASC), possess the capacity for homing, immunomodulation, promotion of repair, and direct regeneration of damaged tissues, which make them promising therapeutic interventions for numerous diseases. ASCs also produce soluble factors and extravascular vesicles (exosomes and microvesicles) that have been demonstrated to have therapeutic effects. The cargo contained within microvesicles is a complex mix of proteins, lipids, DNA, mRNAs, microRNAs, tRNA, and non-coding RNAs that are delivered to other cells and can have biological impacts. SVF, ASC, and ASC-derived microvesicles are promising candidates as cellular therapies due to their abundance, ease of isolation, and natural mechanisms for mediating tissue regeneration. Pre-clinical and clinical studies indicate that each of them have the potential to improve disease outcomes.
This Topical Collection focused on Adipose Stem Cells seeks original research and review articles with a focus on:
- Biological characterization of cell populations with regeneration potential isolated from adipose tissue: SVF and ASCs;
- Biological characterization and therapeutic application of microvesicles produced by ASCs;
- Studies on mechanisms of action of cells and microvesicles;
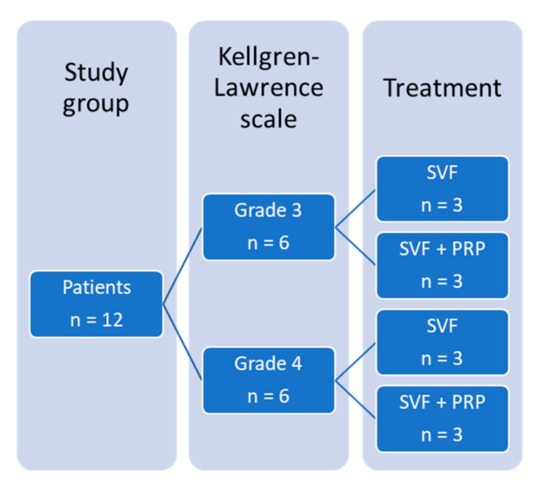
- Therapeutic applications in pre-clinical models and clinical trials.
Prof. Dr. Bruce A. Bunnell
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Adipose stem cells (ASCs)
- Stromal vascular fraction (SVF)
- Microvesicles
- Basic biology
- Mechanisms of action
- Therapeutics