Microbiota-Dependent Effects of IL-22
Abstract
1. Introduction
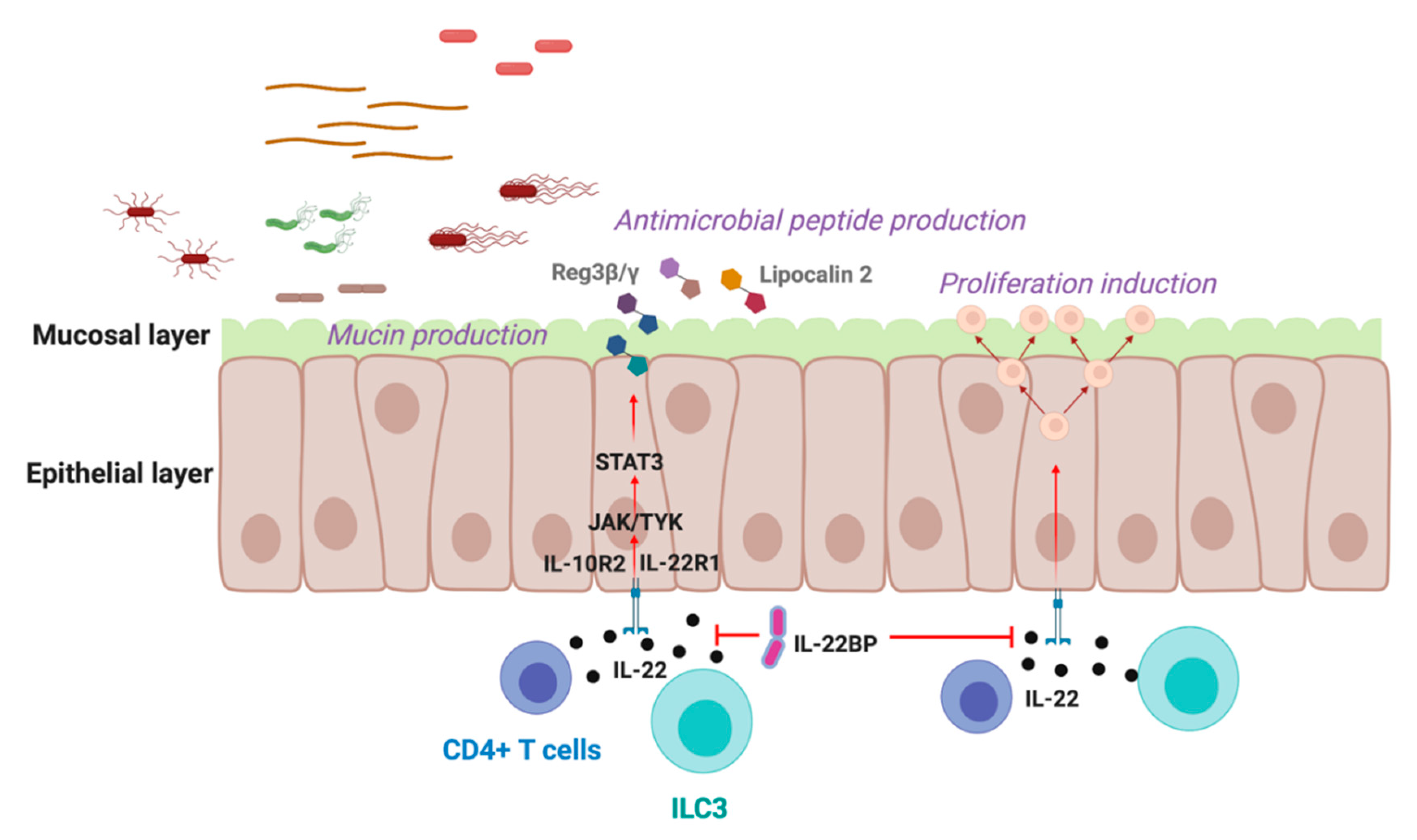
2. IL-22 in the Gastrointestinal Tract
3. IL-22 in the Liver
4. IL-22 in the Skin
5. IL-22 in the Respiratory Tract
6. IL-22 in the Female Genital Tract
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sorini, C.; Cardoso, R.F.; Gagliani, N.; Villablanca, E.J. Commensal Bacteria-Specific CD4(+) T Cell Responses in Health and Disease. Front. Immunol. 2018, 9, 2667. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, F.; Schaltenberg, N.; Villablanca, E.J.; Huber, S.; Gagliani, N. Dietary Habits and Intestinal Immunity: From Food Intake to CD4(+) T H Cells. Front. Immunol. 2018, 9, 3177. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Chen, P.; Schnabl, B. Host-microbiome interactions in alcoholic liver disease. Gut Liver 2014, 8, 237–241. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalova, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e1412. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Zenewicz, L.A. IL-22: There Is a Gap in Our Knowledge. Immunohorizons 2018, 2, 198–207. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef]
- Pelczar, P.; Witkowski, M.; Garcia Perez, L.; Kempski, J.; Hammel, A.G.; Brockmann, L.; Kleinschmidt, D.; Wende, S.; Haueis, C.; Bedke, T.; et al. A pathogenic role for T-cell derived IL-22BP in inflammatory bowel disease. Science 2016, 354, 358–362. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Kempski, J.; Brockmann, L.; Gagliani, N.; Huber, S. TH17 Cell and Epithelial Cell Crosstalk during Inflammatory Bowel Disease and Carcinogenesis. Front. Immunol. 2017, 8, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Yin, X.; Wang, G.; Elinav, E.; Hao, L.; Zhao, L.; Flavell, R.A. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 2013, 190, 5306–5312. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Dumoutier, L.; Van Roost, E.; Colau, D.; Renauld, J.C. Human interleukin-10-related T cell-derived inducible factor: Molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. USA 2000, 97, 10144–10149. [Google Scholar] [CrossRef]
- Xie, M.H.; Aggarwal, S.; Ho, W.H.; Foster, J.; Zhang, Z.; Stinson, J.; Wood, W.I.; Goddard, A.D.; Gurney, A.L. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000, 275, 31335–31339. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Carcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef]
- Pearson, C.; Uhlig, H.H.; Powrie, F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012, 33, 289–296. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Trifari, S.; Kaplan, C.D.; Tran, E.H.; Crellin, N.K.; Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009, 10, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Gagliani, N.; Flavell, R.A. Life, death, and miracles: Th17 cells in the intestine. Eur. J. Immunol. 2012, 42, 2238–2245. [Google Scholar] [CrossRef]
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002, 277, 33676–33682. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Suzuki, K.; Karibe, T.; Fujii, S.; Ichikawa, K.; Tomita, S.; Imura, J.; Shiratori, K.; Chiba, T.; et al. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab. Investig. 2010, 90, 496–505. [Google Scholar] [CrossRef]
- Andoh, A.; Zhang, Z.; Inatomi, O.; Fujino, S.; Deguchi, Y.; Araki, Y.; Tsujikawa, T.; Kitoh, K.; Kim-Mitsuyama, S.; Takayanagi, A.; et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005, 129, 969–984. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Zenewicz, L.A.; Huber, F.J.; Bosurgi, L.; Hu, B.; Hedl, M.; Zhang, W.; O’Connor, W., Jr.; Murphy, A.J.; et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012, 491, 259–263. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Izotova, L.S.; Mirochnitchenko, O.V.; Esterova, E.; Dickensheets, H.; Donnelly, R.P.; Pestka, S. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 2001, 166, 7096–7103. [Google Scholar] [CrossRef]
- Kleinschmidt, D.; Giannou, A.D.; McGee, H.M.; Kempski, J.; Steglich, B.; Huber, F.J.; Ernst, T.M.; Shiri, A.M.; Wegscheid, C.; Tasika, E.; et al. A Protective Function of IL-22BP in Ischemia Reperfusion and Acetaminophen-Induced Liver Injury. J. Immunol. 2017, 199, 4078–4090. [Google Scholar] [CrossRef]
- Wei, C.C.; Ho, T.W.; Liang, W.G.; Chen, G.Y.; Chang, M.S. Cloning and characterization of mouse IL-22 binding protein. Genes Immun. 2003, 4, 204–211. [Google Scholar] [CrossRef]
- Xu, W.; Presnell, S.R.; Parrish-Novak, J.; Kindsvogel, W.; Jaspers, S.; Chen, Z.; Dillon, S.R.; Gao, Z.; Gilbert, T.; Madden, K.; et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl. Acad. Sci. USA 2001, 98, 9511–9516. [Google Scholar] [CrossRef] [PubMed]
- Dumoutier, L.; Lejeune, D.; Colau, D.; Renauld, J.C. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 2001, 166, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.C.; Logsdon, N.J.; Walter, M.R. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 2008, 16, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, E.; Hoffmann, U.; Doecke, W.D.; Endesfelder, S.; Asadullah, K.; Sterry, W.; Volk, H.D.; Wittig, B.M.; Sabat, R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: A potential systemic role of IL-22 in Crohn’s disease. J. Immunol. 2007, 178, 5973–5981. [Google Scholar] [CrossRef] [PubMed]
- Jinnohara, T.; Kanaya, T.; Hase, K.; Sakakibara, S.; Kato, T.; Tachibana, N.; Sasaki, T.; Hashimoto, Y.; Sato, T.; Watarai, H.; et al. IL-22BP dictates characteristics of Peyer’s patch follicle-associated epithelium for antigen uptake. J. Exp. Med. 2017, 214, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Beriou, G.; Heslan, M.; Bossard, C.; Jarry, A.; Abidi, A.; Hulin, P.; Menoret, S.; Thinard, R.; Anegon, I.; et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2016, 9, 539–549. [Google Scholar] [CrossRef]
- Wittkopf, N.; Neurath, M.F.; Becker, C. Immune-epithelial crosstalk at the intestinal surface. J. Gastroenterol. 2014, 49, 375–387. [Google Scholar] [CrossRef]
- Couturier-Maillard, A.; Froux, N.; Piotet-Morin, J.; Michaudel, C.; Brault, L.; Le Berichel, J.; Senechal, A.; Robinet, P.; Chenuet, P.; Jejou, S.; et al. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol. 2018, 11, 1181–1190. [Google Scholar] [CrossRef]
- Castleman, M.J.; Dillon, S.M.; Purba, C.M.; Cogswell, A.C.; Kibbie, J.J.; McCarter, M.D.; Santiago, M.L.; Barker, E.; Wilson, C.C. Commensal and Pathogenic Bacteria Indirectly Induce IL-22 but Not IFNgamma Production From Human Colonic ILC3s via Multiple Mechanisms. Front. Immunol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Seshadri, S.; Allan, D.S.J.; Carlyle, J.R.; Zenewicz, L.A. Bacillus anthracis lethal toxin negatively modulates ILC3 function through perturbation of IL-23-mediated MAPK signaling. PLoS Pathog. 2017, 13, e1006690. [Google Scholar] [CrossRef]
- Hrdy, J.; Alard, J.; Couturier-Maillard, A.; Boulard, O.; Boutillier, D.; Delacre, M.; Lapadatescu, C.; Cesaro, A.; Blanc, P.; Pot, B.; et al. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci. Rep. 2020, 10, 5345. [Google Scholar] [CrossRef]
- Pham, T.A.; Clare, S.; Goulding, D.; Arasteh, J.M.; Stares, M.D.; Browne, H.P.; Keane, J.A.; Page, A.J.; Kumasaka, N.; Kane, L.; et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 2014, 16, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Fatkhullina, A.R.; Peshkova, I.O.; Dzutsev, A.; Aghayev, T.; McCulloch, J.A.; Thovarai, V.; Badger, J.H.; Vats, R.; Sundd, P.; Tang, H.Y.; et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 2018, 49, 943–957.e949. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Kitamoto, H.; Leslie, J.L.; Kitamoto, S.; Jin, C.; Thomsson, K.A.; Gillilland, M.G., 3rd; Kuffa, P.; Goto, Y.; Jenq, R.R.; Ishii, C.; et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 2020, 26, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.; De Luca, A.; Romani, L. IL-22 in antifungal immunity. Eur. J. Immunol. 2011, 41, 270–275. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Lo, B.C.; Shin, S.B.; Canals Hernaez, D.; Refaeli, I.; Yu, H.B.; Goebeler, V.; Cait, A.; Mohn, W.W.; Vallance, B.A.; McNagny, K.M. IL-22 Preserves Gut Epithelial Integrity and Promotes Disease Remission during Chronic. J. Immunol. 2019, 202, 956–965. [Google Scholar] [CrossRef]
- Zheng, M.; Horne, W.; McAleer, J.P.; Pociask, D.; Eddens, T.; Good, M.; Gao, B.; Kolls, J.K. Therapeutic Role of Interleukin 22 in Experimental Intra-abdominal Klebsiella pneumoniae Infection in Mice. Infect. Immun. 2016, 84, 782–789. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, M.S.; Kuo, S.H.; Liao, F. IL-22 negatively regulates Helicobacter pylori-induced CCL20 expression in gastric epithelial cells. PLoS ONE 2014, 9, e97350. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Miki, T.; Holst, O.; Hardt, W.D. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J. Biol. Chem. 2012, 287, 34844–34855. [Google Scholar] [CrossRef] [PubMed]
- van Ampting, M.T.; Loonen, L.M.; Schonewille, A.J.; Konings, I.; Vink, C.; Iovanna, J.; Chamaillard, M.; Dekker, J.; van der Meer, R.; Wells, J.M.; et al. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect. Immun. 2012, 80, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Seeley, R.J. Reg3 Proteins as Gut Hormones? Endocrinology 2019, 160, 1506–1514. [Google Scholar] [CrossRef]
- Behnsen, J.; Jellbauer, S.; Wong, C.P.; Edwards, R.A.; George, M.D.; Ouyang, W.; Raffatellu, M. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 2014, 40, 262–273. [Google Scholar] [CrossRef]
- Muñoz, M.; Heimesaat, M.M.; Danker, K.; Struck, D.; Lohmann, U.; Plickert, R.; Bereswill, S.; Fischer, A.; Dunay, I.R.; Wolk, K.; et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 2009, 206, 3047–3059. [Google Scholar] [CrossRef]
- Wilson, M.S.; Feng, C.G.; Barber, D.L.; Yarovinsky, F.; Cheever, A.W.; Sher, A.; Grigg, M.; Collins, M.; Fouser, L.; Wynn, T.A. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol. 2010, 184, 4378–4390. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Greten, T.F. Gut microbiome in HCC—Mechanisms, diagnosis and therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Shen, F.; Cao, H.X.; Ding, W.J.; Chen, Y.W.; Fan, J.G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017, 7, 1529. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.N.; et al. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe 2020, 27, 25–40.e26. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Park, O.; Wang, H.; Weng, H.; Feigenbaum, L.; Li, H.; Yin, S.; Ki, S.H.; Yoo, S.H.; Dooley, S.; Wang, F.S.; et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011, 54, 252–261. [Google Scholar] [CrossRef]
- Radaeva, S.; Sun, R.; Pan, H.N.; Hong, F.; Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004, 39, 1332–1342. [Google Scholar] [CrossRef]
- Liang, S.C.; Nickerson-Nutter, C.; Pittman, D.D.; Carrier, Y.; Goodwin, D.G.; Shields, K.M.; Lambert, A.J.; Schelling, S.H.; Medley, Q.G.; Ma, H.L.; et al. IL-22 induces an acute-phase response. J. Immunol. 2010, 185, 5531–5538. [Google Scholar] [CrossRef]
- Ki, S.H.; Park, O.; Zheng, M.; Morales-Ibanez, O.; Kolls, J.K.; Bataller, R.; Gao, B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology 2010, 52, 1291–1300. [Google Scholar] [CrossRef]
- Ren, X.; Hu, B.; Colletti, L.M. IL-22 is involved in liver regeneration after hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G74–G80. [Google Scholar] [CrossRef]
- Wu, Y.; Min, J.; Ge, C.; Shu, J.; Tian, D.; Yuan, Y.; Zhou, D. Interleukin 22 in Liver Injury, Inflammation and Cancer. Int. J. Biol. Sci. 2020, 16, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Sellau, J.; Alvarado, C.F.; Hoenow, S.; Mackroth, M.S.; Kleinschmidt, D.; Huber, S.; Jacobs, T. IL-22 dampens the T cell response in experimental malaria. Sci. Rep. 2016, 6, 28058. [Google Scholar] [CrossRef] [PubMed]
- Sertorio, M.; Hou, X.; Carmo, R.F.; Dessein, H.; Cabantous, S.; Abdelwahed, M.; Romano, A.; Albuquerque, F.; Vasconcelos, L.; Carmo, T.; et al. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology 2015, 61, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Gkolfakis, P.; Dimitriadis, G.; Triantafyllou, K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis. Int. 2015, 14, 572–581. [Google Scholar] [CrossRef]
- Meighani, A.; Alimirah, M.; Ramesh, M.; Salgia, R. Fecal Microbiota Transplantation for Clostridioides Difficile Infection in Patients with Chronic Liver Disease. Int. J. Hepatol. 2020, 2020, 1874570. [Google Scholar] [CrossRef]
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Starkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef]
- Hendrikx, T.; Duan, Y.; Wang, Y.; Oh, J.H.; Alexander, L.M.; Huang, W.; Starkel, P.; Ho, S.B.; Gao, B.; Fiehn, O.; et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019, 68, 1504–1515. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Robek, M.D. Protective and pathological properties of IL-22 in liver disease: Implications for viral hepatitis. Am. J. Pathol. 2013, 182, 21–28. [Google Scholar] [CrossRef]
- Khawar, M.B.; Azam, F.; Sheikh, N.; Abdul Mujeeb, K. How Does Interleukin-22 Mediate Liver Regeneration and Prevent Injury and Fibrosis? J. Immunol. Res. 2016, 2016, 2148129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cobleigh, M.A.; Lian, J.Q.; Huang, C.X.; Booth, C.J.; Bai, X.F.; Robek, M.D. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011, 141, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, Y.K.; Zhao, Q.; Wang, C.Z.; Hu, Y.Z.; Wu, B.Y. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2012, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dambacher, J.; Beigel, F.; Zitzmann, K.; Heeg, M.H.; Goke, B.; Diepolder, H.M.; Auernhammer, C.J.; Brand, S. The role of interleukin-22 in hepatitis C virus infection. Cytokine 2008, 41, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.X.; Tang, J.; Wang, X.Y.; Wu, F.R.; Ge, J.F.; Chen, F.H. Role of interleukin-22 in liver diseases. Inflamm. Res. 2014, 63, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.H.; Pei, Z.; Blaser, M.J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Egeberg, A.; Mallbris, L.; Warren, R.B.; Bachelez, H.; Gislason, G.H.; Hansen, P.R.; Skov, L. Association between psoriasis and inflammatory bowel disease: A Danish nationwide cohort study. Br. J. Dermatol. 2016, 175, 487–492. [Google Scholar] [CrossRef]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2016, 232, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2018, 27, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 2013, 72, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, A.B.; de Heusch, M.; Lemaire, M.M.; Hendrickx, E.; Warnier, G.; Dunussi-Joannopoulos, K.; Fouser, L.A.; Renauld, J.C.; Dumoutier, L. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 2012, 188, 462–469. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Orfali, R.L.; da Silva Oliveira, L.M.; de Lima, J.F.; de Carvalho, G.C.; Ramos, Y.A.L.; Pereira, N.Z.; Pereira, N.V.; Zaniboni, M.C.; Sotto, M.N.; da Silva Duarte, A.J.; et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci. Rep. 2018, 8, 6665. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Yoon, J.; Leyva-Castillo, J.M.; Galand, C.; Archer, N.; Miller, L.S.; Geha, R.S. IL-22 derived from gammadelta T cells restricts Staphylococcus aureus infection of mechanically injured skin. J. Allergy Clin. Immunol. 2016, 138, 1098–1107 e1093. [Google Scholar] [CrossRef] [PubMed]
- Voglis, S.; Moos, S.; Kloos, L.; Wanke, F.; Zayoud, M.; Pelczar, P.; Giannou, A.D.; Pezer, S.; Albers, M.; Luessi, F.; et al. Regulation of IL-22BP in psoriasis. Sci. Rep. 2018, 8, 5085. [Google Scholar] [CrossRef]
- Lindahl, H.; Martini, E.; Brauner, S.; Nikamo, P.; Gallais Serezal, I.; Guerreiro-Cacais, A.O.; Jagodic, M.; Eidsmo, L.; Stahle, M.; Olsson, T. IL-22 binding protein regulates murine skin inflammation. Exp. Dermatol. 2017, 26, 444–446. [Google Scholar] [CrossRef]
- Fukaya, T.; Fukui, T.; Uto, T.; Takagi, H.; Nasu, J.; Miyanaga, N.; Arimura, K.; Nakamura, T.; Koseki, H.; Choijookhuu, N.; et al. Pivotal Role of IL-22 Binding Protein in the Epithelial Autoregulation of Interleukin-22 Signaling in the Control of Skin Inflammation. Front. Immunol. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Prince, A. Participation of the IL-10RB Related Cytokines, IL-22 and IFN-lambda in Defense of the Airway Mucosal Barrier. Front. Cell. Infect. Microbiol. 2020, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Renneson, J.; Fontaine, J.; Barthelemy, A.; Paget, C.; Fernandez, E.M.; Blanc, F.; De Trez, C.; Van Maele, L.; Dumoutier, L.; et al. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 2013, 87, 6911–6924. [Google Scholar] [CrossRef]
- Kumar, P.; Thakar, M.S.; Ouyang, W.; Malarkannan, S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013, 6, 69–82. [Google Scholar] [CrossRef]
- Abood, R.N.; McHugh, K.J.; Rich, H.E.; Ortiz, M.A.; Tobin, J.M.; Ramanan, K.; Robinson, K.M.; Bomberger, J.M.; Kolls, J.K.; Manni, M.L.; et al. IL-22-binding protein exacerbates influenza, bacterial super-infection. Mucosal Immunol. 2019, 12, 1231–1243. [Google Scholar] [CrossRef]
- Barthelemy, A.; Sencio, V.; Soulard, D.; Deruyter, L.; Faveeuw, C.; Le Goffic, R.; Trottein, F. Interleukin-22 Immunotherapy during Severe Influenza Enhances Lung Tissue Integrity and Reduces Secondary Bacterial Systemic Invasion. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Hebert, K.D.; McLaughlin, N.; Galeas-Pena, M.; Zhang, Z.; Eddens, T.; Govero, A.; Pilewski, J.M.; Kolls, J.K.; Pociask, D.A. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 2020, 13, 64–74. [Google Scholar] [CrossRef]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef]
- Gessner, M.A.; Werner, J.L.; Lilly, L.M.; Nelson, M.P.; Metz, A.E.; Dunaway, C.W.; Chan, Y.R.; Ouyang, W.; Brown, G.D.; Weaver, C.T.; et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect. Immun. 2012, 80, 410–417. [Google Scholar] [CrossRef]
- Rampersaud, R.; Randis, T.M.; Ratner, A.J. Microbiota of the upper and lower genital tract. Semin. Fetal Neonatal Med. 2012, 17, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Makinde, H.M.; Zariffard, R.; Mirmonsef, P.; Novak, R.M.; Jarrett, O.; Landay, A.L.; Spear, G.T. IL-22 levels are associated with Trichomonas vaginalis infection in the lower genital tract. Am. J. Reprod. Immunol. 2013, 70, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dambaeva, S.; Schneiderman, S.; Jaiswal, M.K.; Agrawal, V.; Katara, G.K.; Gilman-Sachs, A.; Hirsch, E.; Beaman, K.D. Interleukin 22 prevents lipopolysaccharide- induced preterm labor in mice. Biol. Reprod. 2018, 98, 299–308. [Google Scholar] [CrossRef]
- Male, V.; Hughes, T.; McClory, S.; Colucci, F.; Caligiuri, M.A.; Moffett, A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 2010, 185, 3913–3918. [Google Scholar] [CrossRef] [PubMed]
- Mwatelah, R.; McKinnon, L.R.; Baxter, C.; Abdool Karim, Q.; Abdool Karim, S.S. Mechanisms of sexually transmitted infection-induced inflammation in women: Implications for HIV risk. J. Int. AIDS Soc. 2019, 22 (Suppl. 6), e25346. [Google Scholar] [CrossRef] [PubMed]
- Buckner, L.R.; Amedee, A.M.; Albritton, H.L.; Kozlowski, P.A.; Lacour, N.; McGowin, C.L.; Schust, D.J.; Quayle, A.J. Chlamydia trachomatis Infection of Endocervical Epithelial Cells Enhances Early HIV Transmission Events. PLoS ONE 2016, 11, e0146663. [Google Scholar] [CrossRef]
- Jha, R.; Srivastava, P.; Salhan, S.; Finckh, A.; Gabay, C.; Mittal, A.; Bas, S. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect. 2011, 13, 167–178. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, D.; Ye, J.; Li, X.; Wang, Z.; Zhang, L.; Xu, W. The potential protective role of the combination of IL-22 and TNF-alpha against genital tract Chlamydia trachomatis infection. Cytokine 2015, 73, 66–73. [Google Scholar] [CrossRef]
- Frazer, L.C.; Scurlock, A.M.; Zurenski, M.A.; Riley, M.M.; Mintus, M.; Pociask, D.A.; Sullivan, J.E.; Andrews, C.W., Jr.; Darville, T. IL-23 induces IL-22 and IL-17 production in response to Chlamydia muridarum genital tract infection, but the absence of these cytokines does not influence disease pathogenesis. Am. J. Reprod. Immunol. 2013, 70, 472–484. [Google Scholar] [CrossRef]
- Yano, J.; Kolls, J.K.; Happel, K.I.; Wormley, F.; Wozniak, K.L.; Fidel, P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE 2012, 7, e46311. [Google Scholar] [CrossRef]
- Feinen, B.; Russell, M.W. Contrasting Roles of IL-22 and IL-17 in Murine Genital Tract Infection by Neisseria gonorrhoeae. Front. Immunol. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Doisne, J.M.; Balmas, E.; Boulenouar, S.; Gaynor, L.M.; Kieckbusch, J.; Gardner, L.; Hawkes, D.A.; Barbara, C.F.; Sharkey, A.M.; Brady, H.J.; et al. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J. Immunol. 2015, 195, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rubens, C.E.; Sadovsky, Y.; Muglia, L.; Gravett, M.G.; Lackritz, E.; Gravett, C. Prevention of preterm birth: Harnessing science to address the global epidemic. Sci. Transl. Med. 2014, 6, 262sr5. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Gotsch, F.; Pineles, B.; Kusanovic, J.P. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 2007, 65, S194–S202. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, C.O.; Murugappan, G.; Lathi, R.B. Time to next pregnancy in spontaneous pregnancies versus treatment cycles in fertile patients with recurrent pregnancy loss. Fertil Res. Pract. 2015, 1, 5. [Google Scholar] [CrossRef]
- Kamoi, M.; Fukui, A.; Kwak-Kim, J.; Fuchinoue, K.; Funamizu, A.; Chiba, H.; Yokota, M.; Fukuhara, R.; Mizunuma, H. NK22 Cells in the Uterine Mid-Secretory Endometrium and Peripheral Blood of Women with Recurrent Pregnancy Loss and Unexplained Infertility. Am. J. Reprod. Immunol. 2015, 73, 557–567. [Google Scholar] [CrossRef]
- Gilman-Sachs, A.; Dambaeva, S.; Salazar Garcia, M.D.; Hussein, Y.; Kwak-Kim, J.; Beaman, K. Inflammation induced preterm labor and birth. J. Reprod. Immunol. 2018, 129, 53–58. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Liao, B.; Qiao, J.; Pang, Y. The therapeutic effect of interleukin-22 in high androgen-induced polycystic ovary syndrome. J. Endocrinol. 2020, 245, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.; Gronke, K.; Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018, 48, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Gronke, K.; Hernandez, P.P.; Zimmermann, J.; Klose, C.S.N.; Kofoed-Branzk, M.; Guendel, F.; Witkowski, M.; Tizian, C.; Amann, L.; Schumacher, F.; et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.G.; Kempski, J.; McGee, H.M.; Pelzcar, P.; Agalioti, T.; Giannou, A.; Konczalla, L.; Brockmann, L.; Wahib, R.; Xu, H.; et al. TGF-beta signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat. Commun. 2020, 11, 2608. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Dauden, E.; Canete, J.D.; Pablos, J.L.; Martin, A.O.; Juanatey, C.G.; Adan, A.; Montalban, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969. [Google Scholar] [CrossRef]
- Feng, D.; Park, O.; Radaeva, S.; Wang, H.; Yin, S.; Kong, X.; Zheng, M.; Zakhari, S.; Kolls, J.K.; Gao, B. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int. J. Biol. Sci. 2012, 8, 249–257. [Google Scholar] [CrossRef]
- erusina Lanfranca, M.; Zhang, Y.; Girgis, A.; Kasselman, S.; Lazarus, J.; Kryczek, I.; Delrosario, L.; Rhim, A.; Koneva, L.; Sartor, M.; et al. Interleukin 22 Signaling Regulates Acinar Cell Plasticity to Promote Pancreatic Tumor Development in Mice. Gastroenterology 2020, 158, 1417–1432.e1411. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Dickson, I. Microbiome promotes pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 328. [Google Scholar] [CrossRef]
- Takayama, S.; Takahashi, H.; Matsuo, Y.; Okada, Y.; Manabe, T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepatogastroenterology 2007, 54, 2387–2391. [Google Scholar]
- Staffas, A.; Burgos da Silva, M.; van den Brink, M.R. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017, 129, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Wilson, R.; Bealmear, P.M. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat. Res. 1971, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- van Bekkum, D.W.; Roodenburg, J.; Heidt, P.J.; van der Waaij, D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J. Natl. Cancer Inst. 1974, 52, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Lampert, I.A.; Moore, R.H.; Huby, R.; Cohen, J. Observations on the role of endotoxin in graft-versus-host disease. Prog. Clin. Biol. Res. 1988, 272, 351–359. [Google Scholar] [PubMed]
- Vossen, J.M.; Guiot, H.F.; Lankester, A.C.; Vossen, A.C.; Bredius, R.G.; Wolterbeek, R.; Bakker, H.D.; Heidt, P.J. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS ONE 2014, 9, e105706. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Fredricks, D.N. The gut microbiota and graft-versus-host disease. J. Clin. Investig. 2019, 129, 1808–1817. [Google Scholar] [CrossRef]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.R.; Sun, Y.; Rossi, C.; et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Lamarthee, B.; Malard, F.; Saas, P.; Mohty, M.; Gaugler, B. Interleukin-22 in Graft-Versus-Host Disease after Allogeneic Stem Cell Transplantation. Front. Immunol. 2016, 7, 148. [Google Scholar] [CrossRef]
- Hanash, A.M.; Dudakov, J.A.; Hua, G.; O’Connor, M.H.; Young, L.F.; Singer, N.V.; West, M.L.; Jenq, R.R.; Holland, A.M.; Kappel, L.W.; et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 2012, 37, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, D.; Huang, D.; Yin, L.; Chen, C.; Pan, B.; Wu, Q.; Li, Z.; Yao, Y.; Shen, E.; et al. Interleukin-22 aggravates murine acute graft-versus-host disease by expanding effector T cell and reducing regulatory T cell. J. Interferon Cytokine Res. 2014, 34, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Munneke, J.M.; Bjorklund, A.T.; Mjosberg, J.M.; Garming-Legert, K.; Bernink, J.H.; Blom, B.; Huisman, C.; van Oers, M.H.; Spits, H.; Malmberg, K.J.; et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 2014, 124, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Bruggen, M.C.; Klein, I.; Greinix, H.; Bauer, W.; Kuzmina, Z.; Rabitsch, W.; Kalhs, P.; Petzelbauer, P.; Knobler, R.; Stingl, G.; et al. Diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood 2014, 123, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, E.; Powell, N. Group 3 ILCs: Peacekeepers or Troublemakers? What’s Your Gut Telling You?! Front. Immunol. 2019, 10, 676. [Google Scholar] [CrossRef]

| Organ (System) | IL-22-Associated Microbe | Impact of IL-22 | Impact of IL-22 on Microbiota | Cellular Source |
|---|---|---|---|---|
| Gastrointestinal Tract | Gram-positive/negative | ↑ IL-22 [39] | ILC3 | |
| Bacillus anthracis | ↓ IL-22 [40] | ILC3 | ||
| Lactobacillus spp. | ↑ IL-22 ↑ IL-17 [41] | CD4+ T cells | ||
| Il-22−/− [13] | ↑Helicobacter spp. | |||
| ↓Lactobacillus | ||||
| ↑ IL-22 [44] | ↓Clostridium difficile ↑Phascolarctobacterium spp. | |||
| ↑ IL-22 [45] | ↓Candida albicans | |||
| ↑ IL-22 [47] | ↓Citrobacter rodentium | |||
| ↑ IL-22 [58] ↑ IL-22 [59] | ↑Salmonella typhimurium ↑Toxoplasma gondii | |||
| Liver | Schistosoma spp. | ↑ IL-22 [73] | ||
| Plasmodium spp. | ↑ IL-22 [72] | γδ T cells | ||
| Christensenellaceae and Lactobacillus | ↑ IL-22 in NASH 1 [63] | |||
| Roseburia intestinalis | ↑ IL-22 in ALD 2 [64] | |||
| Hepatitis B virus | ↑ IL-22 [83] | CD4+ T cells | ||
| Hepatitis C virus | ↑ IL-22 [85] | |||
| Skin | Staphylococcus aureus | ↓ IL-22 [96] | CD4+ T cells | |
| ↑ IL-22 [96] | CD8+ T cells | |||
| ↑ IL-22 [97] | γδ T cells | |||
| Respiratory tract | Influenza A virus | ↑ IL-22 [104] | αβ T cells, γδ T cells and ILCs | |
| Female genital tract | Chlamydia trachomatis | ↑ IL-22 [112] | ||
| Trichomonas vaginalis | ↑ IL-22 [112] | |||
| Neisseria gonorrhea | ↑ IL-22 [112] | |||
| Escherichia coli | ↑ IL-22 ↑ IL-17 [123] | MAIT cells 3 | ||
| ↑ IL-22 [113] | ↓Gram-negative(LPS) 4 | uNK cells 5 | ||
| Bacteriodes vulgaris | ↓ IL-22 in PCOS 6 [130] | ILC3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabihi, M.; Böttcher, M.; Pelczar, P.; Huber, S. Microbiota-Dependent Effects of IL-22. Cells 2020, 9, 2205. https://doi.org/10.3390/cells9102205
Sabihi M, Böttcher M, Pelczar P, Huber S. Microbiota-Dependent Effects of IL-22. Cells. 2020; 9(10):2205. https://doi.org/10.3390/cells9102205
Chicago/Turabian StyleSabihi, Morsal, Marius Böttcher, Penelope Pelczar, and Samuel Huber. 2020. "Microbiota-Dependent Effects of IL-22" Cells 9, no. 10: 2205. https://doi.org/10.3390/cells9102205
APA StyleSabihi, M., Böttcher, M., Pelczar, P., & Huber, S. (2020). Microbiota-Dependent Effects of IL-22. Cells, 9(10), 2205. https://doi.org/10.3390/cells9102205







