BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Surface Marker Analysis by Flow Cytometry
2.3. Immunoblotting
2.4. Presto Blue Assay
2.5. Zymography Assay
2.6. Mammosphere Assay
2.7. Chicken Embryo in Vivo Growth Assay
2.8. Limiting Dilution Assay in the in Vivo Mice Model
2.9. Primary Breast Cancer Series
2.10. Breast Cancer Brain Metastases Series
2.11. Immunohistochemistry
2.12. Immunohistochemical Evaluation
2.13. Statistical Analysis
3. Results
3.1. Brain-Tropic Metastatic Breast Cancer Cells Show Increased Stem Cell Activity and Tumorigenic Potential
3.2. Human Breast Cancer Brain Metastases Are Enriched in BCSC Markers
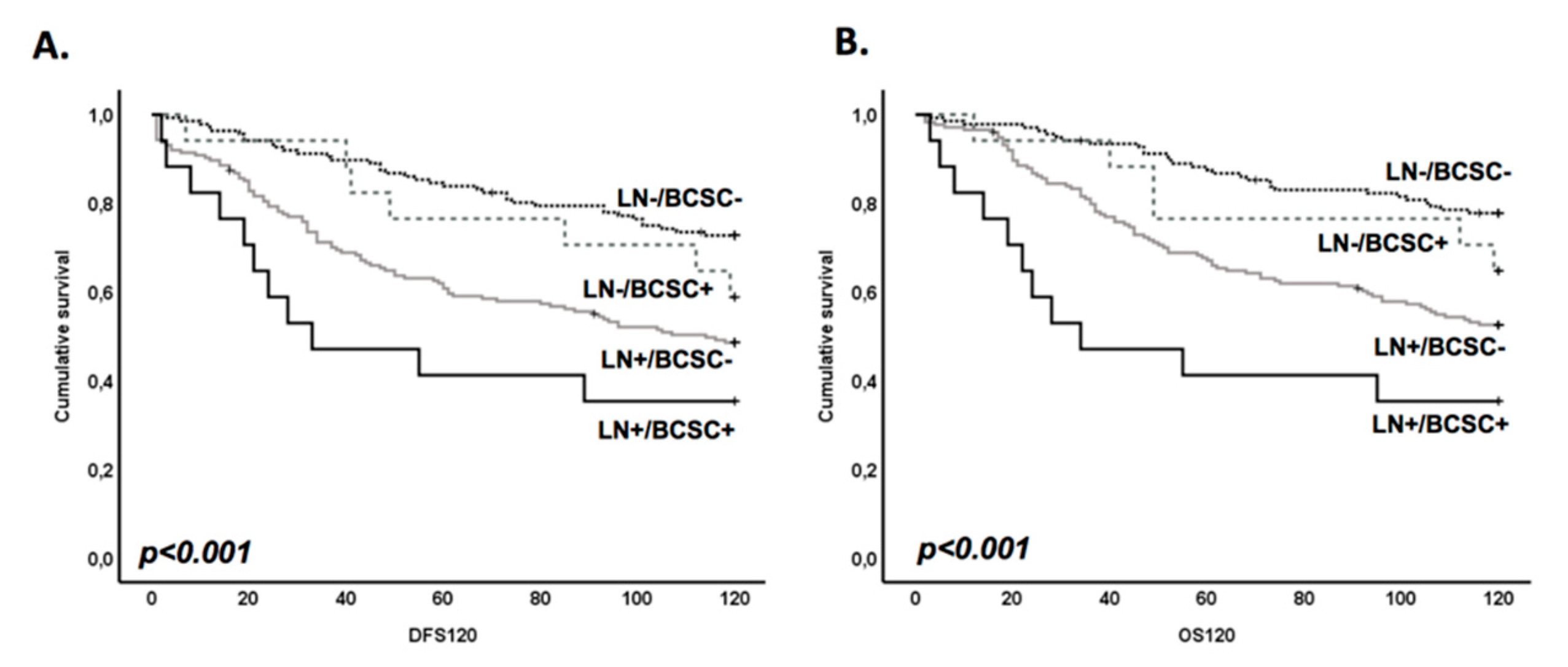
3.3. BCSC Signature Enriched in Brain Metastasis Is Significantly Associated with a Patient’s Worse Prognosis
3.4. BR-BCSC Signature Strongly Predicts a Poor Overall Survival in Lymph Node-Positive Breast Cancer Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 231.Br | MDA-MB-231.BR |
| 231.Br.HER2 | MB-231.BR.HER2 overexpressing cells |
| ALDH | Aldehyde dehydrogenase |
| BCSC | Breast cancer stem cells |
| BMFS | Brain metastases-free survival |
| BR-BCSC | Brain breast cancer stem cell signature |
| CAM | Chicken embryo chorioallantoic membrane model |
| CNS | Central nervous system |
| CSC | Cancer stem cells |
| DFS | Disease free survival |
| HER 2 | Human epidermal growth factor receptor 2 |
| MFE | Mammosphere forming efficiency |
| OS | Overall patient survival |
| REMARK | REporting recommendations for tumor MARKer prognostic studies |
| TNBC | Triple-negative breast cancer |
References
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Müller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Gonçalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar] [PubMed]
- Yau, T.; Swanton, C.; Chua, S.; Sue, A.; Walsh, G.; Rostom, A.; Johnston, S.R.; O’Brien, M.E.R.; Smith, I.E. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006, 45, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Witzel, I.; Kantelhardt, E.J.; Milde-Langosch, K.; Ihnen, M.; Zeitz, J.; Harbeck, N.; Jänicke, F.; Müller, V. Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie 2011, 34, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.A.; Isikdogan, A.; Koca, D.; Kucukoner, M.; Gumussoy, O.; Yildiz, R.; Dayan, A.; Demir, L.; Geredeli, C.; Koçer, M.; et al. Biological subtypes and survival outcomes in breast cancer patients with brain metastases (study of the Anatolian Society of Medical Oncology). Oncology 2012, 83, 141–150. [Google Scholar] [CrossRef]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2013, 36, 377–382. [Google Scholar] [CrossRef]
- McPherson, C.M.; Suki, D.; Feiz-Erfan, I.; Mahajan, A.; Chang, E.; Sawaya, R.; Lang, F.F. Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases. Neuro Oncol. 2010, 12, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Niwińska, A.; Murawska, M.; Pogoda, K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer 2010, 116, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Gao, S.L. Management of breast cancer brain metastases: Focus on human epidermal growth factor receptor 2-positive breast cancer. Chronic Dis. Transl. Med. 2017, 3, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ma, H.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y. Unraveling the roles of Cd44/Cd24 and Aldh1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef] [Green Version]
- Theodoropoulos, P.A.; Polioudaki, H.; Agelaki, S.; Kallergi, G.; Saridaki, Z.; Mavroudis, D.; Georgoulias, V. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010, 288, 99–106. [Google Scholar] [CrossRef]
- Wang, J.; Cao, M.G.; You, C.Z.; Wang, C.L.; Liu, S.L.; Kai, C.; Dou, J. A preliminary investigation of the relationship between circulating tumor cells and cancer stem cells in patients with breast cancer Cell. Mol. Biol. (Noisy-le-grand) 2012, 58, OL1641–OL1645. [Google Scholar]
- Giordano, A.; Gao, H.; Cohen, E.N.; Anfossi, S.; Khoury, J.; Hess, K.; Krishnamurthy, S.; Tin, S.; Cristofanilli, M.; Hortobagyi, G.N.; et al. Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann. Oncol. 2013, 24, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H. CD44+/CD24− Breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell 2014, 14, 306–321. [Google Scholar] [CrossRef] [Green Version]
- McGowan, P.M.; Simedrea, C.; Ribot, E.J.; Foster, P.J.; Palmieri, D.; Steeg, P.S.; Allan, A.L.; Chambers, A.F. Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Mol. Cancer Res. 2011, 9, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.M.; Foekens, J.A.; Martens, J.W.M. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef] [Green Version]
- Jhanwar-Uniyal, M.; Labagnara, M.; Friedman, M.; Kwasnicki, A.; Murali, R. Glioblastoma: Molecular pathways, stem cells and therapeutic targets. Cancers 2015, 7, 538–555. [Google Scholar] [CrossRef] [Green Version]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Doheny, D.; Zhu, D.; Aguayo, N.R.; Xing, F.; Chan, M.; Ruiz, J.; Metheny-Barlow, L.J.; et al. TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment. Oncogene 2019, 39, 64–78. [Google Scholar] [CrossRef]
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nat. Cell Biol. 2006, 439, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.; Eaves, C.J.; Zandieh, I.; Emerman, J.T. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 2001, 67, 93–109. [Google Scholar] [CrossRef]
- Eirew, P.; Stingl, J.; Raouf, A.; Turashvili, G.; Aparicio, S.; Emerman, J.T.; Eaves, C.J. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat. Med. 2008, 14, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Ribeiro, A.S.; Dionisio, M.R.; Sousa, B.; Nobre, A.R.; Albergaria, A.; Santiago-Gomez, A.; Mendes, N.; Gerhard, R.; Schmitt, F.; et al. P-cadherin signals through the laminin receptor alpha6beta4 integrin to induce stem cell and invasive properties in basal-like breast cancer cells. Oncotarget 2014, 5, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercurio, A.M.; Bachelder, R.E.; Chung, J.; O’Connor, K.L.; Rabinovitz, I.; Shaw, L.M.; Tani, T. Integrin laminin receptors and breast carcinoma progression. J. Mammary Gland. Biol. Neoplasia 2001, 6, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Rabinovitz, I.; Wang, H.H.; Toker, A.; Mercurio, A.M. Activation of phosphoinositide 3-oh kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997, 91, 949–960. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, K.; Ruiz, P.; Franke, F.; Gille, I.; Terpe, H.J.; A Imhof, B. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995, 55, 901–906. [Google Scholar]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004, 64, 5818–5824. [Google Scholar] [CrossRef] [Green Version]
- Hase, T.; Sato, M.; Yoshida, K.; Girard, L.; Takeyama, Y.; Horio, M.; Elshazley, M.; Oguri, T.; Sekido, Y.; Shames, D.S.; et al. Pivotal role of epithelial cell adhesion molecule in the survival of lung cancer cells. Cancer Sci. 2011, 102, 1493–1500. [Google Scholar] [CrossRef] [Green Version]
- Hiraga, T.; Ito, S.; Nakamura, H. EpCAM expression in breast cancer cells is associated with enhanced bone metastasis formation. Int. J. Cancer 2015, 138, 1698–1708. [Google Scholar] [CrossRef] [Green Version]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Croker, A.K.; Rodriguez-Torres, M.; Xia, Y.; Pardhan, S.; Leong, H.S.; Lewis, J.D.; Allan, A.L. Differential functional roles of ALDH1A1 and ALDH1A3 in mediating metastatic behavior and therapy resistance of human breast cancer cells. Int. J. Mol. Sci. 2017, 18, 2039. [Google Scholar] [CrossRef]
- Vieira, A.F.; Ricardo, S.; Ablett, M.P.; Dionisio, M.R.; Mendes, N.; Albergaria, A.; Farnie, G.; Gerhard, R.; Cameselle-Teijeiro, J.F.; Seruca, R.; et al. P-cadherin is co-expressed with Cd44 and Cd49f and mediates stem cell properties in basal-like breast cancer. Stem Cells 2012, 30, 854–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.-Y.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. [Google Scholar] [CrossRef]
- Paredes, J.; Albergaria, A.; Oliveira, J.T.; Jerónimo, C.; Milanezi, F.; Schmitt, F.C. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin. Cancer Res. 2005, 11, 5869–5877. [Google Scholar] [CrossRef] [Green Version]
- Paredes, J.; Lopes, N.; Milanezi, F.; Schmitt, F.C. P-cadherin and cytokeratin 5: Useful adjunct markers to distinguish basal-like ductal carcinomas in situ. Virchows Arch. 2007, 450, 73–80. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Albergaria, A.; Sousa, B.; Correia, A.L.; Bracke, M.; Seruca, R.; Schmitt, F.C.; Paredes, J. Extracellular cleavage and shedding of P-cadherin: A mechanism underlying the invasive behaviour of breast cancer cells. Oncogene 2010, 29, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.F.; Paredes, J. P-cadherin and the journey to cancer metastasis. Mol. Cancer 2015, 14, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner. Res. 2001, 16, 1486–1495. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Sousa, B.; Carreto, L.; Mendes, N.; Nobre, A.R.; Ricardo, S.; Albergaria, A.; Cameselle-Teijeiro, J.F.; Gerhard, R.; Söderberg, O.; et al. P-cadherin functional role is dependent on E-cadherin cellular context: A proof of concept using the breast cancer model. J. Pathol. 2013, 229, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef]
- Sousa, B.; Paredes, J.; Milanezi, F.; Lopes, N.; Martins, D.; Dufloth, R.; Vieira, D.; Albergaria, A.; Veronese, L.; Carneiro, V.; et al. P-cadherin, vimentin and CK14 for identification of basal-like phenotype in breast carcinomas: An immunohistochemical study. Histol. Histopathol. 2010, 25, 963–974. [Google Scholar]
- Kamal, N.M.; Salem, H.M.; Dahmoush, H.M. Immunohistochemical expression of epithelial cell adhesion molecule (EpCAM) in mucoepidermoid carcinoma compared to normal salivary gland tissues. Arch. Oral Biol. 2017, 79, 87–94. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Qu, J.; He, L.; Peng, H.; Chen, P.; Zhou, Y. Alpha 6-integrin alternative splicing: Distinct cytoplasmic variants in stem cell fate specification and niche interaction. Stem Cell Res. Ther. 2018, 9, 122–128. [Google Scholar] [CrossRef]
- Hu, Y.; Smyth, G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef]
- Li, F.; Tiede, B.; Massagué, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2006, 17, 3–14. [Google Scholar] [CrossRef]
- Morris, V.L.; Koop, S.; Macdonald, I.C.; Schmidt, E.E.; Grattan, M.; Percy, D.; Chambers, A.F.; Groom, A.C. Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin. Exp. Metastasis 1994, 12, 357–367. [Google Scholar] [CrossRef]
- Ren, D.; Zhu, X.; Kong, R.; Zhao, Z.; Sheng, J.; Wang, J.; Xu, X.; Liu, J.; Cui, K.; Zhang, X.H.-F.; et al. Targeting brain-adaptive cancer stem cells prohibits brain metastatic colonization of triple-negative breast cancer. Cancer Res. 2018, 78, 2052–2064. [Google Scholar] [CrossRef] [Green Version]
- Sousa, B.; Ribeiro, A.S.; Nobre, A.R.; Lopes, N.; Martins, D.; Pinheiro, C.; Vieira, A.F.; Albergaria, A.; Gerhard, R.; Schmitt, F.; et al. The basal epithelial marker P-cadherin associates with breast cancer cell populations harboring a glycolytic and acid-resistant phenotype. BMC Cancer 2014, 14, 734. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.S.; Paredes, J. P-cadherin linking breast cancer stem cells and invasion: A promising marker to identify an “Intermediate/Metastable” EMT state. Front. Oncol. 2014, 4, 371. [Google Scholar] [CrossRef]
- Jeevan, D.S.; Cooper, J.B.; Braun, A.; Murali, R.; Jhanwar-Uniyal, M. Molecular pathways mediating metastases to the brain via epithelial-to-mesenchymal transition: Genes, proteins, and functional analysis. Anticancer Res. 2016, 36, 523–532. [Google Scholar]
- Auvinen, P.; Tammi, R.; Kosma, V.-M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2012, 132, 531–539. [Google Scholar] [CrossRef]
- Narkhede, A.A.; Crenshaw, J.H.; Manning, R.M.; Rao, S.S. The influence of matrix stiffness on the behavior of brain metastatic breast cancer cells in a biomimetic hyaluronic acid hydrogel platform. J. Biomed. Mater. Res. Part A 2018, 106, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, inflammation, and breast cancer progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Soysal, S.D.; Muenst, S.; Barbie, T.; Fleming, T.; Gao, F.; Spizzo, G.; Oertli, D.; Viehl, C.T.; Obermann, E.C.; E Gillanders, W. EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2+, basal-like, and HER2 intrinsic subtypes of breast cancer. Br. J. Cancer 2013, 108, 1480–1487. [Google Scholar] [CrossRef]
- Chen, E.I.; Hewel, J.; Krueger, J.S.; Tiraby, C.; Weber, M.R.; Kralli, A.; Becker, K.; Yates, J.R.; Felding-Habermann, B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007, 67, 1472–1486. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra48. [Google Scholar] [CrossRef] [Green Version]
- Brennan, N.J.; Jirström, K.; Kronblad, Å.; Millikan, R.C.; Landberg, G.; Duffy, M.J.; Gallagher, W.M.; O’Brien, S.L.; Rydén, L. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin. Cancer Res. 2006, 12, 6421–6431. [Google Scholar] [CrossRef] [Green Version]




| Cell Model | 1M | 100K | 10K | 1k | Stem Cell Frequency (ELDA) | p-Value (vs 231) * | p-Value ** |
|---|---|---|---|---|---|---|---|
| 231 | 18/18 (100%) | 5/9 (55.6%) | 2/10 (20%) | 3/8 (37.5%) | 1/68769 | - | 7.31 × 10−5 |
| 231.Br | 18/18 (100%) | 10/10 (100%) | 4/10 (40%) | 3/10 (30%) | 1/12215 | 0.0007 | |
| 231.Br.HER2 | 15/15 (100%) | 10/10 (100%) | 6/10 (60%) | 1/9 (11.1%) | 1/10535 | 0.0003 |
| Breast Cancer Brain Metastases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Metastasis Free Survival | Overall Survival | |||||||||
| 10-Year BMFS | 10-Year OS | |||||||||
| Mean | Std dev | 95% CI | p-Value | Mean | Std dev | 95% CI | p-Value | |||
| Inferior | Superior | Inferior | Superior | |||||||
| CD44 | ||||||||||
| Negative (n = 7) | 68.5 | 21.8 | 25.8 | 111.3 | 0.029 | 81.5 | 16.0 | 50.1 | 112.8 | 0.045 |
| Positive (n = 49) | 33.4 | 4.5 | 24.6 | 42.2 | 53.4 | 4.7 | 44.2 | 62.6 | ||
| CD49F | ||||||||||
| Negative (n = 19) | 41.9 | 10.5 | 21.2 | 62.6 | 0.552 | 61.2 | 8.7 | 44.2 | 78.1 | 0.488 |
| Positive (n = 37) | 35.6 | 5.2 | 25.4 | 45.9 | 54.6 | 5.6 | 43.7 | 65.6 | ||
| P-cadherin | ||||||||||
| Negative (n = 27) | 41.6 | 7.1 | 27.6 | 55.6 | 0.144 | 64.7 | 7.1 | 50.8 | 78.6 | 0.067 |
| Positive (n = 27) | 30.5 | 5.4 | 19.9 | 41.1 | 47.9 | 5.9 | 36.4 | 59.4 | ||
| EPCAM | ||||||||||
| Negative (n = 27) | 46.8 | 8.3 | 30.6 | 63.1 | 0.07 | 65.0 | 7.3 | 50.7 | 79.2 | 0.091 |
| Positive (n = 29) | 29.3 | 5.3 | 19.0 | 39.6 | 49.5 | 5.8 | 38.1 | 60.9 | ||
| ALDH1 | ||||||||||
| Negative (n = 57) | 33.8 | 4.1 | 25.8 | 41.8 | 0.313 | 53.8 | 4.4 | 45.1 | 62.5 | 0.535 |
| Positive (n = 9) | 53.4 | 23.1 | 8.2 | 98.6 | 55.7 | 12.9 | 30.4 | 81.0 | ||
| BR-BCSC | ||||||||||
| 0–2 BCSS markers (n = 24) | 48.1 | 8.1 | 32.1 | 64.0 | 0.008 | 68.8 | 7.6 | 54.0 | 83.7 | 0.02 |
| 3–5 BCSS markers (n = 29) | 26.7 | 4.3 | 18.2 | 35.1 | 46.2 | 5.4 | 35.6 | 56.8 | ||
| Disease-Free Survival | Overall Survival | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Tumor | 5-Year Survival | 10-Year Survival | 5-Year Survival | 10-Year Survival | |||||||||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||||||
| Inferior | Superior | Inferior | Superior | Inferior | Superior | Inferior | Superior | ||||||||||
| Univariate analysis | LN/BR-BCSC | ||||||||||||||||
| Negative/Negative (n = 136, ref.) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | |||||||||
| Negative/Positive (n = 17) | 1.62 | 0.55 | 4.75 | 0.38 | 1.57 | 0.70 | 3.52 | 0.27 | 2.08 | 0.69 | 6.21 | 0.19 | 1.68 | 0.70 | 4.04 | 0.25 | |
| Positive/Negative (n = 174) | 2.94 | 1.78 | 4.85 | 0.00 | 2.36 | 1.61 | 3.46 | 0.00 | 3.13 | 1.79 | 5.45 | 0.00 | 2.60 | 1.71 | 3.95 | 0.00 | |
| Positive/Positive (n = 17) | 5.71 | 2.67 | 12.21 | 0.00 | 3.80 | 1.94 | 7.46 | 0.00 | 7.89 | 3.58 | 17.42 | 0.00 | 5.10 | 2.55 | 10.19 | 0.00 | |
| Multivariate analysis | LN/BR-BCSC | ||||||||||||||||
| Negative/Negative (n = 136, ref.) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | |||||||||
| Negative/Positive (n = 17) | 1.16 | 0.39 | 3.49 | 0.79 | 1.20 | 0.52 | 2.74 | 0.67 | 1.35 | 0.44 | 4.12 | 0.60 | 1.25 | 0.51 | 3.07 | 0.62 | |
| Positive/Negative (n = 174) | 2.33 | 1.35 | 4.02 | 0.00 | 1.89 | 1.25 | 2.85 | 0.00 | 2.15 | 1.19 | 3.88 | 0.01 | 1.98 | 1.27 | 3.10 | 0.00 | |
| Positive/Positive (n = 17) | 3.49 | 1.56 | 7.83 | 0.00 | 2.34 | 1.15 | 4.73 | 0.02 | 4.13 | 1.80 | 9.51 | 0.00 | 3.04 | 1.47 | 6.29 | 0.00 | |
| Histological grade | |||||||||||||||||
| Grade I (n = 54, ref.) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | |||||||||
| Grade II (n = 94) | 0.91 | 0.39 | 2.13 | 0.84 | 1.21 | 0.63 | 2.32 | 0.56 | 1.11 | 0.43 | 2.86 | 0.83 | 1.19 | 0.61 | 2.34 | 0.61 | |
| Grade III (n = 175) | 1.85 | 0.87 | 3.92 | 0.11 | 1.85 | 1.02 | 3.37 | 0.04 | 2.00 | 0.85 | 4.71 | 0.11 | 1.62 | 0.87 | 3.02 | 0.13 | |
| Tumor size | |||||||||||||||||
| T1: <2 cm (n = 82, ref.) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | |||||||||
| T2: 2–5 cm (n = 189) | 1.86 | 0.93 | 3.73 | 0.08 | 1.65 | 0.98 | 2.77 | 0.06 | 2.70 | 1.13 | 6.43 | 0.03 | 1.88 | 1.06 | 3.35 | 0.03 | |
| T3: >5 cm (n = 52) | 3.21 | 1.49 | 6.91 | 0.00 | 2.99 | 1.66 | 5.39 | 0.00 | 5.11 | 2.02 | 12.92 | 0.00 | 3.45 | 1.81 | 6.59 | 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionísio, M.R.; Vieira, A.F.; Carvalho, R.; Conde, I.; Oliveira, M.; Gomes, M.; Pinto, M.T.; Pereira, P.; Pimentel, J.; Souza, C.; et al. BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer. Cells 2020, 9, 2442. https://doi.org/10.3390/cells9112442
Dionísio MR, Vieira AF, Carvalho R, Conde I, Oliveira M, Gomes M, Pinto MT, Pereira P, Pimentel J, Souza C, et al. BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer. Cells. 2020; 9(11):2442. https://doi.org/10.3390/cells9112442
Chicago/Turabian StyleDionísio, Maria Rita, André F. Vieira, Rita Carvalho, Inês Conde, Mónica Oliveira, Madalena Gomes, Marta T. Pinto, Pedro Pereira, José Pimentel, Cristiano Souza, and et al. 2020. "BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer" Cells 9, no. 11: 2442. https://doi.org/10.3390/cells9112442