TRPM Channels in Human Diseases
Abstract
:1. Introduction
2. TRPM1/TRPM3
2.1. General Properties and Distribution
2.2. Activation and Inhibition: Endogenous Modulators
2.3. Physiological Function and Role in Diseases
3. TRPM2/TRPM8
3.1. General Properties and Distribution
3.2. Activation and Inhibition: Endogenous Modulators
3.3. Physiological Function and Role in Diseases
4. TRPM4/TRPM5
4.1. General Properties and Distribution
4.2. Activation and Inhibition: Endogenous Modulators
4.3. Physiological Function and Role in Diseases
5. TRPM6-TRPM7
5.1. General Properties and Distribution
5.2. Activation and Inhibition: Endogenous Modulators
5.3. Physiological Function and Role in Diseases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hübner, C.A.; Jentsch, T.J. Ion channel diseases. Hum. Mol. Genet. 2002, 11, 2436–2447. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, B.A.; Mery, L.; Zawar, C.; Suckow, A.; Monje, F.; Pardo, L.A.; Stühmer, W.; Flockerzi, V.; Hoth, M. Ion channels in health and disease. EMBO Rep. 2001, 2, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef] [PubMed]
- Varela, D.; Simon, F.; Riveros, A.; Jørgensen, F.; Stutzin, A. NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J. Biol. Chem. 2004, 279, 13301–13304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, F.; Varela, D.; Luisa Eguiguren, A.; Díaz, L.F.; Sala, F.; Stutzin, A.; Stutzin Hydroxyl, A. Hydroxyl radical activation of a Ca2+-sensitive nonselective cation channel involved in epithelial cell necrosis. Am. J. Physiol. 2004, 287, 963–970. [Google Scholar] [CrossRef]
- Tai, Y.S.; Yang, S.C.; Hsieh, Y.C.; Huang, Y.B.; Wu, P.C.; Tsai, M.J.; Tsai, Y.H.; Lin, M.W. A novel model for studying voltage-gated ion channel gene expression during reversible ischemic stroke. Int. J. Med. Sci. 2019, 16, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Vien, T.; Duan, J.; Sheu, S.H.; DeCaen, P.G.; Clapham, D.E. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 2018, 7, e33183. [Google Scholar] [CrossRef]
- Ko, J.H.; Ko, E.A.; Gu, W.; Lim, I.; Bang, H.; Zhou, T. Expression profiling of ion channel genes predicts clinical outcome in breast cancer. Mol. Cancer 2013, 12, 106. [Google Scholar] [CrossRef] [Green Version]
- Metz, K.A.; Teng, X.; Coppens, I.; Lamb, H.M.; Wagner, B.E.; Rosenfeld, J.A.; Chen, X.; Zhang, Y.; Kim, H.J.; Meadow, M.E.; et al. KCTD7 deficiency defines a distinct neurodegenerative disorder with a conserved autophagy-lysosome defect. Ann. Neurol. 2018, 84, 766–780. [Google Scholar] [CrossRef]
- Simon, F.; Varela, D.; Cabello-Verrugio, C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell. Signal. 2013, 25, 1614–1624. [Google Scholar] [CrossRef]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion channels as therapeutic targets: A drug discovery perspective. J. Med. Chem. 2013, 56, 593–624. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Yu, Y.; Platoshyn, O.; Zhang, S.; McDaniel, S.S.; Yuan, J.X.J. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L144–L155. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Kannan, K.B.; Livingston, D.H.; Deitch, E.A.; Fekete, Z.; Hauser, C.J. Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J. Immunol. 2002, 168, 4063–4069. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Wakamori, M.; Miyakawa, T.; Hermosura, M.; Hara, Y.; Nishida, M.; Hirose, K.; Mizushima, A.; Kurosaki, M.; Mori, E.; et al. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J. Exp. Med. 2002, 195, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Obukhov, A.G.; Nowycky, M.C. TRPC4 can be activated by G-protein-coupled receptors and provides sufficient Ca2+ to trigger exocytosis in neuroendocrine cells. J. Biol. Chem. 2002, 277, 16172–16178. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zagranichnaya, T.K.; Gurda, G.T.; Eves, E.M.; Villereal, M.L. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J. Biol. Chem. 2004, 279, 43392–43402. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Fatherazi, S.; Presland, R.B.; Belton, C.M.; Izutsu, K.T. TRPC channel expression during calcium-induced differentiation of human gingival keratinocytes. J. Dermatol. Sci. 2005, 40, 21–28. [Google Scholar] [CrossRef]
- Tabuchi, K.; Suzuki, M.; Mizuno, A.; Hara, A. Hearing impairment in TRPV4 knockout mice. Neurosci. Lett. 2005, 382, 304–308. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Cenac, N.; Altier, C.; Chapman, K.; Liedtke, W.; Zamponi, G.; Vergnolle, N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 2008, 135, 937–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koba, S.; Hayes, S.G.; Sinoway, L.I. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H201–H213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, D.F.; King, J.A.; Weber, D.; Addison, E.; Liedtke, W.; Townsley, M.I. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ. Res. 2006, 99, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, N.; Dietrich, A.; Fuchs, B.; Kalwa, H.; Ay, M.; Dumitrascu, R.; Olschewski, A.; Storch, U.; y Schnitzler, M.M.; Ghofrani, H.A.; et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc. Natl. Acad. Sci. USA 2006, 103, 19093–19098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilo, F.; Scholze, A.; Liu, D.Y.; Zidek, W.; Tepel, M. Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch. Biochem. Biophys. 2008, 471, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, D.Y.; Chung, E.S.; Oh, U.T.; Kim, S.U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J. Neurosci. 2005, 25, 662–671. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, J.M.; Falardeau, J.; Sun, M.; Kanazirska, M.; Brown, E.M.; Slaugenhaupt, S.A.; Vassilev, P.M. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett. 2002, 532, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Pocock, T.M.; Foster, R.R.; Bates, D.O. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1015–H1026. [Google Scholar] [CrossRef] [Green Version]
- Hutter, M.M.; Wick, E.C.; Day, A.L.; Maa, J.; Zerega, E.C.; Richmond, A.C.; Jordan, T.H.; Grady, E.F.; Mulvihill, S.J.; Bunnett, N.W.; et al. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R). Pancreas 2005, 30, 260–265. [Google Scholar] [CrossRef]
- Reiser, J.; Polu, K.R.; Möller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. Medicine: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Scholze, A.; Zhu, Z.; Krueger, K.; Thilo, F.; Burkert, A.; Streffer, K.; Holz, S.; Harteneck, C.; Zidek, W.; et al. Transient receptor potential channels in essential hypertension. J. Hypertens. 2006, 24, 1105–1114. [Google Scholar] [CrossRef]
- Thebault, S.; Flourakis, M.; Vanoverberghe, K.; Vandermoere, F.; Roudbaraki, M.; Lehen’kyi, V.; Slomianny, C.; Beck, B.; Mariot, P.; Bonnal, J.L.; et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006, 66, 2038–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Phillips, A.M.; Bull, A.; Kelly, L.E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 1992, 8, 631–642. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: Implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993, 16, 371–376. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Jiang, M.; Peyton, M.; Boulay, G.; Hurst, R.; Stefani, E.; Birnbaumer, L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 1996, 85, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Held, K.; Gruss, F.; Aloi, V.D.; Janssens, A.; Ulens, C.; Voets, T.; Vriens, J. Mutations in the voltage-sensing domain affect the alternative ion permeation pathway in the TRPM3 channel. J. Physiol. 2018, 596, 2413–2432. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Chubanov, V.; Gudermann, T.; Montell, C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr. Biol. 2003, 13, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Owsianik, G.; Janssens, A.; Talavera, K.; Nilius, B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007, 3, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, L.; Lee, S.Y. The role of π-helices in TRP channel gating. Curr. Opin. Struct. Biol. 2019, 58, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, U.A.; Gaudet, R. Structural biology of TRP channels. Handb. Exp. Pharmacol. 2014, 223, 963–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett. 2010, 584, 2028–2032. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 2008, 4, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Wortley, M.A.; Birrell, M.A.; Belvisi, M.G. Drugs affecting TRP channels. Handb. Exp. Pharmacol. 2017, 237, 213–241. [Google Scholar]
- Fonfria, E.; Murdock, P.R.; Cusdin, F.S.; Benham, C.D.; Kelsell, R.E.; McNulty, S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal Transduct. 2006, 26, 159–178. [Google Scholar] [CrossRef]
- Winkler, P.A.; Huang, Y.; Sun, W.; Du, J.; Lü, W. Electron cryo-microscopy structure of a human TRPM4 channel. Nature 2017, 552, 200–205. [Google Scholar] [CrossRef]
- Jiang, L.H. Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem. Soc. Trans. 2007, 35, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Perraud, A.L.; Fleig, A.; Dunn, C.A.; Bagley, L.A.; Launay, P.; Schmitz, C.; Stokes, A.J.; Zhu, Q.; Bessman, M.J.; Penner, R.; et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001, 411, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Zouharova, M.; Herman, P.; Hofbauerová, K.; Vondrasek, J.; Bousova, K. TRPM6 N-terminal CaM- and S100A1-binding domains. Int. J. Mol. Sci. 2019, 20, 4430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, Y.; Minor, D.L. X-ray Crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J. Mol. Biol. 2008, 383, 854–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mederos y Schnitzler, M.; Wäring, J.; Gudermann, T.; Chubanov, V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 2008, 22, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Hodeify, R.; Yu, F.; Courjaret, R.; Nader, N.; Dib, M.; Sun, L.; Adap, E.; Hubrack, S.; Machaca, K. Regulation and role of store-operated Ca2+ entry in cellular proliferation. In Calcium Entry Channels in Non-Excitable Cells; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–240. [Google Scholar]
- García-Ávila, M.; Islas, L.D. What is new about mild temperature sensing? A review of recent findings. Temperature 2019, 6, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Smani, T.; Gómez, L.J.; Regodon, S.; Woodard, G.E.; Siegfried, G.; Khatib, A.-M.; Rosado, J.A. TRP Channels in angiogenesis and other endothelial functions. Front. Physiol. 2018, 9, 1731. [Google Scholar] [CrossRef]
- Gao, Y.; Liao, P. TRPM4 channel and cancer. Cancer Lett. 2019, 454, 66–69. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM family channels in cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Belrose, J.C.; Jackson, M.F. TRPM2: A candidate therapeutic target for treating neurological diseases. Acta Pharmacol. Sin. 2018, 39, 722–732. [Google Scholar] [CrossRef] [Green Version]
- Gatica, S.; Villegas, V.; Vallejos, A.; Olivares, P.; Aballai, V.; Lagos-Meza, F.; Echeverria, C.; Cabello-Verrugio, C.; Varela, D.; Simon, F. TRPM7 mediates kidney injury, endothelial hyperpermeability and mortality during endotoxemia. Lab. Investig. 2020, 100, 234–249. [Google Scholar] [CrossRef]
- Lee, N.; Chen, J.; Sun, L.; Wu, S.; Gray, K.R.; Rich, A.; Huang, M.; Lin, J.-H.; Feder, J.N.; Janovitz, E.B.; et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J. Biol. Chem. 2003, 278, 20890–20897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lü, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85, 102111. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.F.J.; Drews, A.; Loch, S.; Mohr, F.; Philipp, S.E.; Lambert, S.; Oberwinkler, J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflug. Arch. 2010, 460, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Obara, T.; Uriu, Y.; Numata, T.; Sanuki, R.; Miyata, K.; Koyasu, T.; Ueno, S.; Funabiki, K.; Tani, A.; et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA 2010, 107, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [Green Version]
- Duncan, L.M.; Deeds, J.; Hunter, J.; Shao, J.; Holmgren, L.M.; Woolf, E.A.; Tepper, R.I.; Shyjan, A.W. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998, 58, 1515–1520. [Google Scholar]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.J.; Shao, J.; Smutko, J.S.; Dussault, B.J.; Nagle, D.L.; Woolf, E.A.; Holmgren, L.M.; Moore, K.J.; Shyjan, A.W. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 1998, 54, 116–123. [Google Scholar] [CrossRef]
- Bellone, R.R.; Brooks, S.A.; Sandmeyer, L.; Murphy, B.A.; Forsyth, G.; Archer, S.; Bailey, E.; Grahn, B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus). Genetics 2008, 179, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeds, J.; Cronin, F.; Duncan, L.M. Patterns of melastatin mRNA expression in melanocytic tumors. Hum. Pathol. 2000, 31, 1346–1356. [Google Scholar] [CrossRef]
- Humphray, S.J.; Oliver, K.; Hunt, A.R.; Plumb, R.W.; Loveland, J.E.; Howe, K.L.; Andrews, T.D.; Searle, S.; Hunt, S.E.; Scott, C.E.; et al. DNA sequence and analysis of human chromosome 9. Nature 2004, 429, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, M. Transient receptor potential channels in the context of nociception and pain—Recent insights into TRPM3 properties and function. Biol. Chem. 2019, 400, 917–926. [Google Scholar] [CrossRef]
- Shaham, O.; Gueta, K.; Mor, E.; Oren-Giladi, P.; Grinberg, D.; Xie, Q.; Cvekl, A.; Shomron, N.; Davis, N.; Keydar-Prizant, M.; et al. Pax6 regulates gene expression in the vertebrate lens through miR-204. PLoS Genet. 2013, 9, e1003357. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Shiels, A. TRPM3_miR-204: A complex locus for eye development and disease. Hum. Genom. 2020, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.; Grimm, C.; Kraft, R.; Goldbaum, O.; Wrede, A.; Nolte, C.; Hanisch, U.-K.; Richter-Landsberg, C.; Brück, W.; Kettenmann, H.; et al. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes: TRPM3 in oligodendrocytes. J. Neurochem. 2010, 114, 654–665. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- Son, A.R.; Yang, Y.M.; Hong, J.H.; Lee, S.I.; Shibukawa, Y.; Shin, D.M. Odontoblast TRP channels and thermo/mechanical transmission. J. Dent. Res. 2009, 88, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Pu, X.-Y.; Wang, X.-H. Distribution profiles of transient receptor potential melastatin-related and vanilloid-related channels in prostatic tissue in rat. Asian J. Androl. 2007, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Miao, F.; Jiang, S.; Shi, Y.; Luo, L.; He, X.; Wan, J.; Xu, S.; Lei, T. Role of the p53-TRPM1/miR-211-MMP9 axis in UVB-induced human melanocyte migration and its potential in repigmentation. Int. J. Mol. Med. 2020, 45, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, C.; Kraft, R.; Schultz, G.; Harteneck, C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-Sphingosine. Mol. Pharmacol. 2005, 67, 798–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhiqi, S.; Soltani, M.H.; Bhat, K.M.R.; Sangha, N.; Fang, D.; Hunter, J.J.; Setaluri, V. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res. 2004, 14, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Castorino, J.J.; Wang, Z.-X.; Maminishkis, A.; Grunwald, G.B.; Philp, N.J. Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J. Biol. Chem. 2012, 287, 20491–20503. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Slominski, A.; Yang, S.-E.; Sheehan, C.; Ross, J.; Carlson, J.A. The correlation of TRPM1 (Melastatin) mRNA expression with microphthalmia-associated transcription factor (MITF) and other melanogenesis-related proteins in normal and pathological skin, hair follicles and melanocytic nevi. J. Cutanepus Pathol. 2010, 37 (Suppl. 1), 26–40. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Ross, S.E.; Trimarchi, J.M.; Aach, J.; Greenberg, M.E.; Cepko, C.L. Identification of molecular markers of bipolar cells in the murine retina. J. Comp. Neurol. 2008, 507, 1795–1810. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Ung, D.; Khafizov, K.; Fiser, A.; Cvekl, A. Gene regulation by PAX6: Structural-functional correlations of missense mutants and transcriptional control of Trpm3/miR-204. Mol. Vis. 2014, 20, 270–282. [Google Scholar]
- Morgans, C.W.; Brown, R.L.; Duvoisin, R.M. TRPM1: The endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays 2010, 32, 609–614. [Google Scholar] [CrossRef]
- Popova, E. Ionotropic GABA receptors and distal retinal ON and OFF responses. Scientifica 2014, 2014, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signalling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesch, A.; Rössler, O.G.; Thiel, G. Extracellular signal-regulated protein kinase, c-Jun N-terminal protein kinase, and calcineurin regulate transient receptor potential M3 (TRPM3) induced activation of AP-1. J. Cell. Biochem. 2017, 118, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Lesch, A.; Rubil, S.; Thiel, G. Activation and inhibition of transient receptor potential TRPM3-induced gene transcription. Br. J. Pharmacol. 2014, 171, 2645–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, S.I.; Müller, I.; Mannebach, S.; Endo, T.; Thiel, G. Signal transduction of pregnenolone sulfate in insulinoma cells: Activation of Egr-1 expression involving TRPM3, voltage-gated calcium channels, ERK, and ternary complex factors. J. Biol. Chem. 2011, 286, 10084–10096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, J.; Li, J.; Milligan, C.J.; Zeng, F.; Sukumar, P.; Hou, B.; Sedo, A.; Yuldasheva, N.; Majeed, Y.; Beri, D.; et al. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 2010, 106, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, O.; da Costa, R.; Gentry, C.; Quallo, T.; Bevan, S.; Andersson, D.A. Promiscuous G-protein-coupled receptor inhibition of transient receptor potential melastatin 3 ion channels by Gβγ subunits. J. Neurosci. 2019, 39, 7840–7852. [Google Scholar] [CrossRef] [Green Version]
- Badheka, D.; Yudin, Y.; Borbiro, I.; Hartle, C.M.; Yazici, A.; Mirshahi, T.; Rohacs, T. Inhibition of transient receptor potential melastatin 3 ion channels by G-protein βγ subunits. eLife 2017, 6, e26147. [Google Scholar] [CrossRef]
- Quallo, T.; Alkhatib, O.; Gentry, C.; Andersson, D.A.; Bevan, S. G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. eLife 2017, 6, e26138. [Google Scholar] [CrossRef] [Green Version]
- Dembla, S.; Behrendt, M.; Mohr, F.; Goecke, C.; Sondermann, J.; Schneider, F.M.; Schmidt, M.; Stab, J.; Enzeroth, R.; Leitner, M.G.; et al. Anti-nociceptive action of peripheral mu-opioid receptors by G-beta-gamma protein-mediated inhibition of TRPM3 channels. eLife 2017, 6, e26280. [Google Scholar] [CrossRef]
- Lambert, S.; Drews, A.; Rizun, O.; Wagner, T.F.J.; Lis, A.; Mannebach, S.; Plant, S.; Portz, M.; Meissner, M.; Philipp, S.E.; et al. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J. Biol. Chem. 2011, 286, 12221–12233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, S.; Markandeya, Y.; Maddodi, N.; Dhingra, A.; Vardi, N.; Balijepalli, R.C.; Setaluri, V. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment Cell Melanoma Res. 2013, 26, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandmeyer, L.S.; Bellone, R.R.; Archer, S.; Bauer, B.S.; Nelson, J.; Forsyth, G.; Grahn, B.H. Congenital stationary night blindness is associated with the leopard complex in the miniature horse. Vet. Ophthalmol. 2012, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yasukochi, Y.; Kato, K.; Oguri, M.; Horibe, H.; Fujimaki, T.; Takeuchi, I.; Sakuma, J. Identification of 26 novel loci that confer susceptibility to early-onset coronary artery disease in a Japanese population. Biomed. Repos. 2018, 9, 383–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, K.; Voets, T.; Vriens, J. TRPM3 in temperature sensing and beyond. Temperature 2015, 2, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, K.; Kichko, T.; De Clercq, K.; Klaassen, H.; Van Bree, R.; Vanherck, J.-C.; Marchand, A.; Reeh, P.W.; Chaltin, P.; Voets, T.; et al. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc. Natl. Acad. Sci. USA 2015, 112, E1363–E1372. [Google Scholar] [CrossRef] [Green Version]
- Vriens, J.; Voets, T. Heat sensing involves a TRiPlet of ion channels. Br. J. Pharm. 2019, 176, 3893–3898. [Google Scholar] [CrossRef]
- Paricio-Montesinos, R.; Schwaller, F.; Udhayachandran, A.; Rau, F.; Walcher, J.; Evangelista, R.; Vriens, J.; Voets, T.; Poulet, J.F.A.; Lewin, G.R. The sensory coding of warm perception. Neuron 2020, 106, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Rubil, S.; Lesch, A.; Mukaida, N.; Thiel, G. Stimulation of transient receptor potential M3 (TRPM3) channels increases interleukin-8 gene promoter activity involving AP-1 and extracellular signal-regulated protein kinase. Cytokine 2018, 103, 133–141. [Google Scholar] [CrossRef]
- Alonso-Carbajo, L.; Alpizar, Y.A.; Startek, J.B.; López-López, J.R.; Pérez-García, M.T.; Talavera, K. Activation of the cation channel TRPM3 in perivascular nerves induces vasodilation of resistance arteries. J. Mol. Cell. Cardiol. 2019, 129, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Okumus, S.; Demiryürek, S.; Gürler, B.; Coskun, E.; Bozgeyik, I.; Oztuzcu, S.; Kaydu, E.; Celik, O.; Erbagcı, I.; Demiryürek, A.T. Association transient receptor potential melastatin channel gene polymorphism with primary open angle glaucoma. Mol. Vis. 2013, 19, 1852–1858. [Google Scholar] [PubMed]
- Bennett, T.M.; Mackay, D.S.; Siegfried, C.J.; Shiels, A. Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS ONE 2014, 9, e104000. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Molecular regulations and functions of the transient receptor potential channels of the islets of langerhans and insulinoma cells. Cells 2020, 9, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyment, D.A.; Terhal, P.A.; Rustad, C.F.; Tveten, K.; Griffith, C.; Jayakar, P.; Shinawi, M.; Ellingwood, S.; Smith, R.; van Gassen, K.; et al. De novo substitutions of TRPM3 cause intellectual disability and epilepsy. Eur. J. Hum. Genet. 2019, 27, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yudin, Y.; Rohacs, T. Disease-associated mutations in the human TRPM3 render the channel overactive via two distinct mechanisms. eLife 2020, 9, e55634. [Google Scholar] [CrossRef]
- Van Hoeymissen, E.; Held, K.; Nogueira Freitas, A.C.; Janssens, A.; Voets, T.; Vriens, J. Gain of channel function and modified gating properties in TRPM3 mutants causing intellectual disability and epilepsy. eLife 2020, 9, e57190. [Google Scholar] [CrossRef]
- Santoni, G.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Marinelli, O.; Amantini, C. Targeting transient receptor potential channels by MicroRNAs drives tumor development and progression. Adv. Exp. Med Biol. 2020, 1131, 605–623. [Google Scholar] [CrossRef]
- Cao, Q.; Bai, P. Role of autophagy in renal cancer. J. Cancer 2019, 10, 2501–2509. [Google Scholar] [CrossRef] [Green Version]
- Santoni, G.; Maggi, F.; Morelli, M.B.; Santoni, M.; Marinelli, O. Transient receptor potential cation channels in cancer therapy. Med. Sci. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacigalupa, Z.A.; Rathmell, W.K. Beyond glycolysis: Hypoxia signaling as a master regulator of alternative metabolic pathways and the implications in clear cell renal cell carcinoma. Cancer Lett. 2020, 489, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.R. Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell. Mol. Life Sci. 2016, 73, 775–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotze, M.T.; Maranchie, J.; Appleman, L. Inhibiting autophagy: A novel approach for the treatment of renal cell carcinoma. Cancer J. 2013, 19, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Zhang, D.; Luo, W.; Liu, R.; Xu, D.; Diao, L.; Liao, L.; Liu, Z. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat. Commun. 2018, 9, 3492. [Google Scholar] [CrossRef]
- Wong, P.-M.; Puente, C.; Ganley, I.G.; Jiang, X. The ULK1 complex: Sensing nutrient signals for autophagy activation. Autophagy 2013, 9, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, F.; Jäättelä, M. Targeting ions-induced autophagy in cancer. Cancer Cell 2014, 26, 599–600. [Google Scholar] [CrossRef] [Green Version]
- Weindling, A.M.; Bamford, F.N.; Whittall, R.A. Health of juvenile delinquents. Br. Med. J. 1986, 292, 447. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.P.; Cost, N.G.; Hegde, S.; Kellner, E.; Mikhaylova, O.; Stratton, Y.; Ehmer, B.; Abplanalp, W.A.; Pandey, R.; Biesiada, J.; et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014, 26, 738–753. [Google Scholar] [CrossRef] [Green Version]
- Cost, N.G.; Czyzyk-Krzeska, M.F. Regulation of autophagy by two products of one gene: TRPM3 and miR-204. Mol. Cell. Oncol. 2015, 2, e1002712. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Setaluri, V. Expression and Up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem. Biophys. Res. Commun. 2000, 279, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; von Bohlen Und Halbach, O.; Hadler, M.D.; Harteneck, C.; Albrecht, D. A novel form of capsaicin-modified amygdala LTD mediated by TRPM1. Neurobiol. Learn. Mem. 2016, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.-H.; Brown, R.L.; Reed, B.; Burke, N.S.; Duvoisin, R.M.; Morgans, C.W. Voriconazole, an antifungal triazol that causes visual side effects, is an inhibitor of TRPM1 and TRPM3 channels. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.; Lee, T.H.; Lee, W.-J.; Shim, W.-S.; Yeo, E.-J.; Kim, S.; Kim, S.Y. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell. Melanoma Res. 2016, 29, 81–91. [Google Scholar] [CrossRef]
- Wagner, T.F.J.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Düfer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar] [CrossRef]
- Vriens, J.; Held, K.; Janssens, A.; Tóth, B.I.; Kerselaers, S.; Nilius, B.; Vennekens, R.; Voets, T. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat. Chem. Biol. 2014, 10, 188–195. [Google Scholar] [CrossRef]
- Majeed, Y.; Tumova, S.; Green, B.L.; Seymour, V.A.L.; Woods, D.M.; Agarwal, A.K.; Naylor, J.; Jiang, S.; Picton, H.M.; Porter, K.E.; et al. Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium 2012, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Meyerhof, W.; Harteneck, C. Fenamates as TRP channel blockers: Mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [Google Scholar] [CrossRef] [Green Version]
- Straub, I.; Mohr, F.; Stab, J.; Konrad, M.; Philipp, S.E.; Oberwinkler, J.; Schaefer, M. Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br. J. Pharmacol. 2013, 168, 1835–1850. [Google Scholar] [CrossRef] [Green Version]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Bidaux, G.; Sgobba, M.; Lemonnier, L.; Borowiec, A.S.; Noyer, L.; Jovanovic, S.; Zholos, A.V.; Haider, S. Functional and modeling studies of the transmembrane region of the TRPM8 channel. Biophys. J. 2015, 109, 1840–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tóth, B.; Szollosi, A.; Chen, J.; Csanády, L. Structure of a TRPM2 channel in complex with Ca2+ explains unique gating regulation. eLife 2018, 7, e36409. [Google Scholar] [CrossRef]
- Hasan, R.; Zhang, X. Ca2+ regulation of TRP ion channels. Int. J. Mol. Sci. 2018, 19, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouron, A.; Kiselyov, K.; Oberwinkler, J. Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflug. Arch. 2015, 467, 1143–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, R.; Mei, Z.Z.; Mao, H.J.; Yang, W.; Dong, L.; Bradley, H.; Beech, D.J.; Jiang, L.H. Identification of pore residues engaged in determining divalent cationic permeation in transient receptor potential melastatin subtype channel 2. J. Biol. Chem. 2008, 283, 27426–27432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earley, S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation 2010, 17, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.A.; Skryma, R.; Bidaux, G.; Magleby, K.L.; Scholfeld, C.N.; McGeown, J.G.; Prevarskaya, N.; Zholos, A.V. Voltage-and cold-dependent gating of single TRPM8 ion channels. J. Gen. Physiol. 2011, 137, 173–195. [Google Scholar] [CrossRef] [Green Version]
- Raddatz, N.; Castillo, J.P.; Gonzalez, C.; Alvarez, O.; Latorre, R. Temperature and voltage coupling to channel opening in transient receptor potential melastatin 8 (TRPM8). J. Biol. Chem. 2014, 289, 35438–35454. [Google Scholar] [CrossRef] [Green Version]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signaling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef]
- Nagamine, K.; Kudoh, J.; Minoshima, S.; Kawasaki, K.; Asakawa, S.; Ito, F.; Shimizu, N. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 1998, 54, 124–131. [Google Scholar] [CrossRef]
- Grolez, G.P.; Gkika, D. TRPM8 puts the chill on prostate cancer. Pharmaceuticals 2016, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Jara-Oseguera, A.; Swartz, K.J. TRPM channels come into focus. Science 2018, 359, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Inamura, K.; Miyake, A.; Mochizuki, S.; Yokoi, H.; Matsushime, H.; Furuichi, K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001, 293, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.B.; Gaudet, R. The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J. Biol. Chem. 2007, 282, 36474–36480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruda, P.R.; Julius, D.; Minor, D.L. Coiled coils direct assembly of a cold-activated TRP channel. Neuron 2006, 51, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolisek, M.; Beck, A.; Fleig, A.; Penner, R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 2005, 18, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fliegert, R.; Watt, J.M.; Schöbel, A.; Rozewitz, M.D.; Moreau, C.; Kirchberger, T.; Thomas, M.P.; Sick, W.; Araujo, A.C.; Harneit, A.; et al. Ligand-induced activation of human TRPM2 requires the terminal ribose of ADPR and involves Arg1433 and Tyr1349. Biochem. J. 2017, 474, 2159–2175. [Google Scholar] [CrossRef]
- McNulty, S.; Fonfria, E. The role of TRPM channels in cell death. Pflug. Arch. 2005, 451, 235–242. [Google Scholar] [CrossRef]
- Cheung, J.Y.; Miller, B.A. Transient receptor potential-melastatin channel family member 2: Friend or foe. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 308–329. [Google Scholar] [PubMed]
- Di, A.; Gao, X.P.; Qian, F.; Kawamura, T.; Han, J.; Hecquet, C.; Ye, R.D.; Vogel, S.M.; Malik, A.B. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 2012, 13, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubisha, O.; Rafty, L.A.; Takanishi, C.L.; Xu, X.; Tong, L.; Perraud, A.L.; Scharenberg, A.M.; Denu, J.M. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J. Biol. Chem. 2006, 281, 14057–14065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiner, I.; Eisfeld, J.; Warnstedt, M.; Radukina, N.; Jüngling, E.; Lückhoff, A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem. J. 2006, 398, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.H.; Li, X.; Syed Mortadza, S.A.; Lovatt, M.; Yang, W. The TRPM2 channel nexus from oxidative damage to Alzheimer’s pathologies: An emerging novel intervention target for age-related dementia. Ageing Res Rev. 2018, 47, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Bauer, I.; Poggi, A.; Mannino, E.; Sturla, L.; Brini, M.; Zocchi, E.; De Flora, A.; Nencioni, A.; Bruzzone, S. NAD+ levels control Ca2+ store replenishment and mitogen-induced increase of cytosolic Ca2+ by cyclic ADP-ribose-dependent TRPM2 channel gating in human T lymphocytes. J. Biol. Chem. 2012, 287, 21067–21081. [Google Scholar] [CrossRef] [Green Version]
- Kheradpezhouh, E.; Barritt, G.J.; Rychkov, G.Y. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Togashi, K.; Inada, H.; Tominaga, M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br. J. Pharmacol. 2008, 153, 1324–1330. [Google Scholar] [CrossRef]
- Kraft, R.; Grimm, C.; Frenzel, H.; Harteneck, C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br. J. Pharmacol. 2006, 148, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.; McNulty, S.; Randall, A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 227–237. [Google Scholar] [CrossRef]
- Hill, K.; Benham, C.D.; McNulty, S.; Randall, A.D. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 2004, 47, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.E.; Jackson, M.F.; Li, H.; Perez, Y.; Sun, H.S.; Kiyonaka, S.; Mori, Y.; Tymianski, M.; Macdonald, J.F. Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J. Physiol. 2009, 587, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, J.; Lückhoff, A. TRPM2. Handb. Exp. Pharmacol. 2007, 179, 237–252. [Google Scholar] [CrossRef]
- Starkus, J.G.; Poerzgen, P.; Layugan, K.; Kawabata, K.G.; Goto, J.I.; Suzuki, S.; Myers, G.; Kelly, M.; Penner, R.; Fleig, A.; et al. Scalaradial is a potent inhibitor of transient receptor potential melastatin 2 (TRPM2) ion channels. J. Nat. Prod. 2017, 80, 2741–2750. [Google Scholar] [CrossRef] [PubMed]
- Fonfria, E.; Marshall, I.C.B.; Benham, C.D.; Boyfield, I.; Brown, J.D.; Hill, K.; Hughes, J.P.; Skaper, S.D.; McNulty, S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br. J. Pharmacol. 2004, 143, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharate, S.S.; Bharate, S.B. Modulation of thermoreceptor TRPM8 by cooling compounds. ACS Chem. Neurosci. 2012, 3, 248–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudin, Y.; Rohacs, T. Regulation of TRPM8 channel activity. Mol. Cell. Endocrinol. 2012, 353, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccoy, D.D.; Knowlton, W.M.; Mckemy, D.D. Scraping through the ice: Uncovering the role of TRPM8 in cold transduction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1278–1287. [Google Scholar] [CrossRef] [Green Version]
- Sherkheli, M.A.; Vogt-Eisele, A.K.; Bura, D.; Beltrán Márques, L.R.; Gisselmann, G.; Hatt, H. Characterization of selective trpm8 l igands and their structureactivity response (S.A.R) relationship. J. Pharm. Pharm. Sci. 2010, 13, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.-Y. Structure of the cold-and menthol-sensing ion channel TRPM8 HHS public access. Science 2018, 359, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Bertamino, A.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Soldovieri, M.V.; Iraci, N.; Fernandez Carvajal, A.; De La Torre-Martinez, R.; et al. Tryptamine-based derivatives as transient receptor potential melastatin type 8 (TRPM8) channel modulators. J. Med. Chem. 2016, 59, 2179–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, C.; Russo, R.; Avagliano, C.; Cristiano, C.; Calignano, A.; Aramini, A.; Bianchini, G.; Allegretti, M.; Brandolini, L. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br. J. Pharmacol. 2018, 175, 1691–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of New TRPM8 modulators in pain perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashinger, E.S.R.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Ren. Physiol. 2008, 295, F803–F810. [Google Scholar] [CrossRef]
- Thiel, G.; Backes, T.M.; Welck, J.; Steinhausen, S.; Fischer, A.L.; Langfermann, D.S.; Ulrich, M.; Wissenbach, U.; Rössler, O.G. Pharmacological inhibition of TRPM8-induced gene transcription. Biochem. Pharmacol. 2019, 170, 113678. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef]
- Zhang, W.; Hirschler-Laszkiewicz, I.; Tong, Q.; Conrad, K.; Sun, S.C.; Penn, L.; Barber, D.L.; Stahl, R.; Carey, D.J.; Cheung, J.Y.; et al. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am. J. Physiol. Cell Physiol. 2006, 290, C1146–C1159. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wang, K.; Da, Y.; Zhou, M.; Yan, H.; Zheng, D.; Zhong, S.; Cai, S.; Zhu, H.; et al. Suppression of TRPM2 reduces renal fibrosis and inflammation through blocking TGF-β1-regulated JNK activation. Biomed. Pharmacother. 2019, 120, 109556. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, Y.; Hu, H.; Zheng, Q.; Luo, X.; Ling, Y.; Ying, Y.; Shen, Z.; Jiang, P.; Shu, Q. TRPM2 channel regulates cytokines production in astrocytes and aggravates brain disorder during lipopolysaccharide-induced endotoxin sepsis. Int. Immunopharmacol. 2019, 75, 105836. [Google Scholar] [CrossRef]
- Ostapchenko, V.G.; Chen, M.; Guzman, M.S.; Xie, Y.F.; Lavine, N.; Fan, J.; Beraldo, F.H.; Martyn, A.C.; Belrose, J.C.; Mori, Y.; et al. The transient receptor potential melastatin 2 (TRPM2) channel contributes to β-amyloid oligomer-related neurotoxicity and memory impairment. J. Neurosci. 2015, 35, 15157–15169. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jackson, M.F.; Xie, Y.F. Glia and TRPM2 channels in plasticity of central nervous system and Alzheimer’s diseases. Neural Plast. 2016, 2016, 1680905. [Google Scholar] [CrossRef] [Green Version]
- Eraslan, E.; Tanyeli, A.; Polat, E.; Polat, E. 8-Br-cADPR, a TRPM2 ion channel antagonist, inhibits renal ischemia–reperfusion injury. J. Cell. Physiol. 2019, 234, 4572–4581. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2012, 16, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; De Castro Reis, F.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Olsen, R.V.; Møller, H.G.; Eskelund, P.W.; Gazerani, P.; Arendt-Nielsen, L. A review of topical high-concentration L-menthol as a translational model of cold allodynia and hyperalgesia. Eur. J. Pain 2014, 18, 315–325. [Google Scholar] [CrossRef]
- Brosseau, L.; Yonge, K.; Welch, V.; Marchand, S.; Judd, M.; Wells, G.A.; Tugwell, P. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst. Rev. 2003, 2003, CD004522. [Google Scholar] [CrossRef]
- Nadler, S.F.; Weingand, K.; Kruse, R.J. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 2004, 7, 395–399. [Google Scholar]
- Eccles, R. Menthol and Related Cooling Compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Menthol: A refreshing look at this ancient compound. J. Am. Acad. Dermatol. 2007, 57, 873–878. [Google Scholar] [CrossRef]
- Dussor, G.; Cao, Y.Q. TRPM8 and migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar] [PubMed]
- Henshall, S.M.; Afar, D.E.H.; Hiller, J.; Horvath, L.G.; Quinn, D.I.; Rasiah, K.K.; Gish, K.; Willhite, D.; Kench, J.G.; Gardiner-Garden, M.; et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003, 63, 4196–4203. [Google Scholar] [PubMed]
- Grolez, G.P.; Gordiendko, D.V.; Clarisse, M.; Hammadi, M.; Desruelles, E.; Fromont, G.; Prevarskaya, N.; Slomianny, C.; Gkika, D. TRPM8-androgen receptor association within lipid rafts promotes prostate cancer cell migration. Cell Death Dis. 2019, 10, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-H.; Wang, X.-H.; Wang, H.-P.; Hu, L.-Q. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J. Androl. 2009, 11, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017, 216, 2107–2130. [Google Scholar] [CrossRef] [Green Version]
- Grolez, G.P.; Hammadi, M.; Barras, A.; Gordienko, D.; Slomianny, C.; Völkel, P.; Angrand, P.O.; Pinault, M.; Guimaraes, C.; Potier-Cartereau, M.; et al. Encapsulation of a TRPM8 Agonist, WS12, in lipid nanocapsules potentiates PC3 prostate cancer cell migration inhibition through channel activation. Sci. Rep. 2019, 9, 7926. [Google Scholar] [CrossRef]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef]
- Robledo-Avila, F.H.; Ruiz-Rosado, J.d.D.; Brockman, K.L.; Partida-Sánchez, S. The TRPM2 Ion channel regulates inflammatory functions of neutrophils during listeria monocytogenes infection. Front. Immunol. 2020, 11, 97. [Google Scholar] [CrossRef]
- Korenaga, D.; Takesue, F.; Kido, K.; Yasuda, M.; Inutsuka, S.; Honda, M.; Nagahama, S. Impaired antioxidant defense system of colonic tissue and cancer development in dextran sulfate sodium-induced colitis in mice. J. Surg. Res. 2002, 102, 144–149. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null Mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partida-Sanchez, S.; Gasser, A.; Fliegert, R.; Siebrands, C.C.; Dammermann, W.; Shi, G.; Mousseau, B.J.; Sumoza-Toledo, A.; Bhagat, H.; Walseth, T.F.; et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by ADP-ribose, the major product generated by the CD38 enzyme reaction. J. Immunol. 2007, 179, 7827–7839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Li, M.; Zhan, K.; Yang, W.; Zhang, L.; Wang, K.W.; Yu, P.; Zhang, L. Selective inhibition of TRPM2 channel by two novel synthesized ADPR analogues. Chem. Biol. Drug Des. 2018, 91, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Dietz, R.M.; Cruz-Torres, I.; Strnad, F.; Garske, A.K.; Moreno, M.; Venna, V.R.; Quillinan, N.; Herson, P.S. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Exp. Neurol. 2016, 283, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Gasser, A.; Glassmeier, G.; Fliegert, R.; Langhorst, M.F.; Meinke, S.; Hein, D.; Krüger, S.; Weber, K.; Heiner, I.; Oppenheimer, N.; et al. Activation of T cell calcium influx by the second messenger ADP-ribose. J. Biol. Chem. 2006, 281, 2489–2496. [Google Scholar] [CrossRef] [Green Version]
- Wehage, E.; Eisfeld, J.; Heiner, I.; Jüngling, E.; Zitt, C.; Lückhoff, A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J. Biol. Chem. 2002, 277, 23150–23156. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Xie, J.; Yue, L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J. Gen. Physiol. 2009, 134, 471–488. [Google Scholar] [CrossRef] [Green Version]
- Starkus, J.G.; Fleig, A.; Penner, R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J. Physiol. 2010, 588, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Wehrhahn, J.; Kraft, R.; Harteneck, C.; Hauschildt, S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J. Immunol. 2010, 184, 2386–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Shimizu, S.; Kiyonaka, S.; Takahashi, N.; Wajima, T.; Hara, Y.; Negoro, T.; Hiroi, T.; Kiuchi, Y.; Okada, T.; et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008, 14, 738–747. [Google Scholar] [CrossRef]
- Liu, B.; Qin, F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005, 25, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Aroke, E.N.; Powell-Roach, K.L.; Jaime-Lara, R.B.; Tesfaye, M.; Roy, A.; Jackson, P.; Joseph, P.V. Taste the Pain: The Role of TRP Channels in Pain and Taste Perception. Int. J. Mol. Sci. 2020, 21, 5929. [Google Scholar] [CrossRef] [PubMed]
- Simard, C.; Hof, T.; Keddache, Z.; Launay, P.; Guinamard, R. The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J. Mol. Cell. Cardiol. 2013, 59, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Launay, P.; Fleig, A.; Perraud, A.L.; Scharenberg, A.M.; Penner, R.; Kinet, J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Li, Z.; Li, J.; Santa-Cruz, A.; Sanchez-Martinez, S.; Zhang, J.; Clapham, D.E. Structure of full-length human TRPM4. Proc. Natl. Acad. Sci. USA 2018, 115, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yu, Y.; Yang, J. Structural biology of TRP channels. In Transient Receptor Potential Channels; Springer: Dordrecht, The Netherlands, 2011; Volume 704, pp. 1–23. [Google Scholar]
- Autzen, H.E.; Myasnikov, A.G.; Campbell, M.G.; Asarnow, D.; Julius, D.; Cheng, Y. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 2018, 359, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, N.D.; Voets, T.; Prenen, J.; Vennekens, R.; Talavera, K.; Droogmans, G.; Nilius, B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 2005, 37, 267–278. [Google Scholar] [CrossRef]
- Prawitt, D.; Enklaar, T.; Klemm, G.; Gärtner, B.; Spangenberg, C.; Winterpacht, A.; Higgins, M.; Pelletier, J.; Zabel, B. Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum. Mol. Genet. 2000, 9, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilius, B.; Prenen, J.; Tang, J.; Wang, C.; Owsianik, G.; Janssens, A.; Voets, T.; Zhu, M.X. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 2005, 280, 6423–6433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousova, K.; Jirku, M.; Bumba, L.; Bednarova, L.; Sulc, M.; Franek, M.; Vyklicky, L.; Vondrasek, J.; Teisinger, J. PIP2 and PIP3 interact with N-terminus region of TRPM4 channel. Biophys. Chem. 2015, 205, 24–32. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Voets, T.; Droogmans, G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflüg. Arch. 2004, 448, 70–75. [Google Scholar] [CrossRef]
- Wilsher, N.E.; Court, W.J.; Ruddle, R.; Newbatt, Y.M.; Aherne, W.; Sheldrake, P.W.; Jones, N.P.; Katan, M.; Eccles, S.A.; Raynaud, F.I. The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17beta-3-methoxyestra-1,3,5-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug Metab. Dispos. 2007, 35, 1017–1022. [Google Scholar] [CrossRef]
- Leitner, M.G.; Michel, N.; Behrendt, M.; Dierich, M.; Dembla, S.; Wilke, B.U.; Konrad, M.; Lindner, M.; Oberwinkler, J.; Oliver, D. Direct modulation of TRPM4 and TRPM3 channels by the phospholipase C inhibitor U73122. Br. J. Pharmacol. 2016, 173, 2555–2569. [Google Scholar] [CrossRef]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, Z.; Liman, E.R. Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3-S4 and S5-S6 extracellular domains. J. Biol. Chem. 2005, 280, 20691–20699. [Google Scholar] [CrossRef] [Green Version]
- Guinamard, R.; Simard, C.; Del Negro, C. Flufenamic acid as an ion channel modulator. Pharmacol. Ther. 2013, 138, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grand, T.; Demion, M.; Norez, C.; Mettey, Y.; Launay, P.; Becq, F.; Bois, P.; Guinamard, R. 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br. J. Pharmacol. 2008, 153, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burris, S.K.; Wang, Q.; Bulley, S.; Neeb, Z.P.; Jaggar, J.H. 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br. J. Pharmacol. 2015, 172, 2459–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozhathil, L.C.; Delalande, C.; Bianchi, B.; Nemeth, G.; Kappel, S.; Thomet, U.; Ross-Kaschitza, D.; Simonin, C.; Rubin, M.; Gertsch, J.; et al. Identification of potent and selective small molecule inhibitors of the cation channel TRPM4. Br. J. Pharmacol. 2018, 175, 2504–2519. [Google Scholar] [CrossRef] [Green Version]
- Delalande, C.; Awale, M.; Rubin, M.; Probst, D.; Ozhathil, L.C.; Gertsch, J.; Abriel, H.; Reymond, J.-L. Optimizing TRPM4 inhibitors in the MHFP6 chemical space. Eur. J. Med. Chem. 2019, 166, 167–177. [Google Scholar] [CrossRef]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Baylie, R.L.; Tavares, M.J.; Brayden, J.E. TRPM4 channels couple purinergic receptor mechanoactivation and myogenic tone development in cerebral parenchymal arterioles. J. Cereb. Blood Flow Metab. 2014, 34, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Gerzanich, V.; Woo, S.K.; Vennekens, R.; Tsymbalyuk, O.; Ivanova, S.; Ivanov, A.; Geng, Z.; Chen, Z.; Nilius, B.; Flockerzi, V.; et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat. Med. 2009, 15, 185–191. [Google Scholar] [CrossRef]
- Echeverría, C.; Montorfano, I.; Cabello-Verrugio, C.; Armisén, R.; Varela, D.; Simon, F. Suppression of transient receptor potential melastatin 4 expression promotes conversion of endothelial cells into fibroblasts via transforming growth factor/activin receptor-like kinase 5 pathway. J. Hypertens. 2015, 33, 981–992. [Google Scholar] [CrossRef]
- Dragún, M.; Gažová, A.; Kyselovič, J.; Hulman, M.; Máťuš, M. TRP Channels expression profile in human end-stage heart failure. Medicina 2019, 55, 380. [Google Scholar] [CrossRef] [Green Version]
- Cáceres, M.; Ortiz, L.; Recabarren, T.; Romero, A.; Colombo, A.; Leiva-Salcedo, E.; Varela, D.; Rivas, J.; Silva, I.; Morales, D.; et al. TRPM4 is a novel component of the adhesome required for focal adhesion disassembly, migration and contractility. PLoS ONE 2015, 10, e0130540. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A.; Echeverría, C.; Varela, D.; Sarmiento, D.; Armisén, R.; Nuñez-Villena, F.; Montecinos, M.; Simon, F. Transient receptor potential melastatin 4 inhibition prevents lipopolysaccharide-induced endothelial cell death. Cardiovasc. Res. 2011, 91, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmiento, D.; Montorfano, I.; Cerda, O.; Cáceres, M.; Becerra, A.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Tapia, P.; Velásquez, L.A.; et al. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc. Res. 2015, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Leiva-Salcedo, E.; Armisén, R.; Riveros, A.; Cerda, O.; Varela, D.; Eguiguren, A.L.; Olivero, P.; Stutzin, A. Hydrogen peroxide removes TRPM4 current desensitization conferring increased vulnerability to necrotic cell death. J. Biol. Chem. 2010, 285, 37150–37158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Owsianik, G.; Freichel, M.; Flockerzi, V.; Nilius, B.; Vennekens, R. TRPM4 regulates migration of mast cells in mice. Cell Calcium 2009, 45, 226–232. [Google Scholar] [CrossRef]
- Vennekens, R.; Olausson, J.; Meissner, M.; Bloch, W.; Mathar, I.; Philipp, S.E.; Schmitz, F.; Weissgerber, P.; Nilius, B.; Flockerzi, V.; et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 2007, 8, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Léger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.-P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef] [Green Version]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.-P. TRPM4 regulates calcium oscillations after T cell activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.S.; Hildner, K.; Murphy, K.M.; Allen, P.M. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J. Immunol. 2010, 185, 2836–2846. [Google Scholar] [CrossRef]
- Serafini, N.; Dahdah, A.; Barbet, G.; Demion, M.; Attout, T.; Gautier, G.; Arcos-Fajardo, M.; Souchet, H.; Jouvin, M.-H.; Vrtovsnik, F.; et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J. Immunol. 2012, 189, 3689–3699. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Wang, S.; Nichols, C.G. On potential interactions between non-selective cation channel TRPM4 and sulfonylurea receptor SUR1. J. Biol. Chem. 2012, 287, 8746–8756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.K.; Kwon, M.S.; Ivanov, A.; Gerzanich, V.; Simard, J.M. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J. Biol. Chem. 2013, 288, 3655–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.I.; Tosun, C.; Ivanova, S.; Tsymbalyuk, N.; Famakin, B.M.; Kwon, M.S.; Castellani, R.J.; Gerzanich, V.; Simard, J.M. Sur1-Trpm4 cation channel expression in human cerebral infarcts. J. Neuropathol. Exp. Neurol. 2015, 74, 835–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinamard, R.; Demion, M.; Magaud, C.; Potreau, D.; Bois, P. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 2006, 48, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demion, M.; Thireau, J.; Gueffier, M.; Finan, A.; Khoueiry, Z.; Cassan, C.; Serafini, N.; Aimond, F.; Granier, M.; Pasquié, J.-L.; et al. Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS ONE 2014, 9, e115256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kecskés, M.; Jacobs, G.; Kerselaers, S.; Syam, N.; Menigoz, A.; Vangheluwe, P.; Freichel, M.; Flockerzi, V.; Voets, T.; Vennekens, R. The Ca2+-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy. Basic Res. Cardiol. 2015, 110, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Takahashi, K.; Piao, H.; Qu, P.; Naruse, K. 9-Phenanthrol, a TRPM4 inhibitor, protects isolated rat hearts from ischemia-reperfusion injury. PLoS ONE 2013, 8, e70587. [Google Scholar] [CrossRef] [Green Version]
- Frede, W.; Medert, R.; Poth, T.; Gorenflo, M.; Vennekens, R.; Freichel, M.; Uhl, S. TRPM4 modulates right ventricular remodeling under pressure load accompanied with decreased expression level. J. Card. Fail. 2020, 26, 599–609. [Google Scholar] [CrossRef]
- Armisén, R.; Marcelain, K.; Simon, F.; Tapia, J.C.; Toro, J.; Quest, A.F.G.; Stutzin, A. TRPM4 enhances cell proliferation through up-regulation of the β-catenin signaling pathway. J. Cell. Physiol. 2011, 226, 103–109. [Google Scholar] [CrossRef]
- Holzmann, C.; Kappel, S.; Kilch, T.; Jochum, M.M.; Urban, S.K.; Jung, V.; Stöckle, M.; Rother, K.; Greiner, M.; Peinelt, C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget 2015, 6, 41783–41793. [Google Scholar] [CrossRef]
- Sagredo, A.I.; Sagredo, E.A.; Cappelli, C.; Báez, P.; Andaur, R.E.; Blanco, C.; Tapia, J.C.; Echeverría, C.; Cerda, O.; Stutzin, A.; et al. TRPM4 regulates Akt/GSK3-β activity and enhances β-catenin signaling and cell proliferation in prostate cancer cells. Mol. Oncol. 2018, 12, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagredo, A.I.; Sagredo, E.A.; Pola, V.; Echeverría, C.; Andaur, R.; Michea, L.; Stutzin, A.; Simon, F.; Marcelain, K.; Armisén, R. TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J. Cell. Physiol. 2019, 234, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Hussain, F.A. TRPM4 is overexpressed in breast cancer associated with estrogen response and epithelial-mesenchymal transition gene sets. PLoS ONE 2020, 15, e0233884. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.; Díaz, N.; Silva, I.; Morales, D.; Lavanderos, B.; Álvarez, A.; Saldías, M.P.; Pulgar, E.; Cruz, P.; Maureira, D.; et al. KCTD5, a novel TRPM4-regulatory protein required for cell migration as a new predictor for breast cancer prognosis. FASEB J. 2020, 34, 7847–7865. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Yu, J.-J. MicroRNA-150 suppresses epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer through the TRPM4-mediated β-catenin signaling pathway. Am. J. Physiol. Cell Physiol. 2019, 316, C463–C480. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Bhardwaj, A.; Singh, S.; Arora, S.; Wang, B.; Grizzle, W.E.; Singh, A.P. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis 2011, 32, 1832–1839. [Google Scholar] [CrossRef]
- Fan, H.; Liu, X.; Zheng, W.-W.; Zhuang, Z.-H.; Wang, C.-D. MiR-150 alleviates EMT and cell invasion of colorectal cancer through targeting Gli1. Eur. Rev. Med. Pharm. Sci. 2017, 21, 4853–4859. [Google Scholar]
- Blanco, C.; Morales, D.; Mogollones, I.; Vergara-Jaque, A.; Vargas, C.; Álvarez, A.; Riquelme, D.; Leiva-Salcedo, E.; González, W.; Morales, D.; et al. EB1- and EB2-dependent anterograde trafficking of TRPM4 regulates focal adhesion turnover and cell invasion. FASEB J. 2019, 33, 9434–9452. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Suzuki, A.; Koga, K.; Miyamoto, C.; Maehata, Y.; Ozawa, S.; Hata, R.-I.; Nagashima, Y.; Nabeshima, K.; Miyazaki, K.; et al. TRPM5 mediates acidic extracellular pH signaling and TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6 melanoma cells. Oncotarget 2017, 8, 78312–78326. [Google Scholar] [CrossRef]
- Palmer, R.K.; Atwal, K.; Bakaj, I.; Carlucci-Derbyshire, S.; Buber, M.T.; Cerne, R.; Cortés, R.Y.; Devantier, H.R.; Jorgensen, V.; Pawlyk, A.; et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev. Technol. 2010, 8, 703–713. [Google Scholar] [CrossRef]
- Han, S.; Koo, H.H.; Lan, Q.; Lee, K.-M.; Park, A.K.; Park, S.K.; Sung, H.; Ahn, H.S.; Shin, H.Y.; Kang, H.J.; et al. Common variation in genes related to immune response and risk of childhood leukemia. Hum. Immunol. 2012, 73, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, C.; Müssig, K.; Heni, M.; Dudziak, K.; Randrianarisoa, E.; Wagner, R.; Machicao, F.; Stefan, N.; Holst, J.J.; Fritsche, A.; et al. Genetic variation within the TRPM5 locus associates with prediabetic phenotypes in subjects at increased risk for type 2 diabetes. Metabolism 2011, 60, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Brixel, L.R.; Monteilh-Zoller, M.K.; Ingenbrandt, C.S.; Fleig, A.; Penner, R.; Enklaar, T.; Zabel, B.U.; Prawitt, D. TRPM5 regulates glucose-stimulated insulin secretion. Pflüg. Arch. 2010, 460, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendinning, J.I.; Gillman, J.; Zamer, H.; Margolskee, R.F.; Sclafani, A. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol. Behav. 2012, 107, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, M.H.; Håkansson, P.; Jansen, F.P.; Magnell, K.; Brodin, P. Ablation of TRPM5 in mice results in reduced body weight gain and improved glucose tolerance and protects from excessive consumption of sweet palatable food when fed high caloric diets. PLoS ONE 2015, 10, e0138373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colsoul, B.; Jacobs, G.; Philippaert, K.; Owsianik, G.; Segal, A.; Nilius, B.; Voets, T.; Schuit, F.; Vennekens, R. Insulin downregulates the expression of the Ca2+-activated nonselective cation channel TRPM5 in pancreatic islets from leptin-deficient mouse models. Pflüg. Arch. 2014, 466, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wu, H.; Li, Q.; Liao, J.; Gao, P.; Sun, F.; Zhang, H.; Lu, Z.; Wei, X.; He, C.; et al. Impairment of bitter taste sensor transient receptor potential channel M5-mediated aversion aggravates high-salt intake and hypertension. Hypertension 2019, 74, 1021–1032. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y.; He, C.; Gao, P.; Li, Q.; Zhang, H.; Jiang, Y.; Hu, Y.; Wei, X.; Lu, Z.; et al. Activation of the bitter taste sensor TRPM5 prevents high salt-induced cardiovascular dysfunction. Sci. China Life Sci. 2020, 63, 1665–1677. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Pavur, K.S.; Petrov, A.N.; Dorovkov, M.V.; Ryazanov, A.G. Novel type of signaling molecules: Protein kinases covalently linked to ion channels. Mol. Biol. 2001, 35, 271–283. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Topala, C.N.; Groenestege, W.T.; Thébault, S.; van den Berg, D.; Nilius, B.; Hoenderop, J.G.; Bindels, R.J. Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium 2007, 41, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.S.; Hermosura, M.C.; Inabe, K.; Perraud, A.-L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.-P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Matsushita, M.; Nairn, A.C.; Kuriyan, J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol. Cell 2001, 7, 1047–1057. [Google Scholar] [CrossRef]
- Runnels, L.W. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Thébault, S.; Cao, G.; Venselaar, H.; Xi, Q.; Bindels, R.J.M.; Hoenderop, J.G.J. Role of the α-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP. J. Biol. Chem. 2008, 283, 19999–20007. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, C.; Dorovkov, M.V.; Zhao, X.; Davenport, B.J.; Ryazanov, A.G.; Perraud, A.-L. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 2005, 280, 37763–37771. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.C.M.; Droogmans, G.; Bindels, R.J.M.; Hoenderop, J.G.J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Bratesco, D.; Brauchi, S.; Gonzalez-Teuber, V.; Steinberg, X.; Valencia, I.; Colenso, C. The different roles of the channel-kinases TRPM6 and TRPM7. Curr. Med. Chem. 2015, 22, 2943–2953. [Google Scholar] [CrossRef]
- Cahalan, M.D. Channels as enzymes. Nature 2001, 411, 542–543. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Drennan, D.; Ryazanov, A.G. Alpha-kinases: Analysis of the family and comparison with conventional protein kinases. Prog. Biophys. Mol. Biol. 2004, 85, 1–32. [Google Scholar] [CrossRef]
- Matsushita, M.; Kozak, J.A.; Shimizu, Y.; McLachlin, D.T.; Yamaguchi, H.; Wei, F.-Y.; Tomizawa, K.; Matsui, H.; Chait, B.T.; Cahalan, M.D.; et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J. Biol. Chem. 2005, 280, 20793–20803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS ONE 2008, 3, e1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijenhuis, T.; Vallon, V.; van der Kemp, A.W.C.M.; Loffing, J.; Hoenderop, J.G.J.; Bindels, R.J.M. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J. Clin. Investig. 2005, 115, 1651–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 Channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yu, H.; Huang, J.; Faouzi, M.; Schmitz, C.; Penner, R.; Fleig, A. The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J. Biol. Chem. 2014, 289, 5217–5227. [Google Scholar] [CrossRef] [Green Version]
- Ferioli, S.; Zierler, S.; Zaißerer, J.; Schredelseker, J.; Gudermann, T.; Chubanov, V. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci. Rep. 2017, 7, 8806. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Du, J.; Yang, W.; Chen, H.-C.; Overton, J.D.; Runnels, L.W.; Yue, L. Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci. Rep. 2011, 1, 146. [Google Scholar] [CrossRef] [Green Version]
- Ikari, A.; Sanada, A.; Okude, C.; Sawada, H.; Yamazaki, Y.; Sugatani, J.; Miwa, M. Up-regulation of TRPM6 transcriptional activity by AP-1 in renal epithelial cells. J. Cell. Physiol. 2010, 222, 481–487. [Google Scholar] [CrossRef]
- Thebault, S.; Alexander, R.T.; Tiel Groenestege, W.M.; Hoenderop, J.G.; Bindels, R.J. EGF Increases TRPM6 activity and surface expression. J. Am. Soc. Nephrol. 2009, 20, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Groenestege, W.M.T.; Thébault, S.; van der Wijst, J.; van den Berg, D.; Janssen, R.; Tejpar, S.; van den Heuvel, L.P.; van Cutsem, E.; Hoenderop, J.G.; Knoers, N.V.; et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Investig. 2007, 117, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, C.; Fujii, N.; Manabe, A.; Matsunaga, T.; Endo, S.; Hasegawa, H.; Ito, Y.; Yamaguchi, M.; Yamazaki, Y.; Ikari, A. Up-regulation of transient receptor potential melastatin 6 channel expression by tumor necrosis factor-α in the presence of epidermal growth factor receptor tyrosine kinase inhibitor: Increase in trpm6 expression by TNF-α. J. Cell. Physiol. 2017, 232, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.V.; Hocher, B.; Verkaart, S.; van Zeeland, F.; Pfab, T.; Slowinski, T.; Chen, Y.-P.; Schlingmann, K.P.; Schaller, A.; Gallati, S.; et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 11324–11329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouras, H.; Roig, S.R.; Kurstjens, S.; Tack, C.J.J.; Kebieche, M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Metformin regulates TRPM6, a potential explanation for magnesium imbalance in type 2 diabetes patients. Can. J. Physiol. Pharmacol. 2020, 98, 400–411. [Google Scholar] [CrossRef]
- Nie, M.; Bal, M.S.; Liu, J.; Yang, Z.; Rivera, C.; Wu, X.-R.; Hoenderop, J.G.J.; Bindels, R.J.M.; Marciano, D.K.; Wolf, M.T.F. Uromodulin regulates renal magnesium homeostasis through the ion channel transient receptor potential melastatin 6 (TRPM6). J. Biol. Chem. 2018, 293, 16488–16502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Baaij, J.H.F.; Blanchard, M.G.; Lavrijsen, M.; Leipziger, J.; Bindels, R.J.M.; Hoenderop, J.G.J. P2X4 receptor regulation of transient receptor potential melastatin type 6 (TRPM6) Mg2+ channels. Pflug. Arch. 2014, 466, 1941–1952. [Google Scholar] [CrossRef]
- Cao, G.; Lee, K.P.; van der Wijst, J.; de Graaf, M.; van der Kemp, A.; Bindels, R.J.M.; Hoenderop, J.G.J. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J. Biol. Chem. 2010, 285, 26081–26087. [Google Scholar] [CrossRef] [Green Version]
- Manabe, A.; Furukawa, C.; Hasegawa, H.; Matsunaga, T.; Endo, S.; Ikari, A. Upregulation of transient receptor potential melastatin 6 channel expression by rosiglitazone and all-trans-retinoic acid in erlotinib-treated renal tubular epithelial cells. J. Cell. Physiol. 2019, 234, 8951–8962. [Google Scholar] [CrossRef]
- Chokshi, R.; Matsushita, M.; Kozak, J.A. Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am. J. Physiol. Cell Physiol. 2012, 302, C1004–C1011. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Inazu, M.; Konishi, M.; Yokoyama, U. Functional expression of TRPM7 as a Ca2+ influx pathway in adipocytes. Physiol. Rep. 2019, 7, e14272. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, R.; Schmitz, C.; Demeuse, P.; Scharenberg, A.M.; Penner, R.; Fleig, A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA 2004, 101, 6009–6014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broertjes, J.; Klarenbeek, J.; Habani, Y.; Langeslag, M.; Jalink, K. TRPM7 residue S1269 mediates cAMP dependence of Ca2+ influx. PLoS ONE 2019, 14, e0209563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaes, N.; Girolami, J.-P. Targeting the ‘Janus face’ of the B2-bradykinin receptor. Expert Opin. Ther. Targets 2013, 17, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kamat, A.; Singh, B.B. Isoproterenol-dependent activation of TRPM7 protects against neurotoxin-induced loss of neuroblastoma cells. Front. Physiol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Murayama, T.; Tashiro, M.; Sakurai, T.; Konishi, M. Mg2+- and ATP-dependent inhibition of transient receptor potential melastatin 7 by oxidative stress. Free Radic. Biol. Med. 2014, 72, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Aarts, M.; Iihara, K.; Wei, W.-L.; Xiong, Z.-G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Sato-Kasai, M.; Kato, T.A.; Ohgidani, M.; Mizoguchi, Y.; Sagata, N.; Inamine, S.; Horikawa, H.; Hayakawa, K.; Shimokawa, N.; Kyuragi, S.; et al. Aripiprazole inhibits polyI:C-induced microglial activation possibly via TRPM7. Schizophr. Res. 2016, 178, 35–43. [Google Scholar] [CrossRef]
- Qin, X.; Yue, Z.; Sun, B.; Yang, W.; Xie, J.; Ni, E.; Feng, Y.; Mahmood, R.; Zhang, Y.; Yue, L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels: SPH and FTY720 are potent TRPM7 blockers. Br. J. Pharmacol. 2013, 168, 1294–1312. [Google Scholar] [CrossRef] [Green Version]
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Schäfer, S.; Linseisen, M.; Sytik, L.; Gudermann, T.; Chubanov, V. Activation of TRPM7 channels by small molecules under physiological conditions. Pflüg. Arch. 2014, 466, 2177–2189. [Google Scholar] [CrossRef]
- Chubanov, V.; Mittermeier, L.; Gudermann, T. Role of kinase-coupled TRP channels in mineral homeostasis. Pharmacol. Ther. 2018, 184, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Mastrototaro, L.; Cittadini, A.; Schweigel, M. Modulation of TRPM6 and Na+/Mg2+ exchange in mammary epithelial cells in response to variations of magnesium availability. J. Cell. Physiol. 2010, 222, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.; Trapani, V. TRPM6 is essential for magnesium uptake and epithelial cell function in the colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chubanov, V.; Waldegger, S.; y Schnitzler, M.M.; Vitzthum, H.; Sassen, M.C.; Seyberth, H.W.; Konrad, M.; Gudermann, T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 2004, 101, 2894–2899. [Google Scholar] [CrossRef] [Green Version]
- van der Wijst, J.; Blanchard, M.G.; Woodroof, H.I.; Macartney, T.J.; Gourlay, R.; Hoenderop, J.G.; Bindels, R.J.; Alessi, D.R. Kinase and channel activity of TRPM6 are co-ordinated by a dimerization motif and pocket interaction. Biochem. J. 2014, 460, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudenberg-Vrenken, T.E.; Sukinta, A.; van der Kemp, A.W.; Bindels, R.J.M.; Hoenderop, J.G.J. Transient receptor potential melastatin 6 knockout mice are lethal whereas heterozygous deletion results in mild hypomagnesemia. Nephron Physiol. 2011, 117, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. eLife 2016, 5, e20914. [Google Scholar] [CrossRef]
- Komiya, Y.; Bai, Z.; Cai, N.; Lou, L.; Al-Saadi, N.; Mezzacappa, C.; Habas, R.; Runnels, L.W. A nonredundant role for the TRPM6 channel in neural tube closure. Sci. Rep. 2017, 7, 15623. [Google Scholar] [CrossRef] [Green Version]
- Faouzi, M.; Kilch, T.; Horgen, F.D.; Fleig, A.; Penner, R. The TRPM7 channel kinase regulates store-operated calcium entry: TRPM7 modulation of SOCE. J. Physiol. 2017, 595, 3165–3180. [Google Scholar] [CrossRef] [Green Version]
- Brauchi, S.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 8304–8308. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Xiong, Z.-G. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc. Res. 2009, 83, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Milla, P.J.; Aggett, P.J.; Wolff, O.H.; Harries, J.T. Studies in primary hypomagnesaemia: Evidence for defective carrier-mediated small intestinal transport of magnesium. Gut 1979, 20, 1028–1033. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef]
- Coulter, M.; Colvin, C.; Korf, B.; Messiaen, L.; Tuanama, B.; Crowley, M.; Crossman, D.K.; McCormick, K. Hypomagnesemia due to two novel TRPM6 mutations. J. Pediatr. Endocrinol. Metab. 2015, 28, 1373–1378. [Google Scholar] [CrossRef]
- Saraç, M.; Önalan, E.; Bakal, Ü.; Tartar, T.; Aydın, M.; Orman, A.; Tektemur, A.; Taşkın, E.; Erol, F.S.; Kazez, A. Magnesium-permeable TRPM6 polymorphisms in patients with meningomyelocele. SpringerPlus 2016, 5, 1703. [Google Scholar] [CrossRef] [Green Version]
- Kekatpure, M.; Gaur, S.; Dash, G.; Kannan, S. TRPM6 mutation: A novel cause of “reversible” infantile epileptic encephalopathy. Neurol. India 2016, 64, 1037. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chen, S.-J.; Cai, N.-H.; Liu, Z.-H.; Zhang, M.; Wang, P.-C.; Zhao, J.-N. Increased risk of post-stroke epilepsy in Chinese patients with a TRPM6 polymorphism. Neurol. Res. 2019, 41, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Takashina, Y.; Manabe, A.; Hasegawa, H.; Matsunaga, T.; Endo, S.; Ikari, A. Sodium citrate increases expression and flux of Mg2+ transport carriers mediated by activation of MEK/ERK/c-Fos pathway in renal tubular epithelial cells. Nutrients 2018, 10, 1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-T.; Lien, Y.-H.H.; Lai, L.-W.; Chen, J.-B.; Lin, C.-R.; Chen, H.-C. Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int. 2006, 69, 1786–1791. [Google Scholar] [CrossRef] [Green Version]
- Yogi, A.; Callera, G.E.; O’Connor, S.E.; He, Y.; Correa, J.W.; Tostes, R.C.; Mazur, A.; Touyz, R.M. Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J. Hypertens. 2011, 29, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhao, R.; Bai, B.; Wu, Y.; Xu, Y.; Lu, S.; Fang, Y.; Wang, Z.; Maswikiti, E.; Zhou, X.; et al. Identification of key tumorigenesis-related genes and their microRNAs in colon cancer. Oncol. Rep. 2018, 40, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Selvaraj, S.; Varma, A.; Derry, S.; Sahmoun, A.E.; Singh, B.B. Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J. Biol. Chem. 2013, 288, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Cai, J.; Zhan, H.; Situ, J.; Li, W.; Mao, Y.; Luo, Y. Suppression of TRPM7 inhibited hypoxia-induced migration and invasion of androgen-independent prostate cancer cells by enhancing RACK1-mediated degradation of HIF-1 α. Oxidative Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.-F.; Zhang, T.; Hou, X.-M.; Feng, M.-H. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Armuzzi, A.; Castri, F.; Benvenuto, R.; Mangoni, A.; Guidi, L.; Gasbarrini, A.; Rapaccini, G.L.; Wolf, F.I.; Trapani, V. TRPM7 is overexpressed in human IBD-related and sporadic colorectal cancer and correlates with tumor grade. Dig. Liver Dis. 2020, 52, 1188–1194. [Google Scholar] [CrossRef]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Sun, H.-S.; Jackson, M.F.; Martin, L.J.; Jansen, K.; Teves, L.; Cui, H.; Kiyonaka, S.; Mori, Y.; Jones, M.; Forder, J.P.; et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009, 12, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Montorfano, I.; Sarmiento, D.; Becerra, A.; Nuñez-Villena, F.; Figueroa, X.F.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Simon, F. Lipopolysaccharide induces a fibrotic-like phenotype in endothelial cells. J. Cell. Mol. Med. 2013, 17, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Montorfano, I.; Hermosilla, T.; Armisén, R.; Velásquez, L.A.; Cabello-Verrugio, C.; Varela, D.; Simon, F. Endotoxin induces fibrosis in vascular endothelial cells through a mechanism dependent on transient receptor protein melastatin 7 activity. PLoS ONE 2014, 9, e94146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmiento, D.; Montorfano, I.; Cáceres, M.; Echeverría, C.; Fernández, R.; Cabello-Verrugio, C.; Cerda, O.; Tapia, P.; Simon, F. Endotoxin-induced vascular endothelial cell migration is dependent on TLR4/NF-κB pathway, NAD(P)H oxidase activation, and transient receptor potential melastatin 7 calcium channel activity. Int. J. Biochem. Cell Biol. 2014, 55, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gatica, S.; Eltit, F.; Santibanez, J.F.; Varela, D.; Cabello-Verrugio, C.; Simon, F. Expression suppression and activity inhibition of TRPM7 regulate cytokine production and multiple organ dysfunction syndrome during endotoxemia: A new target for sepsis. Curr. Mol. Med. 2019, 19, 547–559. [Google Scholar] [CrossRef]
- Liu, A.; Yang, B. Roles of TRPM7 in Renal Ischemia-Reperfusion Injury. Curr. Protein Pept. Sci. 2019, 20, 777–788. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.-Y.; Li, X.-D.; Yang, S.; Liu, X.-Y.; Yang, C.-W.; Liu, Y.-F. The expression of TRPM7 in serum of patients with sepsis, its influences on inflammatory factors and prognosis, and its diagnostic value. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3926–3932. [Google Scholar] [CrossRef]
- Schappe, M.S.; Szteyn, K.; Stremska, M.E.; Mendu, S.K.; Downs, T.K.; Seegren, P.V.; Mahoney, M.A.; Dixit, S.; Krupa, J.K.; Stipes, E.J.; et al. Chanzyme TRPM7 Mediates the Ca2+ influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity 2018, 48, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Nuñez-Villena, F.; Becerra, A.; Echeverría, C.; Briceño, N.; Porras, O.; Armisén, R.; Varela, D.; Montorfano, I.; Sarmiento, D.; Simon, F. Increased expression of the transient receptor potential melastatin 7 channel is critically involved in lipopolysaccharide-induced reactive oxygen species-mediated neuronal death. Antioxid. Redox Signal. 2011, 15, 2425–2438. [Google Scholar] [CrossRef]
- Suzuki, S.; Penner, R.; Fleig, A. TRPM7 contributes to progressive nephropathy. Sci. Rep. 2020, 10, 2333. [Google Scholar] [CrossRef] [Green Version]
- Turlova, E.; Wong, R.; Xu, B.; Li, F.; Du, L.; Habbous, S.; Horgen, F.D.; Fleig, A.; Feng, Z.-P.; Sun, H.-S. TRPM7 Mediates neuronal cell death upstream of calcium/calmodulin-dependent protein kinase II and calcineurin mechanism in neonatal hypoxic-ischemic brain injury. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Aziz, Q.; Harmer, S.C.; Thayyil, S.; Tinker, A.; Munroe, P.B. Genetic variants in TRPM7 associated with unexplained stillbirth modify ion channel function. Hum. Mol. Genet. 2019, 29, 1797–1807. [Google Scholar] [CrossRef]
- Yang, C.-W.; Liu, H.; Li, X.-D.; Sui, S.-G.; Liu, Y.-F. Salvianolic acid B protects against acute lung injury by decreasing TRPM6 and TRPM7 expressions in a rat model of sepsis. J. Cell. Biochem. 2018, 119, 701–711. [Google Scholar] [CrossRef]
- Callera, G.E.; He, Y.; Yogi, A.; Montezano, A.C.; Paravicini, T.; Yao, G.; Touyz, R.M. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J. Hypertens. 2009, 27, 155–166. [Google Scholar] [CrossRef]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef]
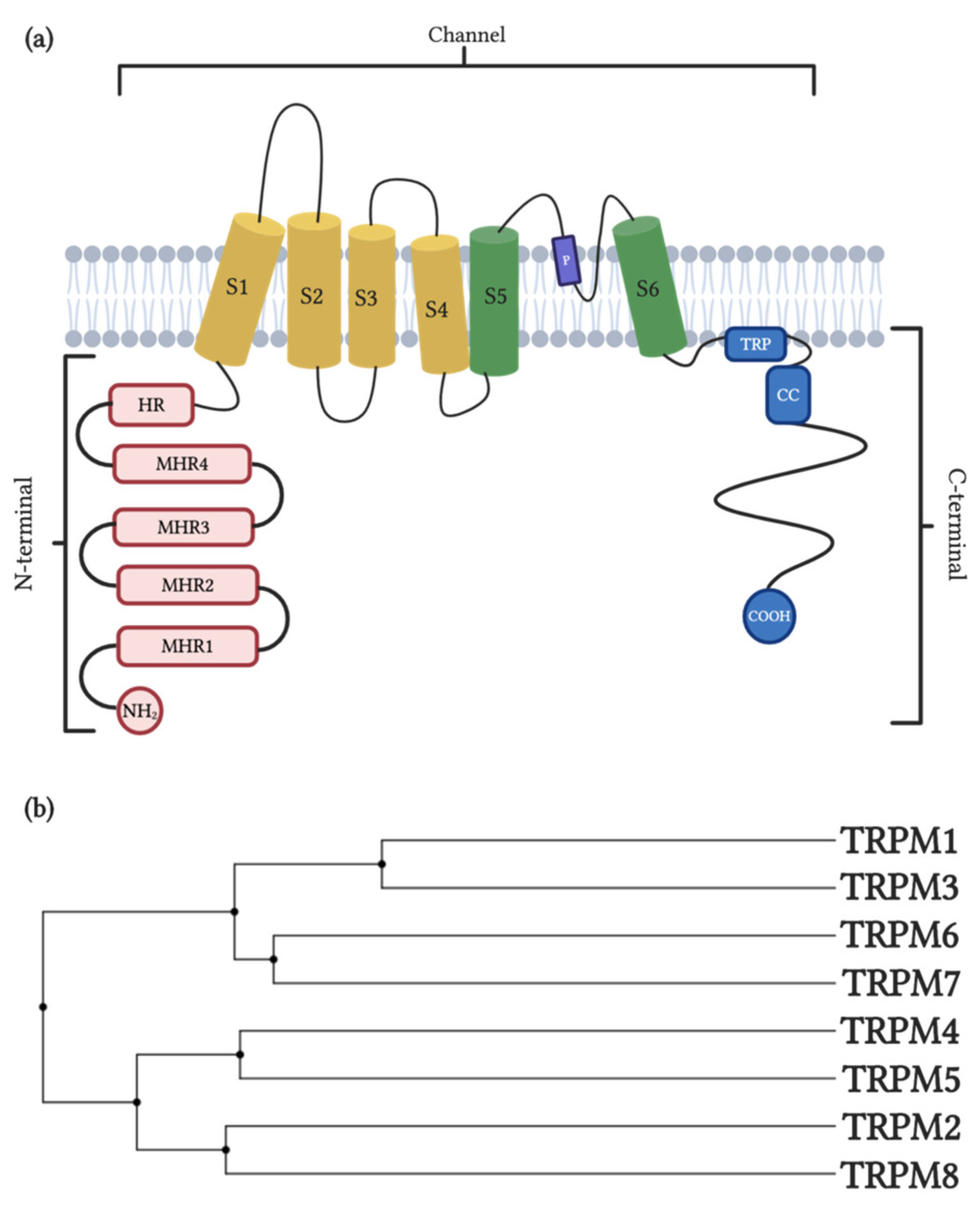

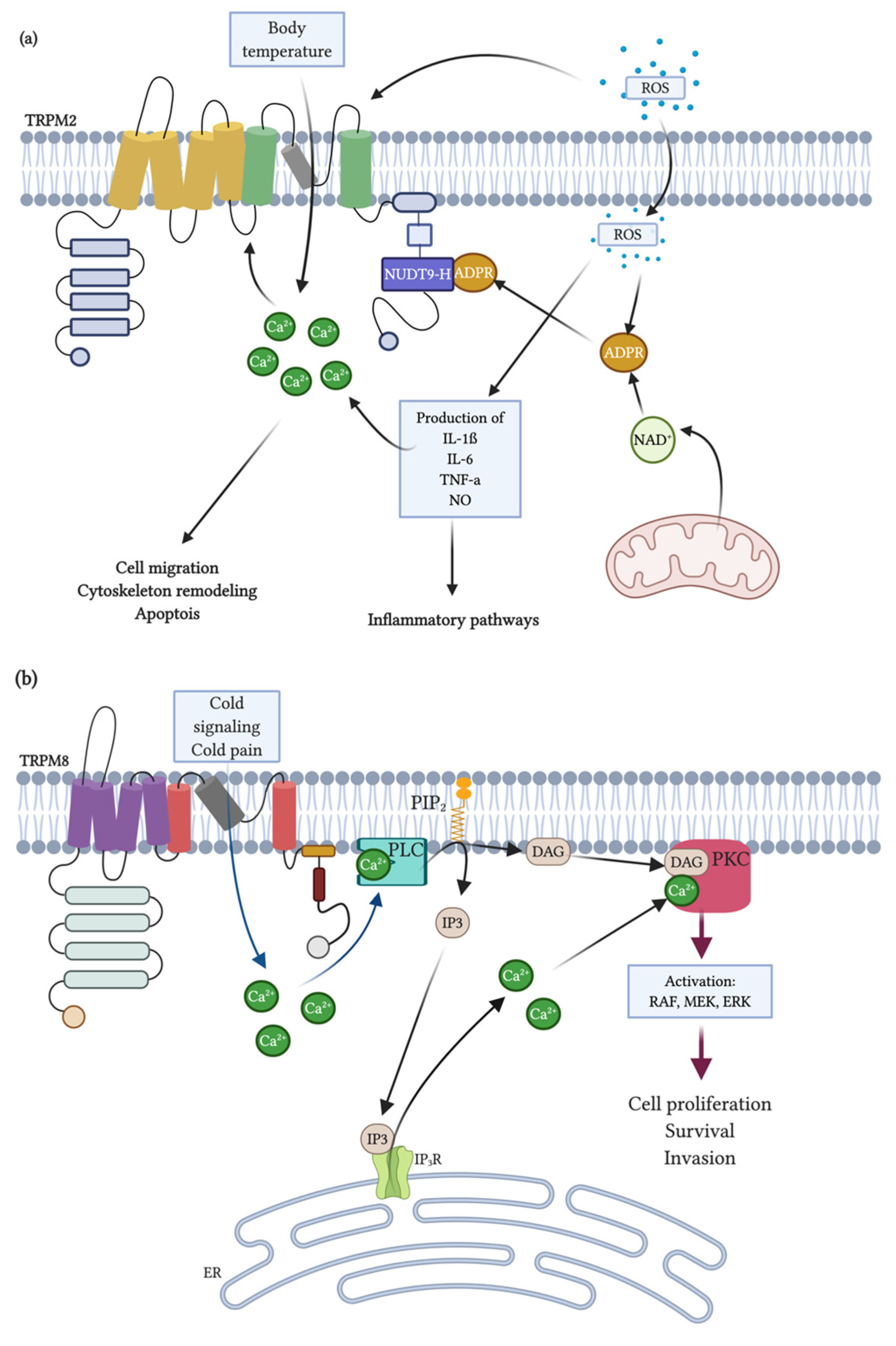
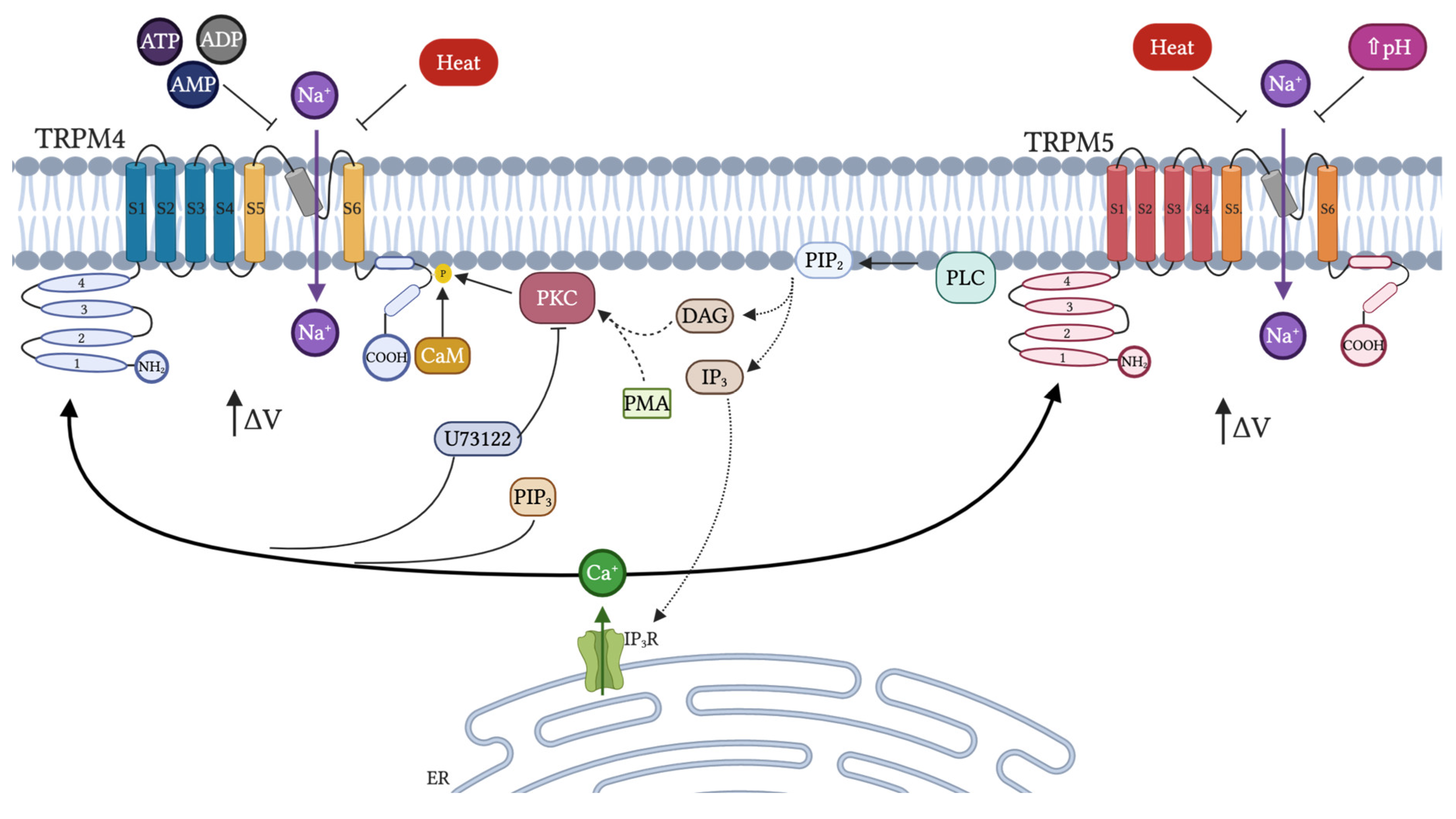

| Channel | Modulator | Inhibition or Activation 1 | Function | Disease, Affection or Objective of Study 2 | Model of Study | Ref. |
|---|---|---|---|---|---|---|
| TRPM1 | Hexamethylene bisacetamide (HMBA) | + | Melanocyte differentiation | Melanoma | Pigmented melanoma cells | [132] |
| Pregnolone sulphate | + | Ca2+ currents | Insulin secretion | HEK-293 cells, Pancreatic | [102] | |
| Capsaicin | + | Field potential | Retinal synaptic plasticity | Mouse model | [133] | |
| Extracellular Zn2+ | - | Ca2+ currents | Insulin secretion | HEK-293 cells, Pancreatic Islets | [102] | |
| Voriconazole | - | ON-bipolar cells in mouse retina | congenital stationary night blindness | ON-bipolar cells of mouse retina | [134] | |
| NED-180 | - | Ca2+ currents | Melanocyte | HEK-293 cells | [135] | |
| TRPM3 | Low intracellular Ca2+ | + | Ca2+ entry | PC | HEK-293 cells | [62] |
| Heat (40 °C) | + | Ca2+ currents | PC | HEK-293 Neurons (DRG, TGN) | [116] | |
| Hypotonicity | + | Channel activation | Kidney function | HEK-293 cells | [66] | |
| Derythro-sphingosine, N,N-dimethyl-d-erythro-sphingosine | + | Ca2+ currents | Channel activation | HEK-293 cells | [85] | |
| Pregonolone sulphate | + | Mg2+ currents | Neuro endotoxin- induces loss of neuron cells | HEK-293 Pancreatic β-cells | [136] | |
| Pregnolone | + | Ca2+ currents | Nociception | HEK-293 Neurons (DRG, TGN), mouse models. | [137] | |
| Dehydroepiandrosterone (DHEA) | + | Ca2+ currents | Nociception | HEK-293 Neurons (DRG, TGN), mouse models. | [137] | |
| Epiallopregnanolone sulfate | + | Ca2+ currents | Insulin secretion | HEK-293, Pancreatic islets | [102] | |
| Intracellular Mg2+ | - | Ca2+ currents | Insulin secretion | Pancreatic β-cells | [64] | |
| La3+ | - | Ca2+ currents | PC | HEK-293 Neurons (DRG, TGN) | [108] | |
| Gd3+ | - | Partial inhibition of Ca2+ entry | Channel activation/inhibition | HEK-293 cells | [107] | |
| Clotrimazole | + | Ca2+ currents | Anoxic neural damage | HEK-293 Neurons (DRG, TGN), pancreatic islet. | [108] | |
| Nifedipine | - | Ca2+, Mg2+ currents and mortality | PC | HEK-293 Pancreatic b-cells | [136] | |
| CIM0216 | + | Ca2+ | Pain and insulin secretion | HEK-293 Neurons (DRG, TGN), pancreatic islet. | [108] | |
| Progesterone | - | Ca2+ current | Cardiovascular function | HEK-293 and Vascular smooth muscle cells | [138] | |
| Cholesterol | - | Ca2+ current | Cardiovascular function | Vascular smooth muscle cells | [97] | |
| Fenamates | - | Ca2+ current and insulin secretion | Pancreatic function | HEK-293, Insulin secreting cells | [139] | |
| Fenamates | - | Ca2+ current and insulin secretion | Pancreatic function | HEK-293, Insulin secreting cells | [139] | |
| Citrus fruit flavonoids | - | Intracellular Ca2+ | Nociception | HEK293, Neurons (DRG) | [140] |
| Channel | Modulator | Inhibition or Activation 1 | Function | Disease, Affection or Objective of Study 2 | Model of Study | Ref. |
|---|---|---|---|---|---|---|
| TRPM2 | ADPR | + | Ca2+ currents | PC | HEK293 cells and Jurkat T lymphocytes | [160,219] |
| ROS | + | Cell death | PC | HEK293 cells | [187,220] | |
| pH | - | Ca2+ currents | PC | HEK293 cells | [221,222] | |
| Curcumin | - | Production of ROS | Hepatic damage | Hooded Wistar rat | [168] | |
| 2-APB | - | Ca2+ currents | PC | HEK293 cells | [169] | |
| Flufenamic acid | - | Ca2+ currents | PC | HEK293 cells | [170] | |
| Imidazoles clotrim-azole and econazole | - | Ca2+ currents | PC | HEK293 and CRI-G1 | [171] | |
| Cacospongiasp. OrganicExtract | - | Ca2+ currents | PC | HEK293 cells | [175] | |
| PJ34 and DPQ | Inhibition of poly (ADP-ribose) polymerase | PC | HEK293 cells | [176] | ||
| TRPM2-knockou and siRNA | - | Ca2+ currents and JNK pathway activation | Renal fibrosis | C57BL/6J mice and HK-2 | [189] | |
| shRNA | - | Production of inflammatory mediators and decreased apoptosis-related protein expressions | Sepsis | Human primary monocytes | [223] | |
| siRNA and Trpm2-deficient mice | - | CXCL2 expression, neutrophil infiltration | Inflammation | Mouse monocytes | [224] | |
| TRPM2-KO | - | Neuronal toxicity and memory impairment | β-amyloid-mediated neuronal toxicity | APP/PS1 mice | [191] | |
| 8-Br-cADPR | - | Inhibition of renal ischemia–reperfusion injury | renal ischemia–reperfusion injury | Wistar rat | [193] | |
| TRPM8 | Cold and Menthol | + | Ca2+ currents | PC | CHO-K1/FRT cells | [141,177] |
| Icilin | + | Ca2+ currents | PC | Trigeminal ganglia from newborn Sprague-Dawley rats | [154,177] | |
| Menthol derivates CPS-368, CPS-369, CPS-125, WS-5 and WS-12 | + | Ca2+ currents | PC | Xenopus oocyte system and channel activity assayed | [180] | |
| 2-(1H-Indol-3-yl)-N-(4-phenoxybenzyl)ethanamine | + | Ca2+ currents | PC | HEK293 | [182] | |
| PIP2 | + | Ca2+ currents | PC | HEK293 and Xenopusoocytes | [225] | |
| N,N-Dibenzyl-2-(1H-indol-3-yl)ethanamine | - | Ca2+ currents | PC | HEK293 | [182] | |
| DFL23693 and DFL23448 | - | Ca2+ currents | Induction of orofacial and neuropathic pain | HEK293, Sprague-Dawley rats | [183] | |
| IGM-18 | - | Ca2+ currents and pain reduction | Induction of orofacial and neuropathic pain | Sprague-Dawley rats | [184,226] | |
| IGM-5 | + | Increase in body temperature | Induction of orofacial and neuropathic pain | Sprague-Dawley rats | [184,226] | |
| TRPM8-KO | - | Sensing temperature | Thermosensation | C57BL/6 mice | [212,214] |
| Channel | Modulator | Inhibition or Activation 1 | Function | Disease, Affection or Objective of Study 2 | Model of Study | Ref. |
|---|---|---|---|---|---|---|
| TRPM4 | PLC > PIP2 > IP3 > Ca2+ rise | + | Channel activation | PC | 293T, HEK 293 and rosetta cells | [41,228,230,232,237] |
| ATP PKC | - + | Ca2+ sensitivity | PC | HEK 293 cells | [228] | |
| ADP, ATP, AMP and adenosine | - | Channel activation | PC | HEK 293 cells | [232,238] | |
| CAM | - | Ca2+ sensitivity, activation and shift the voltage dependence of activation | PC | HEK293 cells | [235] | |
| Heat | + | Temperature- dependent activation curve | Thermal sensitivity of sweet taste | HEK293 cells | [241] | |
| Flufenamic acid and spermine | - | Channel activation | PC | HEK 293 cells | [232,243] | |
| 9-phenanthrol | - | Channel activity | PC | HEK293 cells | [244,245] | |
| Glibenclamide | - | Channel current | PC | HEK293 cells | [246] | |
| CBA | - | Channel current | PC | HEK293 cells | [246,247] | |
| TRPM5 | PLC > PIP2 > Ca2+ rise | + - | Channel activation, desensitization and sensibilization | PC | CHO-K1 or HEK-293 M1, 293T cells | [41,230,232,236] |
| ATP | + | Channel activation | PC | 293T cells | [41,230] | |
| Heat | + | Temperature- dependent activation curve | Thermal sensitivity of sweet taste | HEK293 cells | [241] | |
| pH 6.2–5.9 | - | Channel activation | Taste transduction | HEK293 cells | [242] | |
| Flufenamic acid and spermine | - | Channel activation | PC | HEK 293 cells | [232,243] |
| Channel | Modulator | Inhibition or Activation 1 | Function | Disease, Affection or Objective of Study 2 | Model of Study | Ref. |
|---|---|---|---|---|---|---|
| TRPM6 | PIP2 and PLC activity | + | Mg2+ currents and homeostasis | HSH | HEK-293 cells | [310] |
| Cytosolic Mg2+ levels | - | Mg2+ currents | Hypomagnesemia | Murine models | [306] | |
| EGF/ERK/AP-1 signaling | + | Mg2+ transport and homeostasis | PC | HEK-293 cells | [311,312] | |
| Insulin | + | Mg2+ homeostasis | Glucose tolerance during pregnancy | Murine model | [315] | |
| Metformin | - | Mg2+ homeostasis and TRPM6 expression | Type 2 Diabetes | HEK-293 and hCaco-2 colon cells | [316] | |
| Uromodulin | + | Renal Mg2+ homeostasis | Low-magnesium diet | Murine model | [317] | |
| P2X4 receptor activity | - | Mg2+ transport and homeostasis | PC | Murine model | [318] | |
| MsrB1 | + | Renal Mg2+ homeostasis | Oxidative Stress | HEK-293 cells | [319] | |
| Pharmacological activity | - | Intestinal and renal Mg2+ absorption | PC | HEK-293 cells | [299] | |
| Cytosolic Mg2+ levels | + | Mg2+ currents | Low-magnesium diet | Mammary epithelial cells | [334] | |
| Pharmacological activity | - | Mg2+ currents | PC | Human colon cells | [335] | |
| TRPM6 depletion | - | Neural tube closure | Embryogenesis | Xenopus laevis | [340] | |
| Homozygous deletion | - | Mg2+ homeostasis | Embryonic development | Murine models | [336,338] | |
| TRPM6 mutation | - | Intestinal and kidney Mg2+ homeostasis | HSH | Human DNA | [349] | |
| TRPM6 mutation | - | Mg2+ homeostasis | Hypomagnesemia | Baby human | [350] | |
| TRPM6 polymorphisms | - | Mg2+ and Ca2+ serological concentration | Meningomyelocele | Human serum | [351] | |
| TRPM6 polymorphisms | - | Mg2+ serological concentration | Post-stroke Epilepsy | Human serum | [353] | |
| Colorectal cancer | - | TRPM6 expression | Colorectal cancer | Colon cancer tissue | [357] | |
| Anti-EGFR drugs; GW-9662 and LE135 | +/- | TRPM6 expression and kidney reabsorption | Cancer treatment | NRK-52E cells | [320] | |
| SA-B | - | TRPM6 expression and pro-inflammatory cytokines secretion | ALI during sepsis | Rat model | [375] | |
| TRPM7 | Intracellular pH and Mg2+ | - | Ca2+ currents | PC | HEK-293 and Jurkat T cells | [321] |
| cAMP/PKA signaling | + | Ca2+ currents | PC | HEK-293 cells | [323] | |
| Isoproterenol | + | Mg2+ currents | Neuro endotoxin-induces loss of neuron cells | Neuroblastoma SHSY-5Y cells | [326] | |
| ROS | + | Ca2+ currents | Anoxic neural damage | HEK-293 and Cortical neurons | [328] | |
| ATP | + | Ca2+ currents | PC | CHO-K1 cells | [296] | |
| Mg-ATP and Mg-GTP | - | Ca2+, Mg2+ currents and mortality | PC | HEK-293 and DT-40 B lymphocytes | [294] | |
| S1296 residue from TRPM7 TRP domain by PKA | - | Ca2+ currents | PC | Neuroblastoma cells | [324] | |
| Reduced Mg2+ intracellular, H2O2 and pharmacological activity | + | Ca2+ and Mg2+ currents | Function in adipocytes | Adipocytes | [322] | |
| Kinase domain mutation and H2O2 response | - | Intracellular levels and Mg2+ currents | Oxidative stress | HEK-293 cells and murine models | [327] | |
| Pharmacological activity | + | Ca2+ currents by SOCE and Proliferation | PC | DT-40 B lymphocytes | [341] | |
| Mg-ATP, Gd3+ and pharmacological activity | - | Growth and Proliferation | Low Mg2+ and Ca2+ environment | EC | [343] | |
| Kinase domain deficient TRPM7 channel | - | Mg2+ homeostasis | Embryonic development | Murine model | [345] | |
| Bradykinin | + | Upregulation of proinflammatory proteins and Mg2+ currents | Proinflammatory signaling | Smooth muscle cells | [356,376] | |
| PIP2 hydrolysis and PLC activity | - | Ca2+ and Mg2+ currents | PC | HEK-293T and CHO-K1 cells | [377] | |
| LPS | + | Ca2+ current and endothelial fibrosis | Endotoxic Condition | EC | [364,365] | |
| LPS | + | Endothelial migration | Endotoxic condition | EC | [366] | |
| LPS in plasma | + | Secretion of pro-inflammatory cytokines, metabolic dysfunction and organ failure | Endotoxemia | Rat model | [367] | |
| LPS in plasma | + | Vascular permeability, kidney damage and increased mortality | Endotoxemia | Rat model | [61] | |
| TRPM7 polymorphisms | - | Mg2+ currents | Arrhythmia associated to unexplained stillbirth | HEK-293, CHO-K1 and hiPSC-derived cardiomyocytes cells | [374] | |
| Aripiprazole | - | Ca2+ current | Inflammation | Microglia primary culture from mice models | [329] | |
| Carvacrol, FTY720, Sphingosine, 2-APB | - | Ca2+, Mg2+ currents | PC | HEK-293 and Jurkat T lymphocytes | [321,330,331] | |
| Small drug-like compounds | + | Ca2+ current | PC | HEK-293 cells and murine models | [332] | |
| LPS in plasma | + | TLR4 endocytosis, Ca2+ current, NF-κB and IRF3 transcription and translocation | Endotoxemia | Mouse model | [370] | |
| LPS | + | Neuronal cell death | Endotoxic conditions | Primary hippocampal and PC12 neurons | [371] | |
| Inactive TRPM7-kinase | - | Proinflammatory cytokines secretion, lymphocytes differentiation to Th17 | PC and acute graft-versus-host disease | Mouse model | [362] | |
| siRNA | - | Membrane voltage and vesicle fusion | PC | PC12 cells | [342] | |
| siRNA | - | Neuron survival and memory retention | DND after ischemic injury | CA1 neurons and rat model | [363] | |
| Ca2+/Mg2+ external ratio and hypoxia | + | Ca2+ current, cell proliferation, HIF-1α accumulation and RACK1 phosphorylation | Prostate cancer | Prostate cancer cells | [358,359] | |
| TRPM7 expression | + | Tumor development, morphology and proliferation | Inflammatory bowel disease and CRC | Tumor tissue and colorectal cancer cells | [360,361] | |
| Fibrosis stimulus | + | Kidney atrophy, tubular formation and cell proliferation | Nephropathy | Unilateral ureteral obstruction mouse model | [372] | |
| Hypoxic and ischemic physiopathology | + | Protein expression, brain injury and outcome score | Hypoxic-ischemic encephalopathy | Hypoxic-ischemic brain cell death model | [373] | |
| SA-B | - | TRPM7 expression and pro-inflammatory cytokines secretion | ALI during sepsis | Rat model | [375] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, I.; Prado, Y.; Marchant, F.; Otero, C.; Eltit, F.; Cabello-Verrugio, C.; Cerda, O.; Simon, F. TRPM Channels in Human Diseases. Cells 2020, 9, 2604. https://doi.org/10.3390/cells9122604
Jimenez I, Prado Y, Marchant F, Otero C, Eltit F, Cabello-Verrugio C, Cerda O, Simon F. TRPM Channels in Human Diseases. Cells. 2020; 9(12):2604. https://doi.org/10.3390/cells9122604
Chicago/Turabian StyleJimenez, Ivanka, Yolanda Prado, Felipe Marchant, Carolina Otero, Felipe Eltit, Claudio Cabello-Verrugio, Oscar Cerda, and Felipe Simon. 2020. "TRPM Channels in Human Diseases" Cells 9, no. 12: 2604. https://doi.org/10.3390/cells9122604






