Influence of ARHGAP29 on the Invasion of Mesenchymal-Transformed Breast Cancer Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Generation of Mesenchymal-Transformed MCF-7 and T-47D Cells
2.3. Small Interfering RNA Transfection
2.4. Transwell Co-Culture Invasion Assay
2.5. AlamarBlue Assay
2.6. Western Blot Analysis
2.7. Real-Time Quantitative PCR Analysis
2.8. Data-Based Analysis
2.9. Statistical Analysis
3. Results
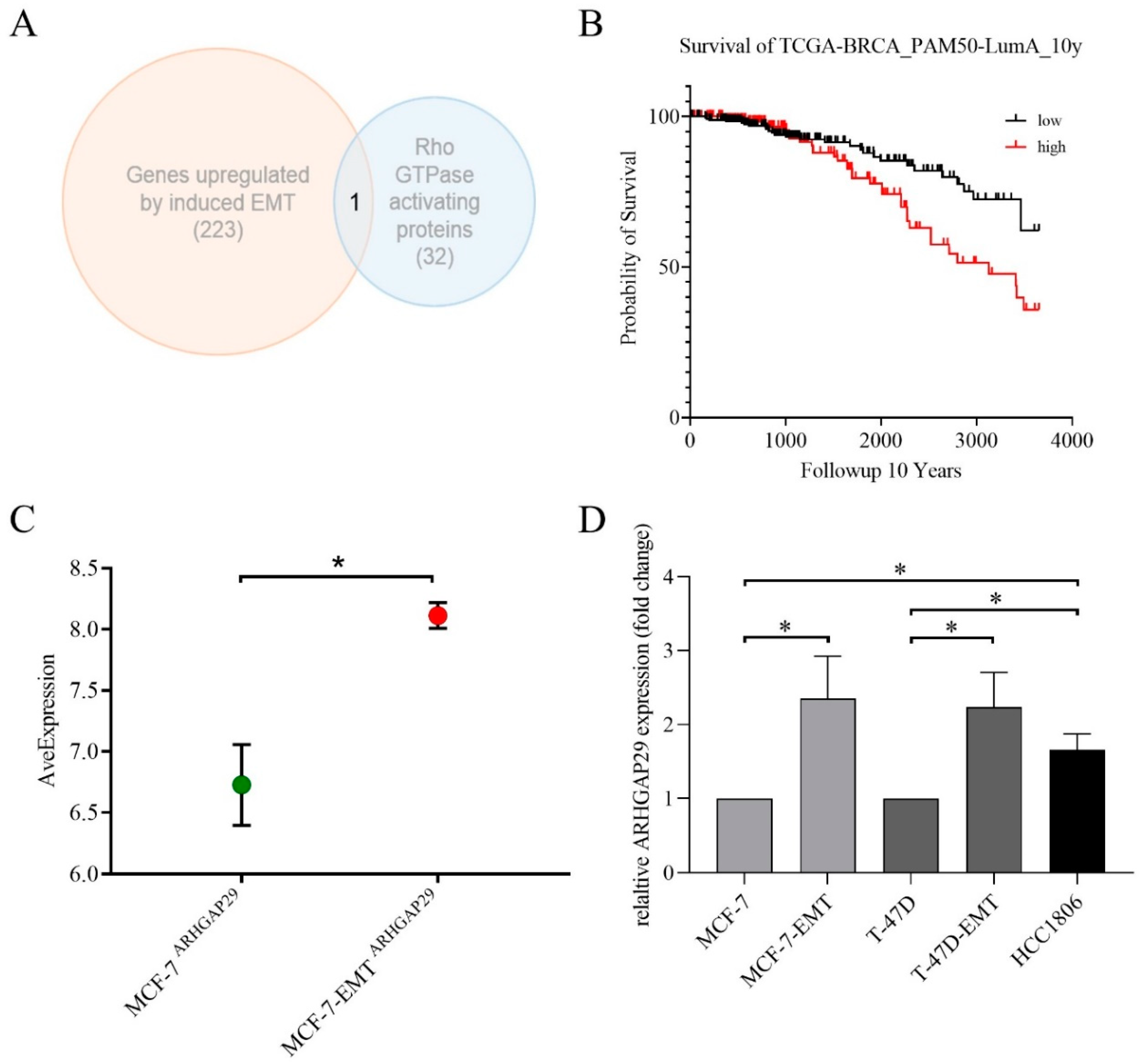
3.1. ARHGAP29 Expression in Mesenchymal-Transformed Breast Cancer Cells
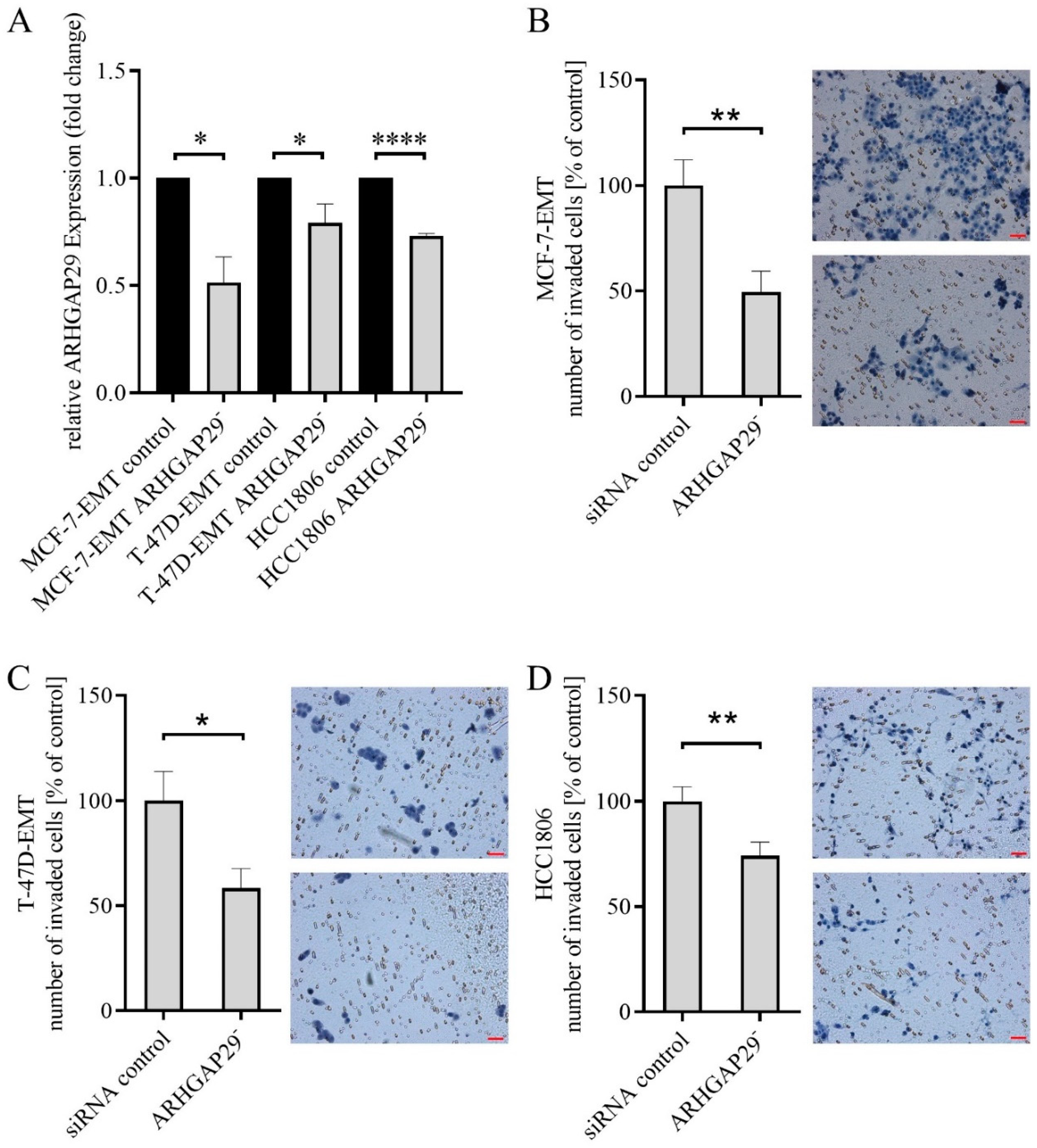
3.2. Reduced Invasion after Knock-Down of ARHGAP29 Expression
3.3. Increased Proliferation after Knock-Down of ARHGAP29 Expression
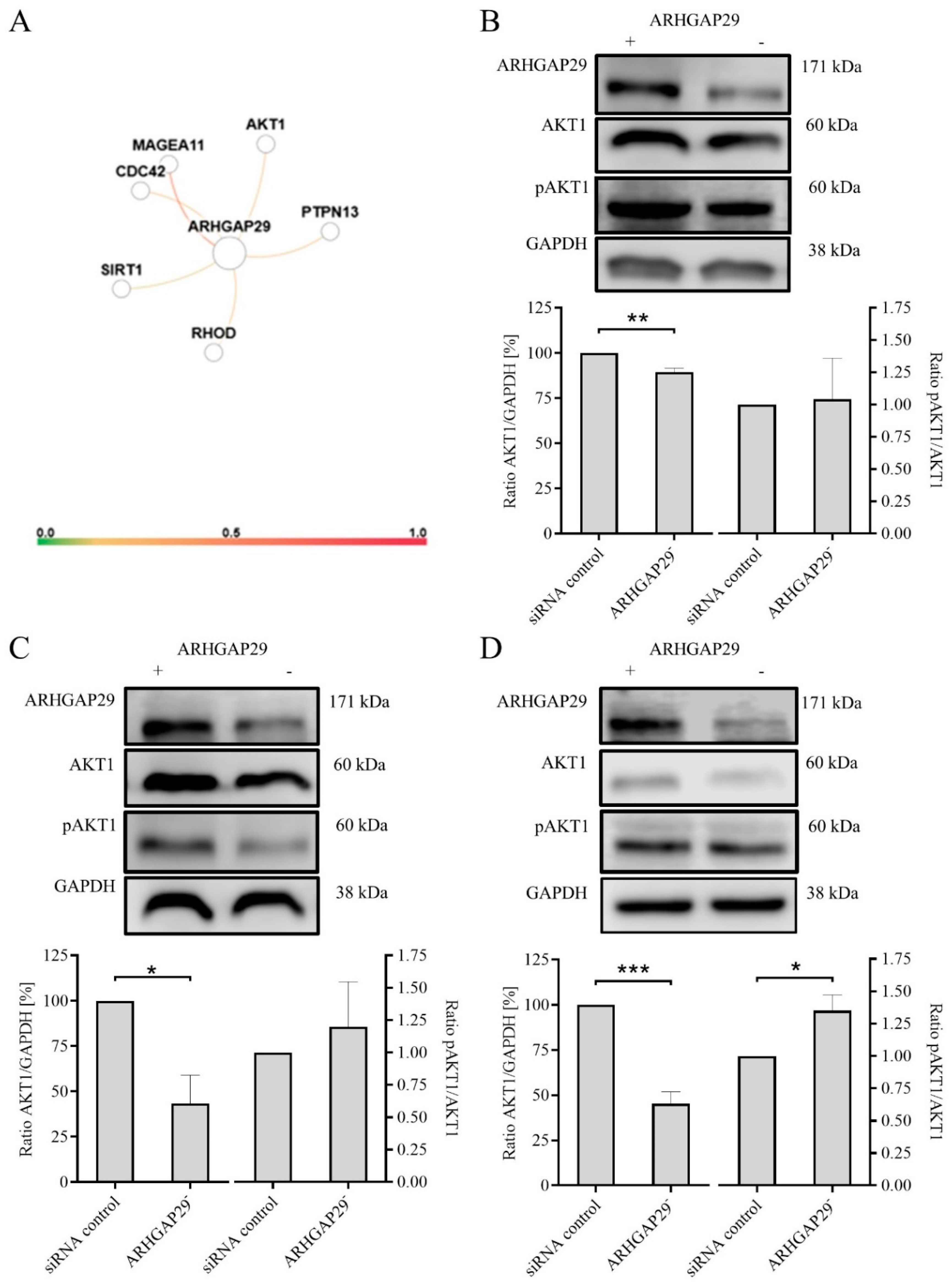
3.4. Decreased Expression of AKT1 after Knock-Down of ARHGAP29 Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing: Petersburg, FL, USA, 2020. [Google Scholar] [PubMed]
- World Health Organization. Cancer Today-Breast Cancer Fact Sheet. The Global Cancer Observatory, International Agency for Research on Cancer. 2019. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 30 July 2020).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. (Eds.) SEER Cancer Statistics Review 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 30 July 2020).
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. Context-specific roles of EMT programmes in cancer cell dissemination. Nat. Cell Biol. 2017, 19, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Saras, J.; Franzén, P.; Aspenström, P.; Hellman, U.; Gonez, L.J.; Heldin, C.-H. A Novel GTPase-activating protein for Rho interacts with a PDZ domain of the protein-tyrosine phosphatase PTPL1. J. Biol. Chem. 1997, 272, 24333–24338. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.C.; Naridze, R.L.; DeMali, K.A.; Lusche, D.F.; Kuhl, S.; Soll, D.R.; Schutte, B.C.; Dunnwald, M. Interferon regulatory factor 6 regulates keratinocyte migration. J. Cell Sci. 2014, 127, 2840–2848. [Google Scholar] [CrossRef]
- Miyazaki, J.; Ito, K.; Fujita, T.; Matsuzaki, Y.; Asano, T.; Hayakawa, M.; Asano, T.; Kawakami, Y. Progression of human renal cell carcinoma via inhibition of RhoA-ROCK axis by PARG1. Transl. Oncol. 2017, 10, 142–152. [Google Scholar] [CrossRef]
- Xu, K.; Sacharidou, A.; Fu, S.; Chong, D.C.; Skaug, B.; Chen, Z.J.; Davis, G.E.; Cleaver, O. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell 2011, 20, 526–539. [Google Scholar] [CrossRef]
- Leinhos, L.; Peters, J.; Krull, S.; Helbig, L.; Vogler, M.; Levay, M.; van Belle, G.J.; Ridley, A.J.; Lutz, S.; Katschinski, D.M.; et al. Hypoxia suppresses myofibroblast differentiation by changing RhoA activity. J. Cell Sci. 2019, 132, jcs223230. [Google Scholar] [CrossRef]
- Post, A.; Pannekoek, W.-J.; Ross, S.H.; Verlaan, I.; Brouwer, P.M.; Bos, J. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc. Natl. Acad. Sci. USA 2013, 110, 11427–11432. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, J.; Lim, Y.B.; Finch-Edmondson, M.L.; Seshachalam, V.P.; Qin, L.; Jiang, T.; Low, B.C.; Singh, H.; Lim, C.T.; et al. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 2017, 19, 1495–1502. [Google Scholar] [CrossRef]
- Tagashira, T.; Fukuda, T.; Miyata, M.; Nakamura, K.; Fujita, H.; Takai, Y.; Hirata, K.-I.; Rikitake, Y. Afadin facilitates vascular endothelial growth factor–induced network formation and migration of vascular endothelial cells by inactivating Rho-associated kinase through ArhGAP29. Arter. Thromb. Vasc. Biol. 2018, 38, 1159–1169. [Google Scholar] [CrossRef]
- Myagmar, B.-E.; Umikawa, M.; Asato, T.; Taira, K.; Oshiro, M.; Hino, A.; Takei, K.; Uezato, H.; Kariya, K.-I. PARG1, a protein-tyrosine phosphatase-associated RhoGAP, as a putative Rap2 effector. Biochem. Biophys. Res. Commun. 2005, 329, 1046–1052. [Google Scholar] [CrossRef]
- Xu, Q.; Duan, H.; Gan, L.; Liu, X.; Chen, F.; Shen, X.; Tang, Y.-Q.; Wang, S. MicroRNA-1291 promotes endometrial fibrosis by regulating the ArhGAP29-RhoA/ROCK1 signaling pathway in a murine model. Mol. Med. Rep. 2017, 16, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Beaudry, C.; McDonough, W.S.; Hoelzinger, D.B.; DeMuth, T.; Ross, K.R.; Berens, T.; Coons, S.W.; Watts, G.; Trent, J.M.; et al. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: A cDNA microarray analysis. J. Neurooncol. 2001, 53, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Lamar, J.; Xiao, Y.; Lamar, J. YAP/TAZ activation as a target for treating metastatic cancer. Cancers 2018, 10, 115. [Google Scholar] [CrossRef]
- Cao, L.; Sun, P.-L.; Yao, M.; Jia, M.; Gao, H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum. Pathol. 2017, 68, 166–174. [Google Scholar] [CrossRef]
- Yu, O.M.; Brown, J.H. G protein–coupled receptor and RhoA-stimulated transcriptional responses: Links to inflammation, differentiation, and cell proliferation. Mol. Pharmacol. 2015, 88, 171–180. [Google Scholar] [CrossRef]
- Moroishi, T.; Hansen, C.G.; Guan, K.-L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef]
- Xia, H.; Dai, X.; Yu, H.; Zhou, S.; Fan, Z.; Wei, G.; Tang, Q.; Gong, Q.; Bi, F. EGFR-PI3K-PDK1 pathway regulates YAP signaling in hepatocellular carcinoma: The mechanism and its implications in targeted therapy. Cell Death Dis. 2018, 9, 269. [Google Scholar] [CrossRef]
- Ziegler, E.; Hansen, M.-T.; Haase, M.; Emons, G.; Gründker, C. Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res. Treat. 2014, 148, 269–277. [Google Scholar] [CrossRef]
- von Alten, J.; Fister, S.; Schulz, H.; Viereck, V.; Frosch, K.-H.; Emons, G.; Gründker, C. GnRH analogs reduce invasiveness of human breast cancer cells. Breast Cancer Res. Treat. 2006, 100, 13–21. [Google Scholar] [CrossRef]
- Xu, S.; Feng, Y.; Zhao, S. Proteins with evolutionarily hypervariable domains are associated with immune response and better survival of basal-like breast cancer patients. Comput. Struct. Biotechnol. J. 2019, 17, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.S.; Krishnan, A.; Wong, A.K.; Ricciotti, E.; Zelaya, R.A.; Himmelstein, D.S.; Zhang, R.; Hartmann, B.M.; Zaslavsky, E.; Sealfon, S.C.; et al. Understanding multicellular function and disease with human tissue-specific networks. Nat. Genet. 2015, 47, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, J.W.; Schömel, F.; Buse, J.V.; Lenz, C.; Bauerschmitz, G.; Emons, G.; Gründker, C. Identification of drivers of breast cancer invasion by secretome analysis: Insight into CTGF signaling. Sci. Rep. 2020, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, J.W.; Hüchel, S.; Goetz, L.; Bauerschmitz, G.; Emons, G.; Gründker, C. Inhibition of CYR61-S100A4 axis limits breast cancer invasion. Front. Oncol. 2019, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Gründker, C.; Bauerschmitz, G.; Schubert, A.; Emons, G. Invasion and increased expression of S100A4 and CYR61 in mesenchymal transformed breast cancer cells is downregulated by GnRH. Int. J. Oncol. 2016, 48, 2713–2721. [Google Scholar] [CrossRef]
- Kalpana, G.; Figy, C.; Yeung, M.; Yeung, K.C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Humphries, B.; Wang, Z.; Li, Y.; Jhan, J.-R.; Jiang, Y.; Yang, C. ARHGAP18 downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Res. 2017, 77, 4051–4064. [Google Scholar] [CrossRef]
- Lawson, C.D.; Fan, C.; Mitin, N.; Baker, N.M.; George, S.D.; Graham, D.M.; Perou, C.M.; Burridge, K.; Der, C.J.; Rossman, K.L. Rho GTPase transcriptome analysis reveals oncogenic roles for Rho GTPase-activating proteins in basal-like breast cancers. Cancer Res. 2016, 76, 3826–3837. [Google Scholar] [CrossRef]
- Simpson, K.J.; Dugan, A.S.; Mercurio, A.M. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004, 64, 8694–8701. [Google Scholar] [CrossRef]
- Chan, C.-H.; Lee, S.-W.; Li, C.-F.; Wang, J.; Yang, W.-L.; Wu, C.-Y.; Wu, J.; Nakayama, K.I.; Kang, H.-Y.; Huang, H.-Y.; et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat. Cell Biol. 2010, 12, 457–467. [Google Scholar] [CrossRef]
- Daubriac, J.; Han, S.; Grahovac, J.; Smith, E.; Hosein, A.; Buchanan, M.; Basik, M.; Boucher, Y. The crosstalk between breast carcinoma-associated fibroblasts and cancer cells promotes RhoA-dependent invasion via IGF-1 and PAI-1. Oncotarget 2017, 9, 10375–10387. [Google Scholar] [CrossRef] [PubMed]
- Pillé, J.-Y.; Denoyelle, C.; Varet, J.; Bertrand, J.-R.; Soria, J.; Opolon, P.; Lu, H.; Pritchard, L.-L.; Vannier, J.-P.; Malvy, C.; et al. Anti-RhoA and Anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 2005, 11, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005, 33, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Wolf, K.; Mazo, I.B.; Leung, H.; Engelke, K.; von Andrian, U.H.; Deryugina, E.I.; Strongin, A.Y.; Bröcker, E.-B.; Friedl, P. Compensation mechanism in tumor cell migration. J. Cell Biol. 2003, 160, 267–277. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, Y.; Yu, H.; Yu, X.; Zhou, J.; Xiao, J. RhoA protects the podocytes against high glucose-induced apoptosis through YAP and plays critical role in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2018, 504, 949–956. [Google Scholar] [CrossRef]
- Liu, D.; Mei, X.; Wang, L.; Yang, X. RhoA inhibits apoptosis and increases proliferation of cultured SPCA1 lung cancer cells. Mol. Med. Rep. 2017, 15, 3963–3968. [Google Scholar] [CrossRef]
- Zhu, S.; Korzh, V.; Gong, Z.; Low, B.C. RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene 2007, 27, 1580–1589. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.-J.; Bao, Q.-C.; Wang, L.; Guo, T.-K.; Chen, W.-L.; Xu, L.-L.; Zhou, H.-S.; Bian, J.-L.; Yang, Y.-R.; et al. NRF2 promotes breast cancer cell proliferation and metastasis by increasing RhoA/ROCK pathway signal transduction. Oncotarget 2016, 7, 73593–73606. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.F.; Jakubowicz, T.; Pitossi, F.J.; Maurer, F.; Hemmings, B.A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 1991, 88, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- López-Knowles, E.; O’Toole, S.A.; McNeil, C.M.; Millar, E.K.A.; Qiu, M.R.; Crea, P.; Daly, R.J.; Musgrove, E.A.; Sutherland, R.L. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int. J. Cancer 2009, 126, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef] [PubMed]
- Kazerounian, S.; Gerald, D.; Huang, M.; Chin, Y.R.; Udayakumar, D.; Zheng, N.; O’Donnell, R.; Perruzzi, C.; Mangiante, L.; Pourat, J.; et al. RhoB differentially controls Akt function in tumor cells and stromal endothelial cells during breast tumorigenesis. Cancer Res. 2012, 73, 50–61. [Google Scholar] [CrossRef]
- Yang, E.; Boire, A.; Agarwal, A.; Nguyen, N.; O’Callaghan, K.; Tu, P.; Kuliopulos, A.; Covic, L. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009, 69, 6223–6231. [Google Scholar] [CrossRef]
- Choi, J.-A.; Jung, Y.S.; Kim, J.Y.; Kim, H.M.; Lim, I.K. Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 2015, 35, 83–93. [Google Scholar] [CrossRef]
- Joglekar, M.; Elbazanti, W.O.; Weitzman, M.D.; Lehman, H.L.; van Golen, K.L. Caveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPase. J. Cell. Biochem. 2015, 116, 923–933. [Google Scholar] [CrossRef]
- Ooms, L.M.; Binge, L.C.; Davies, E.M.; Rahman, P.; Conway, J.R.; Gurung, R.; Ferguson, D.T.; Papa, A.; Fedele, C.G.; Vieusseux, J.L.; et al. The inositol polyphosphate 5-Phosphatase PIPP regulates AKT1-dependent breast cancer growth and metastasis. Cancer Cell 2015, 28, 155–169. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Xu, H.; Yang, Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem. Biophys. Res. Commun. 2016, 469, 189–195. [Google Scholar] [CrossRef]
- Hohensee, I.; Chuang, H.-N.; Grottke, A.; Werner, S.; Schulte, A.; Horn, S.; Lamszus, K.; Bartkowiak, K.; Witzel, I.; Westphal, M.; et al. PTEN mediates the cross talk between breast and glial cells in brain metastases leading to rapid disease progression. Oncotarget 2016, 8, 6155–6168. [Google Scholar] [CrossRef] [PubMed]
- Stambolic, V.; Suzuki, A.; de la Pompa, J.L.; Brothers, G.M.; Mirtsos, C.; Sasaki, T.; Ruland, J.; Penninger, J.M.; Siderovski, D.P.; Mak, T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95, 29–39. [Google Scholar] [CrossRef]
- Huber, M.; Helgason, C.D.; Damen, J.E.; Scheid, M.; Duronio, V.; Liu, L.; Ware, M.D.; Humphries, R.K.; Krystal, G. The role of SHIP in growth factor induced signalling. Prog. Biophys. Mol. Biol. 1999, 71, 423–434. [Google Scholar] [CrossRef]
- Gao, T.; Furnari, F.; Newton, A.C. PHLPP: A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell 2005, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ugi, S.; Imamura, T.; Maegawa, H.; Egawa, K.; Yoshizaki, T.; Shi, K.; Obata, T.; Ebina, Y.; Kashiwagi, A.; Olefsky, J.M. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 2004, 24, 8778–8789. [Google Scholar] [CrossRef]
- Hinz, N.; Jücker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019, 17, 1–29. [Google Scholar] [CrossRef]
- Park, B.K.; Zeng, X.; I Glazer, R. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001, 61, 7647–7653. [Google Scholar]
- Lehman, H.L.; Van Laere, S.J.; van Golen, C.M.; Vermeulen, P.B.; Dirix, L.Y.; van Golen, K.L. Regulation of inflammatory breast cancer cell invasion through Akt1/PKB phosphorylation of RhoC GTPase. Mol. Cancer Res. 2012, 10, 1306–1318. [Google Scholar] [CrossRef]
- Wu, J.-X.; Zhang, D.-G.; Zheng, J.; Pei, D.-S. Rap2a is a novel target gene of p53 and regulates cancer cell migration and invasion. Cell. Signal. 2015, 27, 1198–1207. [Google Scholar] [CrossRef]
- Ju, X.; Katiyar, S.; Wang, C.; Liu, M.; Jiao, X.; Li, S.; Zhou, J.; Turner, J.; Lisanti, M.P.; Russell, R.G.; et al. Akt1 governs breast cancer progression in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 7438–7443. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Nelson, C.M.; Zhang, H.; Fata, J.E.; Roth, R.A.; Bissell, M.J. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. USA 2006, 103, 4134–4139. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolb, K.; Hellinger, J.; Kansy, M.; Wegwitz, F.; Bauerschmitz, G.; Emons, G.; Gründker, C. Influence of ARHGAP29 on the Invasion of Mesenchymal-Transformed Breast Cancer Cells. Cells 2020, 9, 2616. https://doi.org/10.3390/cells9122616
Kolb K, Hellinger J, Kansy M, Wegwitz F, Bauerschmitz G, Emons G, Gründker C. Influence of ARHGAP29 on the Invasion of Mesenchymal-Transformed Breast Cancer Cells. Cells. 2020; 9(12):2616. https://doi.org/10.3390/cells9122616
Chicago/Turabian StyleKolb, Katharina, Johanna Hellinger, Maike Kansy, Florian Wegwitz, Gerd Bauerschmitz, Günter Emons, and Carsten Gründker. 2020. "Influence of ARHGAP29 on the Invasion of Mesenchymal-Transformed Breast Cancer Cells" Cells 9, no. 12: 2616. https://doi.org/10.3390/cells9122616
APA StyleKolb, K., Hellinger, J., Kansy, M., Wegwitz, F., Bauerschmitz, G., Emons, G., & Gründker, C. (2020). Influence of ARHGAP29 on the Invasion of Mesenchymal-Transformed Breast Cancer Cells. Cells, 9(12), 2616. https://doi.org/10.3390/cells9122616






